Events of regional-scale vegetation mortality appear to be increasing in a variety of biomes throughout the Earth and are frequently associated with increased temperatures, droughts, and often (but not always) with outbreaks of biotic agents such as insects and pathogens (for review, see Allen et al., 2010; Supplemental Information S1). The precise physiological mechanisms underlying plant mortality are poorly understood, which limits prediction of where, when, and how vegetation will change with future climatic variation (for review, see Supplemental Information S2). In particular, hypotheses regarding carbon starvation (failure to maintain metabolism or fend off biotic agents due to prolonged negative carbohydrate balance) and hydraulic failure (desiccation from failed water transport) have stimulated recent debate (Adams et al., 2009a, 2009b; Leuzinger et al., 2009; Sala, 2009; McDowell and Sevanto, 2010; Sala et al., 2010). In this Update, I offer a perspective on the mechanisms of drought-induced mortality by providing an integrated view of carbon metabolism in relation to stress and mortality, linking carbon metabolism with new evidence on the role of hydraulic failure in mortality, and identifying associated uncertainties. Drought is the predominant climate characteristic associated with mortality (Allen et al., 2010), but there are parallels with low temperature, light, and paleo-CO2 concentrations, from which I have cautiously drawn evidence regarding mechanisms of drought-induced mortality. Two novel results emerged from this review. First, well-known carbohydrate partitioning patterns are consistent with observations of carbohydrate concentrations in stressed but surviving plants as well as those that are dying, and these results support carbon starvation as a threat to survival during drought. Second, carbon metabolism and hydraulics are coupled via multiple interactions that are only recently becoming appreciated, including an enhanced risk of mortality in species that maintain narrow margins of hydraulic safety.
CARBON STARVATION
Starve (v): “…to perish or be in process of perishing from lack or insufficiency of food…” (Oxford English Dictionary, 2009). I define the process of carbon starvation as any situation in which carbon supply from photosynthesis and the mobilization of nonstructural carbohydrates (NSC) and autophagy (vacuolar proteolysis) is less than carbon use by respiration, growth, and defense. Death is defined as thermodynamic equilibrium between the organism and the environment, in which plants no longer have energy gradients to drive metabolism or regenerate. According to these definitions, measurable NSC need not be zero at death, but any remaining NSC must be unavailable to metabolism (i.e. “accessible carbon exhaustion”; Sala et al., 2010).
Drought can put plants on the path to carbon starvation, but they may eventually die of another mechanism. Moreover, there are potential interactions between the path to carbon starvation and (1) utilization of energy stores for metabolism and defense; (2) carbohydrate transport processes, including phloem loading, unloading, and transport; and (3) the demand for energy and carbon skeletons needed to maintain turgor and photosynthesis. The importance of these processes (described below), as well as the widespread observations of elevated NSC in plants during periods of stress (Chapin et al., 1990; Körner 2003; for further review, see Supplemental Information S3), has led to recent debate regarding the mechanisms of carbon starvation or whether it even occurs at all. One interpretation of these observations is that the carbon starvation process is unimportant and water stress induces mortality via hydraulic failure, limiting NSC utilization or predisposing plants to lethal attack by biotic agents (McDowell et al., 2008; Sala et al., 2010). I propose an additional interpretation: water stress impacts on hydraulics, NSC transport, and the use of NSC for metabolism and defense accelerate the process of carbon starvation, which feeds back on further starvation for carbon and associated vulnerability to biotic attack.
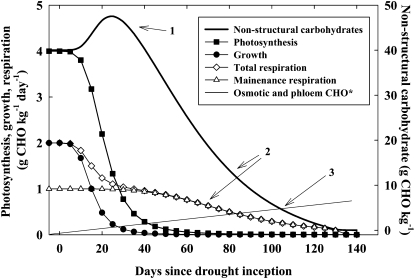
During water stress, plant growth declines before photosynthesis, resulting in a NSC surplus (Luxmoore et al., 1995; Estiarte and Peñuelas, 1999; Kozlowski and Pallardy, 2002; Hummel et al., 2010, Pinheiro and Chaves, 2011). This NSC surplus results because water stress causes greater reductions in growth than photosynthesis (Supplemental Information S4) and greater reductions in photosynthesis than maintenance respiration (Supplemental Information S5). A modeled representation that summarizes these observations is shown in Figure 1 and described in the Supplemental Data (Amthor and McCree 1990). An important result of this simulation is that carbohydrate content increases early in the drought, but if drought causes significantly reduced photosynthesis for a sufficiently long period, then NSC content will eventually decline, as NSC is consumed for the maintenance of cellular survival, including respiratory metabolism and osmotic adjustment. The threshold NSC concentration (per unit of dry matter) and content (per individual) after which mortality will occur are currently unknown (see below). Notably, sampling of the simulated plant in Figure 1 within the first 40 d of drought would reveal elevated NSC relative to predrought conditions, similar to many observations. Likewise, sampling of this plant throughout most of its range of photosynthesis would also show elevated NSC, with concentrations declining only when photosynthesis approaches zero (Supplemental Fig. S1). Thus, the critical physiology associated with mortality may not occur until the later stages of the mortality process.
Figure 1.
Simulated effects of prolonged drought on total NSC content, photosynthesis, growth, and respiration of a generic tree. The model summarizes the general relationships among these carbon pools and fluxes; it is not intended to mimic any particular circumstance. Arrow 1, Based on experimental observations that growth is most sensitive to water stress, followed by photosynthesis, and finally respiration, the carbohydrate pool initially increases. Only after photosynthesis declines to a rate slower than respiration do carbohydrates decline. Arrow 2, Growth respiration becomes minimal and hence respiration is only for maintenance. If maintenance respiration increases with drought and high temperatures, the size of the carbohydrate pool might decline more rapidly (Supplemental Fig. S2). Arrow 3, The simulated NSC pool implicitly includes noncarbohydrate organic compounds such as Pro that can accumulate during drought but does not account for NSC utilization for osmotic adjustment or maintenance of phloem transport. A linear increase in carbohydrate (CHO) consumption is shown for demonstrative purposes (asterisk). The intercept point of NSC with the NSC required to maintain turgor is a hypothetical mortality threshold in the absence of attack by biotic agents. See Supplemental Data for a fuller description of the model.
EVIDENCE REGARDING CARBON METABOLISM AND STARVATION
For clarity, I reiterate here the basic concept of avoidance of hydraulic failure at the cost of carbon assimilation and then review the evidence for carbon starvation. A consequence of drought is the risk of hydraulic failure or desiccation and subsequent cessation of cellular metabolism if the entire water column becomes irreparably cavitated. Stomatal closure prolongs survival on limited water supply by reducing transpiration and the risk of hydraulic failure, but this avoidance of hydraulic failure also reduces photosynthesis (McDowell et al., 2008; Pinheiro and Chaves, 2011). While photosynthesis is curtailed, plants continue to respire to maintain a minimal level of metabolism (except in rare species that can enter dormancy; Gaff, 1971). An analogous situation is that of shaded environments, in which low photosynthetic rates force NSC consumption for respiration rather than growth, and if light limitation is prolonged or severe enough, plants eventually die (Marshall and Waring, 1985; Kobe, 1997; Fig. 2).
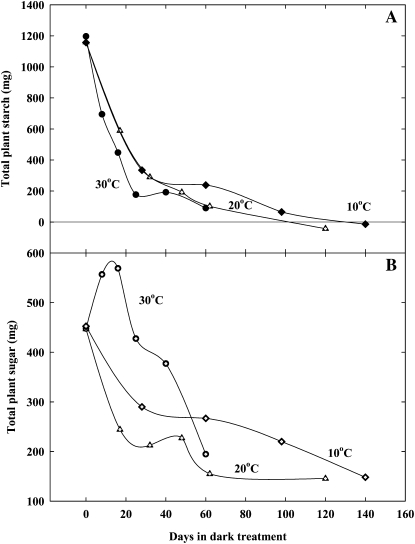
Figure 2.
Observations of whole-plant starch (A) and sugar (B) during shading in Douglas-fir (Pseugostuga menziesii) seedlings. Seedlings received zero light for the duration of the experiment and were maintained at three different temperatures (30°C, 20°C, and 10°C), with lower temperatures resulting in longer survival times. All seedlings were dead at the end of the experiment (J.D. Marshall, personal communication). Control plants received full sunlight, and both starch and sugar contents stayed above the values observed for shaded plants. Whole-plant (shoot, coarse root, new and old fine roots) carbohydrates were calculated from table 3 and figures 2 to 4 in Marshall and Waring (1985).
Components of carbon starvation have been demonstrated. Elevated mortality has been observed with low starch contents (Marshall and Waring, 1985; Kobe, 1997; Bréda et al., 2006; Fig. 2), photosynthetic constraints (McDowell et al., 2010), and faster respiration rates during warm periods (Adams et al., 2009c). Addition of exogenous sugars to water-stressed plants has shown that carbon availability, and not low plant water potential per se, was the causal factor in tissue mortality in maize (Zea mays; Boyle et al., 1991) and Protea (Bieleski et al., 1992). Recent molecular evidence is also consistent with the mechanism of carbon starvation. Using a variety of experiments to reduce photosynthesis, including water, salinity, light, and temperature stresses, it was observed that increased photosynthate partitioning to storage during the early period of stress (Fig. 1) is associated with molecular signals that down-regulate growth and respiration and up-regulate carbohydrate flux to storage (Achard et al., 2006; Cross et al., 2006; Stitt et al., 2007; Gibon et al., 2009; Hummel et al., 2010). Much of the regulation of enzyme activity is posttranslational, consistent with acclimation responses to longer term carbon deficits (e.g. drought or low temperature) rather than responses to short-term environmental changes (e.g. fluctuating light or temperature; Achard et al., 2008; Stitt et al., 2010). An essential point is that these molecular changes respond to carbohydrate availability per se, not to environmental cues (Smith and Stitt, 2007; Harberd et al., 2009). Thus, an initial driver of carbon starvation, declining whole-plant photosynthesis, is in fact a driver of a relative increase in starch production with stress (Gibon et al., 2009; Sulpice et al., 2009). Starch storage finally decreases when whole-plant photosynthesis, rather than per unit leaf area because leaf area often declines during drought (Tyree et al., 1993; Vilagrosa et al., 2003; Limousin et al., 2009; Hummel et al., 2010; Pinheiro and Chaves, 2011), falls to minimum rates and available NSC fails to meet essential metabolic demands (Marshall and Waring, 1985; Bieleski et al., 1992; McLaughlin and Boyer, 2004; Gibon et al., 2009; Figs. 1 and 2). These regulatory responses are similar to the observed up-regulation of fat storage and down-regulation of energy expenditure in starving animals (Wang et al., 2006; Xingsheng et al., 2010). Thus, molecular, tissue, and whole-plant evidence is consistent with observations of elevated carbohydrates in stressed plants and carbon starvation during extended periods of low photosynthesis (Fig. 1).
HOW DO CARBON METABOLISM, DEFENSE, AND TRANSPORT INTERACT DURING WATER STRESS?
The existing evidence shows that plants accumulate carbohydrates when stress affects growth more strongly than photosynthesis (Fig. 1) and, in the limited studies that have killed plants, NSC declines (though rarely to zero values; Fig. 2; Piper et al., 2009; for review, see McDowell and Sevanto, 2010). Water stress constraints on carbohydrate transport, utilization, and defensive mobilization, however, may drive the critical tipping points of mortality (Leuzinger et al., 2009; Sala et al., 2010). These mortality tipping points are largely unstudied and therefore provide ripe opportunities for future research.
The availability of carbohydrates for respiration may be constrained if cellular water stress requires increased sugar utilization to maintain osmotic potential and to prevent and repair oxidative stress and destabilization of macromolecular integrity (Fig. 1; Déjardin et al., 1999; Kozlowski and Pallardy, 2002; Bartels and Sunkar, 2005; Illing et al., 2005; Atkin and Macherel, 2009, Pinheiro and Chaves, 2011). It is unclear how much the use of NSC for osmotic adjustment and repair processes will elevate the intercept point (Fig. 1) of respiration-available NSC and that required to maintain cellular integrity; solutes and organic acids (the latter ultimately derived from photosynthate) also play a large role in osmotic adjustment (Hummel et al., 2010). Autophagy, or the breakdown of proteins, lipids, and other materials, can provide energy sources when NSC reaches threshold minimum values, but this provides only a short-term energy source (Brouquisse et al., 1998; Atkin and Tjoelker, 2003; Lundgren Rose et al., 2006).
Phloem transport of carbohydrates for metabolism or defense could be diminished during drought via low supply of water or photosynthate to the top of the phloem column, as well as by sink limitations (Cernusak et al., 2003; Wang et al., 2003; Hölttä et al., 2009b; Ainsworth and Bush, 2010). This can cut off organs that require NSC from storage sources or from what limited new photosynthate is assimilated. The use of low-Mr sugars to maintain a water potential gradient within the phloem has been observed in droughted plants (Cernusak et al., 2003), but this subsequently elevates the minimum survivable NSC content (Fig. 1); thus, plants must strike a balance between phloem transport and NSC consumption.
Water stress may also constrain defense in multiple ways (Schoenweiss, 1981). Partitioning carbon to defense is expensive because defense compounds are carbon rich (Poorter and Villar, 1997; Langenheim, 2003; Bilgin et al., 2010). Intermediate levels of water stress reduce growth and increase partitioning to defense (Herms and Mattson, 1992; Stamp, 2003; Navarro et al., 2008), but severe or prolonged water stress reduces the production of defensive compounds (Coulson, 1979). This decline in the production of defensive compounds results directly from insufficient NSC (Lewinsohn et al., 1991; Steele et al., 1995; Paine et al., 1997; Guérard et al., 2007). During prolonged drought, plants may be forced to rely on the use of constitutive defense compounds stored during stress-free periods. The ability to mobilize constitutive compounds may be limited by water stress, however, because the compounds cannot be transported to sites of attack (Guérard et al., 2007). Notably, warm temperatures per se reduce plant sugar and starch contents and increase insect attack performance (Zvereva and Kozlov, 2006).
Respiratory consumption of carbohydrates should exacerbate carbon starvation if drought includes elevated temperatures and associated acceleration in maintenance processes (McDowell et al., 2008; Atkin and Macherel, 2009; Pinheiro and Chaves, 2011); however, respiratory acclimation may mitigate this impact (Sala et al., 2010). Respiration sometimes declines during drought, driven in part by reduced growth, substrate availability, and protein content (Fig. 1; Amthor, 1994; Gibon et al., 2009). Reduced respiration during stress is associated with molecular signals that respond to photosynthate availability (Stitt et al., 2010); however, this reduction in respiration provides only a modest slowing of the loss of the carbohydrate pool because it does not balance the large decline in photosynthesis during extended stress (Flexas et al., 2005; Atkin and Macherel, 2009). Thus, a logical hypothesis is that particularly high temperatures, as often occurs during droughts, may further constrain the benefits of respiratory acclimation by elevating respiratory consumption of NSC due to the temperature dependence of respiration (Supplemental Fig. S2; Atkin et al., 2007; Adams et al., 2009c) as well as accelerated cellular repair processes (Atkin and Macherel, 2009).
A REVISED VIEW OF THE INTERACTION OF PLANT HYDRAULICS WITH THE CARBON BUDGET
There is clear evidence of partial and complete mortality associated with hydraulic failure in both isohydric and anisohydric plants (Supplemental Information S6); however, no tests of hydraulic failure have excluded carbon starvation or other processes as mechanisms involved in mortality. New evidence suggests that multiple interactions between these processes may occur during mortality and may vary with hydraulic strategy. Stomatal regulation of water fluxes and xylem water tensions operate across a continuum, with relatively isohydric species tightly regulating water stress and relatively anisohydric species allowing higher transpiration fluxes and tolerating greater water stress (McDowell et al., 2008; Meinzer et al., 2009). Contrary to the paradigm that isohydric species avoid cavitation, however, it has recently been revealed that relatively isohydric species tend to experience far greater cavitation and refilling of xylem on a daily basis than anisohydric species, the benefit of which is enhanced capacitance for use in transpiration (Sperry et al., 2008; Hölttä et al., 2009a; Meinzer et al., 2009) and potentially lower risk of xylem wall implosion as conduits fill with gas rather than experience more severe water potentials (Pratt et al., 2008). Thus, it appears that the binary mortality theory of McDowell et al. (2008), that trees become vulnerable to pests and extreme climate via carbon starvation or hydraulic failure, is overly simplistic; more likely, the two processes are coupled.
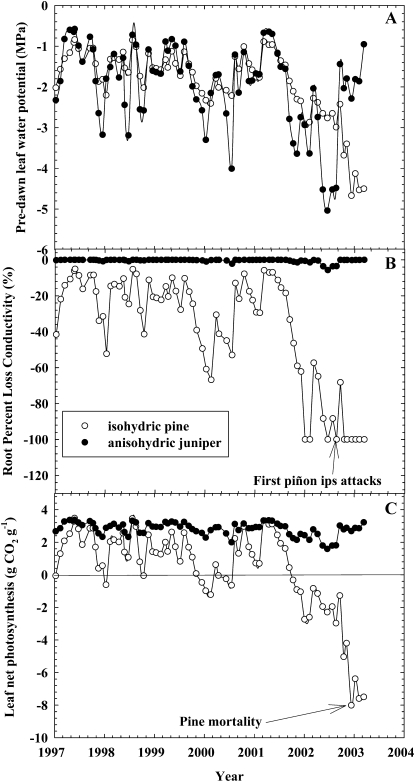
An example of the potential coupling of carbon starvation and hydraulic failure as well as the consequence of the isohydric strategy of maintaining a small hydraulic safety margin is demonstrated using the case study of two sympatric species, piñon pine (Pinus edulis) and one-seed juniper (Juniperus monosperma; Fig. 3). Piñon pine experienced regional-scale mortality in the southwestern United States during a prolonged drought in 2000 to 2003, while juniper largely survived (McDowell et al., 2008). The relatively isohydric piñon pine held water potentials within a much tighter range than the anisohydric juniper prior to the severe drought (1997–1999; Fig. 3A), yet it experienced greater loss of hydraulic conductivity and lower net photosynthesis during this period (Fig. 3, B and C). For approximately 19 months prior to mortality, piñon pine experienced hydraulic conductivity losses of greater than 50% and sustained negative carbon balance. Zero percent hydraulic conductivity was achieved 7 months prior to the first evidence of attacks by the piñon ips (Ips confuses), indicating that plants may survive complete loss of conductivity for some period prior to mortality. This contrasts with the mortality threshold of 50% loss of conductivity shown in potted seedlings of four Australian species (Brodribb and Cochard, 2009) but is similar to observations for other woody species (Vilagrosa et al., 2003; Rice et al., 2004; Adams et al., 2009c). Based on this analysis, it appears that (1) carbon limitation and hydraulic failure are coupled rather than independent, at least in this isohydric species; (2) teasing apart the role of each process cannot be done from these observations alone because the processes co-occurred; (3) in this system, hydraulic failure (and carbon starvation) was more likely in the isohydric than in the anisohydric species; and (4) woody plants in the field may survive prolonged periods of near-zero hydraulic conductance, similar to cacti.
Figure 3.
Observations of predawn water potential (A), loss of hydraulic conductivity of the root system (B), and net photosynthesis (C) of relatively isohydric and anisohydric species (piñon pine and one-seed juniper, respectively) during drought. Predawn water potential measurements are from Breshears et al. (2009). Root conductivity loss due to cavitation and net photosynthesis are shown for both species during this period using published species-specific equations for percentage loss of root hydraulic conductivity and net photosynthesis (McDowell et al., 2008; Willson et al., 2008; B and C). Roots were used rather than stems for B because they are more vulnerable and thus better represent the whole-plant connection to soil water availability and also because predawn water potentials (A) are expected to represent soil water availability. These estimates assume that refilling is possible when soil water potential is more positive than minimum predawn plant water potential, but they are conservative because the net photosynthesis model is for foliage only and does not include carbon losses to respiration of nonphotosynthetic tissues.
If drought prevents refilling of diel cavitation due to low soil water availability, then the progressive increase in xylem embolism subsequently reduces hydraulic conductivity (Fig. 3; Hölttä et al., 2009b), decreasing photosynthesis and increasing the threat of turgor loss (Brodribb and Holbrook, 2006). This creates a feedback loop (Fig. 4) that increases the reliance on NSC to maintain turgor and leaf function (Vilagrosa et al., 2010), further reducing carbohydrate availability for metabolism. This positive feedback is exacerbated because, in many species, refilling of embolized xylem is an energy-requiring process that depends on the conversion of starch to sugars for aquaporin regulation and for the creation of an osmotic gradient within embolized conduits (Bucci et al., 2003; Salleo et al., 2009; Zwieniecki and Holbrook, 2009). A feedback of carbon starvation on vulnerability to further hydraulic failure could thus occur if carbohydrates are unavailable to facilitate refilling (Fig. 4), subsequently pushing plants faster toward the threshold for either hydraulic failure or carbon starvation. It remains to be tested if hydraulic failure in the period prior to mortality results from turgor loss driven by outright desiccation or exhaustion of available carbohydrates and solutes, and it is unclear if hydraulic failure even results in mortality (Fig. 3B).
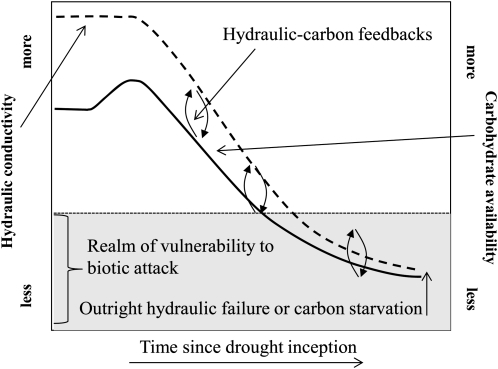
Figure 4.
Hypothetical relationships of whole-plant hydraulic conductivity and carbohydrate availability during drought. Carbohydrate availability is plotted using the simulation from Figure 1, and hydraulic conductivity follows a typical pattern from Brodribb and Cochard (2009). The threats of hydraulic failure, biotic attack, or outright carbon starvation are enhanced by the interdependent feedbacks between conductivity and carbohydrates. The abundance of biotic agents that kill their hosts varies with species, climate, and region, so a flat line is used to indicate their general presence rather than a specific attack agent. If these agents are present, their attack is more likely to result in mortality after the threshold is exceeded.
The feedback loop between carbon availability and hydraulics is likely to be further exacerbated by multiple mechanisms. These include fine root loss, reduced xylem transport efficiency (Eilmann et al., 2009), cavitation fatigue (Hacke et al., 2001), leaf shedding (Tyree et al., 1993; Vilagrosa et al., 2003; Limousin et al., 2009; Hummel et al., 2010), reduced phloem transport (Hölttä et al., 2009b), and turgor loss (particularly in isohydric species; Brodribb and Holbrook, 2006). Nutrient and mineral uptake is reduced during drought due to low microbial activity (Sardans and Peñuelas, 2005) and reduced transport to the canopy due to low transpiration rates (Hu and Schmidhalter, 2005), while the energetic costs of nitrogen conversion into usable forms increases during drought (Farooq et al., 2009; Pinheiro and Chaves, 2011). Nutrient limitations also drive stoichiometric (i.e. carbon-nitrogen ratio) and metabolic constraints on carbohydrate utilization (Rufty et al., 1988; Chapin et al., 1990; Millard et al., 2007; Pinheiro and Chaves, 2011). Lastly, the feedback may continue if pathogens impair hydraulic function, leading to further carbon starvation or hydraulic failure (i.e. fungal blockage of stem xylem; Wullschleger et al., 2004; Fukuda et al., 2007). Thus, plant hydraulic-carbon feedbacks may exacerbate the likelihood of the three mortality mechanisms (carbon starvation, hydraulic failure, and biotic attack) by reducing photosynthesis and impeding refilling and turgor maintenance.
A PATH FORWARD: CRITICAL QUESTIONS AND NEEDED TESTS
Integration of ecological, physiological, and molecular knowledge on carbohydrate metabolism and hydraulics provides a framework for improving our understanding of plant mortality (Fig. 4). The essential hypotheses that must be tested are as follows: (1) outright carbon starvation occurs, in the absence of biotic attack, when an extended negative carbon budget results in failure to maintain metabolism (Fig. 1); (2) hydraulic failure occurs due to progressive increases in nonrefilled xylem embolism (Fig. 3); (3) a negative carbon budget facilitates progressive xylem embolism, and vice versa (Fig. 4); and (4) a negative carbon budget and xylem embolism promote declines in defensive capability (Fig. 4).
There are multiple components of carbon starvation, hydraulic failure, and their interdependency that are currently unconstrained by experimental measurements. The states and fluxes in Figures 1 to 4 should be quantified in plants that are in the process of dying. In particular, we must determine the minimum required availability of substrates for the maintenance of metabolism and defense, including, at a minimum, starch and sugars, as well as defensive function. Ideally, other metabolites and defense compounds that aid in survival should also be studied across the survival-to-mortality continuum to identify metabolite thresholds associated with mortality. The functioning and interaction of the water and carbon transport pathways is a particularly poorly understood process that may be critical to plant mortality. The maximum sustained (nonrefilled) loss of xylem hydraulic conductance that can be survived, regulation of refilling, tolerance of reduced hydraulic conductance, and associated linkages to phloem transport during the period leading up to death must be resolved (Figs. 3 and 4). Future studies should focus on the interactions between plant hydraulics and carbon metabolism at the whole-plant scale because tissues vary in carbohydrate storage (Hoch et al., 2003; Tschaplinski and Hanson, 2003; Würth et al., 2005; Millard et al., 2007) and cavitation resistance (for review, see Meinzer et al., 2010).
Mortality studies will achieve significant benefits from employing experimental approaches that have proven useful for understanding other molecular and whole-plant responses to climate. Experiments may be observational (i.e. sampling of plants that are in the process of dying or have already died) but should include control plants that survive (Piper et al., 2009; McDowell et al., 2010). Mechanisms may be better identified via manipulation of causal factors in mortality such as altered water, temperature, light, and atmospheric CO2 (Supplemental Information S7) and through the use of realistic field experiments rather than greenhouse studies (Pinheiro and Chaves, 2011). The most direct cause-and-effect studies might involve direct addition of exogenous carbohydrates or water to the vascular system of plants grown under experimental control of soil moisture, allowing decoupling of carbohydrate exhaustion from hydraulic failure. Finally, future experiments must be designed to interrogate the mechanisms of mortality per se, rather than relying on ad hoc analyses of experiments and measurements designed with different objectives (McDowell and Sevanto, 2010).
CONCLUSION
Integration of available evidence from disparate research fields provides an internally consistent theory on the physiological mechanisms underlying vegetation mortality and survival during environmental stress while simultaneously opening new avenues of research. Carbon starvation results when carbon acquisition and storage mobilization fail to meet consumption for metabolic maintenance. Observations of carbon metabolism from both stressed plants that survive and from plants that die are internally consistent; thus, future research regarding carbon starvation should move forward to understand the tipping points of mortality. Carbon starvation is tightly interdependent on both the avoidance and occurrence of hydraulic failure through impacts on maintenance metabolism, phloem transport, defense, and the dynamics of hydraulic conductance and refilling. The threshold minimum carbohydrate content at which plants die and its positive and negative responses to the interdependent carbohydrate metabolism-hydraulic processes is unknown. Notably, the isohydric strategy of daily cavitation and refilling may expose plants to greater risk of both carbon starvation and hydraulic failure than has been appreciated previously (McDowell et al., 2008).
It is well established that plants have evolved to survive environmental stress, but the outstanding question regarding future climate impacts on vegetation mortality is, can plants acclimate or adapt their survival mechanisms as quickly as the rate of increase in drought frequency, severity, or length? The consistent predictions of a warming climate punctuated with more frequent and severe droughts (Supplemental Information S8), coupled with the effects of heat and drought on vegetation mortality, indicate that widespread mortality events are a likely future phenomenon. Accurately forecasting these events will depend on our ability to integrate knowledge and experimental systems from a broad set of research disciplines to determine the fundamental mechanisms of mortality and survival.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Modeled relationship of growth and nonstructural carbohydrate storage to photosynthesis during drought.
Supplemental Figure S2. Potential effect of drought-induced increase in maintenance respiration on whole-plant NSC.
Supplemental Information S1. Some references documenting mortality as well as earlier attempts at theoretical integration.
Supplemental Information S2. Some references highlighting challenges associated with modeling future vegetation mortality.
Supplemental Information S3. Some references documenting increased NSC during periods of stress.
Supplemental Information S4. Some references showing greater sensitivity of growth than photosynthesis to water stress.
Supplemental Information S5. Some references showing greater sensitivity of photosynthesis than respiration to water stress.
Supplemental Information S6. Some references showing evidence of hydraulic failure leading to partial or complete mortality.
Supplemental Information S7. Potential experimental manipulations that could be applied to understand mortality mechanisms.
Supplemental Information S8. Some references suggesting future climate will be globally warmer and regionally drier.
Supplementary Material
Acknowledgments
This paper benefited from discussions with too many colleagues to name them all. Rick Meinzer provided valuable discussions regarding hydraulic failure. Jeff Amthor, Craig Allen, Donald Ort, Sanna Sevanto, and three anonymous reviewers provided useful comments on earlier drafts of the manuscript.
References
- Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP. (2006) Integration of plant responses to environmentally activated phytohormonal signals. Science 311: 91–94 [DOI] [PubMed] [Google Scholar]
- Achard P, Gong F, Cheminant S, Alioua M, Hedden P, Genschik P. (2008) The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 20: 2117–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams HD, Guardiola-Claramonte M, Barron-Gafford G, Camilo Villegasa J, Breshears D, Zou C, Troch P, Huxman T. (2009a) Reply to Sala: Temperature sensitivity in drought-induced tree mortality hastens the need to further resolve a physiological model of death. Proc Natl Acad Sci USA 106: E69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams HD, Guardiola-Claramonte M, Barron-Gafford G, Camilo Villegasa J, Breshears D, Zou C, Troch P, Huxman T. (2009b) Reply to Leuzinger et al.: Drought-induced tree mortality temperature sensitivity requires pressing forward with best available science. Proc Natl Acad Sci USA 106: E107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams HD, Guardiola-Claramonte M, Barron-Gafford GA, Villegas JC, Breshears DD, Zou CB, Troch PA, Huxman TE. (2009c) Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proc Natl Acad Sci USA 106: 7063–7066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth EA, Bush DR. (2010) Carbohydrate export from the leaf: a highly regulated process and target to enhance photosynthesis and productivity. Plant Physiol 155: 64–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Macalady A, Chenchouni H, Bachelet D, McDowell N, Vennetier M, Gonzales P, Hogg T, Rigling A, Breshears D, et al. (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manag 259: 660–684 [Google Scholar]
- Amthor JS. (1994) Plant respiratory responses to the environment and their effects on the carbon balance. Wilkinson RE, , Plant-Environment Interactions. Marcel Dekker, New York, pp 501–554 [Google Scholar]
- Amthor JS, McCree KJ. (1990) Carbon balance of stressed plants: a conceptual model for integrating research results. Alscher RG, Cumming JR, , Stress Responses in Plants: Adaptation and Acclimation Mechanisms. Wiley-Liss, New York, pp 1–15 [Google Scholar]
- Atkin OK, Macherel D. (2009) The crucial role of plant mitochondria in orchestrating drought tolerance. Ann Bot (Lond) 103: 581–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin OK, Scheurwater I, Pons TL. (2007) Respiration as a percentage of daily photosynthesis in whole plants is homeostatic at moderate, but not high, growth temperatures. New Phytol 174: 367–380 [DOI] [PubMed] [Google Scholar]
- Atkin OK, Tjoelker MG. (2003) Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci 8: 343–351 [DOI] [PubMed] [Google Scholar]
- Bartels D, Sunkar R. (2005) Drought and salt tolerance in plants. Crit Rev Plant Sci 24: 23–58 [Google Scholar]
- Bieleski RL, Ripperda J, Newman JP, Reid MS. (1992) Carbohydrate changes and leaf blackening in cut flower stems of Protea eximia. J Am Soc Hortic Sci 117: 124–127 [Google Scholar]
- Bilgin DD, Zavala JA, Zhu J, Clough SJ, Ort DR, DeLucia EH. (2010) Biotic stress globally downregulates photosynthesis genes. Plant Cell Environ 33: 1597–1613 [DOI] [PubMed] [Google Scholar]
- Boyle MG, Boyer JS, Morgan PW. (1991) Stem infusion of liquid culture medium prevents reproductive failure of maize at low water potential. Crop Sci 31: 1246–1252 [Google Scholar]
- Bréda N, Huc R, Granier A, Dreyer E. (2006) Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Ann Sci 63: 625–644 [Google Scholar]
- Breshears DD, Myers OB, Meyer CW, Barnes FJ, Zou CB, Allen CD, McDowell NG, Pockman WT. (2009) Tree die-off in response to global-change-type drought: mortality insights from a decade of plant water potential measurements. Front Ecol Environ 7: 185–189 [Google Scholar]
- Brodribb TJ, Cochard H. (2009) Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol 149: 575–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM. (2006) Declining hydraulic efficiency as transpiring leaves desiccate: two types of response. Plant Cell Environ 29: 2205–2215 [DOI] [PubMed] [Google Scholar]
- Brouquisse R, Gaudillére JP, Raymond P. (1998) Induction of a carbon-starvation-related proteolysis in whole maize plants submitted to light/dark cycles and to extended darkness. Plant Physiol 117: 1281–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci SJ, Scholz FG, Goldstein G, Meinzer FC, Sternberg L, Da SL. (2003) Dynamic changes in hydraulic conductivity in petioles of two savanna tree species: factors and mechanisms contributing to the refilling of embolized vessels. Plant Cell Environ 26: 1633–1645 [Google Scholar]
- Cernusak LA, Arthur DJ, Pate JS, Farquhar GD. (2003) Water relations link carbon and oxygen isotope discrimination to phloem sap sugar concentration in Eucalyptus globulus. Plant Physiol 131: 1544–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin FS, III, Schulze ED, Mooney HA. (1990) The ecology and economics of storage in plants. Annu Rev Ecol Syst 21: 423–447 [Google Scholar]
- Coulson RN. (1979) Population dynamics of bark beetles. Annu Rev Entomol 24: 417–447 [Google Scholar]
- Cross JM, von Korff M, Altmann T, Bartzetko L, Sulpice R, Gibon Y, Palacios N, Stitt M. (2006) Variation of enzyme activities and metabolite levels in 24 Arabidopsis accessions growing in carbon-limited conditions. Plant Physiol 142: 1574–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Déjardin A, Sokolov LN, Kleczkowski LA. (1999) Sugar/osmoticum levels modulate differential abscisic acid-independent expression of two stress-responsive sucrose synthase genes in Arabidopsis. Biochem J 344: 503–509 [PMC free article] [PubMed] [Google Scholar]
- Eilmann B, Zweifel R, Buchmann N, Fonti P, Rigling A. (2009) Drought-induced adaptation of the xylem in Scots pine and pubescent oak. Tree Physiol 29: 1011–1020 [DOI] [PubMed] [Google Scholar]
- Estiarte M, Peñuelas J. (1999) Excess carbon: the relationship with phenotypical plasticity in storage and defense functions of plants. Orsis 14: 159–203 [Google Scholar]
- Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA. (2009) Plant drought stress: effects, mechanisms and management. Sust Agric 29: 185–212 [Google Scholar]
- Flexas J, Galmes J, Ribas-Carbo M, Medrano H. (2005) The effects of water stress on plant respiration. Lambers H, Ribas-Carbo M, , Plant Respiration. Springer, Dordrecht, The Netherlands, pp 85–94 [Google Scholar]
- Fukuda K, Utsuzawa S, Sakaue D. (2007) Correlation between acoustic emission, water status and xylem embolism in pine wilt disease. Tree Physiol 27: 969–976 [DOI] [PubMed] [Google Scholar]
- Gaff DF. (1971) Desiccation-tolerant flowering plants in southern Africa. Science 174: 1033–1034 [DOI] [PubMed] [Google Scholar]
- Gibon Y, Pyl ET, Sulpice R, Lunn JE, Höhne M, Günther M, Stitt M. (2009) Adjustment of growth, starch turnover, protein content and central metabolism to a decrease of the carbon supply when Arabidopsis is grown in very short photoperiods. Plant Cell Environ 32: 859–874 [DOI] [PubMed] [Google Scholar]
- Guérard N, Maillard P, Brechet C, Lieutier F, Dreyer E. (2007) Do trees use reserve or newly assimilated carbon for their defense reactions? A 13C labeling approach with young Scots pines inoculated with a bark-beetle-associated fungus (Ophiostoma brunneo ciliatum). Ann Sci 64: 601–608 [Google Scholar]
- Hacke UG, Stiller V, Sperry JS, Pittermann J, McCulloh KA. (2001) Cavitation fatigue: embolism and refilling cycles can weaken the cavitation resistance of xylem. Plant Physiol 125: 779–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harberd NP, Belfield E, Yasumura Y. (2009) The angiosperm gibberellin-GID1-DELLA growth regulatory mechanism: how an “inhibitor of an inhibitor” enables flexible response to fluctuating environments. Plant Cell 21: 1328–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herms DA, Mattson WJ. (1992) The dilemma of plants: to grow or defend. Q Rev Biol 67: 282–335 [Google Scholar]
- Hoch G, Richter A, Körner C. (2003) Non-structural carbon compounds in temperate forest trees. Plant Cell Environ 26: 1067–1081 [Google Scholar]
- Hölttä T, Cochard H, Nikinmaa E, Mencuccini M. (2009a) Capacitive effect of cavitation in xylem conduits: results from a dynamic model. Plant Cell Environ 32: 10–21 [DOI] [PubMed] [Google Scholar]
- Hölttä T, Mencuccini M, Nikinmaa E. (2009b) Linking phloem function to structure: analysis with a coupled xylem-phloem transport model. J Theor Biol 259: 325–337 [DOI] [PubMed] [Google Scholar]
- Hu Y, Schmidhalter U. (2005) Drought and salinity: a comparison of their effects on the mineral nutrition of plants. J Plant Nutr Soil Sci 168: 541–549 [Google Scholar]
- Hummel I, Pantin F, Sulpice R, Piques M, Rolland G, Dauzat M, Christophe A, Pervent M, Bouteillé M, Stitt M, et al. (2010) Arabidopsis plants acclimate to water deficit at low cost through changes of carbon usage: an integrated perspective using growth, metabolite, enzyme, and gene expression analysis. Plant Physiol 154: 357–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illing N, Denby KJ, Collett H, Shen A, Farrant JM. (2005) The signature of seeds in resurrection plants: a molecular and physiological comparison of desiccation tolerance in seeds and vegetative tissues. Integr Comp Biol 45: 771–787 [DOI] [PubMed] [Google Scholar]
- Kobe K. (1997) Carbohydrate allocation to storage as a basis of interspecific variation in sapling survivorship and growth. Oikos 80: 226–233 [Google Scholar]
- Körner C. (2003) Carbon limitation in trees. J Ecol 91: 4–17 [Google Scholar]
- Kozlowski TT, Pallardy SG. (2002) Acclimation and adaptive responses of woody plants to environmental stresses. Bot Rev 68: 270–334 [Google Scholar]
- Langenheim JH. (2003) Plant Resins: Chemistry, Evolution, Ecology and Ethnobotany. Timber Press, Portland, OR [Google Scholar]
- Leuzinger S, Bigler C, Wolf A, Körner C. (2009) Poor methodology for predicting large-scale tree die-off. Proc Natl Acad Sci USA 106: E106; author reply E107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn E, Gijzen M, Croteau R. (1991) Defense mechanisms of conifers : differences in constitutive and wound-induced monoterpene biosynthesis among species. Plant Physiol 96: 44–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limousin JM, Rambal S, Ourcival JM, Rocheteau A, Joffre R, Rodriguez-Cortinta R. (2009) Long-term transpiration change with rainfall decline in a Mediterranean Quercus ilex forest. Glob Change Biol 15: 2163–2175 [Google Scholar]
- Luxmoore RJ, Oren R, Sheriff DW, Thomas RB. (1995) Source-sink-storage relationships of conifers. Smith WK, Hinckley TM, , Resource Physiology of Conifers: Acquisition, Allocation and Utilization. Academic Press, San Diego, pp 179–216 [Google Scholar]
- Marshall JD, Waring RH. (1985) Predicting fine root production and turnover by monitoring root starch and soil temperature. Can J Res 15: 791–800 [Google Scholar]
- McDowell NG, Allen CG, Marshall L. (2010) Growth, carbon isotope discrimination, and mortality across a ponderosa pine elevation transect. Glob Change Biol 16: 399–415 [Google Scholar]
- McDowell NG, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, et al. (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178: 719–739 [DOI] [PubMed] [Google Scholar]
- McDowell NG, Sevanto S. (2010) The mechanisms of carbon starvation: how, when, or does it even occur at all? New Phytol 186: 264–266 [DOI] [PubMed] [Google Scholar]
- McLaughlin JE, Boyer JS. (2004) Glucose localization in maize ovaries when kernel number decreases at low water potential and sucrose is fed to the stems. Ann Bot (Lond) 94: 75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer FC, Johnson DM, Lachenbruch B, McCulloh KA, Woodruff DR. (2009) Xylem hydraulic safety margins in woody plants: coordination of stomatal control of xylem tension with hydraulic capacitance. Funct Ecol 23: 922–930 [Google Scholar]
- Meinzer FC, McCulloh KA, Lachenbruch B, Woodruff DR, Johnson DM. (2010) The blind men and the elephant: the impact of context and scale in evaluating conflicts between plant hydraulic safety and efficiency. Oecologia 164: 287–296 [DOI] [PubMed] [Google Scholar]
- Millard P, Sommerkorn M, Grelet GA. (2007) Environmental change and carbon limitation in trees: a biochemical, ecophysiological and ecosystem appraisal. New Phytol 175: 11–28 [DOI] [PubMed] [Google Scholar]
- Navarro L, Bari R, Achard P, Lisón P, Nemri A, Harberd NP, Jones JD. (2008) DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr Biol 18: 650–655 [DOI] [PubMed] [Google Scholar]
- Oxford English Dictionary (2009) Oxford University Press, Oxford [Google Scholar]
- Paine TD, Raffa KF, Harrington TC. (1997) Interactions among Scolytid bark beetles, their associated fungi, and live host conifers. Annu Rev Entomol 42: 179–206 [DOI] [PubMed] [Google Scholar]
- Pinheiro C, Chaves MM. (2011) Photosynthesis and drought: can we make metabolic connections from available data? J Exp Bot 62: 869–882 [DOI] [PubMed] [Google Scholar]
- Piper FI, Reyes-Diáz EM, Corcuera LJ, Lusk CH. (2009) Carbohydrate storage, survival, and growth of two evergreen Nothofagus species in two contrasting light environments. Ecol Res 24: 1233–1241 [Google Scholar]
- Poorter H, Villar R. (1997) The fate of acquired carbon in plants: chemical composition and construction costs. Bazzaz FA, Jones B, , Plant Resource Allocation. Academic Press, New York, pp 39–72 [Google Scholar]
- Pratt RB, Jacobsen AL, North GB, Sack L, Schenk HJ. (2008) Plant hydraulics: new discoveries in the pipeline. New Phytol 179: 590–593 [DOI] [PubMed] [Google Scholar]
- Rice KJ, Matzner SL, Byer W, Brown JR. (2004) Patterns of tree dieback in Queensland, Australia: the importance of drought stress and the role of resistance to cavitation. Oecologia 139: 190–198 [DOI] [PubMed] [Google Scholar]
- Rose TL, Bonneau L, Der C, Marty-Mazars D, Marty F. (2006) Starvation-induced expression of autophagy-related genes in Arabidopsis. Biol Cell 98: 53–67 [DOI] [PubMed] [Google Scholar]
- Rufty TW, Huber SC, Volk RJ. (1988) Alterations in leaf carbohydrate metabolism in response to nitrogen stress. Plant Physiol 88: 725–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala A. (2009) Lack of direct evidence for the carbon-starvation hypothesis to explain drought-induced mortality in trees. Proc Natl Acad Sci USA 106: E68; author reply E69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala A, Piper F, Hoch G. (2010) Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol 186: 274–281 [DOI] [PubMed] [Google Scholar]
- Salleo S, Trifilo P, Esposito S, Nardini A, LoGullo M. (2009) Starch-to-sugar conversion in wood parenchyma of field-growing Laurus nobilis plants: a component of the signal pathway for embolism repair? Funct Plant Biol 36: 815–825 [DOI] [PubMed] [Google Scholar]
- Sardans J, Peñuelas J. (2005) Drought decreases soil enzyme activity in a Mediterranean Quercus ilex L. forest. Soil Biol Biochem 37: 455–461 [Google Scholar]
- Schoenweiss DF. (1981) Water stress as a predisposing factor in plant disease. Kozlowski TT, , Water Deficits and Plant Growth. Academic Press, New York, pp 61–99 [Google Scholar]
- Smith AM, Stitt M. (2007) Coordination of carbon supply and plant growth. Plant Cell Environ 30: 1126–1149 [DOI] [PubMed] [Google Scholar]
- Sperry JS, Meinzer FC, McCulloh KA. (2008) Safety and efficiency conflicts in hydraulic architecture: scaling from tissues to trees. Plant Cell Environ 31: 632–645 [DOI] [PubMed] [Google Scholar]
- Stamp N. (2003) Out of the quagmire of plant defense hypotheses. Q Rev Biol 78: 23–55 [DOI] [PubMed] [Google Scholar]
- Steele CL, Lewinsohn E, Croteau R. (1995) Induced oleoresin biosynthesis in grand fir as a defense against bark beetles. Proc Natl Acad Sci USA 92: 4164–4168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Gibon Y, Lunn JE, Piques M. (2007) Multilevel genomics analysis of carbon signalling during low carbon availability: coordinating the supply and utilization of carbon in a fluctuating environment. Funct Plant Biol 34: 526–549 [DOI] [PubMed] [Google Scholar]
- Stitt M, Lunn J, Usadel B. (2010) Arabidopsis and primary photosynthetic metabolism: more than the icing on the cake. Plant J 61: 1067–1091 [DOI] [PubMed] [Google Scholar]
- Sulpice R, Pyl ET, Ishihara H, Trenkamp S, Steinfath M, Witucka-Wall H, Gibon Y, Usadel B, Poree F, Piques MC, Von, et al. (2009) Starch as a major integrator in the regulation of plant growth. Proc Natl Acad Sci USA 106: 10348–10353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschaplinski TJ, Hanson PJ. (2003) Dormant season nonstructural carbohydrate storage. Hanson PJ, Wullschleger SD, , North American Temperate Deciduous Forest Responses to Changing Precipitation Regimes. Springer, New York, pp 67–84 [Google Scholar]
- Tyree MT, Cochard H, Cruiziat P, Sinclair B, Ameglio T. (1993) Drought-induced leaf shedding in walnut: evidence for vulnerability segmentation. Plant Cell Environ 16: 879–882 [Google Scholar]
- Vilagrosa A, Bellot J, Vallejo VR, Gil-Pelegrin E. (2003) Cavitation, stomatal conductance, and leaf dieback in seedlings of two co-occurring Mediterranean shrubs during an intense drought. J Exp Bot 54: 2015–2024 [DOI] [PubMed] [Google Scholar]
- Vilagrosa A, Morales F, Abadía A, Bellot J, Cochard H, Gil-Pelegrin E. (2010) Are symplast tolerance to intense drought conditions and xylem vulnerability to cavitation coordinated? An integrated analysis of photosynthetic, hydraulic and leaf level processes in two Mediterranean drought-resistant species. Environ Exp Bot 69: 233–242 [Google Scholar]
- Wang T, Hung CCY, Randall DJ. (2006) The comparative physiology of food deprivation: from feast to famine. Annu Rev Physiol 68: 223–251 [DOI] [PubMed] [Google Scholar]
- Wang ZP, Deloire A, Carbonneau A, Federspiel B, Lopez F. (2003) An in vivo experimental system to study sugar phloem unloading in ripening grape berries during water deficiency stress. Ann Bot (Lond) 92: 523–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson CJ, Manos PS, Jackson RB. (2008) Hydraulic traits are influenced by phylogenetic history in the drought-resistant, invasive genus Juniperus (Cupressaceae). Am J Bot 95: 299–314 [DOI] [PubMed] [Google Scholar]
- Wullschleger SD, McLaughlin SB, Ayres MP. (2004) High-resolution analysis of stem increment and sap flow for loblolly pine trees attacked by southern pine beetle. Can J Res 34: 2387–2393 [Google Scholar]
- Würth MKR, Peláez-Riedl S, Wright SJ, Körner C. (2005) Non-structural carbohydrate pools in a tropical forest. Oecologia 143: 11–24 [DOI] [PubMed] [Google Scholar]
- Xingsheng L, Cope MB, Johnson MS, Smith DL, Jr, Nagy TR. (2010) Mild calorie restriction induces fat accumulation in female C57BL/6J mice. Obesity (Silver Spring) 18: 456–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvereva EL, Kozlov MV. (2006) Consequences of simultaneous elevation of carbon dioxide and temperature for plant-herbivore interactions: a metaanalysis. Glob Change Biol 12: 27–41 [Google Scholar]
- Zwieniecki MA, Holbrook NM. (2009) Confronting Maxwell’s demon: biophysics of xylem embolism repair. Trends Plant Sci 14: 530–534 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.