Abstract
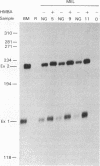
A high-titer amphotropic retroviral vector containing the neomycin resistance gene and a hybrid gamma-beta genomic human globin gene has been constructed. Mouse erythroleukemia cells infected with this virus were found to contain the full transcriptional unit of the transferred human globin gene by Southern blot analysis. These cells contain normally initiated, spliced, and terminated human globin mRNA. The human globin mRNA level increased 5- to 10-fold upon induction of the mouse erythroleukemia cells. Human globin chains were produced but only in a fraction of the cells as detected by immunofluorescent staining. A similar retrovirus containing a human beta-globin gene was used to transduce mouse erythroleukemia cells resulting in much higher levels of human globin synthesis than detected in mouse erythroleukemia cells transduced with the gamma-beta globin virus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnou N. P., Karlsson S., Moulton A. D., Keller G., Nienhuis A. W. Promoter sequences required for function of the human gamma globin gene in erythroid cells. EMBO J. 1986 Jan;5(1):121–126. doi: 10.1002/j.1460-2075.1986.tb04185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson W. F. Prospects for human gene therapy. Science. 1984 Oct 26;226(4673):401–409. doi: 10.1126/science.6093246. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cone R. D., Weber-Benarous A., Baorto D., Mulligan R. C. Regulated expression of a complete human beta-globin gene encoded by a transmissible retrovirus vector. Mol Cell Biol. 1987 Feb;7(2):887–897. doi: 10.1128/mcb.7.2.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Eglitis M. A., Kantoff P., Gilboa E., Anderson W. F. Gene expression in mice after high efficiency retroviral-mediated gene transfer. Science. 1985 Dec 20;230(4732):1395–1398. doi: 10.1126/science.2999985. [DOI] [PubMed] [Google Scholar]
- Gruber H. E., Finley K. D., Hershberg R. M., Katzman S. S., Laikind P. K., Seegmiller J. E., Friedmann T., Yee J. K., Jolly D. J. Retroviral vector-mediated gene transfer into human hematopoietic progenitor cells. Science. 1985 Nov 29;230(4729):1057–1061. doi: 10.1126/science.3864246. [DOI] [PubMed] [Google Scholar]
- Hock R. A., Miller A. D. Retrovirus-mediated transfer and expression of drug resistance genes in human haematopoietic progenitor cells. Nature. 1986 Mar 20;320(6059):275–277. doi: 10.1038/320275a0. [DOI] [PubMed] [Google Scholar]
- Huszar D., Balling R., Kothary R., Magli M. C., Hozumi N., Rossant J., Bernstein A. Insertion of a bacterial gene into the mouse germ line using an infectious retrovirus vector. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8587–8591. doi: 10.1073/pnas.82.24.8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Constitutive and conditional suppression of exogenous and endogenous genes by anti-sense RNA. Science. 1985 Jul 26;229(4711):345–352. doi: 10.1126/science.2990048. [DOI] [PubMed] [Google Scholar]
- Jähner D., Haase K., Mulligan R., Jaenisch R. Insertion of the bacterial gpt gene into the germ line of mice by retroviral infection. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6927–6931. doi: 10.1073/pnas.82.20.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson S., Humphries R. K., Gluzman Y., Nienhuis A. W. Transfer of genes into hematopoietic cells using recombinant DNA viruses. Proc Natl Acad Sci U S A. 1985 Jan;82(1):158–162. doi: 10.1073/pnas.82.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson S., Nienhuis A. W. Developmental regulation of human globin genes. Annu Rev Biochem. 1985;54:1071–1108. doi: 10.1146/annurev.bi.54.070185.005231. [DOI] [PubMed] [Google Scholar]
- Karlsson S., Van Doren K., Schweiger S. G., Nienhuis A. W., Gluzman Y. Stable gene transfer and tissue-specific expression of a human globin gene using adenoviral vectors. EMBO J. 1986 Sep;5(9):2377–2385. doi: 10.1002/j.1460-2075.1986.tb04507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Kim S. K., Wold B. J. Stable reduction of thymidine kinase activity in cells expressing high levels of anti-sense RNA. Cell. 1985 Aug;42(1):129–138. doi: 10.1016/s0092-8674(85)80108-2. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Ong E. S., Rosenfeld M. G., Verma I. M., Evans R. M. Infectious and selectable retrovirus containing an inducible rat growth hormone minigene. Science. 1984 Sep 7;225(4666):993–998. doi: 10.1126/science.6089340. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Trauber D. R., Buttimore C. Factors involved in production of helper virus-free retrovirus vectors. Somat Cell Mol Genet. 1986 Mar;12(2):175–183. doi: 10.1007/BF01560664. [DOI] [PubMed] [Google Scholar]
- Panganiban A. T. Retroviral DNA integration. Cell. 1985 Aug;42(1):5–6. doi: 10.1016/s0092-8674(85)80092-1. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T. H., Lindsley D., Kurachi S., Lewison K., Hemenway T., Melis M., Anagnou N. P., Najfeld V. Adult and fetal human globin genes are expressed following chromosomal transfer into MEL cells. Proc Natl Acad Sci U S A. 1985 Feb;82(3):780–784. doi: 10.1073/pnas.82.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Temin H. M. Loss of intervening sequences in genomic mouse alpha-globin DNA inserted in an infectious retrovirus vector. Nature. 1982 Sep 16;299(5880):265–268. doi: 10.1038/299265a0. [DOI] [PubMed] [Google Scholar]
- Sorge J., Wright D., Erdman V. D., Cutting A. E. Amphotropic retrovirus vector system for human cell gene transfer. Mol Cell Biol. 1984 Sep;4(9):1730–1737. doi: 10.1128/mcb.4.9.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatoyannopoulos G., Farquhar M., Lindsley D., Brice M., Papayannopoulou T., Nute P. E. Monoclonal antibodies specific for globin chains. Blood. 1983 Mar;61(3):530–539. [PubMed] [Google Scholar]
- Tsapis A., Hinard N., Testa U., Dubart A., Vainchenker W., Rouyer-Fessard P., Beuzard Y., Rosa J. Globin-chain affinity chromatography on Sepharose-haptoglobin: a new method of study of hemoglobin synthesis in reticulocytes, in bone marrow and in colonies of erythroid precursors. Eur J Biochem. 1980 Dec;112(3):513–519. doi: 10.1111/j.1432-1033.1980.tb06114.x. [DOI] [PubMed] [Google Scholar]
- Varmus H. E. Form and function of retroviral proviruses. Science. 1982 May 21;216(4548):812–820. doi: 10.1126/science.6177038. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Lemischka I. R., Nathan D. G., Mulligan R. C. Introduction of new genetic material into pluripotent haematopoietic stem cells of the mouse. Nature. 1984 Aug 9;310(5977):476–480. doi: 10.1038/310476a0. [DOI] [PubMed] [Google Scholar]
- Wright S., Rosenthal A., Flavell R., Grosveld F. DNA sequences required for regulated expression of beta-globin genes in murine erythroleukemia cells. Cell. 1984 Aug;38(1):265–273. doi: 10.1016/0092-8674(84)90548-8. [DOI] [PubMed] [Google Scholar]
- Yu S. F., von Rüden T., Kantoff P. W., Garber C., Seiberg M., Rüther U., Anderson W. F., Wagner E. F., Gilboa E. Self-inactivating retroviral vectors designed for transfer of whole genes into mammalian cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3194–3198. doi: 10.1073/pnas.83.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Putten H., Botteri F. M., Miller A. D., Rosenfeld M. G., Fan H., Evans R. M., Verma I. M. Efficient insertion of genes into the mouse germ line via retroviral vectors. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6148–6152. doi: 10.1073/pnas.82.18.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]