Abstract
Aim
To investigate urban-rural differences in the distribution of risk factors for breast cancer.
Methods
We analyzed the data from the first round of the “Mamma” population based-screening program conducted in Croatia between 2007 and 2009 and self-reported questionnaire results for 924 patients with histologically verified breast cancer. Reproductive and anthropometric characteristics, family history of breast cancer, history of breast disease, and prior breast screening history were compared between participants from the city of Zagreb (n = 270) and participants from 13 counties with more than 50% of rural inhabitants (n = 654).
Results
The screen-detected breast cancer rate was 4.5 per 1000 mammographies in rural counties and 4.6 in the city of Zagreb, while the participation rate was 61% in rural counties and 59% in Zagreb. Women from Zagreb had significantly more characteristics associated with an increased risk of breast cancer (P < 0.001 in all cases): no pregnancies (15% vs 7%), late age of first pregnancy (≥30 years) (10% vs 4%), and the most recent mammogram conducted 2-3 years ago (32% vs 14%). Women from rural counties were more often obese (41% vs 28%) and had early age of first live birth (<20 years) (20% vs 7%, P < 0.001 for both).
Conclusion
Identification of rural-urban differences in mammography use and their causes at the population level can be useful in designing and implementing interventions targeted at the reduction of inequalities and modifiable risk factors.
Significant differences in breast cancer frequency have been identified in different socioeconomic groups, ethnic groups, and between urban and rural populations (1,2). Living in rural areas may be associated with lower access to health care and mammography screening (3), as well as with late-stage diagnosis (4). This often means that patients need to travel great distances to receive care (5). Blair et al found that people in rural and urban areas were diagnosed with breast cancer at similar stages of the disease, although those from rural communities lacked basic cancer information because they did not have access to cancer education programs offered in urban areas (6). Robbins et al explained the higher breast cancer incidence in the San Francisco Bay Area than in other regions by known risk factors: parity, age at first full-term pregnancy, breast-feeding, age at menarche, and age at menopause (7). In Croatia, Polašek et al found that in a period without a national cancer screening program access to health care was the strongest cancer screening utilization predictor in adult rural population (8).
Risk factors for breast cancer are mostly those related to the reproductive life of women (9,10): menarche, nulliparity or late age at first birth, late menopause, as well as hormonal factors, be they endogenous or exogenous (eg, term use of oral contraceptives or menopausal hormonal replacement). Other risk factors related to hormonal status include obesity and a diet characterized by a high caloric intake, low intake of fruits and vegetables, and lack of physical activity (11). Radiation, in particular during breast development, was also found to be a risk factor (12), while the role of contaminants, such as xenoestrogens and certain pesticides, remains controversial. Four- to 5-fold risk of developing breast cancer was associated with epithelial proliferative lesions, particularly atypical ductal or lobular hyperplasia (11).
In Croatia, breast cancer is the leading cancer among women, amounting to 27% of new female cancer cases; moreover, the incidence rate in 2007 was 17% higher than in the previous year (13). In 2007, cancer incidence by county and age-standardized rates per 100 000 women varied considerably: from 273.1 (Šibensko-kninska county) to 437.7 (the city of Zagreb), but the prevalence of breast cancer risk factors remains unknown. A government-funded mammography screening program was established in October 2006 and has since been implemented in 21 counties, including the city of Zagreb (14).
Population-based screening for breast cancer is conducted through mammographic examination of all women of a specified age at prescribed time intervals. The implementation of population-based screening requires technical resources and trained personnel for double reading of mammograms, as well as a major media campaign (15).
Within a more extensive study of breast cancer risk factors, this study investigated urban-rural differences in reproductive, anthropometric, and family history of breast cancer and personal history of breast disease among women aged 50-69 from 13 rural counties and the city of Zagreb who participated in the first round of population-based mammography screening in Croatia.
Materials and methods
“Mamma” screening program
Organized population-based screening program in Croatia started in November 2006 on a target population of women aged 50-69. Coordinators from the Public Health Institutes from 21 counties distributed the invitations and coordinated the program at the county level. A separate database was formed for each county and the central unit had access to each of these databases through a common server located at the Croatian Ministry of Health and Social Welfare. The program is centrally coordinated by the Croatian National Institute of Public Health and includes 81 mammography units and more than 200 radiologists. Double reading is obligatory; if the result is normal, women are sent a letter of invitation to another routine screening in 2 years. If the result is abnormal, women and their family physicians are informed about the need for further assessment (14).
We used the Organisation for Economic Co-operation and Development definition of rural and urban from the Croatian Rural Development Strategy 2008-2013 of the Croatian Ministry of Agriculture, Fisheries, and Rural Development (16). Rural counties were considered those with a population density of 150 people or fewer per square mile and more than 50% of rural inhabitants (16). Out of 21 counties, we identified 13 rural counties with a total population of 1 926 219 people: Bjelovarsko-bilogorska, Brodsko-posavska, Karlovačka, Koprivničko-križevačka, Krapinsko-zagorska, Ličko-senjska, Požeško-slavonska, Sisačko-moslavačka, Šibensko-kninska, Virovitičko-podravska, Vukovarsko-Srijemska, Zadarska, and Zagrebačka county. From the group of urban counties, we selected the county of city of Zagreb (population: 779 145), which is the county with the highest population density. We excluded 5 counties with fewer than 50% rural inhabitants and Varaždin county due to lack of data (16).
Participants
The first round of the “Mamma” screening program included 80 092 women aged 50-69 from the city of Zagreb and 184 425 from 13 rural counties. Participation in the screening program was free and based on an invitation. Of 204 352 women screened from 2007-2009, 924 were found to have breast cancer. Of these, 270 were from the city of Zagreb and 654 from rural counties: Bjelovarsko-bilogorska, 56; Brodsko-posavska, 64; Karlovačka, 42; Koprivničko-križevačka, 47; Krapinsko-zagorska, 41; Ličko-senjska, 16; Požeško-slavonska, 32; Sisačko-moslavačka, 45; Šibensko-kninska, 31; Virovitičko-podravska, 30; Vukovarsko-srijemska, 96; Zadarska, 70; and Zagrebačka, 84.
Screening was performed in women who lacked breast physical examination abnormalities, including nipple discharge, lumps, or thickening. Women with previous breast cancer and women who had not answered the questions addressing the studied risk factors were excluded.
Questionnaire. Women involved in the program were sent the questionnaire with an invitation letter to their home address. The invitation list was generated based on records of the Croatian Health Insurance Institute and the Ministry of Interior Affairs. The oldest women invited into the program were born between 1937 and 1941. The participants at the time of mammography completed the questionnaire and reported their age, age at menarche, number of pregnancies and deliveries, age at first live-birth, history and duration of breast feeding, use and duration of use of birth control pills (oral contraceptive), use and duration of hormone replacement therapy, menopausal status, age at menopause, personal or family history of breast cancer (defined as having first-degree or second-degree relative with breast cancer), breast symptoms (pain, tenderness, and swelling), breast procedures, weight, height, and the time of their last mammogram. To increase the response rate, reminders were made by telephone and field nurses motivated women to attend the screening.
BMI was calculated as self-reported current weight in kilograms divided by height in meters squared (kg/m2) and divided into three categories: lean weight (BMI≤25), overweight (25<BMI<30), and obesity (BMI≥30) (17).
The patients gave informed consent when they filled in the questionnaire. The study was approved by the Ethics Committee of the Medical School, University of Zagreb.
Statistical analysis
Descriptive analysis and tabulations for all variables were performed using χ2 test with Yates correction when necessary and two-sided t-tests to compare predictive variables for urban-rural differences (18,19). Statistical calculations were performed using Statistica, version 9.0 (StatSoft, Tulsa, OK, USA).
Results
The first round of the population-based screening program included 204 352 women: 146 110 from 13 rural counties and 58 242 from the city of Zagreb (Figure 1, Table 1). We identified 924 women with breast cancer: 654 (0.45%) from rural areas and 270 (0.46%) from Zagreb. The detected cancer rate was 4.5 per 1000 mammographies in rural counties and 4.6 per 1000 mammographies in Zagreb (Table 1).
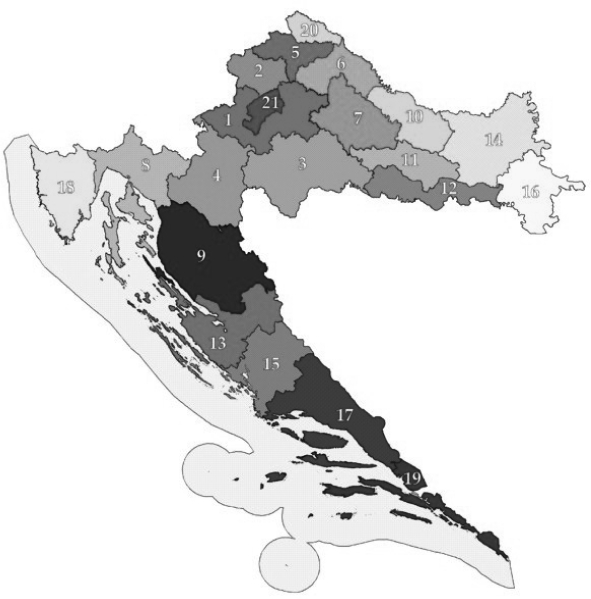
Figure 1.
The map of Croatia with 21 counties. 1 – Zagrebačka; 2 – Krapinsko-zagorska; 3 – Sisačko-moslavačka; 4 – Karlovačka; 5 – Varaždinska (excluded due to lack of data); 6 – Koprivničko-križevačka;7 – Bjelovarsko-bilogorska; 8 – Primorsko-goranska*; 9 – Ličko-senjska; 10 – Virovitičko-podravska; 11 – Požeško-slavonska; 12 – Brodsko-posavska; 13 – Zadarska; 14 – Osiječko-baranjska*; 15 – Šibensko-kninska; 16 – Vukovarsko-srijemska; 17 – Splitsko-dalmatinska*; 18 – Istarska*; 19 – Dubrovačko-neretvanska*; 20 – Međimurska*; 21 – City of Zagreb. Asterisk indicates excluded counties with <50% rural inhabitants.
Table 1.
The rate of screen-detected breast cancer per 1000 mammographies in 13 rural counties and the city of Zagreb in the first round of the “Mamma” screening program (2007-2009)
| County of residence | Participating women, N (%) | Screened women | Screen-detected cancers | Cancer/per 1000 mammographies |
|---|---|---|---|---|
| Rural counties: |
18 4 425 (60.6) |
146 110 |
654 |
4.5 |
| Vukovarsko-srijemska |
18 446 (59.1) |
15 122 |
96 |
6.3 |
| Brodsko-posavska |
14 112 (53.3) |
12 406 |
64 |
5.1 |
| Bjelovarsko-bilogorska |
16 164 (78.1) |
12 061 |
56 |
4.6 |
| Zadarska |
20 906 (69.9) |
15 299 |
70 |
4.6 |
| Zagrebačka |
21 355 (47.0) |
18 203 |
84 |
4.6 |
| Koprivničko-križevačka |
11 642 (63.8) |
10 633 |
47 |
4.4 |
| Požeško-slavonska |
10 312 (74.6) |
7296 |
32 |
4.4 |
| Ličko-senjska |
4880 (47.9) |
3451 |
16 |
4.4 |
| Virovitičko-podravska |
9044 (61.1) |
6938 |
30 |
4.3 |
| Karlovačka |
12 436 (51.2) |
9662 |
42 |
4.3 |
| Krapinsko-zagorska |
13 438 (68.3) |
11 598 |
41 |
3.5 |
| Sisačko-moslavačka |
18 998 (54.9) |
13 712 |
45 |
3.3 |
| Šibensko-kninska |
12 692 (59.2) |
9729 |
31 |
3.2 |
| Urban county |
||||
| City of Zagreb |
80 092 (58.5) |
58 242 |
270 |
4.6 |
| Total | 264 517 (59.6) | 204 352 | 924 | 4.5 |
The screening program involved 66dedicated screening facilities (47 in rural counties and 19 in Zagreb), with specialized equipment and trained staff who performed screening and/or for further assessment in cases when an abnormality was detected. There were only slight rural-urban differences in the number of screening facilities per 10 000 invited women when all rural counties were considered, although there were great differences between individual rural counties, from 0.8 in Brodsko-posavska county to 3.9 in Ličko-senjska county (Table 2).
Table 2.
The number and rate of dedicated screening facilities per 10 000 invited women in 13 rural counties and the city of Zagreb in the “Mamma” screening program
| County of residence | Invited women | Screening facilities | Rate of screening facility per 10 000 invited women |
|---|---|---|---|
| Rural counties: |
310 415 |
47 |
1.6 |
| Ličko-senjska |
10 176 |
4 |
3.9 |
| Zagrebačka |
45 421 |
11 |
2.4 |
| Virovitičko-podravska |
14 779 |
3 |
2.0 |
| Krapinsko-zagorska |
19 657 |
4 |
2.0 |
| Šibensko-kninska |
21 256 |
4 |
1.9 |
| Požeško-slavonska |
13 815 |
2 |
1.4 |
| Sisačko-moslavačka |
34 649 |
4 |
1.2 |
| Koprivničko-križevačka |
18 253 |
2 |
1.1 |
| Zadarska |
29 889 |
3 |
1.0 |
| Bjelovarsko-bilogorska |
20 675 |
2 |
1.0 |
| Vukovarsko-srijemska |
31 113 |
3 |
1.0 |
| Karlovačka |
24 289 |
3 |
1.0 |
| Brodsko-posavska |
26 443 |
2 |
0.8 |
| Urban county |
|||
| City of Zagreb |
136 261 |
19 |
1.4 |
| Total | 446 676 | 66 | 1.5 |
The questionnaires were collected at the time of mammography screening for 913 of the 924 women (99%) (Table 3). Compared with women from rural counties, significantly more women from Zagreb had the characteristics that increased the risk of breast cancer: no pregnancies (15% vs 7%), late age of first pregnancy (≥30-year) (10% vs 4%), and the last mammogram conducted 2-3 years ago (32% vs 14%) (P < 0.001 for all comparisons). Compared with women from Zagreb, women from rural counties were more often obese – BMI>30 (41% vs 28%, respectively) and had early age of first live birth (<20 years) (20% vs 7%, P < 0.001 for both comparisons, Table 3).
Table 3.
Characteristics of women with screen-detected breast cancer who participated in the first round of population-based screening in Croatia, 2007-2009*
| Characteristic |
Breast cancer patients, n (%) |
df |
χ2 |
P† |
|
|---|---|---|---|---|---|
| rural | urban | ||||
| Age at screening, years: |
654 (100.0) |
268 (99.3) |
3 |
0.2487 |
0.969 |
| 50-54 |
91 (13.9) |
40 (14.8) |
|||
| 55-59 |
150 (22.9) |
61 (22.6) |
|||
| 60-64 |
143 (21.9) |
60 (22.2) |
|||
| 65-69 |
270 (41.3) |
107 (39.6) |
|||
| mean age±SD |
58.3 ± 11.4 |
61.2 ± 9.4 |
|||
| Age at menarche, years: |
635 (97.1) |
267 (98.9) |
2 |
6.226 |
0.044 |
| <12 |
38 (5.8) |
26 (9.6) |
|||
| 12-13 |
251 (38.4) |
115 (42.6) |
|||
| ≥14 |
346 (52.9) |
126 (46.7) |
|||
| mean age±SD |
13.7 ± 3.6) |
13.5 ± 3.5 |
|||
| Current menstrual status: |
647 (98.9) |
267 (98.9) |
1 |
0.5584 |
0.455 |
| pre-menopausal |
37 (5.6) |
12 (4.4) |
|||
| peri/postmenopausal |
610 (93.3) |
255 (94.4) |
|||
| Age at menopause, years: |
598 (91.4) |
252 (93.3) |
3 |
4.0369 |
0.257 |
| <45 |
75 (12.3) |
36 (14.1) |
|||
| 45-49 |
159 (26.1) |
57 (22.3) |
|||
| 50-54 |
296 (48.5) |
138 (54.1) |
|||
| ≥55 |
68 (11.1) |
21 (8.2) |
|||
| mean age±SD |
49.0 ± 6.8 |
46.7 ± 6.9 |
|||
| Use of OC (ever): |
652 (99.7) |
267 (98.9) |
1 |
1.2244 |
0.268 |
| no |
538 (82.3) |
212 (78.5) |
|||
| yes |
114 (17.4) |
55 (20.4) |
|||
| Duration of OC use, years: |
114 (17.4) |
53 (20.4) |
2 |
4.5337 |
0.104 |
| <5 |
64 (55.4) |
35 (63.6) |
|||
| 5-9 |
32 (28.6) |
7 (12.7) |
|||
| ≥10 |
18 (16.1) |
11 (20.0) |
|||
| mean months±SD |
52.6 ± 7.9 |
50.4 ± 7.4 |
|||
| Use of HRT (ever): |
652 (99.7) |
267 (98.9) |
1 |
9.5575 |
0.002 |
| no |
593 (90.9) |
224 (83.0) |
|||
| yes |
59 (9.0) |
43 (16.0) |
|||
| Duration of HRT use, years: |
59 |
43 |
2 |
1.0429 |
0.594 |
| <5 |
19 (32.2) |
18 (41.9) |
|||
| 5-9 |
25 (42.4) |
15 (34.9) |
|||
| ≥10 |
15 (25.4) |
10 (23.2) |
|||
| mean months±SD |
78.8 ± 8.3 |
74.7 ± 8.4 |
|||
| Pregnancy history: |
644 (98.5) |
267 (98.9) |
1 |
14.2571 |
<0.001 |
| never pregnant |
45 (6.9) |
40 (14.8) |
|||
| one pregnancy |
113 (17.3) |
43 (15.9) |
|||
| ≥2 pregnancies |
486 (74.3) |
184 (68.1) |
|||
| median, range |
2.0 (1-20) |
2.0 (1-7) |
|||
| Parity: |
640 (97.8) |
267 (98.9) |
2 |
21.6220 |
<0.001 |
| nulliparous |
48 (7.3) |
41 (15.2) |
|||
| uniparous |
134 (20.5) |
74 (27.4) |
|||
| multiparous |
458 (70.0) |
152 (56.3) |
|||
| median (range) |
2.0 (1-7) |
2.0 (1-4) |
|||
| Age at first live birth, years: |
592 (90.5) |
255 (94.4) |
3 |
45.4571 |
<0.001 |
| nulliparous |
48 (7.3) |
41 (15.2) |
|||
| <20 |
132 (20.2) |
20 (7.4) |
|||
| 20-29 |
388 (59.3) |
166 (61.5) |
|||
| ≥30 |
24 (3.7) |
28 (10.4) |
|||
| mean age±SD |
20.4 (4.6) |
24.8 (4.9) |
|||
| Period of breastfeeding, months: |
632 (96.6) |
259 (95.9) |
2 |
11.5929 |
0.003 |
| 0 (include no live birth) |
108 (16.5) |
60 (22.2) |
|||
| ≤12 |
387 (59.2) |
166 (61.5) |
|||
| >12 |
137 (20.9) |
33 (12.2) |
|||
| mean months±SD |
10.8 ± 3.2 |
8.8 ± 3.0 |
|||
| Family history of breast cancer: |
652 (99.7) |
255 (94.4) |
2 |
8.1097 |
0.017 |
| none |
579 (88.5) |
222 (82.2) |
|||
| second degree |
30 (4.6) |
16 (5.9) |
|||
| first degree |
43 (6.6) |
32 (11.8) |
|||
| History of benign disease: |
651 (99.5) |
253 (93.7) |
1 |
7.1417 |
0.007 |
| no |
568 (85.5) |
203 (75.2) |
|||
| yes |
83 (12.7) |
50 (18.5) |
|||
| Body mass index: |
632 (96.6) |
270 (100) |
2 |
21.3414 |
<0.001 |
| <25 |
100 (15.0) |
69 (25.5) |
|||
| 25.0-29.9 |
261 (40.2) |
125 (46.3) |
|||
| ≥30 |
271 (41.4) |
76 (28.1) |
|||
| median weight (kg), range |
78.0 (40-140) |
75.5 (48-115) |
|||
| Height, cm |
630 (96.3) |
270 (100) |
3 |
0.5630 |
0.905 |
| <159 |
151 (23.3) |
67 (28.1) |
|||
| 160-164 |
192 (29.6) |
76 (28.1) |
|||
| 165-169 |
178 (27.1) |
77 (28.5) |
|||
| >170 |
109 (16.2) |
50 (18.4) |
|||
| mean±SD | 163.4 (12.4) | 163.9 (12.8) | |||
*Abbreviations: OC – oral contraceptive; SD – standard deviation; HRT – hormonal replacement therapy; df – degrees of freedom.
†χ2 used to test categorical variables for statistical significance; t-tests were used on continuous variables.
The participation rate was 59% in the city of Zagreb and 61% in rural counties, ranging between 47% in Zagrebačka county and 78% in Bjelovarsko-bilogorska county (Table 1). The number of screened women and reasons for non-compliance with breast cancer screening recommendations varied between urban and rural counties (Table 4) and from county to county (Figure 2).
Table 4.
The number of screened women and reasons for non-compliance with screening program after receiving an invitation, 2007-2009
| No. (%) of women from |
||
|---|---|---|
| rural counties | city of Zagreb | |
| Screened women |
146 110
(47.1) |
58 242
(42.7) |
|
Reasons for non-compliance: |
||
| mammography <12mo |
16 261
(5.2) |
15 673
(11.5) |
| already receiving therapy |
5482
(1.8) |
303
(0.2) |
| other reason |
7851
(2.5) |
1014
(0.7) |
| deceased |
4336
(1.4) |
877
(0.6) |
| temporarily out of place of residence |
7673
(2.5) |
550
(0.4) |
| not-attended |
122 702
(39.5) |
59 602
(43.7) |
| Invited women | 310 415 (100.0) | 136 261 (100.0) |
Figure 2.
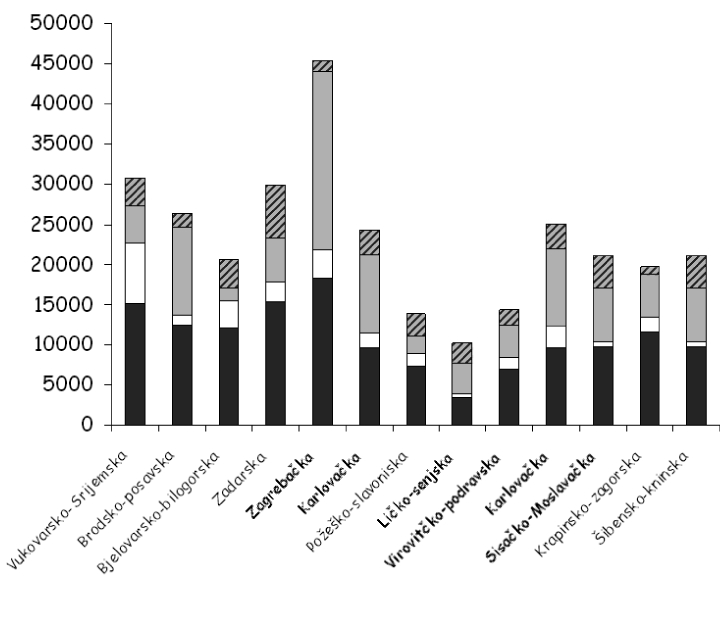
Differences between counties among invited women in the first round of breast cancer screening in Croatia, 2007-2009. Black – number of screened women; gray – women who did not attend screening; white – women who performed mammography in the past 12 months; diagonal lines – deceased women, women with wrong address, and women temporarily out of Croatia.
Women with breast cancer in Zagreb had lower parity than women in rural counties (91% vs 85%; P < 0.001). They also had significantly greater median age of first live birth (25 vs 19 years; P < 0.001) (Table 3).
Most of the women with breast cancer in our study were postmenopausal (93% of rural and 94% of urban women; P = 0.455) (Table 3). There was no urban-rural difference in mean age at menarche (13.7 years in rural and 13.5 years in urban women; P = 0.044) and at menopause (49 years in rural and 47 in urban women; P = 0.257). Fifteen percent of urban women and 7% of rural women had never been pregnant (P < 0.001; Table 3).
More women in rural counties had an average BMI>30 (41% vs 28%; P < 0.001) and there was no difference in height between urban and rural women (163.4 vs 163.9 cm, respectively) (P = 0.905) (Table 4).
Breast feeding was reported by 80% of rural women and 74% of urban women (P = 0.039), and more rural women breast fed for longer than 12 months (21% vs 12%; P = 0.003) (Table 4).
There was no difference in the prevalence of postmenopausal breast cancer patients between rural and urban women (93% vs 94%, respectively; P = 0.455) (Table 3). The history of hormonal replacement therapy use was more common among urban than among rural women (16% vs 9%, respectively; P = 0.003) but there was no difference in the history of oral contraceptive use (20% in urban women vs 17% in rural women; P = 0.338). Significantly more rural women had early age of the first live birth (<20 years) (20% vs 7%; P < 0.001). There was a significant urban-rural difference in having performed a mammogram in the last 2 years (31% vs 14%, respectively; P < 0.001) (Table 5). More urban than rural women had a first-degree relative with breast cancer history (12% vs 7%; P = 0.011), and similar number of women from both groups had a second degree relative with breast cancer history (5% of rural vs 6% of urban women; P = 0.493) (Table 5).
Table 5.
The proportions of breast cancer risk factors among 924 patients with detected breast cancer who participated in the first round of the “Mamma” program
| No. (%) of breast cancer patients |
|||||
|---|---|---|---|---|---|
| Characteristics | rural (n = 654) | urban (n = 270) | Difference (95%confidence interval) | χ2 | P |
| Body mass index >30 (kg/m2) |
271 (41.4) |
76 (28.1) |
13.31 (6.52 to 19.66) |
13.403 |
<0.001 |
| Menarche: |
|||||
| <12 |
38 (5.9) |
26 (9.7) |
3.45 (0.45 to 7.34) |
3.058 |
0.080 |
| ≥14 |
346 (52.9) |
126 (46.5) |
6.30 (-0.77 to 13.37) |
2.778 |
0.095 |
| Parity |
590 (90.3) |
231 (85.6) |
4.76 (-0.01 to 9.53) |
3.928 |
0.047 |
| First live birth <20 y |
132 (20.2) |
20 (7.4) |
12.78 (8.4 to 17.16) |
21.794 |
<0.001 |
| Breast-feeding |
524 (80.1) |
199 (73.7) |
6.42 (0.34 to 12.5) |
4.258 |
0.039 |
| Exogenous hormones: |
|||||
| oral contraceptive use (% ever) |
114 (17.4) |
55 (20.4) |
2.94 (-2.67 to 8.5) |
0.917 |
0.338 |
| hormonal replacement therapy use (% ever) |
59 (9.0) |
43 (15.9) |
6.90 (-2.01 to 11.78) |
8.577 |
0.003 |
| Breast cancer history: |
|||||
| in first-degree relative |
43 (6.5) |
32 (11.8) |
5.28 (0.98 to 9.58) |
6.456 |
0.011 |
| in second-degree relative |
30 (4.6) |
15 (5.9) |
1.34 (-1.9 to 4.58) |
0.470 |
0.493 |
| Prior screening before 2-3 y |
94 (14.4) |
85 (31.5) |
17.11 (10.95 to 23.27) |
34.735 |
<0.001 |
| Breast symptoms | 126 (19.2) | 50 (18.6) | 0.62 (4.92 to 6.16) | 0.016 | 0.899 |
Discussion
Our study investigated urban-rural differences in the distribution of risk factors for breast cancer among women participating in the first round of the population-based screening program in Croatia. We found that women from Zagreb had significantly more characteristics associated with Western lifestyle that increased the risk of breast cancer (20), including no pregnancies, late age of first pregnancy (≥30 years), and mammographic examination in the last 2-3 years, while women from rural counties were more often obese and had younger age of first live birth (<20 years).
Possible explanations for the less frequent use of preventive services in rural counties than in Zagreb include greater distances to medical facilities, less access to services, and lower socioeconomic status (1). Indeed, we found that older age, living temporarily out of the place of residence, and greater distance from health services may be significant barriers to the use of preventive health care services in rural areas. Economic concerns were not relevant to our study, since all women were invited to the free screening regardless of whether they were insured.
We found a similar level of screen-detected breast cancer rate per 1000 mammographies (4.6 vs 4.5) when all rural counties were considered, although our findings, as well as previous cancer incidence data in Croatia (13), showed great variation among rural counties, from 3.2 in Šibensko-kninska county to 6.3 in Vukovarsko-srijemska county.
After the first invitation round, the participation rate in Croatia stabilized at around 60%, which is lower than the 70% rate that the EU Guidelines for Quality Assurance in Mammography Screening recommend as acceptable and the 75% rate that they recommend as desirable (21). The coverage varies considerably from program to program and from country to country (21-23). Attendance is naturally a strong predictor of the program’s impact, and the attendance rate of 61% in rural counties and 59% in Zagreb indicates that the program is well accepted, with no urban-rural gradient in screening participation, but efforts have to be taken to achieve desirable participation rate (14). The participation rate showed a considerable variation between the counties, from 48% in Ličko-senjska county to 78% in Bjelovarsko-bilogorska county. This could be explained by the fact that Ličko-senjska county has more older residents and residents living temporarily out of their permanent place of residence and far from mammography service facilities. Our results are similar to the study by McElroy, who found no significant difference in early detection of cancer between urban and rural communities (1). On the other hand, the Norwegian Breast Cancer Screening program showed that rural areas had a greater attendance rate than Oslo (90% vs 79%), which most probably reflected different access to private mammography services (23). Screening attendance might be affected by false-positive mammography and overdiagnosis in organized mammography screening (24,25).
There are no uniformly accepted definitions of rural and urban areas and this makes the comparison between studies difficult (26). Also, within a single county there are heterogeneous populations and environments, which is likely to mask trends at smaller geographical levels. However, a few studies have evaluated patterns of urban/rural risk even at such levels (27,28). Future studies should examine rural/urban differences in conjunction with other risk factors at different geographical levels, such as neighborhood block, tract, or city (29).
Contrary to our findings, Chelpin et al (23) found a higher coverage in the mixed urban-rural area in Fyn (20%) than in Copenhagen; Thurfjell et al (30) found a lower participation rate in Stockholm than in rural Sweden; and Vizcaino et al (31) found a lower participation rate in Valencia than in Navarra.
On the other hand, Blair et al found substantial differences in the distribution of breast cancer and probable risk factors (parity, age at first full-term pregnancy, breast-feeding, age at menarche, age at menopause, and alcohol consumption) between the urban San Francisco Bay Area and rural regions (6). Increased breast cancer incidence rate in the San Francisco Bay Area could be completely accounted for by regional differences in known risk factors (2). Studies on migration, acculturation, and breast cancer incidence demonstrate that incidence rates increase in women who migrate from low-incidence to high-incidence countries (32).
Our study found a significantly higher proportion of obesity among postmenopausal rural women (BMI≥30), which is in accordance with previous results (33-39). This difference may be explained by a high-fat diet and lower socio-economic status in rural women (40).
A family history of breast cancer has long been recognized as a risk factor for the disease, and the risk of developing breast cancer is increased 1.5- to 3-fold if a woman has mother or sister with breast cancer (41). Our study found a higher proportion of first degree relatives with breast cancer among urban women. These women are at higher risk of breast cancer than general population because of shared genetic factors and possibly because of shared exposures to environmental and lifestyle factors (42). The recent identification of common genetic variants, however, has not heralded the arrival of personalized prevention measures of breast cancer, although it has been recommended that these women undergo annual mammography screening beginning with the age of 40 years (43).
Our findings suggest that mammographic screening has played a major role in the increase in incidence of breast cancer in Croatia (13), but the increase had started well before the screening became widely available (44). The increasing trends observed before 1995 can be attributed to greater disease awareness, greater detection by physical breast examination (either self-examination or examination by physician or a nurse), changes in reproductive factors, increasing use of hormone treatment after menopause, and increasing rates of obesity (39).
Epidemiological studies have consistently identified a number of breast cancer risk factors associated with increased exposure to endogenous estrogens (41,43-47). Our findings suggest that the observed differences between urban and rural women could be substantially reduced by changing the lifestyle, reducing obesity, and promoting breast feeding. It is important to educate the public and health care professionals in order to promote mammography screening (48,49), including the “Mamma” program. Finally, obesity, which increases the risk of many adverse health conditions including breast cancer, needs to be addressed through effective community interventions.
This study has several strengths: population-based design, the response rate of 99%, and availability of information on many established and probable risk factors that may influence breast cancer, with no recall bias. However, one of its limitations is that the questionnaire did not distinguish among types of hormone replacement therapy. Also the data on socioeconomic status, such as education, income, type of occupation, and in some populations, ethnicity, and religion were missing (32). Besides this, the data were self-reported and therefore not verified by objective observers. Future studies should take into account both the women's attitude toward screening and the consistency of women's behavioral pattern (23,50-52). In addition, most research examines rural-urban residence at the time of the diagnosis, but does not examine exposures at critical life stages. Future research should examine residential history to analyze the critical exposures or timing of exposures that lead to greater breast cancer incidence.
In conclusion, our study identified several reproductive and anthropometric risk factors for breast cancer that are modifiable and can reduce inequalities between urban and rural areas. Although there are some effective programs that may reduce some of the preventable risk factors, the availability of these programs may need to be improved in several remote rural areas. Future studies should implement the Gail breast cancer risk prediction model (53,54) to explore how the distribution of established risk factors could explain the high incidence of breast cancer in some counties (Dubrovačko-neretvanska, Zadarska, Primorsko-goranska, and Istarska county) but not in other (Krapinsko-zagorska, Šibensko-kninska, Virovitičko-podravska, and Zagrebačka county). The current county differences in breast cancer incidence may reflect differences in risk factor prevalence but also differences in screening mammography use.
Acknowledgments
The authors thank all coordinators of Public Health Institutes who collaborated with mammographic units and formed a separate database for each county.
Funding: None.
Ethical approval was received from the Ethics Committee of the Zagreb University School of Medicine.
Declaration of authorship: VS worked in the Department for Projects and Programmes in the Ministry of Health and Social Welfare and was a member of the working group that prepared the National Breast Cancer Screening Programme established by the Croatian government in 2006 and is a nominated member of the Committee for the implementation of the program ''Mamma.'' Also this article is a part of her doctoral thesis ''Breast cancer risk factors in population based mammography screening programme of women aged 50-69 in Republic of Croatia'' at the Zagreb University School of Medicine. MS was a leader of working team that prepared the ''National Breast Cancer Screening Programme,'' national coordinator for the implementation of this program at the Croatian National Public Health Institute, and a member of National Committee for the implementation of breast cancer screening program ''Mamma'' at the Ministry of Health and Social Welfare of the Republic of Croatia.
Competing interests: VS has financial support from Ministry of Health and Social Welfare, because she is a member of National Committee for implementation of Breast Cancer Screening Programme ''Mama,'' and is employed at the Ministry of Health and Social Welfare. MS has completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.McElroy JA, Remington PL, Gangnon RE, Hariharan L, Andersen LD. Identifying geographic disparities in the early detection of breast cancer using a geographic information system. Prev Chronic Dis. 2006;3:A10. [PMC free article] [PubMed] [Google Scholar]
- 2.Reynolds P, Hurley SE, Quach AT, Rosen H, Von Behren J, Hertz A, et al. Regional variations in breast cancer incidence among California women, 1988-1997. Cancer Causes Control. 2005;16:139–50. doi: 10.1007/s10552-004-2616-5. [DOI] [PubMed] [Google Scholar]
- 3.Doescher MP, Jackson JE. Trends in cervical and breast cancer screening practices among women in rural and urban areas of the United States (final report). University of Washington. School of Medicine. Final Report, August 2008. Available from: http://depts.washington.edu/uwrhrc/uploads/RHRC_FR121_Doescher.pdf. Accessed: January 17, 2011.
- 4.Hahn KM, Bondy ML, Selvan M, Lund MJ, Liff JM, Flagg EW, et al. Factors associated with advanced disease stage at diagnosis in a population-based study of patients with newly diagnosed breast cancer. Am J Epidemiol. 2007;166:1035–44. doi: 10.1093/aje/kwm177. [DOI] [PubMed] [Google Scholar]
- 5.Liff JM, Chow WH, Greenberg RS. Rural-urban differences in stage at diagnosis. Possible relationship to cancer screening. Cancer. 1991;67:1454–9. doi: 10.1002/1097-0142(19910301)67:5<1454::AID-CNCR2820670533>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 6.Blair SL, Sadler GR, Bristol R, Summers C, Tahar Z, Saltzstein SL. Early cancer detection among rural and urban Californians. BMC Public Health. 2006;6:194. doi: 10.1186/1471-2458-6-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins AS, Brescianini S, Kelsey JL. Regional differences in known risk factors and the higher incidence of breast cancer in San Francisco. J Natl Cancer Inst. 1997;89:960–5. doi: 10.1093/jnci/89.13.960. [DOI] [PubMed] [Google Scholar]
- 8.Polasek O, Kolcic I, Voncina L, Strnad M, Vuletic S, Kern J. Breast, colon, and prostate screening in the adult population of Croatia: does rural origin matter? Rural Remote Health. 2007;7:749. [PubMed] [Google Scholar]
- 9.Rosner B, Colditz GA, Willett WC. Reproductive risk factors in a prospective study of breast cancer: the Nurses' Health Study. Am J Epidemiol. 1994;139:819. doi: 10.1093/oxfordjournals.aje.a117079. [DOI] [PubMed] [Google Scholar]
- 10.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- 11.Autier P, Boffetta P, Boniol M, Boyle P, Ferlay J. Attributable causes of cancer in France in the year 2000. Lyon (France): International Agency for Research on Cancer. IARC press; 2007. [Google Scholar]
- 12.Preston DL, Mattsson A, Holmberg E, Shore R, Hildreth NG, Boice JD., Jr Radiation effects on breast cancer risk: a pooled analysis of eight cohorts. Radiat Res. 2002;158:220–35. doi: 10.1667/0033-7587(2002)158[0220:REOBCR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Cancer incidence in Croatia, Croatian National Cancer Registry. Bulletins No 1-32. Zagreb: Croatian National Institute of Public Health; 2009.
- 14.Croatian Breast Cancer Screening Program. “Mamma.” Ministry of Health and Social Welfare, Republic of Croatia. Available from: http://www.mzss.hr/hr/programi_i_projekti Accessed: January 19, 2011.
- 15.Stewart BW, Kleihues P. International Agency for Research on Cancer. World cancer report. Lyon (France): IARC press; 2003. [Google Scholar]
- 16.Strategy of Rural Development 2008-2013 [in Croatian]. Ministry of Agriculture, Fisheries and Rural Development. Available from: http://www.mps.hr/default.aspx?id=3652. Accessed: January 13, 2011.
- 17.Obesity: preventing and managing the global epidemic. Report of a WHO consultation on obesity. Geneva (Switzerland): World Health Organization; 1988. [PubMed] [Google Scholar]
- 18.dos Santos Silva I. Cancer epidemiology: principles and methods. Lyon (France): IARC press; 1999. [Google Scholar]
- 19.Jekel JF, Elmore JG, Katz DL. Epidemiology, biostatistics and preventive medicine. Philadelphia (PA): WB. Sounders Co; 1996. [Google Scholar]
- 20.Chia KS, Reilly M, Tan CS, Lee J, Pawitan Y, Adami HO, et al. Profound changes in breast cancer incidence may reflect changes into a Westernized lifestyle: a comparative population-based study in Singapore and Sweden. Int J Cancer. 2005;113:302–6. doi: 10.1002/ijc.20561. [DOI] [PubMed] [Google Scholar]
- 21.Broeders M, Nyström L, Ascunce N, Riza E, Becker N, Törnberg S. Epidemiological guidelines for quality assurance in breast cancer screening. In: Perry N, Broeders M, de Wolf C, Törnberg S, Holland R, von Karsa L, et al. European guidelines for quality assurance in breast cancer screening and diagnosis. 4th ed, Luxemburg: European Communities; 2006. p. 15-60. [Google Scholar]
- 22.Wang H, Kĺresen R, Hervik A, Thoresen SŘ. Mammography screening in Norway: results from the first screening round in four counties and cost-effectiveness of a modeled nationwide screening. Cancer Causes Control. 2001;12:39–45. doi: 10.1023/A:1008999403069. [DOI] [PubMed] [Google Scholar]
- 23.von Euler-Chelpin M, Olsen AH, Njor S, Vejborg I, Schwartz W, Lynge E. Women's patterns of participation in mammography screening in Denmark. Eur J Epidemiol. 2006;21:203–9. doi: 10.1007/s10654-006-0002-1. [DOI] [PubMed] [Google Scholar]
- 24.McCann J, Stockton D, Godward S. Impact of false-positive mammography on subsequent screening attendance and risk of cancer. Breast Cancer Res. 2002;4:R11. doi: 10.1186/bcr455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jorgensen KJ, Zahl PH, Gotzsche PC. Overdiagnosis in organised mammography screening in Denmark. A comparative study. BMC Womens Health. 2009;9:36. doi: 10.1186/1472-6874-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall SA, Kaufman JS, Ricketts TC. Defining urban and rural areas in U.S. epidemiologic studies. J Urban Health. 2006;83:162–75. doi: 10.1007/s11524-005-9016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Celaya MO, Berke EM, Onega TL, Gui J, Riddle BL, Cherala SS, et al. Breast cancer stage at diagnosis and geographic access to mammography screening (New Hampshire, 1998-2004). Rural Remote Health. 2010;10:1361. [PMC free article] [PubMed] [Google Scholar]
- 28.Hausauer AK, Keegan TH, Chang ET, Glaser SL, Howe H, Clarke CA. Recent trends in breast cancer incidence in US white women by county-level urban/rural and poverty status. BMC Med. 2009;7:31. doi: 10.1186/1741-7015-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.California Breast Cancer Research Program. Special Research Initiatives. Identifying gaps in breast cancer research: Addressing disparities and the roles of physical and social environment. 2009. Available from: http://www.cbcrp.org/sri/reports/identifyinggaps/gaps_full.pdf. Accessed: January 14, 2011.
- 30.Thurfjell EL, Lindgren JA. Population-based mammography screening in Swedish clinical practice: prevalence and incidence screening in Uppsala County. Radiology. 1994;193:351–7. doi: 10.1148/radiology.193.2.7972742. [DOI] [PubMed] [Google Scholar]
- 31.Vizcaino I, Salas D, Vilar JS, Ruiz-Perales F, Herranz C, Ibanez J. Breast cancer screening: first round in the population-based program in Valencia, Spain. Collaborative Group of Readers of the Breast Cancer Screening Program of the Valencia Community. Radiology. 1998;206:253–60. doi: 10.1148/radiology.206.1.9423680. [DOI] [PubMed] [Google Scholar]
- 32.O'Malley MS, Earp JA, Hawley ST, Schell MJ, Mathews HF, Mitchell J. The association of race/ethnicity, socioeconomic status, and physician recommendation for mammography: who gets the message about breast cancer screening? Am J Public Health. 2001;91:49–54. doi: 10.2105/ajph.91.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vainio H, Bianchin F, editors. Weight control and physical activity IARC handbook on cancer prevention, volume 6. Lyon (France): IARC Press; 2002. [Google Scholar]
- 34.Berclaz G, Li S, Price KN, Coates AS, Castiglione-Gertsch M, Rudenstam CM, et al. Body mass index as a prognostic feature in operable breast cancer: the International Breast Cancer Study Group experience. Ann Oncol. 2004;15:875–84. doi: 10.1093/annonc/mdh222. [DOI] [PubMed] [Google Scholar]
- 35.Huang Z, Hankinson SE, Colditz GA, Stampfer MJ, Hunter DJ, Manson JE, et al. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278:1407–11. doi: 10.1001/jama.278.17.1407. [DOI] [PubMed] [Google Scholar]
- 36.Franceschi S, Favero A, La Vecchia C, Barón AE, Negri E, Dal Maso L, et al. Body size indices and breast cancer risk before and after menopause. Int J Cancer. 1996;67:181–6. doi: 10.1002/(SICI)1097-0215(19960717)67:2<181::AID-IJC5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 37.Potischman N, Swanson CA, Siiteri P, Hoover RN. Reversal of relation between body mass and endogenous estrogen concentrations with menopausal status. J Natl Cancer Inst. 1996;88:756–8. doi: 10.1093/jnci/88.11.756. [DOI] [PubMed] [Google Scholar]
- 38.Hankinson SE, Willett WC, Manson JE, Hunter DJ, Colditz GA, Stampfer MJ, et al. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst. 1995;87:1297–302. doi: 10.1093/jnci/87.17.1297. [DOI] [PubMed] [Google Scholar]
- 39.International Agency for Research on Cancer. IARC – Monographs on the evaluation of carcinogenic risks to humans, volume 96. Lyon (France): IARC Press; 2007. [PMC free article] [PubMed] [Google Scholar]
- 40.Van Loon AJ, Goldbohm RA, Van den Brandt PA. Socioeconomic status and breast cancer incidence: a prospective cohort study. Int J Epidemiol. 1994;23:899–905. doi: 10.1093/ije/23.5.899. [DOI] [PubMed] [Google Scholar]
- 41.Collaborative Group on Hormonal Factors in Breast Cancer Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet. 2001;358:1389–99. doi: 10.1016/S0140-6736(01)06524-2. [DOI] [PubMed] [Google Scholar]
- 42.Wacholder S, Hartge P, Prentice R, Garcia-Closas M, Feigelson HS, Diver WR, et al. Performance of common genetic variants in breast-cancer risk models. N Engl J Med. 2010;362:986. doi: 10.1056/NEJMoa0907727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burstein HJ, Harris JR, Morrow M. Malignant tumors of the breast. In: DeVita VT, Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg’s cancer: principles and practice of oncology, volume 2, 8th ed. Philadelphia (PA): Wolters Kluwer /Lippincott Williams and Wilking. Health; 2008. p:1595-606. [Google Scholar]
- 44.Chiu C, Morrell S, Page A, Rickard M, Brassil A, Taylor R. Population-based mammography screening and breast cancer incidence in New South Wales, Australia. Cancer Causes Control. 2006;17:153–60. doi: 10.1007/s10552-005-2368-x. [DOI] [PubMed] [Google Scholar]
- 45.Beral V, Bull D, Doll R, Peto R, Reeves G, Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and abortion: collaborative reanalysis of data from 53 epidemiological studies, including 83000 women with breast cancer from 16 countries. Lancet. 2004;363:1007–16. doi: 10.1016/S0140-6736(04)15835-2. [DOI] [PubMed] [Google Scholar]
- 46.Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial. JAMA. 2003;289:3243–53. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 47.Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and breast feeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries including 50.302 women with breast cancer and 96.973 without the disease. Lancet. 2002;360:187–95. doi: 10.1016/S0140-6736(02)09454-0. [DOI] [PubMed] [Google Scholar]
- 48.Beral V, Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–27. doi: 10.1016/S0140-6736(03)14596-5. [DOI] [PubMed] [Google Scholar]
- 49.Morimoto LM, White E, Chen Z, Chlebowski RT, Hays J, Kuller L, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women's Health Initiative (United States). Cancer Causes Control. 2002;13:741–51. doi: 10.1023/A:1020239211145. [DOI] [PubMed] [Google Scholar]
- 50.Flynn BS, Gavin P, Worden JK, Ashikaga T, Gautam S, Carpenter J. Community education programs to promote mammography participation in rural New York State. Prev Med. 1997;26:102–8. doi: 10.1006/pmed.1997.0110. [DOI] [PubMed] [Google Scholar]
- 51.Carr WP, Maldonado G, Leonard PR, Halberg JU, Church TR, Mandel JH, et al. Mammogram utilization among farm women. J Rural Health. 1996;12:278–90. doi: 10.1111/j.1748-0361.1996.tb00817.x. [DOI] [PubMed] [Google Scholar]
- 52.Bare ML, Montes J, Florensa R, Sentis M, Donoso L. Factors related to non-participation in a population-based breast cancer screening programme. Eur J Cancer Prev. 2003;12:487–94. doi: 10.1097/00008469-200312000-00007. [DOI] [PubMed] [Google Scholar]
- 53.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–86. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 54.Barlow WE, White E, Ballard-Barbash R, Vacek PM, Titus-Ernstoff L, Carney PA, et al. Prospective breast cancer risk prediction model for women undergoing screening mammography. J Natl Cancer Inst. 2006;98:1204–14. doi: 10.1093/jnci/djj331. [DOI] [PubMed] [Google Scholar]