Abstract
In red clover (Trifolium pratense) leaves, phaselic acid (2-O-caffeoyl-l-malate) accumulates to several mmol kg−1 fresh weight and is a crucial component of a natural system that prevents protein breakdown during harvest and storage of this forage crop. Previously, we identified HCT2, a red clover gene encoding a hydroxycinnamoyl-Coenzyme A (CoA) hydroxycinnamoyl transferase capable of transferring p-coumaroyl and caffeoyl moieties from their CoA derivatives to malic acid to form the corresponding hydroxycinnamoyl-malate esters in vitro. Here, we carried out a detailed kinetic analysis of the enzyme and examined its in vivo function in red clover via reverse genetics. The kinetic analysis indicates that in vitro, despite similar Km values for the tested hydroxycinnamoyl-CoA derivatives, HCT2 favors transfer to malate of p-coumaroyl and feruloyl moieties over caffeoyl moieties by greater than 5-fold. Reverse reaction (transfer of hydroxycinnamoyl moieties from malate to CoA) by HCT2 was observed with p-coumaroyl-malate but not phaselic acid. Analysis of red clover plants down-regulated for HCT2 expression via RNA interference showed a significant and substantial correlation between HCT2 mRNA levels and phaselic acid accumulation (P < 0.005). In several of the HCT2-silenced plants, phaselic acid and p-coumaroyl-malate levels were reduced to <5% that of wild-type controls. These reductions resulted in easily observable phenotypes including reduced polyphenol oxidase-mediated browning and a reduction in blue epidermal fluorescence under ultraviolet light. These results demonstrate a crucial role for HCT2 in phaselic acid accumulation in red clover and define a previously undescribed pathway for the biosynthesis of hydroxycinnamoyl-malate esters in plants.
The hydroxycinnamoyl-malate esters phaselic acid (2-O-caffeoyl-l-malate) and 2-O-(p-coumaroyl)-l-malate (hereafter referred to as p-coumaroyl-malate) accumulate in red clover (Trifolium pratense) leaves to relatively high levels. Phaselic acid predominates, accumulating to >5 mmol kg−1 fresh weight, a level generally 3-fold or more higher than that of the p-coumaroyl ester (Winters et al., 2008; Sullivan, 2009; M.L. Sullivan, unpublished data). Phaselic acid appears to be a major substrate for an endogenous polyphenol oxidase (PPO; Hatfield and Muck, 1999; Winters et al., 2008) and, as such, may be involved in protecting red clover against pathogens and insect herbivory (Steffens et al., 1994). Oxidation of o-diphenol compounds like phaselic acid by red clover PPO has also been shown to be responsible for protecting protein from degradation during harvest and storage of this forage crop (Sullivan and Hatfield, 2006), presumably by a mechanism that involves reaction of PPO-generated o-quinones with endogenous plant proteases or proteolytic substrates. In red clover, phaselic acid and other hydroxycinnamoyl-malate esters could also serve as UV protectants as has been described for sinapoyl-malate in Arabidopsis (Arabidopsis thaliana; Landry et al., 1995; Lehfeldt et al., 2000).
Poor utilization of degraded forage protein by ruminant animals results in economic losses to farmers of over $100 million annually and release of excess nitrogen into the environment (Sullivan and Hatfield, 2006). Thus, adapting red clover’s PPO/o-diphenol system of postharvest protein protection to other forage crops could have substantial economic and environmental benefits. Unfortunately, major forage crops like alfalfa (Medicago sativa) appear to lack not only foliar PPO (Sullivan et al., 2008) but also o-diphenol PPO substrates (Jones et al., 1995; Sullivan and Bringe, 2005), so understanding the pathways whereby PPO-utilizable o-diphenol substrates such as phaselic acid are synthesized could be key to adapting red clover’s natural system of postharvest protein protection for use in this and other forage corps.
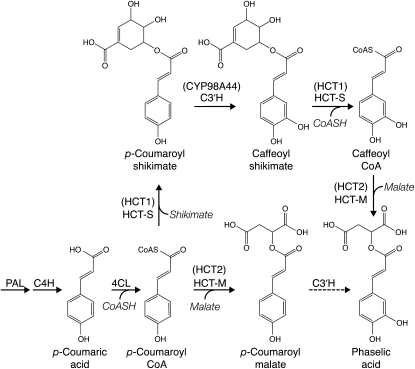
In Arabidopsis and other Brassicaceae, hydroxycinnamoyl-malate esters are synthesized via a hydroxycinnamoyl-Glc:malate hydroxycinnamoyl transferase (in Arabidopsis, the product of the SINAPOYLGLUCOSE ACCUMULATOR1 gene; Lehfeldt et al., 2000). Because there is no genetic evidence for a similar hydroxycinnamoyl-Glc transferase in red clover (Sullivan, 2009), we have proposed that this species makes hydroxycinnamoyl-malate esters such as phaselic acid via the action of a BAHD family (D’Auria, 2006) hydroxycinnamoyl-CoA:malate hydroxycinnamoyl transferase (Sullivan, 2009) in a manner analogous to biosynthesis of chlorogenic acid (caffeoyl-quinate) in tomato (Solanum lycopersicum) and other Solanaceae (Niggeweg et al., 2004). We proposed that in red clover, phaselic acid could be synthesized by two nonmutually exclusive pathways. One pathway would be transfer of a p-coumaroyl moiety to malic acid followed by hydroxylation by a p-coumarate 3′ hydroxylase (C3′H) of the resulting p-coumaroyl-malate to form phaselic acid (Fig. 1, bottom pathway). However, this pathway is at odds with our recent report that red clover C3′H appears to be incapable of hydroxylating p-coumaroyl-malate (Sullivan and Zarnowski, 2010). The second pathway (Fig. 1, top pathway) would require the formation of caffeoyl-CoA by C3′H hydroxylation of a p-coumaroyl-shikimate intermediate (formed by a hydroxycinnamoyl-CoA:shikimate hydroxycinnamoyl transferase). Formation of caffeoyl moieties in this way has been well documented in several plant species (Hoffmann et al., 2003, 2004; Shadle et al., 2007), and we have cloned red clover genes encoding a hydroxycinnamoyl-CoA:shikimate hydroxycinnamoyl transferase (HCT1, GenBank EU861218; Sullivan, 2009) and a C3′H (CYP98A44, GenBank GQ497816; Sullivan, 2009) capable of carrying out these reactions in vitro. Once formed, the caffeoyl-CoA could be a substrate for transfer of the caffeoyl moiety to malate to form phaselic acid.
Figure 1.
Possible pathways for phaselic acid biosynthesis in red clover. Proposed pathway enzymes for production of phaselic acid include Phe ammonia lyase (PAL), cinnamate 4-hydroxylase (C4H), 4-coumarate:CoA ligase (4CL), hydroxycinnamoyl-CoA:shikimate hydroxycinnamoyl transferase (HCT-S), hydroxycinnamoyl-CoA:malate hydroxycinnamoyl transferase (HCT-M), and C3′H. Red clover gene products described here and elsewhere (Sullivan, 2009; Sullivan and Zarnowski, 2010) corresponding to the enzymatic activities are indicated in parentheses. The dashed line for 3′-hydroxylation of p-coumaroyl-malate indicates neither enzyme activity nor its corresponding gene has been detected in red clover. For simplicity, not all reactants and products are shown.
Recently, we cloned a red clover gene (HCT2, GenBank EU861219) encoding a hydroxycinnamoyl-CoA hydroxycinnamoyl transferase capable of transferring p-coumaroyl and caffeoyl moieties to malate in vitro (Sullivan, 2009), an activity consistent with our proposed model of phaselic acid biosynthesis. Here we have further characterized the activities of this transferase in vitro and demonstrate via a reverse-genetic approach its crucial role in the biosynthesis of phaselic acid and p-coumaroyl-malate in vivo in red clover.
RESULTS
Kinetic Parameters of HCT2
To gain insights into the role HCT2 plays and pathways whereby hydroxycinnamoyl-malate esters are synthesized in vivo in red clover, Km and Vmax values were determined for HCT2 expressed in Escherichia coli using various potential substrates. For donor substrates, p-coumaroyl- and caffeoyl-CoA were used, which would correspond to p-coumaroyl- and caffeoyl-malate esters detected in leaves of red clover. Feruloyl-CoA was also tested, even though we have not detected feruloyl-malate in red clover leaves. Donor substrate Km values did not differ markedly, being in the 1 to 2 mm range, with that of p-coumaroyl-CoA being slightly higher than that of caffeoyl- and feruloyl-CoA (Table I). In contrast, Vmax values differed substantially, with those of p-coumaroyl- and feruloyl-CoA being 6- and 8-fold higher than that of caffeoyl-CoA. Km values for malate with either p-coumaroyl- or caffeoyl-CoA were similar (0.64 and 0.67 mm, respectively) and as expected, Vmax for reaction with p-coumaroyl-CoA was approximately 8-fold higher than that of caffeoyl-CoA. Comparison of Vmax/Km values indicate a greater than 5-fold preference of HCT2 for p-coumaroyl- or feruloyl-CoA over caffeoyl-CoA, at least in vitro. These results are consistent with previous specific activity measurements of HCT2 made with 1 mm p-coumaroyl- or caffeoyl-CoA and 3 mm malate, where an approximately 7-fold-higher reaction rate was observed for p-coumaroyl-CoA (Sullivan, 2009).
Table I. Kinetic parameters for HCT2.
| Varying Substrate | Saturating Substratea | Kinetic Parameter |
||
| Km | Vmax | Vmax/Km | ||
| mm | nkat mg−1 | nkat mg−1 mm−1 | ||
| p-Coumaroyl-CoA | Malate | 1.81 ± 0.19 | 82 ± 8 | 45 |
| Caffeoyl-CoA | Malate | 1.26 ± 0.12 | 10 ± 1 | 7.9 |
| Feruloyl-CoA | Malate | 1.17 ± 0.14 | 62 ± 6 | 53 |
| Malate | p-Coumaroyl-CoA | 0.64 ± 0.06 | 56 ± 7 | 87 |
| Malate | Caffeoyl-CoA | 0.67 ± 0.06 | 7.2 ± 1.0 | 11 |
| p-Coumaroyl-malate | CoA | 0.20 ± 0.04 | 0.6 ± 0.1 | 3 |
| Phaselic acidb | CoA | nd | nd | nd |
Substrates were present at the following concentrations: malate, 3 mm; p-coumaroyl-CoA, 4 mm; caffeoyl-CoA, 4 mm; CoA, 3 mm.
nd, Kinetic parameters not determined because no reaction was detected.
Km and Vmax for the reverse reaction (transfer of a hydroxycinnamoyl moiety from a malate ester to CoA) were also determined for p-coumaroyl-malate (Table I). Comparison of Vmax/Km values for the forward and reverse reactions involving the p-coumaroyl derivatives indicate the forward reaction is strongly favored by HCT2. No reverse reaction was detected with phaselic acid as substrate, even under conditions of high enzyme concentration and extended incubation times (up to 24 h), suggesting this may be a metabolic end product in vivo.
Assessment of in Vivo Function of HCT2
To assess the in vivo role of HCT2 in biosynthesis of hydroxycinnamoyl-malate esters, particularly phaselic acid, HCT2 expression was down-regulated by transformation of a hairpin RNA interference gene-silencing construct into two regenerable red clover genotypes, NRC27 and NRC30. Eleven independently derived transgenic red clover plants were identified that had the silencing transgene integrated into their genome (data not shown). These plants were analyzed for HCT2 mRNA levels, phenotypes, and hydroxycinnamoyl-malate ester content.
HCT2 mRNA levels in newly expanded leaves of the 11 transgenic plants and the two corresponding untransformed wild-type controls were assessed by quantitative real-time PCR. As shown in Table II, the two wild-type plants had HCT2 mRNA levels within 2-fold of each other. All transgenic plants carrying the silencing transgene had reduced levels of HCT2 mRNA compared to either of the wild-type controls and six had <10% of wild-type levels of HCT2 mRNA. These results indicate the HCT2-silencing construct was effective at reducing HCT2 mRNA levels.
Table II. HCT2 mRNA levels, phenotypes, and hydroxycinnamoyl-malate ester content of HCT2-silenced red clover plants.
| Planta | Relative HCT2 mRNAb | Browning and Fluorescence | Ester Content |
|
| Phaselic Acid | p-Coumaroyl-Malate | |||
| mmol kg−1 fresh weight | ||||
| NRC27-8 | 0.01 | + | 4.20 | 1.83 |
| NRC27-7 | 0.01 | − | 0.19 | 0.09 |
| NRC27-9 | 0.02 | − | 0.94 | 0.43 |
| NRC30-1 | 0.03 | − | 0.16 | 0.06 |
| NRC27-6 | 0.04 | − | 0.10 | 0.06 |
| NRC27-14 | 0.05 | − | 0.54 | 0.08 |
| NRC30-2 | 0.13 | − | 0.07 | 0.04 |
| NRC27-3 | 0.16 | + | 7.19 | 1.28 |
| NRC27-4 | 0.20 | + | 6.00 | 2.16 |
| NRC27-11 | 0.28 | + | 6.59 | 2.28 |
| NRC27-5 | 0.33 | + | 5.48 | 1.55 |
| NRC30-WT | 0.55 | + | 6.83 | 2.21 |
| NRC27-WT | 1.00 | + | 6.55 | 0.57 |
Plants are designated by background genotype (NRC27 or NRC30). Numbers indicate independently derived transgenic lines. Untransformed wild-type control plants are indicated by WT.
Normalized to actin mRNA levels and expressed relative to NRC27-WT, the plant with the highest measured level of HCT2 mRNA.
Phenotypes of HCT2 Down-Regulated Red Clover Plants
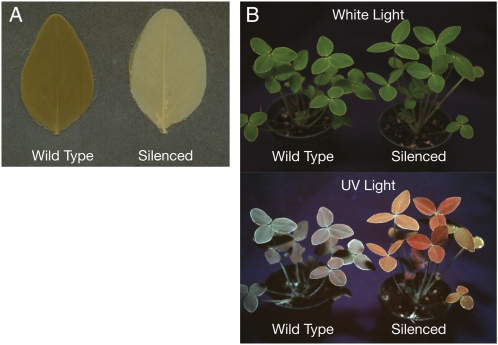
Despite greatly reduced HCT2 mRNA levels in several of the plants transformed with the HCT2-silencing transgene, none had any obvious morphological or developmental phenotypes. However, because phaselic acid is thought to be a major o-diphenol substrate for the PPO activity endogenous to red clover (Hatfield and Muck, 1999; Winters et al., 2008), and because secondary reactions of PPO-generated o-quinones result in postharvest browning in many plant tissues (Steffens et al., 1994), we assessed postharvest browning in red clover plants transformed with the HCT2-silencing construct. Allowing freshly excised leaves to wilt results in release of PPO from chloroplasts so that it can oxidize o-diphenols. To facilitate visualization of the browning products, chlorophyll was extracted from wilted leaves with ethanol. Leaves of six of the plants transformed with the HCT2-silencing construct had dramatic reductions in postharvest browning compared to wild-type plants (Table II). The reduced browning shown for the plant in Figure 2A was typical. With one exception (NRC27-8, discussed in more detail below), plants with the reduced browning phenotype corresponded to those with the lowest levels of HCT2 mRNA. This result strongly suggests the HCT2 gene product is required for the production of a PPO-oxidizable o-diphenol compound in red clover leaves.
Figure 2.
Phenotypes of HCT2 down-regulated plants. A, Leaflets from a wild-type or HCT2-silenced red clover plant were wilted and chlorophyll was extracted with ethanol to reveal extent of browning. B, Wild-type and HCT2-silenced plants were photographed under white or UV light. In the silenced plant, reduction in the levels of blue-fluorescing compounds reveals the pronounced red fluorescence of chlorophyll under UV light.
The strong UV-induced blue fluorescence of hydroxycinnamoyl compounds has been exploited to identify phenylpropanoid pathway mutants in Arabidopsis (Ruegger and Chapple, 2001). In these reduced epidermal fluorescence mutants, levels of blue-fluorescing compounds, particularly sinapoyl-malate, are reduced leading to more pronounced red fluorescence from chlorophyll. Because HCT2 down-regulation would be expected to similarly reduce hydroxycinnamoyl-malate content in red clover leaves if it were involved in making such esters, we examined the transgenic plants under UV light. The plant shown in Figure 2B (compared to its wild-type control) was typical of six of the red clover plants transformed with the HCT2-silencing construct: Blue fluorescence was greatly reduced, revealing bright-red chlorophyll fluorescence. These were the same plants that showed the reduced browning phenotype and, with the exception of NRC27-8, had the lowest levels of HCT2 mRNA. This finding indicates the plants transformed with the HCT2-silencing construct had substantial reductions in blue-fluorescing compounds in their leaves.
Phaselic Acid Content Is Reduced in HCT2 Down-Regulated Plants and Highly Correlated with HCT2 mRNA Levels in Red Clover
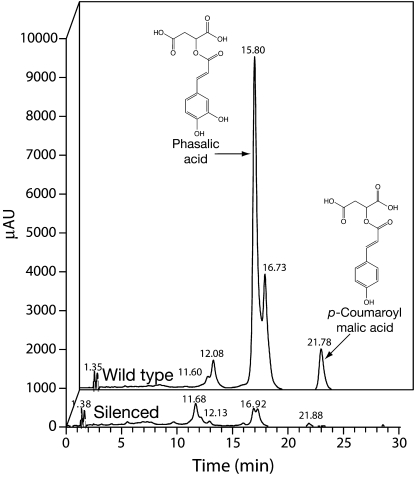
Soluble phenolics present in young leaves were analyzed for all the plants transformed with the HCT2-silencing construct and the two corresponding untransformed genotypes, NRC27 and NRC30. Leaves analyzed for phenolic content were harvested at the same time as leaves used for analysis of HCT2 mRNA. Figure 3 shows a typical HPLC trace for a transgenic plant with reduced browning and fluorescence phenotypes and its wild-type control. Peaks corresponding to phaselic acid and p-coumaroyl-malate were identified by comparing retention times and UV absorption spectra to those of authentic standards and the amount of these hydroxycinnamoyl-malate esters present in leaves were determined for all the plants (Table II). Phaselic acid and p-coumaroyl-malate content for the wild-type plants was similar to that previously reported by us (Sullivan, 2009) and similar to recent measurements we have made for these genotypes in other experiments (4.4–6.9 and 0.7–2.7 mmol kg−1 fresh weight for phaselic acid and p-coumaroyl-malate, respectively), although the p-coumaroyl-malate content measured for the NRC27 control plant here is the lowest level we have measured for a wild-type plant. Of the 11 transgenic plants analyzed, six had phaselic acid levels <15% of that seen for wild-type controls and of these four had <5% of wild-type levels of phaselic acid (Table II). Similar dramatic reductions in p-coumaroyl-malate levels were seen in these plants. The six plants with dramatic reductions in hydroxycinnamoyl-malate content corresponded to the plants with the reduced browning and reduced fluorescence phenotypes. In addition to the caffeoyl- and p-coumaroyl-malate esters, two other peaks, eluting at approximately 12.1 and approximately 16.7 min, showed reduced levels in the six silenced plants (Fig. 3). Although we have been unable to establish the identities of the compounds corresponding to these peaks, we have ruled out cinnamoyl-, feruloyl-, and sinapoyl-malate based on the elution behavior and UV spectra of standards (data not shown). Intermediates expected to precede HCT2 activity such as caffeic and p-coumaric acids or their corresponding CoA thiolesters did not appear to accumulate in the red clover plants transformed with the HCT2-silencing construct.
Figure 3.
Analysis of red clover leaf extracts for phenolic compounds by reverse-phase HPLC. Extracts from equivalent amounts of leaf material from wild-type (background) or HCT2-silenced (foreground) red clover were resolved by reverse-phase HPLC and elution was monitored using a UV/visible photodiode array detector (250–500 nm). Peaks corresponding to phaselic acid and p-coumaroyl-malate were identified by comparing retention times and UV absorption spectra to those of authentic standards.
Interestingly, NRC27-8, which did not exhibit reduced browning or fluorescence phenotypes, despite low measured levels of HCT2 mRNA, had wild-type levels of hydroxycinnamoyl-malate esters (Table II), underscoring the association of these phenotypes with hydroxycinnamoyl-malate content. It is unclear why hydroxycinnamoyl-malate ester content in NRC27-8 was not reduced, and unfortunately the plant was not available for reanalysis to rule out a technical error in mRNA or metabolite measurements. It should also be noted that four of the transgenic plants had moderately reduced HCT2 mRNA levels relative to wild type, but still had wild-type levels of hydroxycinnamoyl-malate esters and wild-type browning and fluorescence phenotypes.
Despite the outlying nature of the NRC27-8 plant, a best-fit logarithmic curve of HCT2 mRNA level versus phaselic acid content (not shown) has an r2 value of 0.55, indicating a highly significant (P < 0.005) correlation between HCT2 mRNA levels and phaselic acid content. A similar correlation was observed for HCT2 mRNA levels versus p-coumaroyl-malate content (r2 = 0.22, P < 0.11). Although the relationship is not as strong as that seen for phaselic acid, the weaker correlation between HCT2 mRNA levels and p-coumaroyl-malate content here is largely due to the NRC27-8 values. Exclusion of this outlier from the analysis suggests a highly significant correlation as well (r2 = 0.42, P < 0.03).
DISCUSSION
Red clover leaves accumulate phaselic acid (caffeoyl-malate) to levels >5 mmol kg−1 fresh weight. The related ester, p-coumaroyl-malate, accumulates as well, but usually to 3-fold or more lower levels (Winters et al., 2008; Sullivan, 2009). In Arabidopsis and other Brassicaceae, hydroxycinnamoyl-malate esters are synthesized via a hydroxycinnamoyl-Glc:malate hydroxycinnamoyl transferase (Grawe et al., 1992; Lehfeldt et al., 2000). Red clover appears to lack a similar hydroxycinnamoyl-Glc transferase, leading to the hypothesis that this species makes hydroxycinnamoyl-malate esters via the action of a hydroxycinnamoyl-CoA:malate hydroxycinnamoyl transferase (Sullivan, 2009) in a manner similar to that described for biosynthesis of chlorogenic acid (caffeoyl-quinate) in some solanaceous species (Niggeweg et al., 2004). We previously described a hydroxycinnamoyl-CoA hydroxycinnamoyl transferase from red clover capable of transferring p-coumaroyl and caffeoyl moieties to malate to form p-coumaroyl-malate and phaselic acid, respectively, in vitro, an activity consistent with our proposed model of phaselic acid biosynthesis (Sullivan, 2009). We sought to further characterize the activities of this transferase and establish its in vivo role in phaselic acid biosynthesis.
In a kinetic analysis of red clover HCT2, while Km values were similar for p-coumaroyl, caffeoyl-, and feruloyl-CoA acyl donors, Vmax/Km values were greater than 5-fold higher for transfer of the hydroxycinnamoyl moiety from feruloyl- and p-coumaroyl-CoA compared to that of caffeoyl-CoA (Table I). An 8-fold-higher Vmax/Km for p-coumaroyl versus caffeoyl-CoA was also seen in experiments measuring the Km of HCT2 for malate. These results are consistent with previous measurements that HCT2 shows an approximately 7-fold-higher specific activity for p-coumaroyl- versus caffeoyl-CoA (Sullivan, 2009). Although the enzyme is capable of transferring feruloyl moieties from the corresponding CoA derivative to malate in vitro, we have not detected feruloyl-malate in red clover leaves, suggesting that a limiting pool of feruloyl-CoA exists in red clover leaves, that feruloyl-CoA might be more readily utilized in a competing reaction, or that feruloyl-malate is readily metabolized in vivo.
Potential for reverse reaction with p-coumaroyl-malate or phaselic acid in the presence of CoA was also examined. In the case of p-coumaroyl substrates, Vmax/Km was 15-fold higher for the forward reaction than the reverse reaction. For phaselic acid, no reverse reaction was detected, even under conditions of high enzyme concentration and incubations up to 24 h. Together, these results suggest the role of HCT2 is primarily in the biosynthesis of malate esters rather than their degradation.
The in vitro enzymatic activities of HCT2 are consistent with a role in phaselic acid biosynthesis in vivo, so we directly tested this hypothesis by transforming red clover with a hairpin RNA interference gene-silencing construct. Analysis of the 11 independent transgenic and two wild-type control plants indicated that HCT2 mRNA levels are highly correlated (P < 0.005) with phaselic acid content. This correlation provides strong evidence that the HCT2 transferase plays a key role in the biosynthesis of phaselic acid in red clover, and establishes a pathway not previously described in plants for the biosynthesis of hydroxycinnamoyl-malate esters. This pathway, utilizing a hydroxycinnamoyl-CoA:malate rather than a hydroxycinnamoyl-Glc:malate transferase, may not be widespread among plants, as BLAST searches of GenBank fail to identify sequences encoding proteins with >60% identity to HCT2. Interestingly, although several of the plants in our study had moderately reduced levels of HCT2 mRNA, they had wild-type levels of hydroxycinnamoyl-malate esters (Table II), which may indicate that in vivo HCT2 transferase activity is in excess and another biosynthetic step may be limiting. One transgenic plant in our analysis, NRC27-8, had greatly reduced HCT2 mRNA levels, but wild-type levels of phaselic acid and p-coumaroyl-malate. It seems most likely this observation is the result of a technical error. Unfortunately NRC27-8 died before it could be reanalyzed. Nonetheless, the correlation between HCT2 mRNA levels and phaselic acid accumulation was highly significant (P < 0.005), even with inclusion of this outlying data point.
In our previously proposed model for phaselic acid biosynthesis, we suggested that phaselic acid could be synthesized in vivo by two nonmutually exclusive pathways (Sullivan, 2009). The more direct pathway (lower pathway of Fig. 1) would be transfer of a p-coumaroyl moiety to malic acid followed by hydroxylation by a C3′H of the resulting p-coumaroyl-malate to form phaselic acid. Although HCT2 is capable of transferring p-coumaroyl moieties to malate, red clover appears to lack a C3′H capable of hydroxylating p-coumaroyl-malate (Sullivan and Zarnowski, 2010). Still, the p-coumaroyl transferase activity of HCT2 likely explains accumulation of p-coumaroyl-malate in vivo. This idea is strongly supported by the observation that HCT2 down-regulation results in reductions in p-coumaroyl-malate as well as in phaselic acid levels (Table II).
The second pathway (top pathway of Fig. 1) whereby phaselic acid could be formed is less direct and would require formation of a p-coumaroyl-shikimate intermediate, possibly by HCT1 (Sullivan, 2009). Hydroxylation of this intermediate by C3′H would form the caffeoyl moieties that would ultimately be transferred to malate by HCT2. Although in vitro HCT2 appears to favor p-coumaroyl-CoA over caffeoyl-CoA as the acyl donor substrate (Table I; Sullivan, 2009), measurements of p-coumaroyl-CoA transferase activities in extracts of red clover leaves indicate that activity for transfer of p-coumaroyl moieties is 8-fold higher to shikimate than to malate (Sullivan, 2009). This finding suggests that in vivo, p-coumaroyl moieties are more likely to be transferred to shikimate where they could be subsequently hydroxylated to form caffeoyl moieties and ultimately transferred to malate by HCT2 to form phaselic acid. Thus, despite the apparent in vitro preference of HCT2 for p-coumaroyl-CoA, in vivo the relative ratio of transferase activity capable of transferring p-coumaroyl moieties to shikimate (HCT1) versus malate (HCT2) may determine the relative accumulation of phaselic acid versus p-coumaroyl-malate. In this scenario, down-regulation of HCT1 might result in decreased levels of phaselic acid (and perhaps increased levels of p-coumaroyl-malate). In the HCT2 down-regulation experiments carried out here, it is unlikely HCT1 mRNA levels would have been affected directly by the HCT2 gene-silencing construct since these share only very limited sequence identity (<50% with no stretches longer than 10 nucleotides). As noted above, we detected low but measurable activity for the reverse reaction for p-coumaroyl-malate but not for phaselic acid, suggesting once formed, phaselic acid may accumulate while p-coumaroyl-malate may be metabolized, which could also contribute to the higher relative accumulation of the caffeoyl versus the p-coumaroyl ester.
No obvious accumulation of metabolites preceding the HCT2 reaction (p-coumaric and caffeic acids and/or their CoA derivatives) was detected in HCT2 down-regulated plants (Fig. 3). This suggests levels of these intermediates may be regulated elsewhere in the pathway such that they do not accumulate when HCT2 activity is absent, or that they are readily utilized as substrates in other pathways in the absence of HCT2. Although our metabolite analysis would be expected to detect related phenylpropanoid compounds, it might have been too limited in scope to identify other newly accumulating metabolites.
HCT2 down-regulated plants that had reduced levels of phaselic acid showed reduced postharvest browning, supporting previous assertions that phaselic acid is one of the major o-diphenol compounds that is oxidized by PPO in red clover (Hatfield and Muck, 1999; Winters et al., 2008). In red clover, oxidation of endogenous o-diphenols by PPO prevents loss of true protein during harvest and storage by ensiling (Sullivan et al., 2004; Sullivan and Hatfield, 2006). Degraded protein is poorly utilized by ruminant animals such as dairy cows, leading to higher production costs due to the need to supplement rations with true protein and release of excess nitrogen into the environment. Because major forage crops like alfalfa lack both foliar PPO and o-diphenol substrates such as phaselic acid (Sullivan and Hatfield, 2006; Sullivan et al., 2008), HCT2 may be able to serve as a crucial component in engineering an o-diphenol biosynthetic pathway in such forage crops. Conversely, since browning is sometimes viewed as undesirable, for example in red clover hay production for horses, HCT2 could be an attractive genetic target to make varieties less prone to postharvest browning. It is possible, however, that reducing levels of hydroxycinnamoyl-malate esters in vivo could make the plants more susceptible to UV damage or other abiotic or biotic stresses in the field. The HCT2 down-regulated plants produced in this study could help address questions related to the in vivo roles of the high levels of phaselic acid that accumulate in red clover.
MATERIALS AND METHODS
Reagents
All purchased reagents were of molecular biology or higher grade. 2-O-(p-coumaroyl)-l-malate was prepared by the method described by Hemmerle et al. (1997). The NMR spectrum of the prepared compound was consistent with the assigned structure (1H-NMR [360 MHz, d6-acetone]: δ 2.95 [m, 2H, AB portion of an ABX pattern, δA = 3.20, δB = 2.92, JAB = 16.74 Hz], 5.54 [m, 1H, X portion of an ABX pattern, δX = 5.54, JAX = 3.85 Hz, JBX = 8.88 Hz], 6.39 [d, 1H, J = 15.9 Hz], 6.89 [d, 2H, J = 8.4 Hz], 7.57 [d, 2H, J = 8.4 Hz], 7.66 [d, 1H, J = 15.9 Hz]; 13C-NMR [90 MHz, d6-acetone]: δ δ 36.5, 69.1, 114.6, 116.7, 126.8, 131.1, 145.6, 160.7, 166.6, 170.6, 170.9). 2-O-caffeoyl-l-malate (phaselic acid) was so readily oxidized during its chemical synthesis that the authentic standard prepared in this manner was unavailable for this study. 2-O-caffeoyl-l-malate prepared enzymatically using HCT2 and whose identity was confirmed by UV spectroscopy and mass spectrometry was prepared as previously described (Sullivan, 2009).
Caffeoyl-, p-coumaroyl-, and feruloyl-CoA thiolesters were prepared using recombinant Arabidopsis (Arabidopsis thaliana) 4CL1 protein (Lee et al., 1995) produced in Escherichia coli using the pET30 expression vector (Novagen) as previously detailed (Sullivan, 2009). Concentrations of the thiolesters were determined spectrophotometrically using extinction coefficients of 21, 18, and 19 mm−1cm−1 for p-coumaroyl- (λmax = 333 nm), caffeoyl- (λmax = 346 nm), and feruloyl-CoA (λmax = 346 nm), respectively (Stoeckigt and Zenk, 1975).
Plant Material
For experiments with transgenic red clover (Trifolium pratense), two highly regenerable genotypes (designated NRC27 and NRC30) derived from a population of NewRC germplasm (Smith and Quesenberry, 1995) were used. These red clover genotypes were transformed via Agrobacterium-mediated transformation as previously described (Sullivan and Quesenberry, 2006). Wild-type (untransformed) and transformed red clover were grown in a greenhouse with temperatures maintained between 20°C and 30°C and light intensities between 400 and 1,000 μmol m−2 s−1. Supplemental lighting was used when day length was <13 h per day. Plants were fertilized weekly with Peter’s soluble 20-10-20 (Scott’s). For analysis of HCT2 mRNA levels and hydroxycinnamoyl ester content, newly expanded leaves with leaflets approximately 1.5 cm, were harvested, frozen in liquid nitrogen, and stored at −80°C until needed.
Determination of HCT2 Kinetic Parameters
Red clover HCT2 was produced in E. coli as previously described (Sullivan, 2009) and used in reaction mixtures containing 100 mm sodium phosphate (pH 6.5) and 25 mm ascorbate incubated at 30°C. All reactions were run in triplicate. For forward reactions (formation of hydroxycinnamoyl-malate esters), p-coumaroyl-, caffeoyl-, or feruloyl-CoA were used at concentrations ranging from 0.5 to 3 mm with 3 mm malate or malate used at concentrations of 0.05 to 3 mm with 4 mm p-coumaroyl- or caffeoyl-CoA. The highest concentration achievable for preparation of hydroxycinnamoy-CoA thiolesters limited the upper range of this component in these reactions. HCT2 protein was present at 11.2 μg mL−1 in reactions with p-coumaroyl- or feruloyl-CoA and 112 μg mL−1 for reactions with caffeoyl-CoA. For reverse reactions (formation of hydroxycinnamoyl-CoA from hydroxycinnamoyl-malate esters), p-coumaroyl-malate or phaselic acid were used at concentrations ranging from 0.05 to 3 mm with 3 mm CoA. HCT2 protein was present at 112 μg mL−1 for reactions with p-coumaroyl-malate and up to 1.12 mg mL−1 for reactions with phaselic acid. At various time points following addition of HCT2 to reaction mixtures, aliquots were removed from reaction mixtures and stopped by the addition of one-tenth volume 10% (v/v) acetic acid in water. Samples were processed, resolved by reverse-phase HPLC, and reaction products quantified using p-coumaric, caffeic, or ferulic acid standards as previously described (Sullivan, 2009). In the case of the reverse reactions, reaction progress was monitored by loss of the hydroxycinnamoyl-malate substrates. Reaction velocity values were determined from the initial linear portions of the reaction progress curves. The Km and Vmax values with their sds were calculated for all the enzyme assays by a nonlinear least-squares regression fit of the measured velocity and substrate concentration values to a hyperbola using Prism software (GraphPad Software). Standard deviations calculated were, in general, less than 12% of derived parameter values, indicating a good fit of the data to the Michaelis-Menten relationship.
Generation of HCT2 Down-Regulated Red Clover Plants
Approximately 700-bp fragments from the coding region of the red clover HCT2 gene were generated from a cloned cDNA (Sullivan, 2009) by PCR using the primer pairs 5′-GGGGGATCCCCAAGGCTTTGCACTTGGTG-3′ and 5′-GGGATCGATCCTGTCCAACTAGCTACTTG-3′ (to produce the sense arm) or 5′-GGGCTCGAGCCAAGGCTTTGCACTTGGTG-3′ and 5′-GGGGGTACCCCTGTCCAACTAGCTACTTG-3′ (to produce the antisense arm). The resulting fragments were digested with either XhoI and KpnI or BamHI and ClaI and cloned into the sense and antisense arms, respectively, of the intron-containing gene-silencing vector pHANNIBAL (Wesley et al., 2001). The silencing cassette of this pHANNIBAL construct was subcloned as a NotI fragment into the NotI site of the binary vector pART27 (Gleave, 1992) such that the promoters for the hairpin-silencing RNA and the nptII selectable marker of pATR27 were divergently transcribed. The resulting plasmid was transferred to Agrobacterium tumefaciens strain EHA101 (Hood et al., 1993) by triparental mating (Rogers et al., 1986) for transformation of NewRC red clover genotypes as described above. Red clover plants transformed with the silencing transgene were identified by PCR using the primers 5′-AGTTGGGAAATTGGGTTCGAAATCG-3′ and 5′-TCATTAAAGCAGGACTCTAGAGGATC-3′ that anneal to the pdk intron and OCS terminator, respectively, of the pHANNIBAL silencing cassette to amplify the intervening HCT2 antisense target sequence.
Qualitative and Quantitative Assessment of HCT2 Down-Regulated Red Clover
To assess PPO-mediated browning in red clover, leaflets were excised from plants and wilted in a 37°C incubator for 1 h. Following wilting, chlorophyll was extracted from the leaflets by incubation with several changes of ethanol.
Fluorescence of red clover leaves was assessed by illumination with a handheld UV lamp (365 nm) and digital photography using a yellow filter essentially as described by Ruegger and Chapple (2001).
To assess HCT2 mRNA levels, cDNA was prepared from RNA of newly expanded leaves and quantitative real-time PCR was carried out using SYBR Green PCR master mix (Applied Biosystems) as described previously (Sullivan, 2009) except 5′-TTGGACAGGAATGCCCTTTT-3′ and 5′-GTGGAGACACACCAGCATAACC-3′ were used as the HCT2-specific primers and signals were normalized to actin mRNA levels determined using the primers 5′-GTGTGAGTCACACTGTGCCAATC-3′ and 5′-ACGGCCAGCAAGATCCAA-3′.
Levels of phaselic acid and p-coumaroyl-malate in newly expanded leaves were determined as previously described (Sullivan, 2009). To assess the correlation of HCT2 mRNA levels and hydroxycinnamoyl-malate content, phaselic acid or p-coumaroyl-malate content was plotted as a function of HCT2 mRNA level for 11 independent transgenic plants containing the HCT2-silencing transgene and the two untransformed genotypes from which the transgenic plants were derived. The best-fit curve and corresponding correlation coefficient were determined using Excel (Microsoft). Significance of the correlation was determined using Student’s t test (Samuels, 1989).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers EU861219 and EU861218.
Acknowledgments
We thank Lisa Koch and Jed Becker for excellent technical assistance; Paul Schatz for providing hydroxycinnamoyl ester standards; Clint Chapple, Jo Cusumano, and Nick Bonawitz for providing the 4CL expression construct and helpful advice; Jane Maria and Ron Hatfield for technical advice; and Julian Verdonk and Heather Green for helpful comments on the manuscript. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
References
- D’Auria JC. (2006) Acyltransferases in plants: a good time to be BAHD. Curr Opin Plant Biol 9: 331–340 [DOI] [PubMed] [Google Scholar]
- Gleave AP. (1992) A versatile binary vector system with a T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol 20: 1203–1207 [DOI] [PubMed] [Google Scholar]
- Grawe W, Bachhuber P, Mock HP, Strack D. (1992) Purification and characterization of sinapolyglucose—malate sinapoyltransferase from Raphanus-sativus L. Planta 187: 236–241 [DOI] [PubMed] [Google Scholar]
- Hatfield RD, Muck R. (1999) Characterizing proteolytic inhibition in red clover silage. XIIth International Silage Conference. Swedish University of Agriculture and Sciences, Uppsala, pp 147–148 [Google Scholar]
- Hemmerle H, Burger HJ, Below P, Schubert G, Rippel R, Schindler PW, Paulus E, Herling AW. (1997) Chlorogenic acid and synthetic chlorogenic acid derivatives: novel inhibitors of hepatic glucose-6-phosphate translocase. J Med Chem 40: 137–145 [DOI] [PubMed] [Google Scholar]
- Hoffmann L, Besseau S, Geoffroy P, Ritzenthaler C, Meyer D, Lapierre C, Pollet B, Legrand M. (2004) Silencing of hydroxycinnamoyl-coenzyme A shikimate/quinate hydroxycinnamoyltransferase affects phenylpropanoid biosynthesis. Plant Cell 16: 1446–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann L, Maury S, Martz F, Geoffroy P, Legrand M. (2003) Purification, cloning, and properties of an acyltransferase controlling shikimate and quinate ester intermediates in phenylpropanoid metabolism. J Biol Chem 278: 95–103 [DOI] [PubMed] [Google Scholar]
- Hood EE, Gelvin SB, Melchers LS, Hoekema A. (1993) New Agrobacterium helper plasmids for gene-transfer to plants. Transgenic Res 2: 208–218 [Google Scholar]
- Jones BA, Hatfield RD, Muck RE. (1995) Screening legume forages for soluble phenols, polyphenol oxidase and extract browning. J Sci Food Agric 67: 109–112 [Google Scholar]
- Landry LG, Chapple CCS, Last RL. (1995) Arabidopsis mutants lacking phenolic sunscreens exhibit enhanced ultraviolet-B injury and oxidative damage. Plant Physiol 109: 1159–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Ellard M, Wanner LA, Davis KR, Douglas CJ. (1995) The Arabidopsis thaliana 4-coumarate:CoA ligase (4CL) gene: stress and developmentally regulated expression and nucleotide sequence of its cDNA. Plant Mol Biol 28: 871–884 [DOI] [PubMed] [Google Scholar]
- Lehfeldt C, Shirley AM, Meyer K, Ruegger MO, Cusumano JC, Viitanen PV, Strack D, Chapple C. (2000) Cloning of the SNG1 gene of Arabidopsis reveals a role for a serine carboxypeptidase-like protein as an acyltransferase in secondary metabolism. Plant Cell 12: 1295–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggeweg R, Michael AJ, Martin C. (2004) Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat Biotechnol 22: 746–754 [DOI] [PubMed] [Google Scholar]
- Rogers SG, Horsch RB, Fraley RT. (1986) Gene-transfer in plants—production of transformed plants using Ti-plasmid vectors. Methods Enzymol 118: 627–640 [Google Scholar]
- Ruegger M, Chapple C. (2001) Mutations that reduce sinapoylmalate accumulation in Arabidopsis thaliana define loci with diverse roles in phenylpropanoid metabolism. Genetics 159: 1741–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels ML. (1989) Statistics for the Life Sciences. Dellen Publishing, San Francisco, p 472 [Google Scholar]
- Shadle G, Chen F, Srinivasa Reddy MS, Jackson L, Nakashima J, Dixon RA. (2007) Down-regulation of hydroxycinnamoyl CoA: shikimate hydroxycinnamoyl transferase in transgenic alfalfa affects lignification, development and forage quality. Phytochemistry 68: 1521–1529 [DOI] [PubMed] [Google Scholar]
- Smith RR, Quesenberry KH. (1995) Registration of NewRC red-clover germplasm. Crop Sci 35: 295 [Google Scholar]
- Steffens JC, Harel E, Hunt MD. (1994) Polyphenol oxidase. Ellis BE, Kuroki GW, Stafford HA, , Genetic Engineering of Plant Secondary Metabolism, Vol 28. Plenum Press, New York, pp 275–312 [Google Scholar]
- Stoeckigt J, Zenk MH. (1975) Chemical synthesis and properties of hydroxycinnamoyl-coenzyme A derivatives. Z Naturforsch C 30C: 352–358 [DOI] [PubMed] [Google Scholar]
- Sullivan ML. (2009) A novel red clover hydroxycinnamoyl transferase has enzymatic activities consistent with a role in phaselic acid biosynthesis. Plant Physiol 150: 1866–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan ML, Bringe A. (2005) A plate-based browning assay to screen alfalfa germplasm for polyphenol oxidase activity and o-diphenols. 2004 Research Report. U.S. Dairy Forage Research Center, Madison, WI, pp 13–15 [Google Scholar]
- Sullivan ML, Hatfield RD. (2006) Polyphenol oxidase and o-diphenols inhibit postharvest proteolysis in red clover and alfalfa. Crop Sci 46: 662–670 [Google Scholar]
- Sullivan ML, Hatfield RD, Samac DA. (2008) Cloning of an alfalfa polyphenol oxidase gene and evaluation of its potential in preventing postharvest protein degradation. J Sci Food Agric 88: 1406–1414 [Google Scholar]
- Sullivan ML, Hatfield RD, Thoma SL, Samac DA. (2004) Cloning and characterization of red clover polyphenol oxidase cDNAs and expression of active protein in Escherichia coli and transgenic alfalfa. Plant Physiol 136: 3234–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan ML, Quesenberry KH. (2006) Red clover (Trifolium pratense). Wang K, , Agrobacterium Protocols, Ed 2 Humana Press, Totowa, NJ, pp 369–383 [DOI] [PubMed] [Google Scholar]
- Sullivan ML, Zarnowski R. (2010) Red clover coumarate 3′-hydroxylase (CYP98A44) is capable of hydroxylating p-coumaroyl-shikimate but not p-coumaroyl-malate: implications for the biosynthesis of phaselic acid. Planta 231: 319–328 [DOI] [PubMed] [Google Scholar]
- Wesley SV, Helliwell CA, Smith NA, Wang MB, Rouse DT, Liu Q, Gooding PS, Singh SP, Abbott D, Stoutjesdijk PA, et al. (2001) Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J 27: 581–590 [DOI] [PubMed] [Google Scholar]
- Winters AL, Minchin FR, Michaelson-Yeates TPT, Lee MRF, Morris P. (2008) Latent and active polyphenol oxidase (PPO) in red clover (Trifolium pratense) and use of a low PPO mutant to study the role of PPO in proteolysis reduction. J Agric Food Chem 56: 2817–2824 [DOI] [PubMed] [Google Scholar]