Abstract
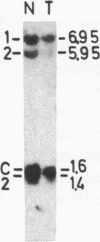
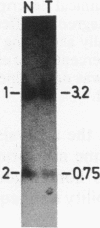
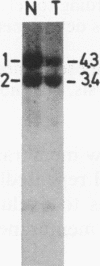
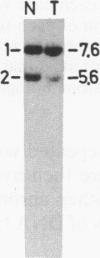
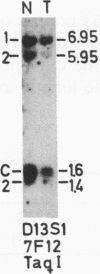
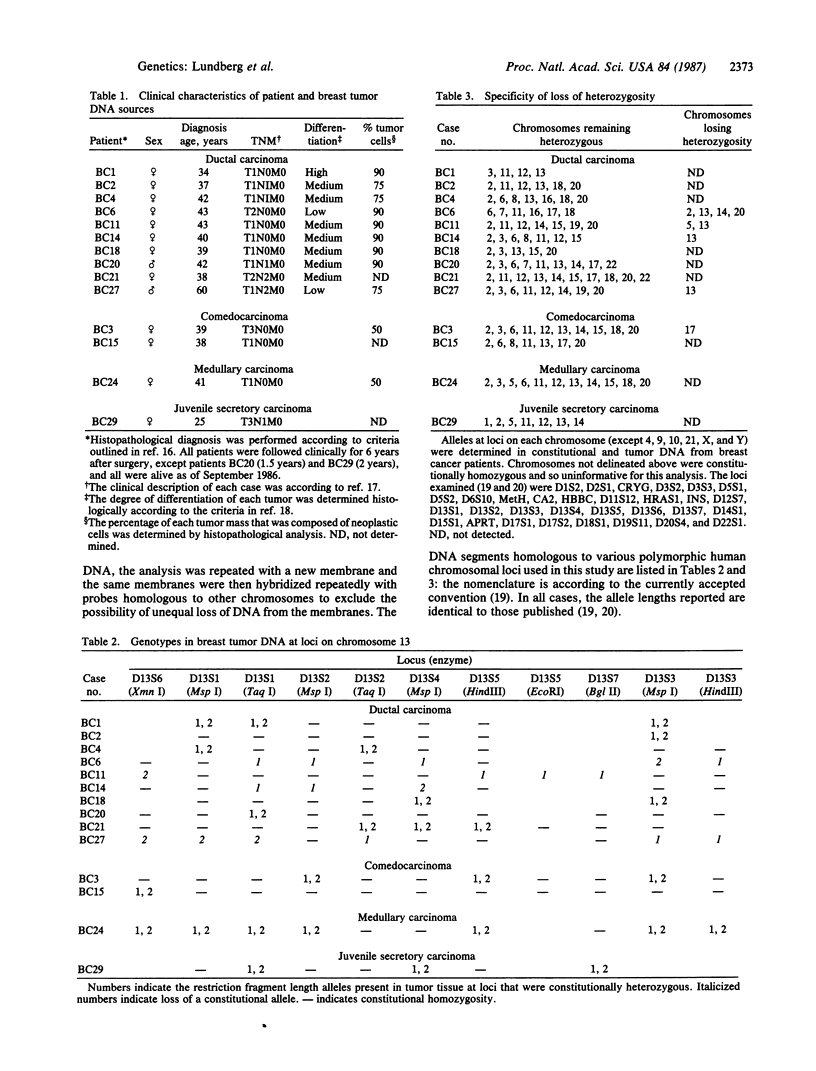
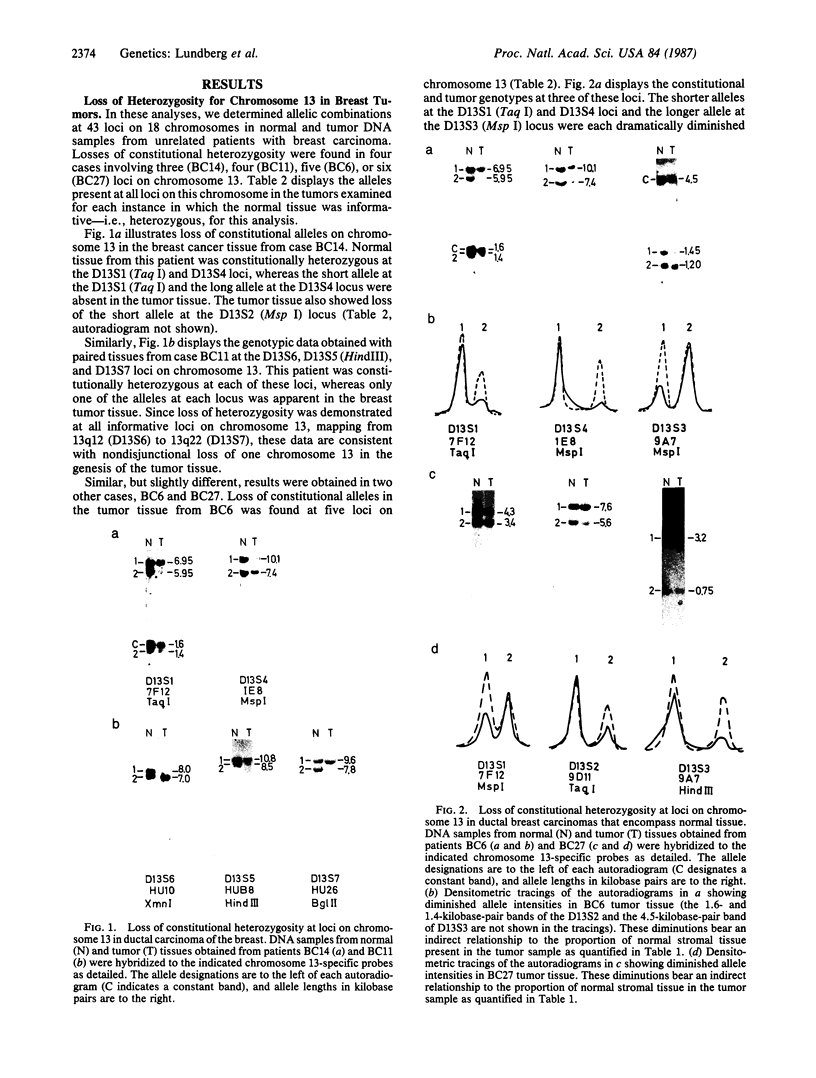
The genotypes at chromosomal loci defined by recombinant DNA probes revealing restriction fragment length polymorphisms were determined in constitutional and tumor tissue from 10 cases of ductal breast cancer: eight premenopausal females and two males. Somatic loss of constitutional heterozygosity was observed at loci on chromosome 13 in primary tumor tissue from three females and one male. In two cases, specific loss of heterozygosity at three distinct genetic loci along the length of the chromosome was observed. In another case, concurrent loss of alleles at loci on chromosomes 2, 13, 14, and 20 was detected, whereas a fourth case showed loss of heterozygosity for chromosomes 5 and 13. In each instance, the data were consistent with loss of one of the homologous chromosomes by mitotic nondisjunction. Analysis of loci on several other chromosomes showed retention of constitutional heterozygosity suggesting the relative specificity of the events. In contrast, similar analyses of other breast cancers, including comedocarcinoma, medullary carcinoma, and juvenile secretory carcinoma, showed no loss of alleles at loci on chromosome 13. These data indicate that the pathogenesis of ductal breast cancer may, in a substantial proportion of cases, involve unmasking of a recessive locus on chromosome 13 and suggest the involvement of such a locus in heritable forms of this disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson D. H., Ellsworth R. M., Kitchin F. D., Tung G. Second nonocular tumors in retinoblastoma survivors. Are they radiation-induced? Ophthalmology. 1984 Nov;91(11):1351–1355. doi: 10.1016/s0161-6420(84)34127-6. [DOI] [PubMed] [Google Scholar]
- Balaban G., Gilbert F., Nichols W., Meadows A. T., Shields J. Abnormalities of chromosome #13 in retinoblastomas from individuals with normal constitutional karyotypes. Cancer Genet Cytogenet. 1982 Jul;6(3):213–221. doi: 10.1016/0165-4608(82)90058-9. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Hansen M. F., Nordenskjold M., Kock E., Maumenee I., Squire J. A., Phillips R. A., Gallie B. L. Genetic origin of mutations predisposing to retinoblastoma. Science. 1985 Apr 26;228(4698):501–503. doi: 10.1126/science.3983638. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Murphree A. L., Shull M. M., Benedict W. F., Sparkes R. S., Kock E., Nordenskjold M. Prediction of familial predisposition to retinoblastoma. N Engl J Med. 1986 May 8;314(19):1201–1207. doi: 10.1056/NEJM198605083141901. [DOI] [PubMed] [Google Scholar]
- Dean M., Park M., Le Beau M. M., Robins T. S., Diaz M. O., Rowley J. D., Blair D. G., Vande Woude G. F. The human met oncogene is related to the tyrosine kinase oncogenes. 1985 Nov 28-Dec 4Nature. 318(6044):385–388. doi: 10.1038/318385a0. [DOI] [PubMed] [Google Scholar]
- Dracopoli N. C., Houghton A. N., Old L. J. Loss of polymorphic restriction fragments in malignant melanoma: implications for tumor heterogeneity. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1470–1474. doi: 10.1073/pnas.82.5.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B., Feinberg A. P. Somatic deletion and duplication of genes on chromosome 11 in Wilms' tumours. Nature. 1984 May 10;309(5964):176–178. doi: 10.1038/309176a0. [DOI] [PubMed] [Google Scholar]
- Hansen M. F., Koufos A., Gallie B. L., Phillips R. A., Fodstad O., Brøgger A., Gedde-Dahl T., Cavenee W. K. Osteosarcoma and retinoblastoma: a shared chromosomal mechanism revealing recessive predisposition. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6216–6220. doi: 10.1073/pnas.82.18.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Copeland N. G., Jenkins N. A., Lampkin B. C., Cavenee W. K. Loss of heterozygosity in three embryonal tumours suggests a common pathogenetic mechanism. Nature. 1985 Jul 25;316(6026):330–334. doi: 10.1038/316330a0. [DOI] [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Lampkin B. C., Workman M. L., Copeland N. G., Jenkins N. A., Cavenee W. K. Loss of alleles at loci on human chromosome 11 during genesis of Wilms' tumour. Nature. 1984 May 10;309(5964):170–172. doi: 10.1038/309170a0. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Albano W. A., Heieck J. J., Mulcahy G. M., Lynch J. F., Layton M. A., Danes B. S. Genetics, biomarkers, and control of breast cancer: a review. Cancer Genet Cytogenet. 1984 Sep;13(1):43–92. doi: 10.1016/0165-4608(84)90087-6. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Goldman D. S., Sallan S. E. Development of homozygosity for chromosome 11p markers in Wilms' tumour. Nature. 1984 May 10;309(5964):172–174. doi: 10.1038/309172a0. [DOI] [PubMed] [Google Scholar]
- Reeve A. E., Housiaux P. J., Gardner R. J., Chewings W. E., Grindley R. M., Millow L. J. Loss of a Harvey ras allele in sporadic Wilms' tumour. Nature. 1984 May 10;309(5964):174–176. doi: 10.1038/309174a0. [DOI] [PubMed] [Google Scholar]
- Rodgers C. S., Hill S. M., Hultén M. A. Cytogenetic analysis in human breast carcinoma. I. Nine cases in the diploid range investigated using direct preparations. Cancer Genet Cytogenet. 1984 Oct;13(2):95–119. doi: 10.1016/0165-4608(84)90052-9. [DOI] [PubMed] [Google Scholar]
- Sotelo-Avila C., Gooch W. M., 3rd Neoplasms associated with the Beckwith-Wiedemann syndrome. Perspect Pediatr Pathol. 1976;3:255–272. [PubMed] [Google Scholar]
- Sparkes R. S., Murphree A. L., Lingua R. W., Sparkes M. C., Field L. L., Funderburk S. J., Benedict W. F. Gene for hereditary retinoblastoma assigned to human chromosome 13 by linkage to esterase D. Science. 1983 Feb 25;219(4587):971–973. doi: 10.1126/science.6823558. [DOI] [PubMed] [Google Scholar]
- Strong L. C., Riccardi V. M., Ferrell R. E., Sparkes R. S. Familial retinoblastoma and chromosome 13 deletion transmitted via an insertional translocation. Science. 1981 Sep 25;213(4515):1501–1503. doi: 10.1126/science.7280668. [DOI] [PubMed] [Google Scholar]
- Trent J. M. Cytogenetic and molecular biologic alterations in human breast cancer: a review. Breast Cancer Res Treat. 1985;5(3):221–229. doi: 10.1007/BF01806017. [DOI] [PubMed] [Google Scholar]
- Vogel F. Genetics of retinoblastoma. Hum Genet. 1979 Nov 1;52(1):1–54. doi: 10.1007/BF00284597. [DOI] [PubMed] [Google Scholar]
- Waziri M., Patil S. R., Hanson J. W., Bartley J. A. Abnormality of chromosome 11 in patients with features of Beckwith-Wiedemann syndrome. J Pediatr. 1983 Jun;102(6):873–876. doi: 10.1016/s0022-3476(83)80014-6. [DOI] [PubMed] [Google Scholar]
- Willard H. F., Skolnick M. H., Pearson P. L., Mandel J. L. Report of the Committee on Human Gene Mapping by Recombinant DNA Techniques. Cytogenet Cell Genet. 1985;40(1-4):360–489. doi: 10.1159/000132180. [DOI] [PubMed] [Google Scholar]