Abstract
Nonsymbiotic hemoglobins are ubiquitously expressed in plants and divided into two different classes based on gene expression pattern and oxygen-binding properties. Most of the published research has been on the function of class 1 hemoglobins. To investigate the role of class 2 hemoglobins, transgenic Arabidopsis (Arabidopsis thaliana) plants were generated overexpressing Arabidopsis hemoglobin-2 (AHb2) under the control of a seed-specific promoter. Overexpression of AHb2 led to a 40% increase in the total fatty acid content of developing and mature seeds in three subsequent generations. This was mainly due to an increase in the polyunsaturated C18:2 (ω-6) linoleic and C18:3 (ω-3) α-linolenic acids. Moreover, AHb2 overexpression led to an increase in the C18:2/C18:1 and C18:3/C18:2 ratios as well as in the C18:3 content in mol % of total fatty acids and in the unsaturation/saturation index of total seed lipids. The increase in fatty acid content was mainly due to a stimulation of the rate of triacylglycerol synthesis, which was attributable to a 3-fold higher energy state and a 2-fold higher sucrose content of the seeds. Under low external oxygen, AHb2 overexpression maintained an up to 5-fold higher energy state and prevented fermentation. This is consistent with AHb2 overexpression results in improved oxygen availability within developing seeds. In contrast to this, overexpression of class 1 hemoglobin did not lead to any significant increase in the metabolic performance of the seeds. These results provide evidence for a specific function of class 2 hemoglobin in seed oil production and in promoting the accumulation of polyunsaturated fatty acids by facilitating oxygen supply in developing seeds.
Hemoglobins are an ancient class of oxygen-binding proteins that are ubiquitous in nature and expressed in organisms as different as animals, bacteria, fungi, and plants (Kundu et al., 2003; Vinogradov et al., 2006). The first plant hemoglobins to be discovered were leghemoglobins, which are highly abundant proteins in root nodules of legume plants infected with symbiotic nitrogen-fixing bacteria (Kubo, 1939; Appleby et al., 1988). They have been shown to be indispensable for symbiotic nitrogen fixation, with a role in facilitating oxygen diffusion to nitrogen-fixing bacteria, and to protect bacterial nitrogenase from inactivation (Ott et al., 2005). In contrast to this, nonsymbiotic plant hemoglobins have been found in various plant tissues and plant species, especially in crop plants (Taylor et al., 1994; Andersson et al., 1996). Different classes of nonsymbiotic plant hemoglobins have been identified and divided into class 1 (Hb1) and class 2 (Hb2) based on phylogenetic characteristics, gene expression patterns, and oxygen-binding properties (Trevaskis et al., 1997; Hunt et al., 2001; Hoy and Hargrove, 2008).
Class 1 and class 2 hemoglobins differ markedly in their oxygen-binding affinities, with the respective Km being 1 to 2 nm for Hb1 and 150 nm for Hb2 (Hoy and Hargrove, 2008; Dordas, 2009). The extremely high affinity of Hb1 for oxygen makes it unlikely that this protein is acting as an oxygen sensor, oxygen carrier, oxygen storage, or electron transport molecule (Hill, 1998). Expression of Hb1 is very low under normal conditions, but it is strongly induced by hypoxic stress. Studies using Arabidopsis (Arabidopsis thaliana; Perazzolli et al., 2004), maize (Zea mays; Dordas et al., 2003, 2004), and alfalfa (Medicago sativa) lines (Igamberdiev et al., 2004) overexpressing Hb1 indicate a role of class 1 hemoglobins in scavenging of nitric oxide (NO) that is produced under severe hypoxia. Under these stress conditions, overexpression of Hb1 leads to a decrease in NO, maintenance of the cellular energy status and growth, as well as increased survival of the plants (Sowa et al., 1998; Hunt et al., 2002; Dordas et al., 2004; Perazzolli et al., 2004).
Relatively little is known concerning the function of Hb2 in plants. In Arabidopsis, AHb2 expression is resilient to hypoxic stress but shows induction in response to low temperatures, indicating a possible yet undefined role under cold stress (Trevaskis et al., 1997). AHb2 is also induced in response to the plant hormone cytokinin (Hunt et al., 2001), which has been shown to be mediated by a cytokinin-regulated transcription factor (Ross et al., 2004). Analysis of the expression pattern in plants reveals preferential expression of Hb2 in young developing leaves, vascular bundles, flowers, developing seeds, and immature fruits (Hunt et al., 2002; Wang et al., 2003). Hb2 is also expressed during somatic embryogenesis in chicory (Cichorium intybus; Hendriks et al., 1998). This indicates a possible role of Hb2 in young and developing plant tissues that usually have a high metabolic activity and energy demand. Arabidopsis plants overexpressing AHb2 under the control of a constitutive promoter were mainly analyzed with respect to severe oxygen deprivation. Under these conditions, AHb2 overexpression led to increased survival of the plants, similar to AHb1 overexpression, suggesting that both AHb1 and AHb2 may have overlapping functions, such as scavenging of NO (Hebelstrup et al., 2006). However, a role of AHb2 in scavenging of NO has not been demonstrated yet. Since the oxygen-binding characteristics of AHb2 are comparable to leghemoglobin, with an affinity to oxygen that resembles the Km of cytochrome oxidase, a specific function of Hb2 in facilitating oxygen diffusion cannot be excluded. Therefore, the underlying mechanisms of AHb2-induced responses are still unresolved and may differ from AHb1.
In the following approach, transgenic Arabidopsis plants were generated that overexpress endogenous class 1 or class 2 hemoglobins specifically in developing seeds under the control of a seed-specific promoter. The results show that overexpressing AHb2, but not AHb1, led to an increase in the lipid content of the seeds that was mainly due to an increase in the level of polyunsaturated fatty acids. This was accompanied by an increase in the adenylate energy state and the Suc content of the seeds. These results indicate a specific role of class 2 hemoglobins in promoting the accumulation of polyunsaturated fatty acids, which have important functions in the response of the plant to cold stress and in human health. This may be attributable to a possible role of class 2 hemoglobins in improving oxygen availability in developing seeds.
RESULTS
Generation of Transgenic Arabidopsis Plants Overexpressing Either AHb1 or AHb2 under the Control of a Seed-Specific Promoter
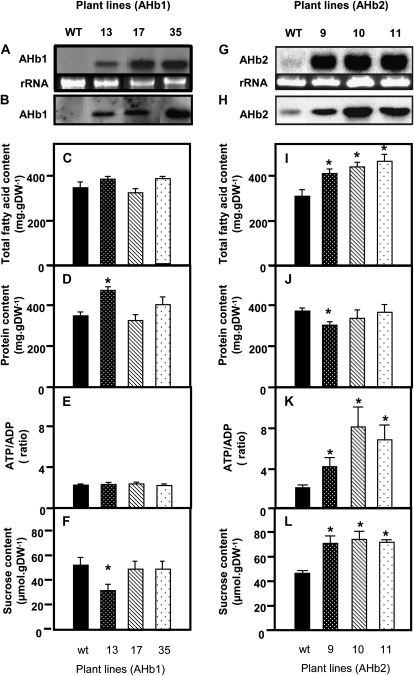
AHb1 or AHb2 cDNA was cloned into a plant binary vector downstream of the USP (for unknown seed protein) promoter, identified to be seed specific in Arabidopsis plants (Bäumlein et al., 1991). The resulting construct was transformed via Agrobacterium tumefaciens into Arabidopsis var C24 plants. Using hygromycin-containing selective medium, three independent transgenic lines showing a significant and reproducible increase in transcript and protein level of either AHb1 (Fig. 1, A and B) or AHb2 (Fig. 1, G and H) were propagated up to the T3 generation for further analyses.
Figure 1.
Increased expression of AHb2 leads to metabolic changes in Arabidopsis seeds. AHb1 (A–F) and AHb2 (G–L) were overexpressed in Arabidopsis seeds under the control of the seed-specific USP promoter. In developing seeds (13–14 DAF) of the T3 generation, the levels of AHb1 transcript (A) and protein (B) and of AHb2 transcript (G) and protein (H) were determined in wild-type plants (WT/wt; black bars) and three independent AHb1 and AHb2 lines (gray bars), respectively. Total fatty acid (C and I) and protein (D and J) contents were analyzed in mature seeds, while ATP-ADP ratio (E and K) and Suc content (F and L) were analyzed in developing seeds (13–14 DAF). Results are means ± se; n = 10 different sets of plants for fatty acid and protein contents, and n = 6 different sets of plants for ATP-ADP ratio and Suc content. Changes that are significantly different from the wild type (based on Student’s t test with P < 0.05) are labeled with asterisks. DW, Dry weight.
Overexpression of AHb2 Leads to an Increase in the Total Fatty Acid Content of Developing and Mature Seeds
The transgenic lines overexpressing AHb1 or AHb2 showed no visible phenotype when grown under normal greenhouse conditions. To investigate the influence of increased expression of these endogenous hemoglobins on storage product accumulation, transgenic lines were further analyzed for total fatty acid content in developing and mature seeds using gas chromatography analysis of fatty acid methyl esters. Overexpression of AHb2 led to a 40% increase in the fatty acid content of mature seeds, both as a percentage of total seed dry weight (Fig. 1I) and in absolute values per seed (Fig. 2A), while the protein content decreased slightly (Fig. 1J). Compared with the wild type, the fatty acid content of mature seeds of AHb2-overexpressing lines was found to be stably increased in three subsequent generations as well as in developing seeds that were harvested 13 to 14 d after flowering (DAF), when rapid rates of lipid synthesis occur (Table I). There were no significant changes in transient starch accumulation during seed development in AHb2-expressing lines compared with the wild type (Table I).
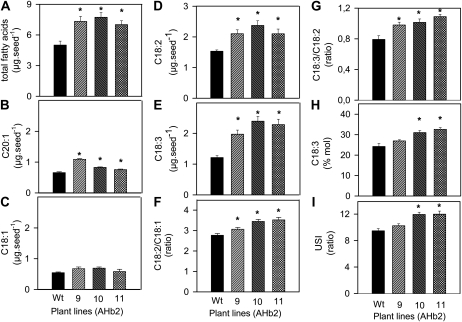
Figure 2.
Increased expression of AHb2 leads to an increase in polyunsaturated fatty acids in Arabidopsis seeds. Mature seeds of wild-type plants (Wt; black bars) and transgenic Arabidopsis plants overexpressing AHb2 in the T3 generation (gray bars) were analyzed to determine total fatty acids in μg seed−1 (A), C20:1 eicosenoic acid (a marker for TAG) in μg seed−1 (B), C18:1 oleic acid in μg seed−1 (C), C18:2 linoleic acid in μg seed−1 (D), C18:3 linolenic acid in μg seed−1 (E), C18:2/C18:1 ratio (F), C18:3/C18:2 ratio (G), C18:3 in mol % of total fatty acids (H), and USI of seed lipids (I) according to Gutierrez et al. (2005). Values are means ± se (n = 10 different sets of plants). Changes that are significantly different from the wild type (based on Student’s t test with P < 0.05) are labeled with asterisks. The weights of mature seeds (in μg seed−1) were as follows: 15.1 ± 0.89, 16.54 ± 0.79, 13.32 ± 1.0, and 16.85 ± 1.6 for the wild type, line 9, line 10, and line 11, respectively.
Table I. Overexpression of AHb2 leads to a specific increase in the seed lipid content of transgenic Arabidopsis lines in three subsequent generations.
Total fatty acid content was determined in mature seeds in T1, T2, and T3 generations and in developing seeds (14 DAF). In the latter, starch content was also measured. Values are means ± se; n = 6 independent measurements. Asterisks represent values significantly different from the wild type (based on Student’s t test with P < 0.05).
| Variable | Storage Product Content |
|||
| Wild Type | Line 9 | Line 10 | Line 11 | |
| mg g−1 dry wt | ||||
| Lipid content in mature seeds | ||||
| T1 generation | 324 ± 12 | 430 ± 20* | 488 ± 79* | 502 ± 34* |
| T2 generation | 383 ± 13 | 451 ± 48 | 499 ± 21* | 507 ± 30* |
| T3 generation | 331 ± 26 | 380 ± 18 | 435 ± 17* | 464 ± 25* |
| Lipid and starch content in developing seeds | ||||
| Lipid content | 128 ± 11 | 245 ± 22* | 224 ± 13* | 208 ± 34* |
| Starch content | 7 ± 1 | 8.6 ± 0.2 | 7 ± 0.2 | 7.2 ± 0.7 |
In contrast to the results with AHb2, overexpression of AHb1 did not lead to any significant changes in the fatty acid content of the seeds (Fig. 1C). Compared with the wild type, AHb1-overexpressing seeds also did not show significant changes in the protein content, except line 13, where the protein content was increased (Fig. 1D).
Overexpression of AHb2 Leads to an Increase in the Specific Triacylglycerol Marker C20:1 Eicosenoic Acid and in the Flux of Suc to Triacylgycerol in Arabidopsis Seeds
In Arabidopsis seeds, almost all of the fatty acids are esterified in the form of triacylglycerols (TAGs; Baud et al., 2002; Li et al., 2006). To investigate whether the AHb2-induced increase in total fatty acid content was attributable to an increase in TAG, the level of C20:1 eicosenoic acid was measured. Eicosenoic acid is a very-long-chain fatty acid that is solely confined to TAG and excluded from polar or membrane lipids in Arabidopsis plants (Lemieux et al., 1990). Therefore, it serves as a specific marker for the formation of storage TAG in Arabidopsis seeds (Lemieux et al., 1990). Changes in the level of eicosenoic acid have been frequently found to correlate with changes in TAG or oil content (Taylor et al., 1992; Germain et al., 2001; Martin et al., 2002; Baud et al., 2003; Rylott et al., 2003; Mu et al., 2008). Overexpression of AHb2 led to a significant 20% to 60% increase in the level of C20:1 eicosenoic acid in mature seeds (Fig. 2B). This indicates that the AHb2-induced increase in total fatty acids is mainly due to an increase in TAG (oil) content.
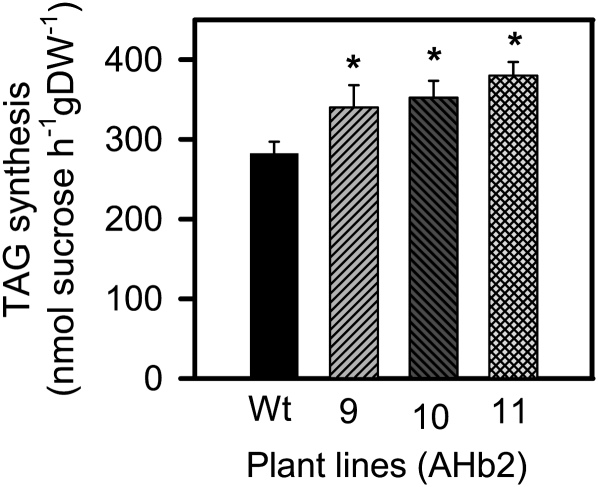
To confirm these results by investigating the rate of TAG synthesis more directly, developing seeds (13–14 DAF) were incubated with [14C]Suc for 2 h to measure label incorporation into TAG. In seeds of the wild type, the flux of Suc into TAG was approximately 280 nmol Suc h−1 g−1 dry weight (Fig. 3), which is in the same range as measured previously for Arabidopsis seeds (Focks and Benning, 1998). Compared with the wild type, there was a significant 30% to 40% increase in the rate of TAG synthesis in the lines overexpressing AHb2 (Fig. 3).
Figure 3.
Increased expression of AHb2 leads to a stimulation in the flux of Suc into TAGs in Arabidopsis seeds. To investigate the effect of AHb2 on the rate of TAG synthesis in developing seeds, wild-type (Wt; black bars) and transgenic (gray bars) siliques that remained attached to their plants were injected with 5 μL of [U-14C]Suc (specific activity of 740 kBq μmol−1) and incubated for 2 h, before they were harvested, rapidly frozen in liquid nitrogen, and seeds dissected to measure label incorporation into TAG. The rate of TAG synthesis was calculated using the specific activity of the labeled Suc. Values are means ± se (n = 4 different sets of plants). Changes that are significantly different from the wild type (based on Student’s t test with P < 0.05) are labeled with asterisks. DW, Dry weight.
Overexpression of AHb2 Leads to an Increase in Polyunsaturated ω-6 Linoleic and ω-3 α-Linolenic Acid Levels and a General Increase in Fatty Acid Desaturation in Mature Seeds
The increase in total fatty acids in response to overexpression of AHb2 was mainly due to an increase in the polyunsaturated C18:2 (ω-6) linoleic and C18:3 (ω-3) α-linolenic acids, the two most abundant fatty acids in Arabidopsis seeds, deriving from C18:1 oleic acid (Fig. 2, compare A with C–E). AHb2 overexpression led to an increase in the C18:2/C18:1 (Fig. 2F) and C18:3/C18:2 (Fig. 2G) ratios as well as in the C18:3 content in mol % of total fatty acids (Fig. 2H). These changes in fatty acid desaturation were also reflected by a general increase in the unsaturation/saturation index (USI) of the seed lipids (ratio between the total number of double bonds and the total content of saturates; Fig. 2I).
Overexpression of AHb2 Leads to an Increase in the Adenylate Energy Status and in the Suc Content of Developing Seeds
In developing oilseeds, Suc derived from photosynthetic leaves is unloaded from the phloem and used as a carbon source for fatty acid synthesis, while energy is supplied by respiratory and photosynthetic processes within the seeds (Rawsthorne, 2002; Hills, 2004; Ruuska et al., 2004). Suc levels and ATP-ADP ratios were measured in developing seeds of wild-type and transgenic plants at a stage that is characterized by a high rate of oil accumulation (13–14 DAF). Overexpression of AHb2 led to a 3-fold higher ATP-ADP ratio and 2-fold-higher Suc content of the seeds, indicating that AHb2 leads to an improved energy and carbon supply for biosynthetic activities (Fig. 1, K and L). In contrast to this, overexpression of AHb1 did not lead to any significant changes in ATP-ADP ratio or Suc levels, with the exception of line 17, where the Suc level decreased (Fig. 1, E and F).
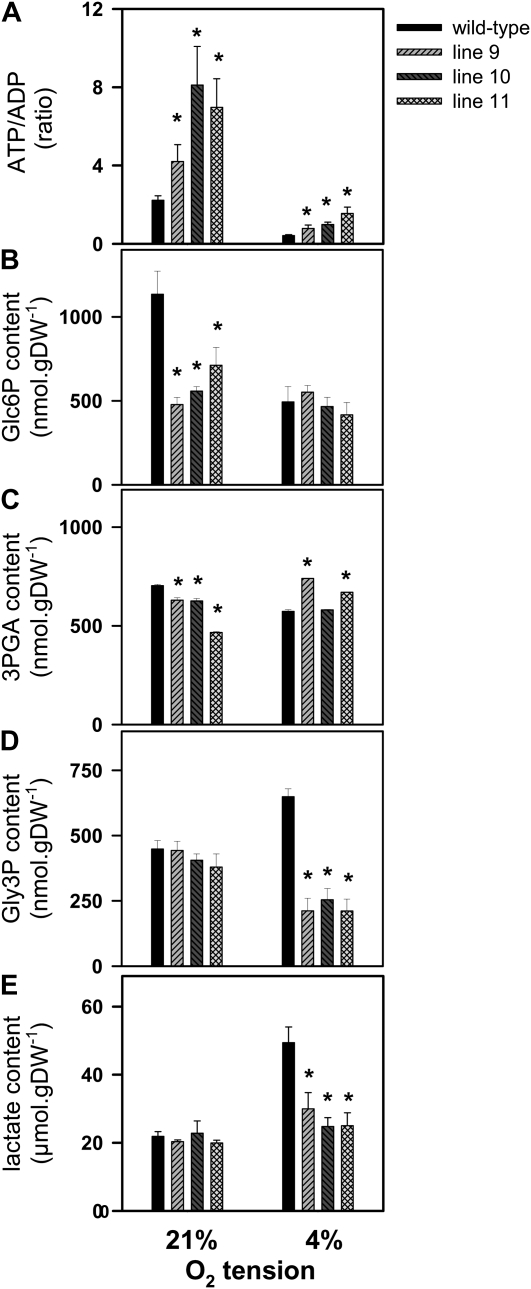
To investigate whether the increase in Suc content and energy state led to changes in the levels of glycolytic intermediates, the contents of Glc-6-P (Glc6P), 3-phosphoglycerate (3PGA), and glycerol-3-phosphate (Gly3P) were measured in developing seeds of AHb2-overexpressing lines compared with the wild type. AHb2 overexpression led to a 2-fold decrease in the level of Glc6P, an up to 20% decrease in the level 3PGA, and a slight decrease in the level of Gly3P (Fig. 4, B–D, left part; 21%). This indicates that AHb2 overexpression stimulated the use of glycolytic intermediates for fatty acid biosynthesis and/or respiration by affecting regulatory sites downstream of these pathways, rather than precursor supply per se.
Figure 4.
Metabolic changes in wild-type and AHb2-overexpressing seeds exposed to low external oxygen concentrations in planta. To investigate the effect of AHb2 on the response of developing seeds to low oxygen, wild-type (black bars) and transgenic (gray bars) siliques attached to the plant were exposed to either 4% or 21% (control) oxygen for 2 h, before they were harvested, rapidly frozen in liquid nitrogen, and seeds dissected to measure ATP-ADP ratio (A), Glc6P (B), 3PGA (C), Gly3P (D), and lactate (E) levels. Values are means ± se (n = 6 different sets of plants). Changes that are significantly different from the wild type (based on Student’s t test with P < 0.05) are labeled with asterisks. DW, Dry weight.
Overexpression of AHb2 Leads to an Increased Energy State and Avoidance of Fermentation When Developing Seeds Are Exposed to Low External Oxygen
Seeds of various species have been found to develop in an internal environment with about 2% to 4% (v/v) oxygen, compared with 21% (v/v) oxygen outside (Porterfield et al., 1999; Gibon et al., 2002; Rolletschek et al., 2002, 2005, 2007; Geigenberger, 2003; Vigeolas et al., 2003, van Dongen et al., 2004). The positive effect of AHb2 on the energy state of the seeds, therefore, may be attributable to a specific role of AHb2 related to the low internal oxygen concentrations within the seeds. To investigate a possible role of AHb2 under low oxygen, external oxygen around the siliques was decreased from 21% to 4% (v/v) for 2 h before adenylate energy state, glycolytic intermediates, and the fermentation products lactate and Gly3P were analyzed in the seeds. In seeds of the wild type, there was a drop in the ATP-ADP ratio from 2 to 0.4 (Fig. 4A) and a decrease in the glycolytic intermediates Glc6P (Fig. 4B) and 3PGA (Fig. 4C), while the fermentation products Gly3P (Fig. 4D) and lactate (Fig. 4E) accumulated under these conditions. This provides evidence that the decrease in external oxygen led to severe limitations in oxygen availability within the seeds, resulting in a shift to anaerobic metabolism. In contrast to this, AHb2 overexpression maintained an up to 5-fold higher ATP-ADP ratio (Fig. 4A) and prevented an increase in the fermentation products Gly3P (Fig. 4D) and lactate (Fig. 4E) as well as a decrease in glycolytic intermediates (Fig. 4, B and C) under these conditions. These data are consistent with AHb2 overexpression results in improved oxygen availability within developing seeds.
DISCUSSION
Nonsymbiotic hemoglobins are widely distributed in plants and divided into two different classes based on gene expression pattern and oxygen-binding properties. Most of the published work has been on the function of class 1 hemoglobins. This study shows a previously unknown function of Arabidopsis class 2 hemoglobin in seed oil production. Overexpression of AHb2 in Arabidopsis seeds resulted in a significant increase in the seed lipid content (Fig. 1; Table I), which was mainly due to an increase in the polyunsaturated (ω-6) C18:2 linoleic and (ω-3) C18:3 α-linolenic acids (Fig. 2). There were both an increase in C20:1 eicosenoic acid, which is a specific marker for TAG (Fig. 2B), and an increase in the rate of TAG biosynthesis (Fig. 3), providing evidence that the increase in total fatty acids in response to AHb2 expression is mainly attributable to an increase in oil content. This was accompanied by an elevated carbon and energy status of the seeds, both under normal conditions (Fig. 1) and in response to low external oxygen (Fig. 4).
To our knowledge, this is the first report of a transgenic approach leading to an increase in both lipid quantity and α-linolenic acid content of oil seeds. Due to the great economic importance of vegetable oils and their expanded use as industrial and nutritional feedstock, there has been considerable interest in engineering plants for high oil quantity and quality. Previous attempts to improve oil quantity have been performed with limited success and concentrated mainly on manipulating enzymes involved in the pathways of fatty acid synthesis (Thelen and Ohlrogge, 2002, Hills, 2004), glycerol-3-phosphate provision (Vigeolas et al., 2007), and TAG assembly (Zou et al., 1997; Jako et al., 2001; Taylor et al., 2002; Zheng et al., 2008). This study shows that increased expression of AHb2, a protein that is not integral to these pathways, leads to a 40% increase in the fatty acid content of the seeds. This is most likely due to the strong increase in seed energy state in response to AHb2 expression (Fig. 1). Previous studies have shown that the energy status is colimiting for the rates of Suc import (Vigeolas et al., 2003; van Dongen et al., 2004) and lipid biosynthesis in developing seeds of different species (Vigeolas et al., 2003; Rolletschek et al., 2005, 2007). Moreover, external conditions, such as light, can have a considerable impact on oil accumulation in seeds by affecting energy and redox parameters (Ruuska et al., 2004; Goffman et al., 2005; Li et al., 2006). Starch synthesis has been found to be more resilient to changes in the seed energy status, since it requires less ATP than lipid synthesis (Neuhaus and Emes, 2000; Vigeolas et al., 2003). This provides a possible explanation why transient starch synthesis was not significantly affected in AHb2-overexpressing seeds (Table I).
Attempts to improve fatty acid composition of seed oil were aimed to increase the production of specific fatty acids with increased industrial or nutritional values (Cahoon et al., 2007; Napier, 2007; Napier and Graham, 2010). One important goal to increase the nutritional value of vegetable oil in food products is to increase the content of polyunsaturated fatty acids. ω-3 polyunsaturated α-linolenic acid is considered an essential fatty acid because it is required for health, but it cannot be synthesized by humans. Vegetable oil is one of the major dietary sources of α-linolenic acid, and a large body of scientific research suggests that increasing the relative abundance of dietary ω-3 fatty acids has a number of clinical benefits for human health through the prevention and management of many diseases (Simopoulos, 1991; Crawford et al., 2000).
Previous approaches were able to increase the α-linolenic acid content of oil in relative terms by ectopic expression of fatty acid desaturases in seeds, but this was often accompanied by severe yield penalties (Kinney et al., 2002; Anai et al., 2003). Other approaches involved the engineering of pathways generating very-long-chain ω-3 polyunsaturated fatty acids, such as eicosapentanoic acid and docosahexaenoic acid in oilseed crops, by introducing genes from diverse organisms that produce these fatty acids (Napier, 2007; Napier and Graham, 2010). While these attempts have met with some degree of success, they did not lead to the production of economically sufficient amounts of these novel fatty acids (Cahoon et al., 2007). Overexpression of AHb2 provides a possible route to achieve a combined increase in α-linolenic acid and total oil content in plants. More studies are needed to investigate whether these results can be transferred from Arabidopsis to economically important oilseeds grown under high-light conditions in the field.
An increase in polyunsaturated fatty acids has also been reported to play a crucial role in the chilling tolerance of plants. Specifically, an increased production of C18:3 α-linolenic acid has been found to be connected with cold acclimation and the protection of plant cells against cold damage (Kodama et al., 1994). Class 2 hemoglobins, therefore, may play an important role in the adaptive response of plants to cold stress by promoting the synthesis of C18:3 α-linolenic acid. This may explain why expression of class 2 hemoglobins is induced by low temperature rather than low oxygen (Trevaskis et al., 1997). In addition to this, it has been reported that an increase in the unsaturation level of C18 fatty acids promotes climacteric fruit ripening by affecting the synthesis of jasmonates (Gutierrez et al., 2005). This provides a possible explanation for the preferential expression of class 2 hemoglobins in immature fruits (Wang et al., 2003). More studies are needed to investigate the importance of Hb2 under chilling stress and during fruit ripening.
To our knowledge, this is also the first report showing that the biosynthetic performance of plants can be improved by overexpressing endogenous hemoglobins. Previous studies in this context were mainly focused on the heterologous expression of the bacterial Vitreoscilla hemoglobin in plants. This led to positive effects on growth and metabolism (Kallio et al., 2001, 2008; Frey and Kallio, 2003, 2005; Jokipii-Lukkari et al., 2009) as well as on the production of different secondary metabolites in tobacco (Nicotiana tabacum; Holmberg et al., 1997), aspen (Populus spp.; Häggman et al., 2003), and Arabidopsis (Wang et al., 2009). Although the underlying mechanisms are not fully resolved yet, Vitreoscilla hemoglobin is mainly considered as an oxygen-binding and -delivering protein with a possible function in facilitating oxygen supply (Jokipii-Lukkari et al., 2009).
In addition to its biotechnological potential, this study provides evidence that class 1 and class 2 hemoglobins have different functions in plants. Previous investigations were mainly focused on class 1 hemoglobins and have led to the conclusion that the main function of these hemoglobins is to metabolize NO formed during severe hypoxia (for review, see Hebelstrup et al., 2007). Under these conditions, Hb1 is strongly induced and serves as part of a NO dioxygenase system, using traces of oxygen to convert NO to nitrate. This not only leads to scavenging of NO but also to the establishment of a connection between NO turnover and the maintenance of redox and energy levels under anaerobic conditions (Sowa et al., 1998; Dordas et al., 2003; Igamberdiev et al., 2004, 2006; Stoimenova et al., 2007). The removal of NO allows nitrite and NAD(P)H-driven ATP synthesis to proceed in anaerobic mitochondria without inhibition of cytochrome oxidase by NO. Overall, this will explain why increased Hb1 expression leads to improved survival under severe hypoxic stress (Hunt et al., 2001).
Here, we show that overexpressing AHb2 has a different effect on seed energy state, Suc level, and fatty acid accumulation than AHb1 (Fig. 1). Under normal growth conditions, overexpression of AHb2 led to an increase in the biosynthetic performance of developing seeds that was not evident for AHb1. This indicates a specific function of AHb2 expression to promote energy-dependent storage metabolism in developing seeds, most probably by improving oxygen supply for mitochondrial respiration. This contrasts with Hb1 having a role to improve plant survival under anaerobic stress conditions by scavenging of NO. The different physiological functions of AHb1 and AHb2 are most likely due to structural differences between the two proteins. First, there are differences in ligand migration properties allowing AHb1 to bind NO and limit NO binding in AHb2. This makes it unlikely that AHb2 plays a role in NO oxidation similar to AHb1 (Bruno et al., 2007; Nienhaus et al., 2010). Second, there are strong differences in the oxygen-binding properties of the two proteins, with AHb2 having a 2 orders of magnitude lower affinity to oxygen than AHb1 (Hoy and Hargrove, 2008). The oxygen affinity of AHb2 is similar to cytochrome oxidase, making it likely that AHb2 may have a specific function in facilitating oxygen supply to mitochondrial respiration. The resulting increase in the energy state will improve the biosynthetic performance of the seeds, which explains the increase in the Suc and fatty acid contents in response to AHb2, but not AHb1, expression (Fig. 1). Internal oxygen concentrations in developing seeds have been frequently reported to be limiting for respiration and biosynthetic performance (Rolletschek et al., 2003, 2005, 2007; Vigeolas et al., 2003; van Dongen et al., 2004).
The increase in polyunsaturated fatty acids is also consistent with a role of AHb2 to improve oxygen availability in the seeds. AHb2 overexpression led to a combined increase in C18:2 linoleic and C18:3 linolenic acids, both in absolute terms and relative to C18:1 oleic acid (Fig. 2), indicating activation of the membrane-bound fatty acid desaturases FAD2 and FAD3 located at the endoplasmic reticulum. Unlike other organisms, plants regulate these fatty acid desaturases at the posttranslational rather than the transcriptional level (Sakamoto and Murata, 2002; Lu et al., 2009). It has been shown for sunflower (Helianthus annuus) seeds that internal oxygen levels act as key regulators for the activities of fatty acid desaturase enzymes (Martínez-Rivas et al., 2003; Rolletschek et al., 2007). Increased oxygen supply raised the energy status of the seeds and produced a dramatic increase in FAD2 activity as well as linoleic acid content. Moreover, low temperature increased the unsaturation degree of sunflower oil by its effect on dissolved oxygen levels in the developing seeds (Rolletschek et al., 2007). Therefore, it is most likely that overexpression of AHb2 led to an increased production of polyunsaturated fatty acids by facilitating the availability of molecular oxygen as a substrate to fatty acid desaturases. Unfortunately, there is no method available at present that allows direct analysis of oxygen concentrations within developing Arabidopsis seeds.
In conclusion, this paper provides evidence for a specific function of class 2 hemoglobins in promoting seed lipid production when overexpressed in developing seeds. It also provides a possible route to improve the production of polyunsaturated fatty acids in oil crops by overexpressing AHb2. Further studies using antisense or RNA interference approaches to knock down AHb2 would be required to establish whether this represents the normal function of AHb2 in seed development rather than a specific function under conditions where its expression is induced (i.e. under cold stress).
MATERIALS AND METHODS
Plant Cultivation, Oxygen Treatments, and Sampling
Arabidopsis (Arabidopsis thaliana) plants were grown in a greenhouse (21°C day and 17°C night, 50% humidity, with a photoperiod of 16-h day/8-h night at a light intensity of 180 μmol photons m−2 s−1). For incubation experiments with low oxygen, silique-bearing stalks were enclosed in a transparent plastic bag through which air with 21% or 4% (v/v) oxygen was circulated. The premixed gases (Messer Griesheim) used for this treatment contained 350 μL L−1 CO2, oxygen concentrations as indicated above, and nitrogen. After 2 h, siliques were harvested and immediately frozen in liquid N2. Seeds were dissected from 13- to 14-d-old lypophilized siliques using a custom-made vacuum sampler as described by Gibon et al. (2002).
Generation and Cultivation of AHb1- and AHb2-Overexpressing Lines
For both AHb1 and AHb2, cDNA was produced by reverse transcription-PCR (SuperScript II; Invitrogen) on total RNA isolated from 6-week-old Arabidopsis plants using the following specific primers: AHb1f (5′-TTTGGTACCATGGAGAGTGAAGGAAAGATTGTG-3′), AHb1r (5′-TTTGGATCCTTAGTTGGAAAGATTCATTTCAGC-3′), AHb2f (5′-TTTGGTACCATGGGAGAGATTGGGTTTACAGAG-3′), and AHb2r (5′-TTTGGATCCTTATGACCTTTCTTGTTTCATCTCGG-3′). The cDNA was cloned in a plant binary vector downstream of the seed-specific USP promoter (Bäumlein et al., 1991). The resulting construct was transformed via Agrobacterium tumefaciens into Arabidopsis var C24 plants. Using hygromycin-containing selective medium, three independent primary USP-AHb1 and four USP-AHb2 transgenic lines were selected and propagated until the third generation, which was used for further analyses.
Analysis of AHb1 and AHb2 mRNA and Protein Levels
Expression levels of AHb1 and AHb2 mRNA levels were analyzed on northern blot. Therefore, total RNA was extracted using Concert RNA Plant Reagent (Invitrogen), and full-length cDNA encoding AHb1 or AHb2 was used as a probe for hybridization. AHb1 and AHb2 protein expression was tested on western blots after hybridization with homolog-specific antibodies raised in rabbit (Hunt et al., 2002) and detection by peroxidase-conjugated anti-rabbit antibodies (Bio-Rad) on x-ray film and enhanced chemiluminescence substrate (CDP Star; Roche). Protein was extracted from 1 mg of ground seed material in 250 μL of extraction buffer (62.5 mm Tris-Cl, pH 6.9, containing 2% SDS, 10% glycerol, 0.02% bromphenol blue, 1 mm benzamidine, 1 mm ε-amino caproic acid, 1 mm EDTA, 1 mm EGTA, and 5 mm dithiothreitol).
Analysis of Metabolites, Lipids, and Protein
Seeds were homogenized using a liquid nitrogen-cooled ball mill, then metabolites were extracted using trichloroacetic acid, and the levels of Suc, Glc6P, 3PGA, glycerol-3-phosphate, ATP, ADP, and lactate were subsequently quantified using enzymic cycling assays as described by Gibon et al. (2002). Total fatty acids were extracted following the method of Bligh and Dyer (1959), and lipid content and composition were determined by gas chromatography of fatty acid methyl esters, using pentadecanoic acid as an internal standard (Benning and Somerville, 1992). The USI of fatty acids is calculated as described by Gutierrez et al. (2005): USI = UI/∑(saturates), with UI = ∑miri, where mi is the number of double bonds and ri is the relative content of each fatty acid. Protein content was determined according to the method described by Bradford (1976) using bovine serum albumin as a standard. Starch content was determined as described by Vigeolas et al. (2003).
Feeding Experiments and Analysis of Label Incorporation into TAG
[U-14C]Suc was provided to developing seeds (13–14 DAF) by injection into the siliques that remained attached to the plant in the light as described by Gibon et al. (2002). Each silique was injected with 5 μL of [U-14C]Suc (specific activity of 740 kBq μmol−1) using a 5-μL Hamilton syringe. After 2 h, siliques were harvested, frozen in liquid nitrogen, and lyophilized, and seeds were separated from the silique wall. TAGs were extracted and separated by thin-layer chromatography as described by Focks and Benning (1998). Silica material containing TAG was transferred from the thin-layer chromatography plate into scintillation cocktail, and radioactivity was determined by scintillation counting.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers NM_127165 and NM_111887.
Acknowledgments
We are very grateful to Peter Hunt and R.A. Watts (Commonwealth Scientific and Industrial Research Organization, Canberra, Australia) for kindly providing the AHb1 and AHb2 hemoglobin antibodies, to Peter Dörmann for help with gas chromatography analysis of fatty acids, to Fabien Poree for help with cloning experiments, and to Mark Stitt and Joost T. van Dongen (all from Max-Planck-Institute of Molecular Plant Physiology, Golm, Germany) for support and for critical readings of the manuscript.
References
- Anai T, Koga M, Tanaka H, Kinoshita T, Rahman SM, Takagi Y. (2003) Improvement of rice (Oryza sativa L.) seed oil quality through introduction of a soybean microsomal omega-3 fatty acid desaturase gene. Plant Cell Rep 21: 988–992 [DOI] [PubMed] [Google Scholar]
- Andersson CR, Jensen EO, Llewellyn DJ, Dennis ES, Peacock WJ. (1996) A new hemoglobin gene from soybean: a role for hemoglobin in all plants. Proc Natl Acad Sci USA 93: 5682–5687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby CA, Bogusz D, Dennis ES, Peacock WJ. (1988) A role for hemoglobin in all plant roots. Plant Cell Environ 1: 359–367 [Google Scholar]
- Baud S, Boutin JP, Miquel M, Lepiniec L, Rochat C. (2002) An integrated overview of seed development in Arabidopsis thaliana ecotype WS. Plant Physiol Biochem 40: 151–160 [Google Scholar]
- Baud S, Guyon V, Kronenberger J, Wuillème S, Miquel M, Caboche M, Lepiniec L, Rochat C. (2003) Multifunctional acetyl-CoA carboxylase 1 is essential for very long chain fatty acid elongation and embryo development in Arabidopsis. Plant J 33: 75–86 [DOI] [PubMed] [Google Scholar]
- Bäumlein H, Boerjan W, Nagy I, Bassüner R, Van Montagu M, Inzé D, Wobus U. (1991) A novel seed protein gene from Vicia faba is developmentally regulated in transgenic tobacco and Arabidopsis plants. Mol Gen Genet 225: 459–467 [DOI] [PubMed] [Google Scholar]
- Benning C, Somerville CR. (1992) Identification of an operon involved in sulfolipid biosynthesis in Rhodobacter sphaeroides. J Bacteriol 174: 6479–6487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917 [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Bruno S, Faggiano S, Spyrakis F, Mozzarelli A, Abbruzzetti S, Grandi E, Viappiani C, Feis A, Mackowiak S, Smulevich G, et al. (2007) The reactivity with CO of AHb1 and AHb2 from Arabidopsis thaliana is controlled by the distal HisE7 and internal hydrophobic cavities. J Am Chem Soc 129: 2880–2889 [DOI] [PubMed] [Google Scholar]
- Cahoon EB, Shockey JM, Dietrich CR, Gidda SK, Mullen RT, Dyer JM. (2007) Engineering oilseeds for sustainable production of industrial and nutritional feedstocks: solving bottlenecks in fatty acid flux. Curr Opin Plant Biol 10: 236–244 [DOI] [PubMed] [Google Scholar]
- Crawford M, Galli C, Visioli F, Renaud S, Simopoulos AP, Spector AA. (2000) Role of plant-derived omega-3 fatty acids in human nutrition. Ann Nutr Metab 44: 263–265 [DOI] [PubMed] [Google Scholar]
- Dordas C. (2009) Nonsymbiotic hemoglobins and stress tolerance in plants. Plant Sci 176: 433–440 [DOI] [PubMed] [Google Scholar]
- Dordas C, Hasinoff BB, Igamberdiev AU, Manac’h N, Rivoal J, Hill RD. (2003) Expression of a stress-induced hemoglobin affects NO levels produced by alfalfa root cultures under hypoxic stress. Plant J 35: 763–770 [DOI] [PubMed] [Google Scholar]
- Dordas C, Hasinoff BB, Rivoal J, Hill RD. (2004) Class-1 hemoglobins, nitrate and NO levels in anoxic maize cell-suspension cultures. Planta 219: 66–72 [DOI] [PubMed] [Google Scholar]
- Focks N, Benning C. (1998) wrinkled1: a novel, low-seed-oil mutant of Arabidopsis with a deficiency in the seed-specific regulation of carbohydrate metabolism. Plant Physiol 118: 91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey AD, Kallio PT. (2003) Bacterial hemoglobins and flavohemoglobins: versatile proteins and their impact on microbiology and biotechnology. FEMS Microbiol Rev 27: 525–545 [DOI] [PubMed] [Google Scholar]
- Frey AD, Kallio PT. (2005) Nitric oxide detoxification: a new era for bacterial globins in biotechnology? Trends Biotechnol 23: 69–73 [DOI] [PubMed] [Google Scholar]
- Geigenberger P. (2003) Response of plant metabolism to too little oxygen. Curr Opin Plant Biol 6: 247–256 [DOI] [PubMed] [Google Scholar]
- Germain V, Rylott EL, Larson TR, Sherson SM, Bechtold N, Carde JP, Bryce JH, Graham IA, Smith SM. (2001) Requirement for 3-ketoacyl-CoA thiolase-2 in peroxisome development, fatty acid beta-oxidation and breakdown of triacylglycerol in lipid bodies of Arabidopsis seedlings. Plant J 28: 1–12 [DOI] [PubMed] [Google Scholar]
- Gibon Y, Vigeolas H, Tiessen A, Geigenberger P, Stitt M. (2002) Sensitive and high throughput metabolite assays for inorganic pyrophosphate, ADPGlc, nucleotide phosphates, and glycolytic intermediates based on a novel enzymic cycling system. Plant J 30: 221–235 [DOI] [PubMed] [Google Scholar]
- Goffman FD, Alonso AP, Schwender J, Shachar-Hill Y, Ohlrogge JB. (2005) Light enables a very high efficiency of carbon storage in developing embryos of rapeseed. Plant Physiol 138: 2269–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez M, Sola MM, Vargas AM. (2005) Fatty acid composition of phospholipids in mesocarp of cherimoya fruit during ripening. Food Chem 90: 341–346 [Google Scholar]
- Häggman H, Frey AD, Ryynänen L, Aronen T, Julkunen-Tiitto R, Tiimonen H, Pihakaski-Maunsbach K, Jokipii S, Chen XW, Kallio PT. (2003) Expression of Vitreoscilla haemoglobin in hybrid aspen (Populus tremula × tremuloides). Plant Biotechnol J 1: 287–300 [DOI] [PubMed] [Google Scholar]
- Hebelstrup KH, Hunt P, Dennis E, Jensen SB, Jensen EO. (2006) Hemoglobin is essential for normal growth of Arabidopsis organs. Physiol Plant 127: 157–166 [Google Scholar]
- Hebelstrup KH, Igamberdiev AU, Hill RD. (2007) Metabolic effects of hemoglobin gene expression in plants. Gene 398: 86–93 [DOI] [PubMed] [Google Scholar]
- Hendriks T, Scheer I, Quillet MC, Randoux B, Delbreil B, Vasseur J, Hilbert JL. (1998) A nonsymbiotic haemoglobin gene is expressed during somatic embryogenesis in Cichorium. Biochim Biophys Acta 1443: 193–197 [DOI] [PubMed] [Google Scholar]
- Hill RD. (1998) What are hemoglobins doing in plants? Can J Bot 76: 707–712 [Google Scholar]
- Hills MJ. (2004) Control of storage-product synthesis in seeds. Curr Opin Plant Biol 7: 302–308 [DOI] [PubMed] [Google Scholar]
- Holmberg N, Lilius G, Bailey JE, Bülow L. (1997) Transgenic tobacco expressing Vitreoscilla hemoglobin exhibits enhanced growth and altered metabolite production. Nat Biotechnol 15: 244–247 [DOI] [PubMed] [Google Scholar]
- Hoy JA, Hargrove MS. (2008) The structure and function of plant hemoglobins. Plant Physiol Biochem 46: 371–379 [DOI] [PubMed] [Google Scholar]
- Hunt PW, Klok EJ, Trevaskis B, Watts RA, Ellis MH, Peacock WJ, Dennis ES. (2002) Increased level of hemoglobin 1 enhances survival of hypoxic stress and promotes early growth in Arabidopsis thaliana. Proc Natl Acad Sci USA 99: 17197–17202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PW, Watts RA, Trevaskis B, Llewelyn DJ, Burnell J, Dennis ES, Peacock WJ. (2001) Expression and evolution of functionally distinct haemoglobin genes in plants. Plant Mol Biol 47: 677–692 [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Seregélyes C, Manac’h N, Hill RD. (2004) NADH-dependent metabolism of nitric oxide in alfalfa root cultures expressing barley hemoglobin. Planta 219: 95–102 [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Stoimenova M, Seregélyes C, Hill RD. (2006) Class-1 hemoglobin and antioxidant metabolism in alfalfa roots. Planta 223: 1041–1046 [DOI] [PubMed] [Google Scholar]
- Jako C, Kumar A, Wei YD, Zou JT, Barton DL, Giblin EM, Covello PS, Taylor DC. (2001) Seed-specific overexpression of an Arabidopsis cDNA encoding a diacylglycerol acyltransferase enhances seed oil content and seed weight. Plant Physiol 126: 861–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokipii-Lukkari S, Frey AD, Kallio PT, Häggman H. (2009) Intrinsic non-symbiotic and truncated haemoglobins and heterologous Vitreoscilla haemoglobin expression in plants. J Exp Bot 60: 409–422 [DOI] [PubMed] [Google Scholar]
- Kallio PT, Frey AD, Bailey JE. (2001) From Vitreoscilla hemoglobin (VHb) to a novel class of growth stimulating hemoglobin proteins. Merten W, Mattanovich D, Lang C, Larsson G, Neubauer P, Posso D, Postma P, Teixeira de Mattos J, Cole JA, , Recombinant Protein Production with Prokaryotic and Eukaryotic Cells: A Comparative View on Host Physiology. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 75–87 [Google Scholar]
- Kinney AJ, Cahoon EB, Hitz WD. (2002) Manipulating desaturase activities in transgenic crop plants. Biochem Soc Trans 30: 1099–1103 [DOI] [PubMed] [Google Scholar]
- Kodama H, Hamada T, Horiguchi G, Nishimura M, Iba K. (1994) Genetic enhancement of cold tolerance by expression of a gene for chloroplast omega-3 fatty acid desaturase in transgenic tobacco. Plant Physiol 105: 601–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo H. (1939) Über Haemoprotein aus den Wurzelknöllchen von Leguminosen. Acta Phytochim 11: 195–200 [Google Scholar]
- Kundu S, Trent JT, III, Hargrove MS. (2003) Plants, humans and hemoglobins. Trends Plant Sci 8: 387–393 [DOI] [PubMed] [Google Scholar]
- Lemieux B, Miquel M, Somerville C, Browse J. (1990) Mutants of Arabidopsis with alterations in seed lipid fatty acid composition. Theor Appl Genet 80: 234–240 [DOI] [PubMed] [Google Scholar]
- Li Y, Beisson F, Pollard M, Ohlrogge J. (2006) Oil content of Arabidopsis seeds: the influence of seed anatomy, light and plant-to-plant variation. Phytochemistry 67: 904–915 [DOI] [PubMed] [Google Scholar]
- Lu C, Xin Z, Ren Z, Miquel M, Browse J. (2009) An enzyme regulating triacylglycerol composition is encoded by the ROD1 gene of Arabidopsis. Proc Natl Acad Sci USA 106: 18837–18842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T, Oswald O, Graham IA. (2002) Arabidopsis seedling growth, storage lipid mobilization, and photosynthetic gene expression are regulated by carbon:nitrogen availability. Plant Physiol 128: 472–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Rivas JM, Sánchez-García A, Sicardo MD, Mancha M. (2003) Oxygen availability regulates microsomal oleate desaturase (FAD2) in sunflower developing seeds by two different mechanisms. Murata N, Yamada M, Nishida I, Okuyama H, Sekiya J, Wada H, , Advanced Research on Plant Lipids. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 109–112 [Google Scholar]
- Mu J, Tan H, Zheng Q, Fu F, Liang Y, Zhang J, Yang X, Wang T, Chong K, Wang XJ, et al. (2008) LEAFY COTYLEDON1 is a key regulator of fatty acid biosynthesis in Arabidopsis. Plant Physiol 148: 1042–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napier JA. (2007) The production of unusual fatty acids in transgenic plants. Annu Rev Plant Biol 58: 295–319 [DOI] [PubMed] [Google Scholar]
- Napier JA, Graham IA. (2010) Tailoring plant lipid composition: designer oilseeds come of age. Curr Opin Plant Biol 13: 330–337 [DOI] [PubMed] [Google Scholar]
- Neuhaus HE, Emes MJ. (2000) Non-photosynthetic metabolism in plastids. Annu Rev Plant Physiol Plant Mol Biol 51: 111–140 [DOI] [PubMed] [Google Scholar]
- Nienhaus K, Dominici P, Astegno A, Abbruzzetti S, Viappiani C, Nienhaus GU. (2010) Ligand migration and binding in nonsymbiotic hemoglobins of Arabidopsis thaliana. Biochemistry 49: 7448–7458 [DOI] [PubMed] [Google Scholar]
- Ott T, van Dongen JT, Günther C, Krusell L, Desbrosses G, Vigeolas H, Bock V, Czechowski T, Geigenberger P, Udvardi MK. (2005) Symbiotic leghemoglobins are crucial for nitrogen fixation in legume root nodules but not for general plant growth and development. Curr Biol 15: 531–535 [DOI] [PubMed] [Google Scholar]
- Perazzolli M, Dominici P, Romero-Puertas MC, Zago E, Zeier J, Sonoda M, Lamb C, Delledonne M. (2004) Arabidopsis nonsymbiotic hemoglobin AHb1 modulates nitric oxide bioactivity. Plant Cell 16: 2785–2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porterfield DM, Kuang A, Smith PJ, Crispi ML, Musgrave ME. (1999) Oxygen-depleted zones inside reproductive structures of Brassicaceae: implications for oxygen control of seed development. Can J Bot 77: 1439–1446 [PubMed] [Google Scholar]
- Rawsthorne S. (2002) Carbon flux and fatty acid synthesis in plants. Prog Lipid Res 41: 182–196 [DOI] [PubMed] [Google Scholar]
- Rolletschek H, Borisjuk L, Koschorreck M, Wobus U, Weber H. (2002) Legume embryos develop in a hypoxic environment. J Exp Bot 53: 1099–1107 [DOI] [PubMed] [Google Scholar]
- Rolletschek H, Borisjuk L, Sánchez-García A, Gotor C, Romero LC, Martínez-Rivas JM, Mancha M. (2007) Temperature-dependent endogenous oxygen concentration regulates microsomal oleate desaturase in developing sunflower seeds. J Exp Bot 58: 3171–3181 [DOI] [PubMed] [Google Scholar]
- Rolletschek H, Radchuk R, Klukas C, Schreiber F, Wobus U, Borisjuk L. (2005) Evidence of a key role for photosynthetic oxygen release in oil storage in developing soybean seeds. New Phytol 167: 777–786 [DOI] [PubMed] [Google Scholar]
- Rolletschek H, Weber H, Borisjuk L. (2003) Energy status and its control on embryogenesis of legumes: embryo photosynthesis contributes to oxygen supply and is coupled to biosynthetic fluxes. Plant Physiol 132: 1196–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross EJH, Stone JM, Elowsky CG, Arredondo-Peter R, Klucas RV, Sarath G. (2004) Activation of the Oryza sativa non-symbiotic haemoglobin-2 promoter by the cytokinin-regulated transcription factor, ARR1. J Exp Bot 55: 1721–1731 [DOI] [PubMed] [Google Scholar]
- Ruuska SA, Schwender J, Ohlrogge JB. (2004) The capacity of green oilseeds to utilize photosynthesis to drive biosynthetic processes. Plant Physiol 136: 2700–2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylott EL, Gilday AD, Graham IA. (2003) The gluconeogenic enzyme phosphoenolpyruvate carboxykinase in Arabidopsis is essential for seedling establishment. Plant Physiol 131: 1834–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, Murata N. (2002) Regulation of the desaturation of fatty acids and its role in tolerance to cold and salt stress. Curr Opin Microbiol 5: 208–210 [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. (1991) Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr 54: 438–463 [DOI] [PubMed] [Google Scholar]
- Sowa AW, Duff SMG, Guy PA, Hill RD. (1998) Altering hemoglobin levels changes energy status in maize cells under hypoxia. Proc Natl Acad Sci USA 95: 10317–10321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoimenova M, Igamberdiev AU, Gupta KJ, Hill RD. (2007) Nitrite-driven anaerobic ATP synthesis in barley and rice root mitochondria. Planta 226: 465–474 [DOI] [PubMed] [Google Scholar]
- Taylor DC, Barton DL, Rioux KP, Mackenzie SL, Reed DW, Underhill EW, Pomeroy MK, Weber N. (1992) Biosynthesis of acyl lipids containing very-long chain fatty acids in microspore-derived and zygotic embryos of Brassica napus L. cv Reston. Plant Physiol 99: 1609–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DC, Katavic V, Zou JT, MacKenzie SL, Keller WA, An J, Friesen W, Barton DL, Pedersen KK, Giblin EM, et al. (2002) Field testing of transgenic rapeseed cv. Hero transformed with a yeast sn-2 acyltransferase results in increased oil content, erucic acid content and seed yield. Mol Breed 8: 317–322 [Google Scholar]
- Taylor ER, Nie XZ, MacGregor AW, Hill RD. (1994) A cereal haemoglobin gene is expressed in seed and root tissues under anaerobic conditions. Plant Mol Biol 24: 853–862 [DOI] [PubMed] [Google Scholar]
- Thelen JJ, Ohlrogge JB. (2002) Metabolic engineering of fatty acid biosynthesis in plants. Metab Eng 4: 12–21 [DOI] [PubMed] [Google Scholar]
- Trevaskis B, Watts RA, Andersson CR, Llewellyn DJ, Hargrove MS, Olson JS, Dennis ES, Peacock WJ. (1997) Two hemoglobin genes in Arabidopsis thaliana: the evolutionary origins of leghemoglobins. Proc Natl Acad Sci USA 94: 12230–12234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen JT, Roeb GW, Dautzenberg M, Froehlich A, Vigeolas H, Minchin PE, Geigenberger P. (2004) Phloem import and storage metabolism are highly coordinated by the low oxygen concentrations within developing wheat seeds. Plant Physiol 135: 1809–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigeolas H, van Dongen JT, Waldeck P, Hühn D, Geigenberger P. (2003) Lipid storage metabolism is limited by the prevailing low oxygen concentrations within developing seeds of oilseed rape. Plant Physiol 133: 2048–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigeolas H, Waldeck P, Zank T, Geigenberger P. (2007) Increasing seed oil content in oil-seed rape (Brassica napus L.) by over-expression of a yeast glycerol-3-phosphate dehydrogenase under the control of a seed-specific promoter. Plant Biotechnol J 5: 431–441 [DOI] [PubMed] [Google Scholar]
- Vinogradov SN, Hoogewijs D, Bailly X, Arrendondo-Peter R, Gough J, Dewilde S, Moens L, Vanfleteren JR. (2006) A phylogenomic profile of globins. BMC Evol Biol 6: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Kochian LV, Doyle JJ, Garvin DF. (2003) Two tomato non-symbiotic haemoglobin genes are differentially expressed in response to diverse changes in mineral nutrition status. Plant Cell Environ 26: 673–680 [Google Scholar]
- Wang Z, Xiao Y, Chen W, Tang K, Zhang L. (2009) Functional expression of Vitreoscilla hemoglobin (VHb) in Arabidopsis relieves submergence, nitrosative, photo-oxidative stress and enhances antioxidant metabolism. Plant Sci 176: 66–77 [Google Scholar]
- Zheng P, Allen WB, Roesler K, Williams ME, Zhang S, Li J, Glassman K, Ranch J, Nubel D, Solawetz W, et al. (2008) A phenylalanine in DGAT is a key determinant of oil content and composition in maize. Nat Genet 40: 367–372 [DOI] [PubMed] [Google Scholar]
- Zou JT, Katavic V, Giblin EM, Barton DL, MacKenzie SL, Keller WA, Hu X, Taylor DC. (1997) Modification of seed oil content and acyl composition in the Brassicaceae by expression of a yeast sn-2 acyltransferase gene. Plant Cell 9: 909–923 [DOI] [PMC free article] [PubMed] [Google Scholar]