Abstract
Jasmonates are ubiquitously occurring plant growth regulators with high structural diversity that mediate numerous developmental processes and stress responses. We have recently identified 12-O-β-d-glucopyranosyljasmonic acid as the bioactive metabolite, leaf-closing factor (LCF), which induced nyctinastic leaf closure of Samanea saman. We demonstrate that leaf closure of isolated Samanea pinnae is induced upon stereospecific recognition of (−)-LCF, but not by its enantiomer, (+)-ent-LCF, and that the nonglucosylated derivative, (−)-12-hydroxyjasmonic acid also displays weak activity. Similarly, rapid and cell type-specific shrinkage of extensor motor cell protoplasts was selectively initiated upon treatment with (−)-LCF, whereas flexor motor cell protoplasts did not respond. In these bioassays related to leaf movement, all other jasmonates tested were inactive, including jasmonic acid (JA) and the potent derivates JA-isoleucine and coronatine. By contrast, (−)-LCF and (−)-12-hydroxyjasmonic acid were completely inactive with respect to activation of typical JA responses, such as induction of JA-responsive genes LOX2 and OPCL1 in Arabidopsis (Arabidopsis thaliana) or accumulation of plant volatile organic compounds in S. saman and lima bean (Phaseolus lunatus), generally considered to be mediated by JA-isoleucine in a COI1-dependent fashion. Furthermore, application of selective inhibitors indicated that leaf movement in S. saman is mediated by rapid potassium fluxes initiated by opening of potassium-permeable channels. Collectively, our data point to the existence of at least two separate JA signaling pathways in S. saman and that 12-O-β-d-glucopyranosyljasmonic acid exerts its leaf-closing activity through a mechanism independent of the COI1-JAZ module.
Jasmonic acid (JA) and its derivatives, collectively referred to as jasmonates, play important roles in controlling growth, development, and responses to environmental changes in higher plants (Devoto and Turner, 2003; Wasternack, 2007; Howe and Jander, 2008; Browse, 2009; Wasternack and Kombrink, 2010). Jasmonates exert their function by activation of a large number of genes mediated in part by the transcription factor MYC2 (Lorenzo et al., 2004; Dombrecht et al., 2007; Fonseca et al., 2009a). Elucidation of the mode of action of jasmonates has long been hampered by the fact that plants contain many jasmonate derivatives, biosynthetic precursors, and JA metabolites, which may differ considerably in their biological activities (Krumm et al., 1995; Kramell et al., 1997; Stintzi et al., 2001; Staswick and Tiryaki, 2004; Taki et al., 2005; Miersch et al., 2008), and the complex structure of the jasmonate target, which is composed of two units, the F-box protein COI1 and a JAZ protein representing the core signaling module (Chini et al., 2007; Thines et al., 2007). The analysis of two Arabidopsis (Arabidopsis thaliana) mutants was essential for unraveling the mode of action of jasmonates, coi1 and jar1, both defective in jasmonate response (Feys et al., 1994; Staswick et al., 2002). While the coi1 mutant was found to be impaired in almost all jasmonate responses (Feys et al., 1994; Katsir et al., 2008), the jar1 mutant was deficient in jasmonoyl-l-Ile (JA-Ile; Staswick and Tiryaki, 2004). This allowed the recent identification of additional signaling components, such as the JAZ repressor proteins that impair MYC2 activity (Lorenzo et al., 2004; Chini et al., 2007; Dombrecht et al., 2007; Thines et al., 2007). The current picture of JA perception and signaling cascade involves the SCFCOI1 complex operating as E3 ubiquitin ligase, which upon binding of JA-Ile targets JAZ repressors for degradation by the 26S-proteasome, thereby releasing the transcription factor MYC2 and allowing activation of gene expression (Fonseca et al., 2009b; Wasternack and Kombrink, 2010). Recently it was demonstrated that coreceptor complex consisting of COI1, JAZ, and inositol pentakisphosphate strongly binds to (+)-7-iso-JA-Ile and thus functions as jasmonate receptor (Sheard et al., 2010). Importantly, only the (+)-7-iso-JA-Ile stereoisomer shows high bioactivity and is therefore considered the most important plant endogenous JA metabolite (Fonseca et al., 2009b).
Other jasmonate derivatives also occur abundantly in plant tissues, including biosynthetic precursors and breakdown products (Miersch et al., 2008). 12-Hydroxyjasmonic acid (12-OH-JA), also known as tuberonic acid, was first isolated from potato (Solanum tuberosum) leaves (Yoshihara et al., 1989), Solanum demissum (Helder et al., 1993), and hairy root culture of tomato (Solanum lycopersicum; Abdala et al., 2003). It was shown to be inactive in mediating typical JA responses, such as tendril coiling (Blechert et al., 1999), inhibition of root growth and seed germination (Miersch et al., 2008), or activation of JA-responsive genes in barley (Hordeum vulgare), tomato, and Arabidopsis (Miersch et al., 1999, 2008; Gidda et al., 2003; Kienow et al., 2008). Thus, 12-OH-JA, as well as its sulfated and glycosylated derivatives, is considered as a by-product of switching off jasmonate signaling by the metabolic conversion of bioactive JA into inactive metabolites, and its broad and abundant occurrence in many different plant species suggests that this is a common mechanism (Miersch et al., 2008). By contrast, 12-OH-JA was also described to function as tuber-inducing factor in Solanaceous species (Yoshihara et al., 1989).
We recently identified 12-O-β-d-glucopyranosyljasmonic acid (12-O-Glc-JA) as the bioactive metabolite, leaf-closing factor (LCF), which induces leaf closure of Samanea saman (Ueda et al., 2000; Ueda and Nakamura, 2006, 2007). S. saman has been used as a model plant in the studies on nyctinastic leaf movement of legumes (Palmer and Asprey, 1958a, 1958b; Satter et al., 1974; Moran, 2007). 12-O-Glc-JA (also referred to as tuberonic acid glucoside), like the aglycon 12-OH-JA showed tuber-inducing activity in potato, and again the (+)-7-iso epimer (cis configuration of substituents at the cyclopenanone ring) is the genuine bioactive form (Yoshihara et al., 1989). Thus, the induction of leaf movement in S. saman and the initiation of tuber formation in potato are two examples of genus- and organ-specific bioactivity of 12-O-Glc-JA.
Using two specific enantiomers of synthetically modified LCF as chemical probes, we have previously identified a membrane protein as putative target of LCF, which we named MTJG (membrane target protein of jasmonate glucoside), in the motor cells of S. saman (Nakamura et al., 2008b). MTJG is expected to be involved in LCF-mediated shrinking of the motor cells, which eventually leads to leaf-closing movement.
In view of the fact that the bioactivity of LCF seems completely different from that of other jasmonates, we wanted to analyze the impact of various jasmonate derivatives on nyctinastic leaf movement in S. saman, and likewise address the question whether or not other jasmonate responses are activated by LCF. In this article, we demonstrate that the leaf-closing activity is a unique property of 12-O-Glc-JA and that other jasmonate derivatives are inactive. Our results imply the existence of an additional signaling pathway or mode of action for jasmonates that is distinct from the established COI1-JA-Ile-JAZ route.
RESULTS
Leaf-Closing Activity Is a Unique Feature of 12-OH-JA Derivatives
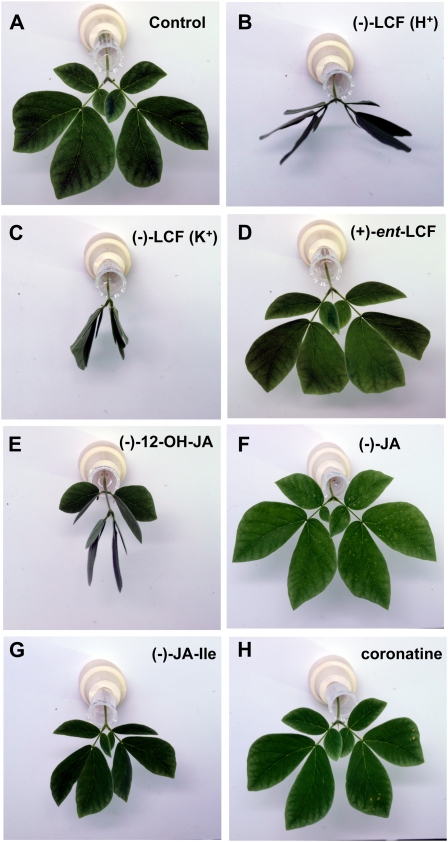
Hydroxylation of JA to 12-OH-JA is considered as one mechanism by which JA is inactivated (Blechert et al., 1999; Miersch et al., 1999, 2008). However, the fact that 12-OH-JA is also a building block of the LCF (12-O-Glc-JA) from S. saman raised the question whether it would display similar bioactivity. We therefore tested leaf-closing activities of 12-OH-JA and of various other jasmonates, including (−)-JA, (−)-JA-Ile, and the jasmonate mimic coronatine (for structures see Fig. 1), in relation to (−)-LCF (as K+-salt and H+-form) and inactive (+)-ent-LCF (Nakamura et al., 2008b). The leaf-closing assay was carried out with pinnae of S. saman containing secondary and tertiary pulvini, which were disconnected from seedlings and conditioned to one more light-dark cycle (16/8 h), during which they continued their leaf opening-closing movement. In the subsequent (second) light period, the pinnae were supplied with solutions of test compounds and leaf movement camera recorded for 48 h. Figure 2 shows the status of each pinna at 10 h of the second light-dark cycle, i.e. 6 h after initiation of treatment. Since the closing movement appeared most prominently on tertiary pulvini, their status was used to evaluate bioactivity. It is obvious that (−)-LCF induced leaf closure most effectively when compared to all jasmonates tested (Fig. 2), irrespective of whether the H+- or K+-form of LCF was used. However, the stereochemistry of LCF strongly affected activity, because (+)-ent-LCF was completely ineffective in this bioassay (Fig. 2D). (−)-12-OH-JA also induced leaf closure (Fig. 2E). In contrast, all other jasmonates tested did not initiate leaf-closing movement, despite the fact that they comprised the most effective activators of other typical jasmonate responses, (−)-JA-Ile and (+)-coronatine (Fig. 2, F–H). These results suggest that the leaf-closing activity is a unique feature of (−)-LCF.
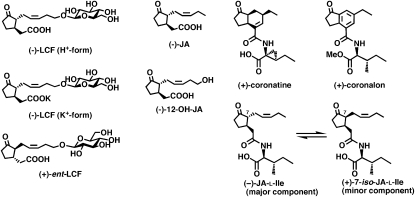
Figure 1.
Structures of (−)-JA, (−)-LCF (as K+-salt and H+-form), (+)-ent-LCF, (−)-12-OH JA, (−)-JA-Ile [shown as a mixture of (−)-JA-Ile and (+)-7-iso-JA-Ile], and (+)-coronatine. JA-Ile is known to exist as a 95:5 ratio of (−)-JA-Ile and (+)-7-iso-JA-Ile, respectively.
Figure 2.
Leaf-closing activities of different jasmonate derivatives using pinnae of S. saman. The leaf-closing assay was carried out using pinna of S. saman containing secondary and tertiary pulvini, which had been isolated from seedlings conditioned to a 16/8-h light-dark cycle for a week. A closing-opening movement of a pair of pinnule continues after they are disconnected from the stem and their behavior was monitored after addition of different jasmonate at 4 h of the second light cycle. The sections shows the status of pinnae at 10 h of the second light cycle after treatment for a period of 6 h with the following compounds: A, Water; B, (−)-LCF (H+-form); C, (−)-LCF (K+-form); D, (+)-ent-LCF; E, (−)-12-OH-JA; F, (−)-JA; G, (−)-JA-Ile (each at 500 μm); or H, (+)-coronatine (at 5 μm). The experiment was repeated three times with three replicates each.
LCF Initiates Volume Changes in Extensor Motor Cell Protoplasts of S. saman
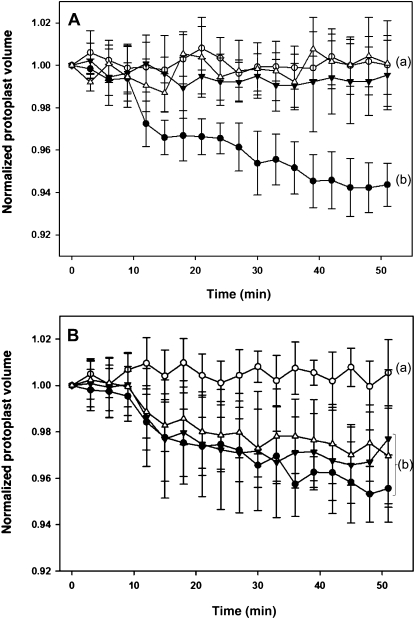
During nyctinastic leaf closure, the extensor motor cells that reside on the adaxial side of tertiary pulvinus shrink, whereas the flexor motor cells that reside on the abaxial side swell (Coté, 1995). This opposite volume change around the vascular bundle is also verified to trigger turgor changes in extensor motor cells for the secondary pulvinus of S. saman (Palmer and Asprey, 1958a; Satter et al., 1974) and laminar pulvinus of lima bean (Phaseolus vulgaris; Millet et al., 1989). Localization studies using a fluorescein isothiocyanate-labeled (−)-LCF have revealed that extensor motor cells in pulvini of S. saman are the targets of (−)-LCF, whereas no binding of (−)-LCF to flexor motor cell was observed (Nakamura et al., 2006). We now addressed the question, whether (−)-LCF has the capacity to induce rapid volume changes in extensor and/or flexor motor cell. Therefore we microscopically monitored the cell volume changes in protoplasts isolated from the extensor or flexor side of tertiary pulvini of S. saman (Gorton and Satter, 1984). As expected from the in vivo studies, (−)-LCF treatment induced shrinking of extensor motor cells, whereas no volume changes were recorded for flexor motor cell protoplasts (Fig. 3A). The shrinking of protoplasts started very rapidly after addition of (−)-LCF and accounted for about 5% in volume change within 40 to 50 min (Fig. 3A). Furthermore, when the concentration dependency was analyzed, we found that (−)-LCF caused significant protoplast shrinkage at a range from 1 to 100 μm (Fig. 3B). Because of their selective response, all subsequent experiments comparing the bioactivity of different jasmonates were carried out with protoplasts prepared from extensor motor cells.
Figure 3.
Cell-shrinking assay using motor cell protoplast of S. saman. Protoplasts were prepared from separated half segments of tertiary pulvini of S. saman, i.e. from the extensor or flexor side, respectively. A, LCF initiates shrinkage only in protoplasts derived from extensor cells but not flexor cells. The volume of extensor cell protoplasts (black circles) or flexor cell protoplasts (black triangles) was monitored for 51 min after treatment with (−)-LCF (100 μm) and compared to the untreated control cells (white circles and white triangles, respectively). B, (−)-LCF was applied at different concentrations to extensor cell protoplasts and their shrinking was monitored for 51 min; 100 μm (−)-LCF (black circles), 10 μm (−)-LCF (black triangles), 1 μm (−)-LCF (white triangles), and water control (white circles). The values represent the normalized mean volume (±sd) of six to 12 protoplasts. Different letters indicate significant differences between the terminal measurement (i.e. t = 51 min; Student-Newman-Keuls post-hoc test: P < 0.05, after univariate ANOVA with treatment as fixed factor and time as random factor: A, F3,25 = 26.99; B, F3,21 = 7.47; P < 0.001).
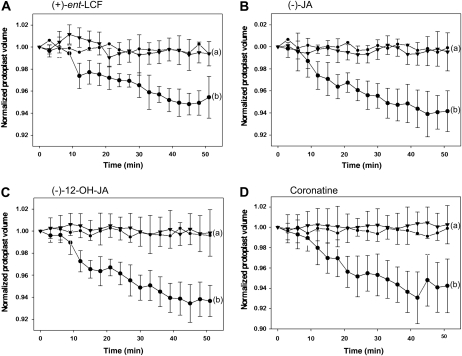
To test for the specificity of the response, we first asked whether the cell-shrinking assay would reflect the stereospecific recognition of active (−)-LCF versus inactive (+)-ent-LCF. Indeed, (+)-ent-LCF did not induce any shrinking of extensor motor cell protoplasts, quite in contrast to (−)-LCF (Fig. 4A). This result strongly supports the involvement of MTJG in stereo-specific recognition of the ligand (−)-LCF (Nakamura et al., 2008a). Next we extended the analysis to other jasmonates and observed that (−)-JA, (−)-12-OH-JA, and (+)-coronatine likewise did not induce shrinking of the extensor motor cells (Fig. 4, B–D). Intriguingly, 12-OH-JA did not cause cell shrinking although it showed some activity in the leaf-closing assay (Fig. 2E). This apparent discrepancy can be explained by different sensitivity and duration of the assays and the lower specific activity of 12-OH-JA, which may be revealed in a long-term experiment by not as a rapid read out. In conclusion, cell-shrinking activity is only associated with (−)-LCF but not with other jasmonates.
Figure 4.
The response of extensor motor cell protoplast of S. saman to various jasmonates. LCF enantiomers differ in their effectiveness to induce extensor cells shrinkage. A, Extensor cell protoplasts were treated with 100 μm (+)-ent-LCF (black triangles), and the response compared to treatment with 100 μm (−)-LCF (black circles) and untreated water control (small black circles). B, Extensor cell protoplast shrinkage in response to 100 μm (−)-JA (black triangles) in comparison to control treatments (as in A). C, Extensor cell protoplast shrinkage in response to 100 μm (−)-12-OH-JA (black triangles) in comparison to control treatments (as in A). D, Extensor cell protoplast shrinkage in response to 5 μm coronatine (black triangles) in comparison to control treatments (as in A). The values represent the normalized mean volume (±sd) of six to 12 protoplasts. Different letters indicate significant differences between the terminal measurement (i.e. t = 51 min; Student-Newman-Keuls post-hoc test: P < 0.05, after univariate ANOVA with treatment as fixed factor and time as random factor: A, F2,19 = 19.99; B, F2,19 = 21.03; C, F2,23 = 32.69; D, F2,24 = 26.31; P < 0.001).
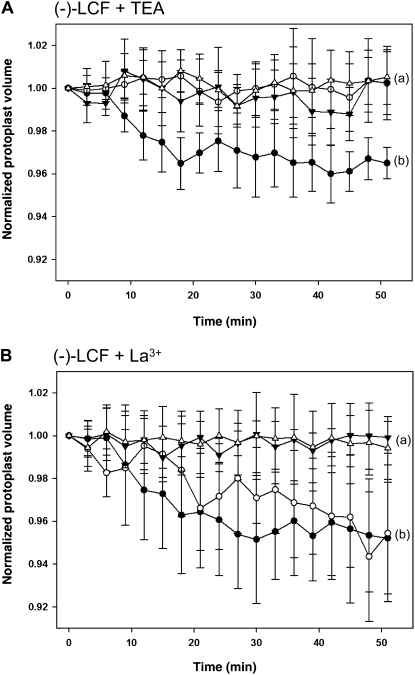
To investigate whether specific ion fluxes are involved in the turgor changes that eventually lead to cell volume changes in motor cells upon (−)-LCF treatment, we performed shrinking assays in the presence of selective inhibitors. Tetraethylammonium (TEA), a commonly used potent K+ channel blocker, impaired the (−)-LCF-induced cell-shrinking process, indicating the involvement of K+ efflux systems in the LCF-mediated pulvini response (Fig. 5A). By contrast, the addition of La3+, a Ca2+ channel blocker, did not inhibit LCF-mediated motor cell shrinkage (Fig. 5B).
Figure 5.
Influence of channel blockers on (−)-LCF-induced shrinkage of extensor motor cell protoplasts. Isolated extensor motor cell protoplasts were preincubated for 30 min with 1 mm TEA (A) or 0.5 mm LaCl3 (B) before 100 μm (−)-LCF was added to initiate protoplasts shrinking. The response of extensor protoplasts to treatment with (−)-LCF and inhibitor (white circles) is compared to the response caused by (−)-LCF only (black circles), untreated control cells (black triangles), and extensor cells treated with inhibitor only (white triangles). The values represent the normalized mean volume (±sd) of six to 12 protoplasts. Different letters indicate significant differences between the terminal measurement (i.e. t = 51 min; Student-Newman-Keuls post-hoc test: P < 0.05, after univariate ANOVA with treatment as fixed factor and time as random factor: A, F3,23 = 13.89; B, F3,22 = 8.40; P < 0.001).
LCF Does Not Activate Typical JA-Responsive Genes
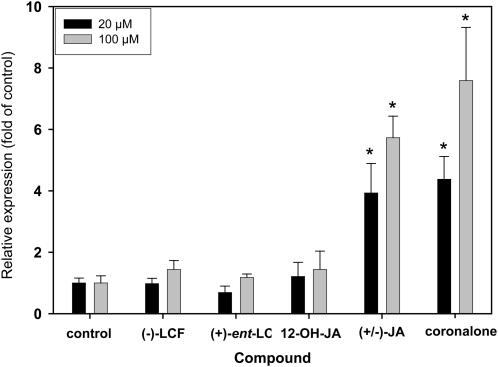
Next we examined whether (−)-LCF is capable of modulating other, typical jasmonate responses, such as activation of specific marker genes. Therefore we employed transgenic Arabidopsis lines harboring the GUS reporter under the control of the LOX2 or the OPCL1 promoter, which have previously been used to assess the activity of different jasmonate derivatives (Kienow et al., 2008). It is obvious from the results presented in Figure 6 that JA and coronalon (for structures see Fig. 1), a powerful synthetic JA analog (Schüler et al., 2004), strongly activated the LOX2p::GUS reporter gene in a concentration-dependent manner. In contrast, (−)-LCF, (+)-ent-LCF, and (−)-12-OH-JA were essentially inactive. Virtually the same result was obtained using the OPCL1p::GUS reporter line (data not shown). Thus, (−)-LCF and (−)-12-OH-JA cannot trigger typical JA-activated processes that were previously shown to be mediated by the COI1-JA-Ile-JAZ signaling module (Chung et al., 2008).
Figure 6.
Activation of the LOX2 promoter by different jasmonates. Arabidopsis plants homozygous for a single insertion of the reporter gene, comprising a 1.8-kb promoter of the LOX2 gene (Jensen et al., 2002), were grown in liquid culture for 2 weeks and subsequently treated with the indicated compounds (at 20 or 100 μm) for 24 h. Control treatment was with DMSO only. GUS activity was quantified in plant extracts using 4-methylumbelliferyl-β-d-glucuronide as substrate in a fluorimetric assay and related to the protein concentration. Activities are normalized to the control treatment (DMSO) and thus represent fold activation. The data represent the average of at least three biological replicates (±sd) and GUS activities that were significantly different compared to control treatment at P < 0.01 (Student’s t test) are indicated (*). Similar values were obtained using an Arabidopsis line harboring the jasmonate-responsive OPCL1p::GUS reporter gene (Kienow et al., 2008).
Comparison of JA and LCF in Mediating Volatile Emission from Plants
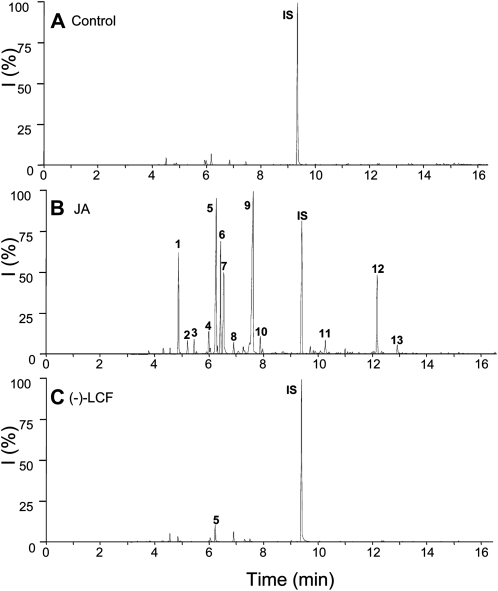
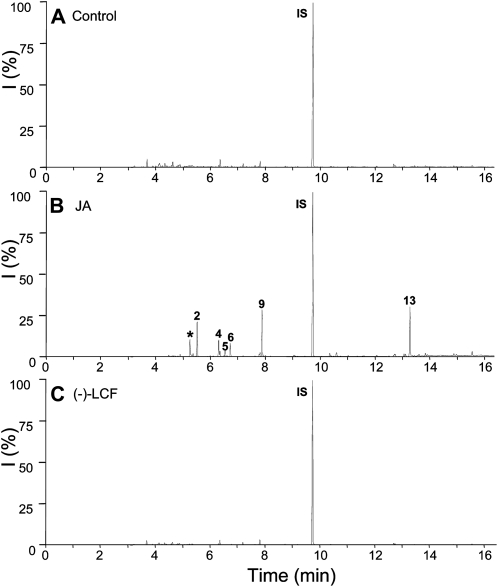
Emission of volatiles from plants under attack by herbivores is another well-documented jasmonate-mediated defense response, which can also be stimulated by exogenous application of jasmonates (Hopke et al., 1994; Boland et al., 1995). We compared the effectiveness of (−)-JA and (−)-LCF to induce volatile emission from S. saman (Fig. 7) and lima bean (Fig. 8). However, in both plants only (−)-JA induced emission of typical volatiles, such as β-ocimene, linalool, 4,8-dimethylnona-1,3,7-triene (DMNT), methyl salicylate, and methyl jasmonate (Figs. 7 and 8). In contrast, (−)-LCF did not activate this particular type of defense response leading to the biosynthesis of specific secondary metabolites. This result underscores that the physiological role and mode of action of (−)-LCF is restricted to controlling leaf closure and thus distinct from that of other jasmonates, such as (−)-JA or JA-Ile.
Figure 7.
Profiles of induced volatile organic compounds emitted from S. saman leaves. S. saman plants were treated either with water as a control (A), JA at 500 μm (B), or (−)-LCF at 500 μm (C). Headspace volatiles were collected for 24 h after initiation of treatment. Compounds were separated by gas chromatography and identified by mass spectrometry: 1, Eucalyptol; 2, β-ocimene; 3, unknown; 4, linalool; 5, DMNT; 6, C10H14; 7, unknown; 8, contamination; 9, methyl salicylate; 10, 3-hexenyl-iso-valerate; 11, β-caryophyllene; 12, 4,8,12-trimethyltrideca-1,3,7,11-tetraene; 13, methyl jasmonate; IS, internal standard (1-bromodecane). All experiments have been conducted at least four times with similar results.
Figure 8.
Profiles of induced volatile organic compounds emitted from lima bean leaves. Lima bean plants were treated either with water as a control (A), JA at 500 μm (B), or (−)-LCF at 500 μm (C). Headspace volatiles were collected for 24 h after initiation of treatment. Compounds were separated by gas chromatography and identified by mass spectrometry: 2, β-ocimene; 4, linalool; 5, DMNT; 6, C10H14; 7, unknown; 9, methyl salicylate; 13, methyl jasmonate; IS, internal standard (1-bromodecane); *, contamination. All experiments have been conducted at least four times with similar results.
DISCUSSION
The Leaf-Closing Activity of 12-O-Glc-JA Is Mediated by Selective Shrinking of Extensor Motor Cells
Rapid leaf movement is a quite common feature within the touch-sensitive Mimosoideae subfamily of the legume family, Fabaceae. It is not known exactly why plants have evolved this trait, but it has been suggested that the plant uses its ability to shrink as a defense from predators. Oscillating leaf movements, controlled by the circadian clock with a cycle of about 24 h, is known as nyctinasty and widely observed in plants including, but not restricted to, leguminous plants such as Mimosa pudica and S. saman, which are established model plants for leaf movement.
Nyctinastic leaf closure of S. saman has been attributed to volume changes in specific motor cells located on opposite side of the vascular bundle: The extensor cells that reside on the adaxial side of tertiary pulvinus shrink, whereas the flexor cells that reside on the abaxial side swell during leaf closure (Gorton and Satter, 1984; Coté, 1995). We have recently identified 12-O-Glc-JA as the bioactive LCF, (−)-LCF, that mediates this leaf movement in S. saman (Ueda et al., 2000; Ueda and Nakamura, 2007). Here we extend this observation by demonstrating that (−)-LCF is also able to selectively induce shrinking of protoplasts isolated from extensor motor cells, whereas flexor motor cell protoplasts was proved to be insensitive to (−)-LCF treatment (Fig. 3A). This is an important and new finding, which is in accordance with previous imunohistochemical labeling studies demonstrating that fluorescence-labeled (−)-LCF likewise selectively bound to extensor motor cells in plant sections (Nakamura et al., 2006). The responsiveness of isolated extensor protoplasts to (−)-LCF allowed additional detailed investigations, such as time and concentration dependence or inhibitor studies. The results demonstrate that (−)-LCF is effective at concentrations as low as 1 μm and that an appreciable decrease in the volume of the protoplasts was established within 10 to 20 min, amounting to a total decrease of approximately 5% within 50 min (Fig. 3). The rapidity of the response suggests that the shrinking of motor cell protoplasts upon (−)-LCF application is the result of an immediate ion channel-mediated process rather than being caused by a metabolism-related mechanism affecting cell turgor.
The physiological mechanisms of leaf movement, such as the effect of light on the leaf closure of S. saman, have been extensively studied (Moran, 2007 and refs. therein). For the dark-promoted leaf closure of S. saman, the involvement of K+ fluxes, driving the hydraulic movement of water, was unequivocally demonstrated using motor cell protoplasts (Moran et al., 1988, 1996; Suh et al., 2000; Yu et al., 2001; Moshelion et al., 2002). This outward-directed K+ efflux from extensor motor cell is mediated by opening of K+-selective channels, which can be specifically blocked by TEA (Moran et al., 1990). Our results likewise point to an involvement of potassium channels in (−)-LCF-induced protoplast shrinking. The specific K+ channel blocker, TEA, completely inhibited this shrinking of extensor cell protoplasts upon treatment with (−)-LCF (Fig. 5A). By contrast, (−)-LCF-dependent cell shrinkage was not impaired by the addition of La3+, a selective inhibitor of Ca2+ channel activity (Fig. 5B). Again, this observation is in accordance with previous reported data, demonstrating that alterations in cytosolic Ca2+ concentrations have only a minor effect on an outward-directed potassium channel (KD) in S. saman (Moshelion and Moran, 2000). Our results are not only consistent with the reported features of Samanea ion channels as obtained by electrophysiological studies, they also indicated that (−)-LCF-induced protoplast shrinkage and dark-promoted leaf closure share a common signaling pathway, at least in part, and that the molecular mechanism involves activation of selective ion channels.
Leaf-Closing Activity and Initiation of Motor Cell Shrinkage Are Unique Properties of (−)-LCF
Disclosure of the chemical nature of Samanea LCF (−)-LCF as 12-O-Glc-(3R,7R)-JA (Ueda et al., 2000; Ueda and Nakamura, 2007) immediately raised two important question: (1) Does the absolute configuration at the chiral centers (C-3 and C-7) of the jasmonate building block affect bioactivity; and (2) do other jasmonate derivatives also display leaf-closing activity? Towards this end we established the structure-activity relationships using the leaf-closing assay with isolated pinnae and the cell-shrinking assay with extensor motor cell protoplasts. Importantly, in both bioassays we found that the activity of LCF was dependent on the stereochemistry with only (−)-LCF causing a response, whereas its stereoisomer (+)-ent-LCF was inactive (Figs. 2 and 4A). These results are consistent with our previous binding study using fluorescein isothiocyanate-labeled LCF stereoisomers, which demonstrated selective recognition and binding of (−)-LCF, but not (+)-ent-LCF, to extensor cells of the Samanea pulvinus (Nakamura et al., 2006).
When we extended our analysis to other jasmonates we observed that neither (−)-JA, (−)-JA-Ile, nor (+)-coronatine could induce leaf closure in S. saman pinnae or cell shrinking of extensor cell protoplasts. However, in the case of (−)-12-OH-JA we observed a differential response, while leaf closure of Samanea pinnae was activated with low efficiency, shrinking of extensor motor cell protoplasts was not affected (Figs. 2 and 4C). The reason for this difference is not clear but may be attributed to the de novo glucosylation of (−)-12-OH-JA to bioactive (−)-LCF in intact pinnae. From these results we conclude that motor activity is a unique feature of (−)-12-O-Glc-JA [(−)-LCF] and not displayed by other jasmonates, including the powerful jasmonate mimic (+)-coronatine, strongly indicating that the leaf-closing mechanism probably operates independent of the COI1-JAZ module. An alternative, conceivable model may include binding of (−)-12-O-Glc-JA to a modified COI1-JAZ complex specific to Samanea and mediating leaf movement. However, direct experimental evidence in support of such molecular mechanism, for example by analysis of knockout mutants for any of the COI1-JAZ components, cannot be obtained, since genetic and molecular approaches have not yet been established for S. saman, M. pudica, or other model plants used for the analysis of rapid leaf movement. In view of these limitations, the obvious approach to unravel the molecular mechanism of (−)-LCF will be the isolation and molecular characterization of its putative membrane-localized target protein, MTJG (Nakamura et al., 2008b).
Plants Respond Differentially to Typical Jasmonates and LCF
In considering the broad range of biological responses that are caused by jasmonate derivatives, it was obviously important to examine whether or not (−)-LCF was also capable of inducing another typical plant response, such as activation of JA-responsive genes or emission of volatile organic compounds. Our results clearly show that the JA-responsive marker genes, composed of the LOX2 or OPCL1 promoters and the GUS reporter, could not be activated by (−)-LCF, (+)-ent-LCF, or 12-OH-JA (Fig. 6). Both reporter genes, as well as the corresponding endogenous genes driven by their native promoters, were previously shown to be strongly up-regulated by several jasmonates including JA, MeJA, 12-oxophytodienoate, and the synthetic JA analog coronalon and indanoyl-Ile (Jensen et al., 2002; Kienow et al., 2008). Because almost all of the jasmonate-activated responses are dependent on the central regulator COI1 and the interacting JAZ repressor proteins (Feys et al., 1994; Wasternack, 2007; Chung et al., 2008; Katsir et al., 2008; Wasternack and Kombrink, 2010), the lacking activation of above reporter genes by (−)-LCF strongly suggests that this compound exerts its leaf-closing activity through an additional, COI1-JAZ-independent signaling pathway.
The emission of organic volatiles is an indirect defense response of plants under attack from herbivores (Mithöfer et al., 2009). It is also under the control of the octadecanoid signaling pathway and can be effectively stimulated by exogenous application of jasmonates (Hopke et al., 1994; Boland et al., 1995). In both plants that were analyzed, S. samana and lima bean, we found a clear difference in responses to treatment with (−)-JA or (−)-LCF. Strikingly, only (−)-JA was capable to initiate volatile emission, whereas (−)-LCF was virtually inactive (Figs. 7 and 8). It is important to note that S. saman can react to JA treatment very similar to other plants, e.g. volatile emission, and in addition has the capacity to specifically respond to the (−)-LCF signal with leaf closure.
The Biological Functions of 12-OH-JA
During the past decade, extensive gene expression studies and comparative mutant analyses have revealed that at least three groups of JA derivative exhibit signaling properties partially different from the parent compound JA (or JAMe). (1) 12-Oxophytodienoate, a biosynthetic precursor, is in many tissues highly abundant (Stintzi et al., 2001; Devoto et al., 2005; Taki et al., 2005; Kienow et al., 2008). (2) JA amino acid conjugates are considered the most active and most important JA derivatives (Kramell et al., 1997; Staswick and Tiryaki, 2004; Fonseca et al., 2009b) and its coreceptor system involved in the COI1-JAZ module was recently identified (Chini et al., 2007; Thines et al., 2007; Sheard et al., 2010). (3) 12-OH-JA and 12-O-Glc-JA, initially identified in Solanaceous plants and described as tuber-inducing factor, have subsequently been found as abundant metabolites in numerous other plants including Arabidopsis and tobacco (Nicotiana tabacum; Yoshihara et al., 1989; Helder et al., 1993; Swiatek et al., 2004; Miersch et al., 2008).
The formation of 12-OH-JA, 12-O-Glc-JA, and 12-HSO4-JA, has been considered a mechanism for switching off JA signaling (Gidda et al., 2003; Miersch et al., 2008) that is tightly associated with simultaneous down-regulation of genes encoding enzymes of JA biosynthesis. Our additional feature of 12-OH-JA and 12-O-Glc-JA [(−)-LCF] is the second example of a genus- and organ-specific bioactivity for these metabolites, in addition to the tuber-inducing activity in Solanaceous species (Yoshihara et al., 1989). However, the analysis of structure-activity relationships revealed that tuber-inducing activity was not a sole feature of 12-OH-JA and 12-O-Glc-JA, but rather that other jasmonates such as JA and JAMe were equally active and coronatine was even 100 to 1,000 times more effective in initiating potato tuberization than 12-O-Glc-JA (Koda et al., 1991, 1996). Obviously, these results do not preclude the involvement of the COI1-JAZ signaling pathway. By contrast, our analysis of Samanea leaf closure and extensor cell protoplast shrinkage clearly revealed that only (−)-12-O-Glc-JA, and (−)-12-OH-JA in case of leaf closure, displayed bioactivity, whereas all other jasmonates tested were ineffective, including the otherwise highly active coronatine (Figs. 2 and 4D). These results also imply the existence of a separate JA signaling pathway in S. saman, operating independently from the COI1-JAZ module and mediating leaf closure via the 12-O-Glc-JA, (−)-LCF, signal. Future work will aim at unraveling the molecular mechanism of (−)-LCF function, including stereospecific recognition by the putative receptor, MTJG, localized on the motor cell membrane and triggering of downstream responses.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Seeds of Samanea saman were purchased from World Flower Service Co., Ltd. Seedlings were grown from the seeds in a growth chamber (Biotron LP1-PH, Nippon medical & chemical instrument Co., Ltd.) under a 16/8-h light/dark regime at an intensity of 160 to 290 μmol m−2 s−1 photosynthetically active radiation (PAR), at 25°C ± 3°C, and 70% relative humidity. Twelve- to 16-d-old lima bean (Phaseolus lunatus cv ‘Ferry Morse’ var. Jackson Wonder Bush) plants, showing two fully developed primary leaves were used for measuring volatile emission upon jasmonate treatment. Individual plants were grown from seed in a plastic pot using sterilized potting soil under the following conditions: a light period of 16 h (270 μmol m−2 s−1) at 23°C and 60% relative humidity.
Preparation of Chemicals
(−)-LCF, (+)-ent-LCF, and 12-OH-JA were synthesized according to our previous report (Nakamura et al., 2008a). (−)-JA was prepared from commercially available (±)-JA by optical resolution as described (Asamitsu et al., 2006). (−)-JA-Ile was prepared essentially as described (Kramell et al., 1988). (±)-JA and (+)-coronatine were purchased from Sigma-Aldrich Co. Ltd. While JA-Ile was supplied as the natural 95:5 ratio of its stereoisomers (−)-JA-Ile and (+)-iso-JA-Ile, respectively, coronatine is the structural and functional mimic of specifically (+)-iso-JA-Ile (Feys et al., 1994; Weiler et al., 1994; Fonseca et al., 2009b).
Leaf-Closing Assay Using Pinna of S. saman
The 8-week-old seedlings of S. saman were conditioned for a week in a growth chamber (Biotron LH-300, Nippon medical & chemical instrument Co., Ltd.) to a 16/8-h light/dark regime at approximately 6 μmol m−2 s−1 PAR, 25°C ± 2°C, and 60% to 70% relative humidity before being using for the leaf-closing assay. Under this condition, the leaves opened for 12 h, starting at 4 h after beginning of the light period, and closed for 12 h.
The pinnae were disconnected from the seedlings just above the primary pulvinus and were placed with their petioles (rachillae) in 1.5-mL plastic tubes filled with distilled water. Under these conditions, the pinnae continued their cycle of opening-closing movement (16-h light, 8-h dark) as before.
The experiments were started at 4 h after beginning of the second light period by supplying the pinnae with the following jasmonate solutions: (−)-LCF (H+-form), (−)-LCF (K+-form), (+)-ent-LCF, (−)-JA, (−)-JA-Ile, (−)-JA-OH (each at the final concentration of 500 μm), or (+)-coronatine (at 5 μm). Subsequently the behavior of each pinna was recorded by digital camera for a period of 48 h. Pictures presented in Figure 2 were taken at 10 h of the second light period, i.e. 6 h after initiation of treatment. Three replicate pinnae with a leaflet pair were used for each compound and the experiment was repeated at least three times with similar results.
Cell-Shrinking Assay Using Motor Cell Protoplast of S. saman
The tertiary pulvini of S. saman were collected from compound leaves on the second or third branch from the short apex. These pulvini were separated under a stereomicroscope into extensor (adaxial) part and flexor (abaxial) part with a sharp razor blade. Protoplasts were prepared from extensor part according to the previously reported method (Gorton and Satter, 1984; Moran et al., 1990; Nakamura et al., 2008b), suspended in wash solution (0.57 m sorbitol, 10 mm KCl, 1 mm CaCl2, 20 mm MES-Tris pH 5.5), and centrifuged at 110g for 5 min. The resulting pellet containing protoplasts was suspended in a small volume of washing solution and the washing step repeated twice.
The freshly prepared protoplasts in 450 μL wash solution were transferred to a glass-bottom petri dish (φ 35 × 12 mm), placed under an inverted microscope (IX-71, Olympus), and monitored at 24°C ± 1°C with continuous irradiation of the light (50 μmol m−2 s−1 PAR) passed through a green filter (43IF550-W45, Olympus).
Following incubation under the microscope for 6 min, 50 μL of different compounds (each at 1 mm dissolved in wash solution) was added to the protoplast suspension, which was sealed with a coverslip. In experiments using inhibitors, such as TEA or lanthanum chloride (La3+), the protoplasts were incubated with these compounds for 30 min before adding LCF. The status of protoplasts was recorded with time-lapse photography (3-min intervals) for 51 min using a digital camera (DP 72, Olympus) and bioimaging analysis software (Lumina Vision, Mitani Co.). The diameter for each magnified image of protoplast was measured precisely by Photoshop software (Adobe Systems). For the volume analysis, protoplasts were selected with regard to roundness and clarity of the margin. Figures 3 to 5 presented the changes in protoplast volumes after addition of each chemical.
Jasmonate Bioactivity Assay Using Arabidopsis GUS Reporter Lines
Transgenic Arabidopsis (Arabidopsis thaliana) lines harboring the GUS reporter under the control of the LOX2 or the OPCL1 promoter, LOX2p::GUS or OPCL1p::GUS, respectively, were generated previously (Jensen et al., 2002; Kienow et al., 2008) and obtained from the European Arabidopsis Stock Centre. Sterile seedlings of these lines were grown in hydroponic culture as previously described (Serrano et al., 2007). Briefly, two to four seeds per well of a 48-well microtiter plate were germinated and grown at 24°C in 0.5× Murashige and Skoog medium with 0.5% Suc. After 2 weeks the medium was replaced by fresh Murashige and Skoog supplemented with jasmonate derivatives (dissolved in dimethyl sulfoxide [DMSO]) at the indicated concentration and incubation continued for 24 h. Seedling extracts were prepared and fluorimetric GUS activities relative to protein concentration determined as previously described (Kienow et al., 2008). The quantitative analysis is based on at least three biological replicates.
Volatile Induction Assay Using S. saman and Lima Bean
Volatiles were collected over a period of 24 h using the highly sensitive closed-loop-stripping method as described by Donath and Boland (1995). Therefore, leaves were detached from plants and placed with their cut petiole into vials containing water (control) or the respective jasmonate solution (500 μm). Each sample was placed separately into an exsiccator connected to a pump, which circulated the air from the headspace through a charcoal trap, thereby continuously collecting the emitted leaf volatiles. Finally, compounds were desorbed from the filters by two washes with methylene chloride (20 μL each) containing 100 μg/mL n-bromodecane as internal standard and the volatiles analyzed using gas chromatography-mass spectrometry (TRACE 2000 series, Finnigan) with a capillary column EC-5 (Alltech).
Acknowledgments
We thank Brigitte Pickel for excellent technical assistance and Christian Kost for help in statistical analyses. We also thank the Experimental Station for Medicinal Plant Studies, Graduate School of Pharmaceutical Sciences, Tohoku University, for cultivation of plants.
References
- Abdala G, Miersch O, Kramell R, Vigliocco A, Agostini E, Forchetti G, Alemano S. (2003) Jasmonate and octadecanoid occurrence in tomato hairy roots: endogenous level changes in response to NaCl. Plant Growth Regul 40: 21–27 [Google Scholar]
- Asamitsu Y, Nakamura Y, Ueda M, Kuwahara S, Kiyota H. (2006) Synthesis and odor description of both enantiomers of methyl 4,5-didehydrojasmonate, a component of jasmin absolute. Chem Biodivers 3: 654–659 [DOI] [PubMed] [Google Scholar]
- Blechert S, Bockelmann C, Füßlein M, v. Schrader T, Stelmach B, Niesel U, Weiler EW. (1999) Structure-activity analyses reveal the existence of two separate groups of active octadecanoids in elicitation of the tendril-coiling response of Bryonia dioica Jacq. Planta 207: 470–479 [Google Scholar]
- Boland W, Hopke J, Donath J, Nüske J, Bublitz F. (1995) Jasmonic acid and coronatin induce odor production in plants. Angew Chem Int Ed Engl 34: 1600–1602 [Google Scholar]
- Browse J. (2009) Jasmonate passes muster: a receptor and targets for the defense hormone. Annu Rev Plant Biol 60: 183–205 [DOI] [PubMed] [Google Scholar]
- Chini A, Fonseca S, Fernández G, Adie B, Chico JM, Lorenzo O, García-Casado G, López-Vidriero I, Lozano FM, Ponce MR, et al. (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448: 666–671 [DOI] [PubMed] [Google Scholar]
- Chung HS, Koo AJK, Gao X, Jayanty S, Thines B, Jones AD, Howe GA. (2008) Regulation and function of Arabidopsis JASMONATE ZIM-domain genes in response to wounding and herbivory. Plant Physiol 146: 952–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coté GG. (1995) Signal transduction in leaf movement. Plant Physiol 109: 729–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto A, Ellis C, Magusin A, Chang HS, Chilcott C, Zhu T, Turner JG. (2005) Expression profiling reveals COI1 to be a key regulator of genes involved in wound- and methyl jasmonate-induced secondary metabolism, defence, and hormone interactions. Plant Mol Biol 58: 497–513 [DOI] [PubMed] [Google Scholar]
- Devoto A, Turner JG. (2003) Regulation of jasmonate-mediated plant responses in Arabidopsis. Ann Bot (Lond) 92: 329–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrecht B, Xue GP, Sprague SJ, Kirkegaard JA, Ross JJ, Reid JB, Fitt GP, Sewelam N, Schenk PM, Manners JM, et al. (2007) MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell 19: 2225–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donath J, Boland W. (1995) Biosynthesis of acyclic homoterpenes: enzyme selectivity and absolute configuration of the nerolidol precursor. Phytochemistry 39: 785–790 [Google Scholar]
- Feys BJF, Benedetti CE, Penfold CN, Turner JG. (1994) Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6: 751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca S, Chico JM, Solano R. (2009a) The jasmonate pathway: the ligand, the receptor and the core signalling module. Curr Opin Plant Biol 12: 539–547 [DOI] [PubMed] [Google Scholar]
- Fonseca S, Chini A, Hamberg M, Adie B, Porzel A, Kramell R, Miersch O, Wasternack C, Solano R. (2009b) (+)-7-iso-jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat Chem Biol 5: 344–350 [DOI] [PubMed] [Google Scholar]
- Gidda SK, Miersch O, Levitin A, Schmidt J, Wasternack C, Varin L. (2003) Biochemical and molecular characterization of a hydroxyjasmonate sulfotransferase from Arabidopsis thaliana. J Biol Chem 278: 17895–17900 [DOI] [PubMed] [Google Scholar]
- Gorton HL, Satter RL. (1984) Extensor and flexor protoplasts from samanea pulvini: I. Isolation and initial characterization. Plant Physiol 76: 680–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helder H, Miersch O, Vreugdenhil D, Sembdner G. (1993) Occurrence of hydroxylated jasmonic acids in leaflets of Solanum demissum plants grown under long- and short-day conditions. Physiol Plant 88: 647–653 [DOI] [PubMed] [Google Scholar]
- Hopke J, Donath J, Blechert S, Boland W. (1994) Herbivore-induced volatiles: the emission of acyclic homoterpenes from leaves of Phaseolus lunatus and Zea mays can be triggered by a β-glucosidase and jasmonic acid. FEBS Lett 352: 146–150 [DOI] [PubMed] [Google Scholar]
- Howe GA, Jander G. (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59: 41–66 [DOI] [PubMed] [Google Scholar]
- Jensen AB, Raventos D, Mundy J. (2002) Fusion genetic analysis of jasmonate-signalling mutants in Arabidopsis. Plant J 29: 595–606 [DOI] [PubMed] [Google Scholar]
- Katsir L, Schilmiller AL, Staswick PE, He SY, Howe GA. (2008) COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc Natl Acad Sci USA 105: 7100–7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienow L, Schneider K, Bartsch M, Stuible HP, Weng H, Miersch O, Wasternack C, Kombrink E. (2008) Jasmonates meet fatty acids: functional analysis of a new acyl-coenzyme A synthetase family from Arabidopsis thaliana. J Exp Bot 59: 403–419 [DOI] [PubMed] [Google Scholar]
- Koda Y, Kikuta Y, Tazaki H, Tsujino Y, Sakamura S, Yoshihara T. (1991) Potato tuber-inducing activities of jasmonic acid and related compounds. Phytochemistry 30: 1435–1438 [Google Scholar]
- Koda Y, Takahashi K, Kikuta Y, Greulich F, Toshima H, Ichihara A. (1996) Similarities of the biological activities of coronatine and coronafacic acid to those of jasmonic acid. Phytochemistry 41: 93–96 [Google Scholar]
- Kramell R, Miersch O, Hause B, Ortel B, Parthier B, Wasternack C. (1997) Amino acid conjugates of jasmonic acid induce jasmonate-responsive gene expression in barley (Hordeum vulgare L.) leaves. FEBS Lett 414: 197–202 [DOI] [PubMed] [Google Scholar]
- Kramell R, Schmidt J, Schneider G, Sembdner G, Schreiber K. (1988) Synthesis of N-(jasmonoyl)amino acid conjugates. Tetrahedron 44: 5791–5807 [Google Scholar]
- Krumm T, Bandemer K, Boland W. (1995) Induction of volatile biosynthesis in the lima bean (Phaseolus lunatus) by leucine- and isoleucine conjugates of 1-oxo- and 1-hydroxyindan-4-carboxylic acid: evidence for amino acid conjugates of jasmonic acid as intermediates in the octadecanoid signalling pathway. FEBS Lett 377: 523–529 [DOI] [PubMed] [Google Scholar]
- Lorenzo O, Chico JM, Sánchez-Serrano JJ, Solano R. (2004) JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 16: 1938–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miersch O, Kramell R, Parthier B, Wasternack C. (1999) Structure-activity relations of substituted, deleted or stereospecifically altered jasmonic acid in gene expression of barley leaves. Phytochemistry 50: 353–361 [Google Scholar]
- Miersch O, Neumerkel J, Dippe M, Stenzel I, Wasternack C. (2008) Hydroxylated jasmonates are commonly occurring metabolites of jasmonic acid and contribute to a partial switch-off in jasmonate signaling. New Phytol 117: 114–127 [DOI] [PubMed] [Google Scholar]
- Millet B, Coillot L, Agosti RD. (1989) The rhythmic leaf movements after regeneration of partially excised pulvinus in Phaseolus vulgaris L. Plant Cell Physiol 30: 643–648 [Google Scholar]
- Mithöfer A, Boland W, Maffei ME. (2009) Chemical ecology of plant-insect interactions. Parker J, , Plant Disease Resistance. Wiley-Blackwell, Chichester, UK, pp 261–291 [Google Scholar]
- Moran N. (1996) Membrane-delimited phosphorylation enables the activation of the outward-rectifying K channels in motor cell protoplasts of Samanea saman. Plant Physiol 111: 1281–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran N. (2007) Osmoregulation of leaf motor cells. FEBS Lett 581: 2337–2347 [DOI] [PubMed] [Google Scholar]
- Moran N, Ehrenstein G, Iwasa K, Mischke C, Bare C, Satter RL. (1988) Potassium channels in motor cells of Samanea saman: a patch-clamp study. Plant Physiol 88: 643–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran N, Fox D, Satter RL. (1990) Interaction of the depolarization-activated K+ channel of Samanea saman with inorganic ions: a patch-clamp study. Plant Physiol 94: 424–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshelion M, Becker D, Czempinski K, Mueller-Roeber B, Attali B, Hedrich R, Moran N. (2002) Diurnal and circadian regulation of putative potassium channels in a leaf moving organ. Plant Physiol 128: 634–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshelion M, Moran N. (2000) Potassium-efflux channels in extensor and flexor cells of the motor organ of Samanea saman are not identical: effects of cytosolic calcium. Plant Physiol 124: 911–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Miyatake R, Inomata S, Ueda M. (2008a) Synthesis and bioactivity of potassium β-D-glucopyranosyl 12-hydroxy jasmonate and related compounds. Biosci Biotechnol Biochem 72: 2867–2876 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Miyatake R, Matsubara A, Kiyota H, Ueda M. (2006) Enantio-differential approach to identify the target cell for glucosyl jasmonate-type leaf-closing factor, by using fluorescence-labeled probe compounds. Tetrahedron 62: 8805–8813 [Google Scholar]
- Nakamura Y, Miyatake R, Ueda M. (2008b) Enantiodifferential approach for the detection of the target membrane protein of the jasmonate glycoside that controls the leaf movement of Albizzia saman. Angew Chem Int Ed Engl 47: 7289–7292 [DOI] [PubMed] [Google Scholar]
- Palmer J, Asprey G. (1958a) Studies in the nyctinastic movement of the leaf pinnae of Samanea saman (Jacq.) Merrill. II. The behaviour of upper and lower half-pulvini. Planta 51: 770–785 [Google Scholar]
- Palmer J, Asprey G. (1958b) Studies in the nyctinastic movement of the leaf pinnae of Samanea saman (Jacq.) Merrill. I. A general description of the effect of light on the nyctinastic rhythm. Planta 51: 757–769 [Google Scholar]
- Satter RL, Geballe GT, Applewhite PB, Galston AW. (1974) Potassium flux and leaf movement in Samanea saman. I. Rhythmic movement. J Gen Physiol 64: 413–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüler G, Mithöfer A, Baldwin IT, Berger S, Ebel J, Santos JG, Herrmann G, Hölscher D, Kramell R, Kutchan TM, et al. (2004) Coronalon: a powerful tool in plant stress physiology. FEBS Lett 563: 17–22 [DOI] [PubMed] [Google Scholar]
- Serrano M, Robatzek S, Torres M, Kombrink E, Somssich IE, Robinson M, Schulze-Lefert P. (2007) Chemical interference of pathogen-associated molecular pattern-triggered immune responses in Arabidopsis reveals a potential role for fatty-acid synthase type II complex-derived lipid signals. J Biol Chem 282: 6803–6811 [DOI] [PubMed] [Google Scholar]
- Sheard LB, Tan X, Mao H, Withers J, Ben-Nissan G, Hinds TR, Kobayashi Y, Hsu FF, Sharon M, Browse J, et al. (2010) Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468: 400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Tiryaki I. (2004) The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16: 2117–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Tiryaki I, Rowe ML. (2002) Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell 14: 1405–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzi A, Weber H, Reymond P, Browse J, Farmer EE. (2001) Plant defense in the absence of jasmonic acid: the role of cyclopentenones. Proc Natl Acad Sci USA 98: 12837–12842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh SJ, Moran N, Lee YS. (2000) Blue light activates potassium-efflux channels in flexor cells from Samanea saman motor organs via two mechanisms. Plant Physiol 123: 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiatek A, Van Dongen W, Esmans EL, Van Onckelen H. (2004) Metabolic fate of jasmonates in tobacco bright yellow-2 cells. Plant Physiol 135: 161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki N, Sasaki-Sekimoto Y, Obayashi T, Kikuta A, Kobayashi K, Ainai T, Yagi K, Sakurai N, Suzuki H, Masuda T, et al. (2005) 12-Oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol 139: 1268–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J. (2007) JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448: 661–665 [DOI] [PubMed] [Google Scholar]
- Ueda M, Nakamura Y. (2006) Metabolites involved in plant movement and ‘memory’: nyctinasty of legumes and trap movement in the Venus flytrap. Nat Prod Rep 23: 548–557 [DOI] [PubMed] [Google Scholar]
- Ueda M, Nakamura Y. (2007) Chemical basis of plant leaf movement. Plant Cell Physiol 48: 900–907 [DOI] [PubMed] [Google Scholar]
- Ueda M, Okazaki M, Ueda K, Yamamura S. (2000) A leaf-closing substance of Albizzia julibrissin Durazz. Tetrahedron 56: 8101–8105 [Google Scholar]
- Wasternack C. (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot (Lond) 100: 681–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C, Kombrink E. (2010) Jasmonates: structural requirements for lipid-derived signals active in plant stress responses and development. ACS Chem Biol 5: 63–77 [DOI] [PubMed] [Google Scholar]
- Weiler EW, Kutchan TM, Gorba T, Brodschelm W, Niesel U, Bublitz F. (1994) The Pseudomonas phytotoxin coronatine mimics octadecanoid signalling molecules of higher plants. FEBS Lett 345: 9–13 [DOI] [PubMed] [Google Scholar]
- Yoshihara T, Omer ESA, Koshino H, Sakamura S, Kikuta Y, Koda Y. (1989) Structure of a tuber-inducing stimulus from potato leaves (Solanum tuberosum L.). Agric Biol Chem 53: 2835–2837 [Google Scholar]
- Yu L, Moshelion M, Moran N. (2001) Extracellular protons inhibit the activity of inward-rectifying potassium channels in the motor cells of Samanea saman pulvini. Plant Physiol 127: 1310–1322 [PMC free article] [PubMed] [Google Scholar]