Abstract
Context: Increased epigenetic variability in the placenta may have evolved in response to its role in mediating the conflicting demands of the mother and fetus. One essential guardian of early pregnancy maintenance is the placental hormone human chorionic gonadotropin (HCG).
Objective: Among the four primate-specific duplicate HCGβ-coding genes, chorionic gonadotropin-β8 (CGB8) and chorionic gonadotropin-β5 (CGB5) jointly contribute 62–82% of the total HCGβ transcript pool. Because these genes share common features with known imprinted placenta-expressed loci, we addressed the role of epigenetic mechanisms affecting their action.
Design and Subjects: Parental origin of CGB5 and CGB8 transcripts and promoter methylation patterns were addressed in trophoblastic tissues from 23 mother-offspring duos and nine mother-father-offspring trios including the following: 1) third-trimester normal delivery at term (n = 14), 2) first-trimester elective termination of uncomplicated pregnancy (n = 10), and 3) first-trimester recurrent (≥3) miscarriage (n = 8).
Results: A normal uncomplicated pregnancy was characterized by balanced, biallelic expression of CGB5 and CGB8. However, in three (two recurrent miscarriage and one early elective termination of uncomplicated pregnancy) of nine genetically informative cases of CGB5, monoallelic expression of maternal alleles and hemimethylated gene promoters were identified.
Conclusion: Our finding may represent a novel methylation allelic polymorphism or gain of imprinting in CGB5 promoter leading to expressional silencing of paternal alleles and increasing susceptibility to pregnancy loss. Aberrant methylation patterns in placenta may result from random reprogramming defects affecting normal implantation process. Alternatively, methylation allelic polymorphism in the placenta favoring the failure of pregnancy may arise as a response to cellular stress caused by, in general, aneuploidy or conditions in placental-maternal interface.
Placental CGB5 gene exhibits a methylation allelic polymorphism, which may lead to expressional silencing of one parental allele, and increase susceptibility to miscarriage.
Pregnancy success is strongly dependent on the quantitative and qualitative expression profile of placenta-specific genes. Placenta-expressed genes contribute to the regulation of several metabolic-, endocrine-, hormonal-, and immunity-related processes and enhance maternal-fetal communication during human embryonic development (1). Genomic imprinting is an epigenetic process resulting in monoallelic expression of certain genes in a parent-of-origin-dependent manner (2). Several genes required for implantation are transcribed only from the alleles inherited from the father (3). It has been suggested that increased epigenetic variability in placenta has evolved in response to its role in mediating the conflicting demands of the mother and fetus (4). In eutherian mammals, the phenomenon of genomic imprinting has been attributed a significant role in affecting the evolution and development of placenta and its function in the control of nutritional resources to the fetus (5).
One of the essential guardians of embryo implantation and the maintenance of early pregnancy is a placenta-specific hormone human chorionic gonadotropin (HCG) (6). Critically low levels of HCG during the first trimester and low transcription of CGB genes represent a sign of either maternal susceptibility to miscarriage, chromosomal anomalies of the fetus, ectopic pregnancy, or failure of in vitro fertilization (7,8). HCG is composed of an α-subunit shared with other glycoprotein hormones and a specific β-subunit coded by a set of primate-specific duplicated CGB genes exhibiting up to 99% sequence identity (9,10). The four HCGβ-coding genes (CGB8, CGB5, CGB, CGB7) together with the ancestral LHβ (LHB) and the HCGβ noncoding genes CGB1 and CGB2 map to a joint gene cluster at chromosome 19q13.33 (Fig. 1A).
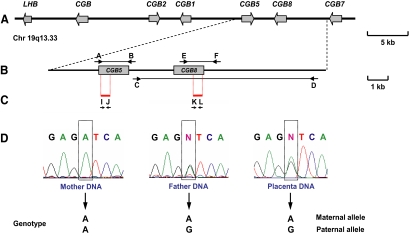
Figure 1.
Determination of the parental origin of SNP alleles in CGB5 and CGB8 loci in placental genomic DNA. Panel A, Schematic presentation of the human LHB/CGB gene cluster at 19q13.33. Panel B, Amplified genomic regions specific to the CGB5 and CGB8 loci using a combination of long-range and nested PCR (connected black arrows, primers A–F in Supplemental Table 1). Panel C, Sequenced segments in the amplified CGB5 and CGB8 loci harboring marker SNPs (short red bars, primers I–L in Supplemental Table 1). Panel D, Example of a genetically informative placental tissue for the parental origin of variants: heterozygosity in placenta and homozygosity at least in one of the parents. Chr, Chromosome; kb, kilobase.
The HCGβ-coding CGB genes have several common features with previously described imprinted placenta-specific genes (11). The LHB/CGB region is characterized by high G+C content (≥55 vs. 41% human genome average); high repeat-content; and the abundance of CpG islands, which are likely targets of DNA methylation (9). Despite the nearly identical sequences, a seminal study has reported significant differences in the DNA methylation patterns of the individual HCGβ genes in human term placenta and tumor cell lines (12). It has been suggested that the accumulation of repeats in eutherian imprinted gene clusters is coincident with, and may have been a potential driving force in, the development of mammalian genomic imprinting (13). Also, consistent with the observation that the imprinted genes tend to cluster in the genome (14), an imprinted paternally expressed gene 3 (PEG3; 19q13.4) has been identified in the vicinity of the LHB/CGB region (15).
From the four HCG β coding loci the chorionic gonadotropin-β5 (CGB5) and the chorionic gonadotropin-β8 (CGB8) genes were used as a model because they contribute together 62–82% of the total pool of HCGβ transcripts (7,16). This study aimed the following: 1) to determine the parental origin of CGB5 and CGB8 transcripts in placentas from cases of uncomplicated and recurrently miscarried pregnancies using single-nucleotide polymorphism (SNP) positions previously described in detail (9); 2) to define the DNA methylation patterns of CGB5 promoter in trophoblastic tissues and blood leukocytes; and 3) to explore the role of potential aberrant methylation of CGB5 promoter in susceptibility to recurrent miscarriage.
The data revealed the significance of biparental expression of CGB8 and CGB5 to guarantee an uncomplicated outcome of human pregnancy, identified the correlation between maternally expressed CGB5 and hemimethylation of the corresponding promoter, and suggested that methylation allelic polymorphism (MAP) or gain of paternal imprinting in CGB5 may be associated with susceptibility to recurrent miscarriage.
Subjects and Methods
Experimental subjects
The study was approved by the Ethics Review Committee of Human Research of the University of Tartu, Estonia (permissions no. 117/9, 16.06.2003 and 126/14, 26.04.2004). A written informed consent to participate in the study was obtained from every family. The study group was recruited at the Women’s Clinic of Tartu University Hospital (2003–2005), and all subjects were of white European ancestry and living in Estonia. The study group comprised 23 mother-offspring duos and nine mother-father-offspring trios. At the recruitment, parental peripheral blood samples and placenta material (representing the offspring’s genome) for the extraction of genomic DNA and mRNA were collected for each family. Placental samples were obtained from females who underwent the following: 1) elective therapeutic abortion during the first trimester of pregnancy (5–13 wk of gestational age, n = 10); 2) normal delivery at term resulting from uncomplicated pregnancy (39–42 wk of gestational age, n = 14); and 3) cervical dilatation and uterine curettage because of recurrent (patients had three or more spontaneous abortions before the case) incomplete or missed abortion (7–18 wk of gestational age, n = 8). Collected tissue samples were snap frozen in liquid nitrogen and stored at −80 C or placed immediately into RNAlater solution (Ambion Inc., Austin, TX) and kept at −20 C until genomic DNA and RNA isolation, respectively. Parental blood samples for DNA isolation were collected on the day of delivery, abortion, or surgery and frozen at −20 C in EDTA.
Identification of genetically informative cases by resequencing of CGB5 and CGB8 in parental-offspring materials
Genomic DNA was extracted from the parental peripheral blood using a protocol based on the salting-out method and from trophoblast tissues (representing the offspring’s genome) with the genomic DNA purification kit (Fermentas Life Sciences, Vilnius, Lithuania). Long-range amplification (Fig. 1B) and resequencing (Fig. 1C) across the genomic regions of CGB5 and CGB8 was performed as described previously (9,17). For resequencing the 5′ untranslated region of CGB5 and CGB8, additional primers were designed using Primer3 software (http://frodo.wi.mit.edu/cgi-bin/primer3_www.cgi), taking into account polymorphic positions (SNPs) in identified genes (9) and testing the uniqueness of a primer sequence by BLAST (http://www.ncbi.nlm.nih.gov/BLAST/). All PCR and resequencing primers are listed in Supplemental Table 1, published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org.
The obtained sequences were resolved on either ABI 3730 X1 or ABI 3730 XL DNA analyzer (Applied Biosystems, Foster City, CA). The carrier status of SNP alleles in the CGB5 (rs710899; ss105107003; rs12610392) and the CGB8 (rs34212754; rs13345685; rs35930240; Fig. 2A) genes (9) was determined using sequence analysis software PolyPhred (version 6.02; http://www.phrap.org/phredphrapconsed.html). All genotypes were confirmed manually to ultimately identify genetically informative cases for the parental origin of CGB5 and CGB8 alleles in placental tissues (Fig. 1D).
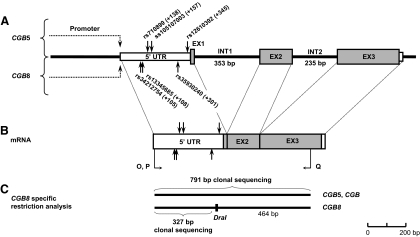
Figure 2.
Localization of marker SNPs used to identify the parental origin of CGB5 and CGB8 transcripts at the genomic (A) and the mRNA (B) sequences (black vertical arrows). RT-PCR primers O–Q are given in Supplemental Table 1. C, Separation of CGB5/CGB and CGB8 transcripts by locus-specific restriction of cDNA using DraI restriction enzyme. Clonal sequencing of the uncut cDNA was performed in the informative cases for CGB5 SNP(s) and the shorter restriction fragment (327 bp) in the informative cases for the CGB8 SNP(s). EX, Exon; INT, intron.
RT-PCR and detection of gene specific transcripts
Total RNA was extracted from 90 to 1000 mg of homogenized placental tissue using TRIzol reagent (Invitrogen, Carlsbad, CA) and purified with a NucleoSpin II isolation kit (Machery-Nagel, Düren, Germany) according to the manufacturer’s protocols. One microgram of total RNA was reverse transcribed to cDNA using a first-strand cDNA synthesis kit and oligo(dT)18 primer (Fermentas) as instructed by the manufacturer. Due to the high-sequence homology in the coding regions of HCGβ-coding genes, the cDNAs of CGB5, CGB, and CGB8 were coamplified from 2 μl of cDNA using a combination of joint primers (CGB5F/CGB8F/CGB8R; Supplemental Table 1) and 1 U of recombinant polymerase (Hot Start Taq; AppliChem, Darmstadt, Germany) in 25 μl reaction mixture following the published protocol (7). CGB8 cDNA sequences were discriminated from CGB5 and CGB (uncut RT-PCR products, 791 bp) using a CGB8 sequence-specific restriction with DraI enzyme (Fermentas Life Sciences) resulting in 327-bp (including marker SNPs) and 464-bp restriction fragments (Fig. 2, B, and C) (18). cDNA sequences of CGB5 (targeted in this study) and CGB (not targeted) were separated based on clonal sequencing and subsequent sequence alignment (ClustalW; http://www.ebi.ac.uk/clustalw/), facilitating the identification CGB5-specific nucleotide positions as described (9). Biallelic expression of CGB5 and CGB8 in genetically informative placental samples was defined in case the sequenced cDNA clones represented transcripts from both parental alleles. Screening of clones was halted at the identification of at least one cDNA clone per parental allele. Monoallelic expression of maternally inherited allele was defined when no paternal transcripts were present in 20 or more sequenced cDNA clones.
Methylation-sensitive amplification (MS)-PCR of the genomic fragment across CGB5 promoter region
Bisulfite modification of 1 μg of genomic DNA was performed using the EpiTect bisulfite kit (QIAGEN, Valencia, CA) according to the manufacturer instructions. The bisulfite-treated DNA samples were subjected to two rounds of PCR amplification of the CGB5 promoter region with the primer pairs specific to either methylated or unmethylated DNA sequences (Supplemental Table 1). To enhance specificity, alternative forward primers (Met_BS_Nest_F, Unmet_BS_Nest_F) were combined with the reverse primers in the nested PCR. The PCR reaction mixture (as described in Ref. 7) contained either 100 ng of bisulfite-converted DNA or 1 μl of the MS-PCR product. Amplification was attained by the GeneAmp PCR System 2700 (Applied Biosystems) thermal cycling for one cycle at 95 C for 3 min to denature and then 30 cycles at 95 C for 20 sec to denature and for 20 sec to anneal and 72 C for 45 sec to extend. The annealing temperature for UNMET-PCR was 61.2 C; for MET-PCR and UNMET-nested PCR, 63.7 C; and MET-nested PCR, 66 C. A final extension step was performed at 72 C for 10 min.
Cloning and sequencing of RT-PCR and MS-nested PCR products
Cloning was performed with gel-puried (UltraClean GelSpin DNA purification kit; MoBio Laboratories, Inc., Solano Beach, CA) CGB5/CGB- (791 bp) and CGB8 (327 bp)-specific cDNA fragments amplified by RT-PCR from placental samples (Fig. 2, B and C and Supplemental Table 1), and methylation-specific nested PCR products of CGB5 promoter (290 bp for UNMET; 281 bp for MET) amplified from bisulfite-treated genomic DNA of blood and placenta (see Fig. 4A and Supplemental Table 1). All inserts were cloned into pCR*II-TOPO vector (TOPO TA cloning kit; Carlsbad, CA) according to the manufacturer’s instructions, and the presence of the insert was confirmed by colony PCR using vector-specific M13F and M13R primers. Plasmid DNA was extracted with a JetQuick plasmid miniprep spin kit (Genomed, Inc., St. Louis, MO), and DNA concentrations were measured using NanoDrop ND-1000 UV-Vis spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE). Cloned inserts were sequenced using M13F and M13R primers and BigDye Terminator version 3.1 cycle sequencing kit (Applied Biosystems). Obtained sequences of inserts were aligned and compared with reference sequences using multiple alignment tool implemented in ClustalW.
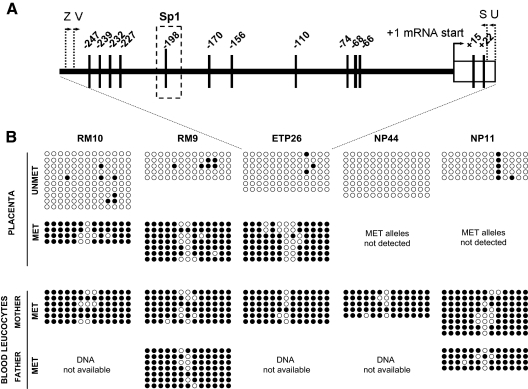
Figure 4.
Fine-scale determination of the methylation status of CGB5 promoter region in placenta and blood using bisulfite sequencing. A, Localization of analyzed CpG sites (vertical lines) in the upstream and transcribed region of CGB5. Positions of primers (Supplemental Table 1) for the amplification and clonal sequencing of methylated (V, S) and unmethylated (Z, U) promoter sequence from bisulfite-modified DNA are indicated. One differentially methylated CpG site is located within the Sp1 binding site, critical in the basal HCGβ expression (19). B, The methylation status of 13 CpG sites within the analyzed region of the CGB5 locus in parental blood DNA (almost complete methylation), placental samples from term pregnancies (NP 44, NP11; almost complete unmethylation) and from placentas with monoallelic expression of the maternal CGB5 allele (RM10, RM9, ETP26; hemimethylation). Four to 10 clones were sequenced per each sample, and the methylation-specific primer combination (UNMET, MET) showed consistent methylation patterns. Filled circle, methylated CpG site; open circle, unmethylated CpG site. Sp1, Selective promoter factor 1.
Screening for genomic rearrangements
Karyotyping of the placental material for the RM10 case was performed during clinical diagnostics procedure for complicated pregnancies in the University of Tartu Clinics (Tartu, Estonia).
Results
Biparental expression of CGB5 and CGB8 is required for pregnancy success
Parental origin of the alleles of SNPs in the CGB5 and the CGB8 loci was addressed in 32 placentas, including eight cases of recurrent miscarriage (RM), 10 cases of elective termination of pregnancy (ETP) during the first trimester, and 14 cases of normal uncomplicated delivery at term (NP). The markers for the parent-of-origin assignment involved three polymorphic positions in the 5′ untranslated region of both genes (CGB5: rs710899, ss105107003, rs12610392; CGB8: rs34212754, rs13345685, rs35930240) (Figs. 1, B and C, and 2A). Genetically informative cases (placental tissues) for determination of parental origin of variants were defined as heterozygous in at least one of the three SNPs, whereas at least one parent carried the homozygous genotype at this SNP position (Fig. 1D). From the 32 analyzed cases, nine and 14 placentas were informative for the parental origin of alleles of the studied CGB5 and CGB8 SNPs, respectively (Tables 1 and 2). These genetically informative cases were explored for the parental origin of expressed alleles by cloning and sequencing the CGB5 and CGB8 mRNA transcripts in placental tissue (Fig. 2, B and C). The majority of placentas revealed biparental expression of CGB5 and CGB8 in first- and third-trimester uncomplicated pregnancies as well as RM cases (Tables 1 and 2). Of the sequenced CGB5 cDNA clones, an equal fraction (50%) represented gene variants inherited from the mother and the father. Among the analyzed CGB8 transcripts, 46% originated from the maternal and 54% from the paternal gene copy.
Table 1.
Parental origin of CGB5 mRNA transcripts in the placental tissue of genetically informative cases
| Informative cases | Gestational agea | Genotype of rs710899b
|
Genotype of ss105107003b
|
Genotype of rs12610392b
|
Origin of expressed allele | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | PL | mRNAc | Mb | F | PL | mRNAc | M | F | PL | mRNAc | |||
| RM | ||||||||||||||
| RM4 | 14 wk + 5 d | AA | AA | AA | A | CC | CC | CC | C | GG | CG | GC | G/C | Maternal/paternal |
| RM9 | 10 wk + 0 d | AA | AA | AA | A | TC | CC | CT | T | GG | GC | GC | G | Maternal |
| RM10 | 11 wk + 6 d | AA | NA | AA | A | CC | NA | CC | C | GG | NA | GC | G | Maternal |
| ETP | ||||||||||||||
| ETP26 | 4 wk + 0 d | AA | NA | AG | A | CC | NA | CC | C | GG | NA | GC | G | Maternal |
| ETP27 | 7 wk + 5 d | AA | NA | AA | A | CC | NA | CC | C | CC | NA | GC | C/G | Maternal/paternal |
| ETP30 | 11 wk + 6 d | AA | NA | AA | A | CC | NA | CC | C | GG | NA | GC | G/C | Maternal/paternal |
| NP | ||||||||||||||
| NP11 | 40 wk + 6 d | AA | AG | AG | A/G | CC | CC | CC | C | GG | CG | GC | G/C | Maternal/paternal |
| NP44 | 40 wk + 4 d | AA | NA | AG | A/G | CC | NA | CC | C | GC | NA | GC | G/C | Maternal/paternal |
| NP45 | 39 wk + 0 d | AA | NA | AG | A/G | CC | NA | CC | C | GG | NA | GC | G/C | Maternal/paternal |
NA, Not available.
Gestational age at the collection of placental tissues is shown in weeks and days.
Genotypes of the mother (M), the father (F), and the offspring (PL) were determined in genomic DNA extracted from peripheral blood (M, F) or from placental tissue (PL). Genetically informative positions are indicated in bold.
Cloned and sequenced placental mRNA transcripts.
Table 2.
Parental origin of CGB8 mRNA transcripts in the placental tissue of genetically informative cases
| Informative cases | Gestational agea | Genotype of rs35930240b
|
Genotype of rs13345685b
|
Genotype of rs34212754b
|
Origin of expressed allele | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | PL | mRNAc | M | F | PL | mRNAc | M | F | PL | mRNAc | |||
| RM | ||||||||||||||
| RM1 | 8 wk + 5 d | AA | AA | AA | A | AA | GA | GA | A/G | CC | CC | CC | C | Maternal/paternal |
| RM2 | 12 wk + 3 d | AA | AA | AA | A | AG | GG | GA | A/G | CC | CC | CC | C | Maternal/paternal |
| RM9 | 10 wk + 0 d | AA | TA | TA | A/T | GG | GA | GG | G | CC | CC | CC | C | Maternal/paternal |
| ETP | ||||||||||||||
| ETP12 | 8 wk + 0 d | AA | NA | AA | A | GG | NA | GA | G/A | CC | NA | CC | C | Maternal/paternal |
| ETP27 | 7 wk + 5 d | AA | NA | AA | A | GG | NA | GA | G/A | CC | NA | CC | C | Maternal/paternal |
| ETP30 | 11 wk + 6 d | AA | NA | AA | A | GG | NA | GA | G/A | CC | NA | CC | C | Maternal/paternal |
| NP | ||||||||||||||
| NP9 | 38 wk + 6 d | AA | AA | AA | A | GG | AA | GA | G/A | CC | CC | CC | C | Maternal/paternal |
| NP12 | 40 wk + 6 d | TA | AA | AT | T/A | GG | GA | GG | G | CC | CC | CC | C | Maternal/paternal |
| NP29 | 40 wk + 6 d | AA | AA | AA | A | AG | GG | GA | A/G | CC | CC | CC | C | Maternal/paternal |
| NP36 | 40 wk + 1 d | AA | NA | AA | A | GG | NA | AG | G/A | CC | NA | CC | C | Maternal/paternal |
| NP37 | 40 wk + 5 d | AA | NA | AA | A | GG | NA | AG | G/A | CC | NA | CC | C | Maternal/paternal |
| NP44 | 40 wk + 4 d | AA | NA | AT | A/T | GA | NA | GA | A/G | CC | NA | CC | C | Maternal/paternal |
| NP45 | 39 wk + 0 d | AA | NA | AA | A | AA | NA | GA | A/G | CC | NA | GC | C/G | Maternal/paternal |
| NP63 | 41 wk + 6 d | AA | NA | AA | A | AA | NA | GA | A/G | CC | NA | CC | C | Maternal/paternal |
NA, Not available.
Gestational age at the collection of placental tissues is shown in weeks and days.
Genotypes of the mother (M), the father (F), and the offspring (PL) were determined in genomic DNA extracted from peripheral blood (M, F) or from placental tissue (PL). Genetically informative positions are indicated in bold.
Cloned and sequenced placental mRNA transcripts.
Notably, in three analyzed cases, monoallelic expression of the maternal copy of CGB5 was detected (Table 1). These cases represent placenta samples from two recurrent miscarriages (RM9, RM10) and one very early first-trimester electively terminated pregnancy (ETP26; gestational age 4 wk). Despite limited statistical power due to small sample size, there was a trend toward an association between RM and monoallelic CGB5 expression (Fischer’s exact test: P = 0.23, all nine samples; P = 0.11, ETP26 was excluded as an outlier of very early gestational age).
Uniparentally expressed CGB5 is accompanied by hemimethylated promoter CpGs
One scenario for the monoallelic expression is transcriptional inactivation of one allele by methylation of CpGs located in gene promoter (2). This is a common mechanism in imprinted genes, whereby loci are expressed in a parent-of-origin manner. The degree of CGB5 promoter methylation in placental tissue was determined in the three cases characterized by the transcription of only maternally derived allele in comparison with term placentas exhibiting biparentally expressed CGB5 (Table 1). The MS-PCR of bisulfite-modified DNA revealed the hemimethylated status of gene promoter in all the three placental samples with uniparentally expressed CGB5 (RM9, RM10, ETP26; Fig. 3). In contrast, term placenta samples (NP44, NP11) were characterized by unmethylated promoter region, whereas all parental DNAs extracted from blood showed almost complete methylation of CGB5 promoter (Fig. 3). This is consistent with the negligible expression of HCGβ genes in blood leukocytes (17).
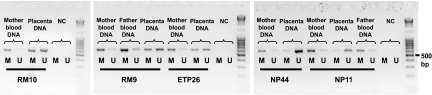
Figure 3.
MS-PCR of the CGB5 promoter region. High degree of methylation (M) was detected in all analyzed parental blood DNAs, almost complete unmethylation (U) in uncomplicated term placenta samples (NP44, NP11), and hemimethylation in cases of uniparentally expressed CGB5 (RM10, RM9, ETP26), M, Methylated DNA-specific PCR (563 bp); U, unmethylated DNA-specific PCR (571 bp); NC, negative control.
Next, the detailed comparative CpG methylation profile of the 13 CpG sites within the CGB5 promoter was addressed by clonal sequencing of bisulfite-converted genomic DNA in the three index and two control cases (Fig. 4A). Consistent with MS-PCR experiments, nearly all the CGB5 promoter CpGs were methylated in blood DNA samples and unmethylated in term placenta, whereas the three placental samples with maternal CGB5 expression revealed both methylated and unmethylated promoter variants (Fig. 4B). All other cases exhibiting weak amplification of CGB5 promoter from placental tissues with methylated DNA specific primers or from blood with unmethylated DNA specific primers were confirmed by clonal sequencing to result from unspecific oligonucleotide annealing (data not shown). Only one CpG site (position −156 from mRNA start) occurred as an outlier of the in consort on-off methylation of the analyzed 13 CGB5 promoter CpGs and maintained relatively high degree of unmethylation in blood DNA as well as within the methylated promoter variant in the placenta of the three index cases. Identification of polymorphic methylation of CGB5 promoter CpGs in placenta contributes to the limited list of known epipolymorphisms. The MAP in CGB5 promoter correlates with obtained gene expression data. Biallelic gene expression was observed in the unmethylated cases, whereas monoallelic expression of the maternal allele was accompanied by the hemimethylated placental DNA. Unfortunately, after bisulfite conversion of genomic DNA, the parental origin of methylated and unmethylated CGB5 alleles was not identifiable. The observed transcription repression may be mediated by the methylation of transcription factor binding sites (2). For example, the CpG within a functional Sp1 binding-site (−198 from mRNA start) (19) is fully hemimethylated in all three cases with uniparentally expressed CGB5 (Fig. 4B).
One case with uniparental placental expression and MAP in CGB5 has X monosomy
One of the three placentas (RM10) with uniparental expression and MAP in CGB5 was identified as a case of Turner syndrome (45, X monosomy; Supplemental Fig. 1).
Discussion
We set forward to study the hypothesis that primate-specific HCGβ-coding genes CGB5 and CGB8 may be imprinted. The hypothesis was based on their genomic location near the known imprinted PEG3 locus within a cluster of duplicated genes enriched with direct and inverted repeats (15), the essential role of HCG in human implantation regulated by several imprinted genes (20) and seminal data on the differential methylation of HCGβ gene copies (12). For the CGB8 gene, the study hypothesis was unequivocally rejected as biallelic expression was identified for 14 of 14 genetically informative cases for the parental origin of alleles. These cases included placental material from uncomplicated first- (ETP; n = 3) and third- (NP; n = 8) trimester pregnancies as well as from cases of RM (n = 3) (Table 2). However, for the neighboring duplicate gene CGB5 (Fig. 1A), monoallelic expression of the maternal allele was detected for three of the nine informative cases (33.3%), whereas six placentas (66.7%) showed transcription of CGB5 from both parental alleles.
Although preferential monoallelic expression can occur also in a non-parent-of-origin pattern (21), we hypothesized that the uniparental expression of the maternal CGB5 allele in the two RM and one ETP cases might result from polymorphic epigenetic silencing of the paternal copy. The observed correlation of monoallelic expression and hemimethylation in CGB5 promoter supported the existence of an epipolymorphism or MAP. MAP has been defined as an on-off type of epigenetic variant resulting from interindividual variations in CpG methylation and contributing to interindividual differences in gene expression and phenotypic variation (22). When occasional silencing of one allele and monoallelic expression of another allele depends on its parental origin, it may represent a case of polymorphic imprinting (23). The first reports on polymorphic imprinting of human genes were on IGF2R (insulin-like growth factor 2 receptor) in placenta (24), WT1 (Wilms’ tumor suppressor gene) in placenta, lymphocytes, and fibroblasts (25,26), IGF2 (insulin-like growth factor 2) in lymphocytes (27), and HTR2A [5-hydroxytryptamine (serotonin) receptor 2A] in adult brain (28). Recently a large-scale study reported epipolymorphisms in WNT2 (wingless-type MMTV integration site family member 2), TUSC3 (tumor suppressor candidate 3), and EPHB4 (ephrin type B receptor 4), which were methylated in 7–25% of human placenta samples, and their promoter methylation was concordant with mRNA allelic expression. In three informative cases, the TUSC3 promoter was methylated on the maternal allele, and it was suggested to represent a polymorphically imprinted gene (22). Unfortunately, in the current study, we were unable to determine the parental origin of the methylated and unmethylated alleles of CGB5 due to limited number of marker polymorphisms and loss of information about their parental origin in bisulfite-treated DNA. Whether the monoallelic maternal gene expression of CGB5 associated with the hemimethylation of the promoter represents a true polymorphic gain of imprinting (GOI), or just a MAP, remains to be determined.
Although polymorphic loss of genomic imprinting has been reported to be common in human first-trimester (29) and term placenta (30), there are few data on GOI. In our study, two of the three cases with hemimethylated promoter and maternally monoallelically expressed CGB5 in placenta were cases of RM, and one represented an early (gestational age 4 wk) ETP. Another pregnancy-related complication, the late-onset preeclampsia displayed in placenta significantly (P < 0.05) more frequent methylation of TUSC3 promoter (GOI: maternal) compared with uncomplicated pregnancies (22). These findings promote to ask whether methylation and/or GOI in loci sensitive to gene dosage in successful placental function represent a mark for adverse pregnancy outcomes. Consistently, in chorionic villus samples of abortions/stillbirth significantly more abnormal methylation values for the nonimprinted APC (adenomatosis polyposis coli tumor suppressor) and the imprinted PEG3 genes were reported (31). In fetal muscle samples, methylation abnormalities in multiple genes were observed in 4% of spontaneous abortions and 18% of stillbirths, whereas none of the induced abortions displayed extreme methylation (32). The phenomenon of polymorphic imprinting in human diseases is not limited to the placenta. In patients with core myopathies, polymorphic epigenetic allele silencing of the maternal allele of RYR1 (ryanodine receptor 1) was reported in skeletal muscle (six of 11 patients), whereas the studied normal adult skeletal muscle samples displayed only biallelic expression (33).
Emergence of an epipolymorphism(s) could be due to either a sequence-dependent allele-specific DNA methylation (cis effect) (34) or affected by trans-acting factors in genome-wide or locus level. The effect of the local DNA sequence on the CGB5 promoter methylation is unlikely because there was no correlation between DNA methylation profile and allelic status of neighboring SNPs (Tables 1 and 2). Our study detected hemimethylated CpG (−198 from mRNA start) in CGB5 promoter coupled with monoallelic gene expression within experimentally defined Sp1 transcription factor binding site (19) (Fig. 4). This Sp1 site appears to be conserved among all HCGβ-coding genes (CGB, CGB5, CGB7, CGB8) (35), and mutations of this sequence were shown to cause a dramatic reduction in the promoter’s basal activity (83%) (19). Another interesting observation was the detection of 45, X monosomy in one of the three cases with the hemimethylated CGB5 promoter. Initiation of X inactivation and parent-of-origin silencing of imprinted genes has evolved in parallel and is mediated by similar molecular mechanisms (36). In the case of Turner syndrome, this entire process may be disturbed. It has been shown that aneuploidy drives to modified gene expression patterns and cellular physiology (37). This may be mediated via epigenetic modifications as shown in cancer tissues, in which aneuploidy and changes in DNA methylation patterns occur hand-in-hand (38). It is also possible that the detected CGB5 MAP is only a tag for methylation-associated functional alteration of another gene(s) primarily increasing susceptibility to miscarriage.
Aberrant locus-specific methylation has also been related to the effect of antisense transcription (23). The LHB/CGB locus includes two HCGβ noncoding genes (CGB1, CGB2) (35). Most intriguingly, the upstream regions distinct to these genes harbor a novel genes snaR (small nuclear factor 90 associated RNA). SnaRs encode noncoding RNA substrates for a nuclear factor 90 protein and are transcribed from an antisense strand in testis, placenta, and brain (39). Whether the transcripts of HCGβ noncoding or snaR genes have any role in modulating the expression of HCGβ genes (CGB5, CGB8, CGB, CGB7) in genomic or mRNA level is unknown.
In summary, we showed that for normal uncomplicated pregnancy biparental expression of major HCGβ genes appears to be optimal. We discovered CGB5 as a novel epipolymorphic gene with possible occasional gain of paternal polymorphic imprinting (GOI). Monoallelic expression of CGB5 and hemimethylation of its promoter were described in the first-trimester placenta in two cases of RM and one case of ETP. Due to delicacy of the recruitment procedure, the limited study sample does not exclude that this observed enrichment of RM cases was a chance finding nor allows to assess whether the detected uniparental expression of one (of four) HCGβ coding genes had a direct effect on maternal serum HCG. The expression of CGB genes and serum HCG levels are dependent on gestational age and course of pregnancy and display high interindividual variation (7,16).
Aberrant methylation patterns in placenta during early pregnancy may represent random reprogramming defects affecting normal implantation process. Alternatively, MAP in placenta directing to the pregnancy failure may arise as a response to cellular stress caused by, in general, aneuploidy or environmental conditions in placental-maternal interface. It has been suggested imprinting may have evolved to act as a genomic cloaking device of either maternal or paternal allele during critical periods in development when selective abortion is possible (40). Placental polymorphic GOI may have developed to serve as a flexible tool affecting the quality of implantation process and increasing the probability of miscarriage in unfavorable developmental conditions for the offspring.
Supplementary Material
Acknowledgments
We thank all the patients who participated in the study, Dr. Kai Muru (United Laboratory of Tartu University Hospital, Estonia) for providing the cytogenetic support, and Dr. Jüri Reimand for critical reading of the manuscript.
Footnotes
This work was supported by Howard Hughes Medical Institute International Scholarship Grant 55005617 and Estonian Science Foundation Grant 7471 (to M.L.). Additional support was provided by Wellcome Trust International Senior Research Fellowship (070191/Z/03/Z) in Biomedical Science in Central Europe (to M.L.) and the Estonian Ministry of Education and Science (Core Grant 0182721s06). L.U. is a recipient of stipends from the Estonian-Revelia Academic Fund, Estonian Student’s Fund in the USA, Inc., and the Estonian World Council, Inc. K.R. is a recipient of the Estonian Women in Science Award financed by European Commission Grant 205419 (ECOGENE) to the Estonian Biocentre. L.N. is recipient of scholarships from the Ernst Jaakson Memorial Fund (University of Tartu Foundation) and the Estonian Student’s Fund in USA, Inc.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 20, 2010
Abbreviations: CGB8, Chorionic gonadotropin-β8; CGB5, chorionic gonadotropin-β5; ETP, elective termination of pregnancy; GOI, gain of imprinting; HCG, human chorionic gonadotropin; LHB, LHβ gene; MAP, methylation allelic polymorphism; MS, methylation-sensitive amplification; NP, normal uncomplicated delivery at term; PEG3, paternally expressed gene 3; RM, recurrent miscarriage; SNP, single-nucleotide polymorphism; TUSC3, tumor suppressor candidate 3; UTR, untranslated region.
References
- Rawn SM, Cross JC 2008 The evolution, regulation, and function of placenta-specific genes. Annu Rev Cell Dev Biol 24:159–181 [DOI] [PubMed] [Google Scholar]
- Reik W, Walter J 2001 Genomic imprinting: parental influence on the genome. Nat Rev Genet 2:21–32 [DOI] [PubMed] [Google Scholar]
- Goshen R, Ben-Rafael Z, Gonik B, Lustig O, Tannos V, de-Groot N, Hochberg AA 1994 The role of genomic imprinting in implantation. Fertil Steril 62:903–910 [PubMed] [Google Scholar]
- Constância M, Kelsey G, Reik W 2004 Resourceful imprinting. Nature 432:53–57 [DOI] [PubMed] [Google Scholar]
- Fowden AL, Sibley C, Reik W, Constância M 2006 Imprinted genes, placental development and fetal growth. Horm Res 65(Suppl 3):50–58 [DOI] [PubMed] [Google Scholar]
- Srisuparp S, Strakova Z, Fazleabas AT 2001 The role of chorionic gonadotropin (CG) in blastocyst implantation. Arch Med Res 32:627–634 [DOI] [PubMed] [Google Scholar]
- Rull K, Laan M 2005 Expression of β-subunit of HCG genes during normal and failed pregnancy. Hum Reprod 20:3360–3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman UH, Tiitinen A, Alfthan H, Valmu L 2006 The classification, functions and clinical use of different isoforms of HCG. Hum Reprod Update 12:769–784 [DOI] [PubMed] [Google Scholar]
- Hallast P, Nagirnaja L, Margus T, Laan M 2005 Segmental duplications and gene conversion: human luteinizing hormone/chorionic gonadotropin β gene cluster. Genome Res 15:1535–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JG, Parsons TF 1981 Glycoprotein hormones: structure and function. Annu Rev Biochem 50:465–495 [DOI] [PubMed] [Google Scholar]
- Bressan FF, De Bem TH, Perecin F, Lopes FL, Ambrosio CE, Meirelles FV, Miglino MA 2009 Unearthing the roles of imprinted genes in the placenta. Placenta 30:823–834 [DOI] [PubMed] [Google Scholar]
- Campain JA, Gutkin DW, Cox GS 1993 Differential DNA methylation of the chorionic gonadotropin beta-subunit multigene family. Mol Endocrinol 7:1331–1346 [DOI] [PubMed] [Google Scholar]
- Pask AJ, Papenfuss AT, Ager EI, McColl KA, Speed TP, Renfree MB 2009 Analysis of the platypus genome suggests a transposon origin for mammalian imprinting. Genome Biol 10:R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verona RI, Mann MR, Bartolomei MS 2003 Genomic imprinting: intricacies of epigenetic regulation in clusters. Annu Rev Cell Dev Biol 19:237–259 [DOI] [PubMed] [Google Scholar]
- Hiby SE, Lough M, Keverne EB, Surani MA, Loke YW, King A 2001 Paternal monoallelic expression of PEG3 in the human placenta. Hum Mol Genet 10:1093–1100 [DOI] [PubMed] [Google Scholar]
- Miller-Lindholm AK, LaBenz CJ, Ramey J, Bedows E, Ruddon RW 1997 Human chorionic gonadotropin-β gene expression in first trimester placenta. Endocrinology 138:5459–5465 [DOI] [PubMed] [Google Scholar]
- Rull K, Hallast P, Uusküla L, Jackson J, Punab M, Salumets A, Campbell RK, Laan M 2008 Fine-scale quantification of HCG β gene transcription in human trophoblastic and non-malignant non-trophoblastic tissues. Mol Hum Reprod 14:23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar V, Diez SG, Laurent A, Giovangrandi Y, Radvanyi F, Chopin D, Bidart JM, Bellet D, Vidaud M 1995 Expression of human chorionic gonadotropin β subunit genes in superficial and invasive bladder carcinomas. Cancer Res 55:3735–3738 [PubMed] [Google Scholar]
- Johnson W, Jameson JL 1999 AP-2 (activating protein 2) and Sp1 (selective promoter factor 1) regulatory elements play distinct roles in the control of basal activity and cyclic adenosine 3′,5′-monophosphate responsiveness of the human chorionic gonadotropin-beta promoter. Mol Endocrinol 13:1963–1975 [DOI] [PubMed] [Google Scholar]
- Glaser RL, Ramsay JP, Morison IM 2006 The imprinted gene and parent-of-origin effect database now includes parental origin of de novo mutations. Nucleic Acids Res 34:D29–D31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastinen T, Sladek R, Gurd S, Sammak A, Ge B, Lepage P, Lavergne K, Villeneuve A, Gaudin T, Brändström H, Beck A, Verner A, Kingsley J, Harmsen E, Labuda D, Morgan K, Vohl MC, Naumova AK, Sinnett D, Hudson TJ 2004 A survey of genetic and epigenetic variation affecting human gene expression. Physiol Genomics 16:184–193 [DOI] [PubMed] [Google Scholar]
- Yuen RK, Avila L, Peñaherrera MS, von Dadelszen P, Lefebvre L, Kobor MS, Robinson WP 2009 Human placental-specific epipolymorphism and its association with adverse pregnancy outcomes. PLoS One 4:e7389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumova AK, Croteau S 2004 Mechanisms of epigenetic variation: polymorphic imprinting. Curr Genomics 5:417–429 [Google Scholar]
- Xu Y, Goodyer CG, Deal C, Polychronakos C 1993 Functional polymorphism in the parental imprinting of the human IGF2R gene. Biochem Biophys Res Commun 197:747–754 [DOI] [PubMed] [Google Scholar]
- Jinno Y, Yun K, Nishiwaki K, Kubota T, Ogawa O, Reeve AE, Niikawa N 1994 Mosaic and polymorphic imprinting of the WT1 gene in humans. Nat Genet 6:305–309 [DOI] [PubMed] [Google Scholar]
- Mitsuya K, Sui H, Meguro M, Kugoh H, Jinno Y, Niikawa N, Oshimura M 1997 Paternal expression of WT1 in human fibroblasts and lymphocytes. Hum Mol Genet 6:2243–2246 [DOI] [PubMed] [Google Scholar]
- Giannoukakis N, Deal C, Paquette J, Kukuvitis A, Polychronakos C 1996 Polymorphic functional imprinting of the human IGF2 gene among individuals, in blood cells, is associated with H19 expression. Biochem Biophys Res Commun 220:1014–1019 [DOI] [PubMed] [Google Scholar]
- Bunzel R, Blümcke I, Cichon S, Normann S, Schramm J, Propping P, Nöthen MM 1998 Polymorphic imprinting of the serotonin-2A (5-HT2A) receptor gene in human adult brain. Brain Res Mol Brain Res 59:90–92 [DOI] [PubMed] [Google Scholar]
- Pozharny Y, Lambertini L, Ma Y, Ferrara L, Litton CG, Diplas A, Jacobs AR, Chen J, Stone JL, Wetmur J, Lee MJ 2010 Genomic loss of imprinting in first-trimester human placenta. Am J Obstet Gynecol 202:391.e1–391.e8 [DOI] [PubMed] [Google Scholar]
- Lambertini L, Diplas AI, Lee MJ, Sperling R, Chen J, Wetmur J 2008 A sensitive functional assay reveals frequent loss of genomic imprinting in human placenta. Epigenetics 3:261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner U, Pliushch G, Schneider E, El Hajj N, Tresch A, Shufaro Y, Seidmann L, Coerdt W, Muller AM, Haaf T 2010 Quantitative methylation analysis of developmentally important genes in human pregnancy losses after ART and spontaneous conception. Mol Hum Reprod 16:704–713 [DOI] [PubMed] [Google Scholar]
- Pliushch G, Schneider E, Weise D, El Hajj N, Tresch A, Seidmann L, Coerdt W, Müller AM, Zechner U, Haaf T 2010 Extreme methylation values of imprinted genes in human abortions and stillbirths. Am J Pathol 176:1084–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Brockington M, Jungbluth H, Monk D, Stanier P, Sewry CA, Moore GE, Muntoni F 2006 Epigenetic allele silencing unveils recessive RYR1 mutations in core myopathies. Am J Hum Genet 79:859–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkel K, Spadola A, Yuan E, Kosek J, Jiang L, Hod E, Li K, Murty VV, Schupf N, Vilain E, Morris M, Haghighi F, Tycko B 2008 Genomic surveys by methylation-sensitive SNP analysis identify sequence-dependent allele-specific DNA methylation. Nat Genet 40:904–908 [DOI] [PubMed] [Google Scholar]
- Hallast P, Rull K, Laan M 2007 The evolution and genomic landscape of CGB1 and CGB2 genes. Mol Cell Endocrinol 260–262:2–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W, Lewis A 2005 Co-evolution of X-chromosome inactivation and imprinting in mammals. Nat Rev Genet 6:403–410 [DOI] [PubMed] [Google Scholar]
- Torres EM, Sokolsky T, Tucker CM, Chan LY, Boselli M, Dunham MJ, Amon A 2007 Effects of aneuploidy on cellular physiology and cell division in haploid yeast. Science 317:916–924 [DOI] [PubMed] [Google Scholar]
- Herrera LA, Prada D, Andonegui MA, Dueñas-González A 2008 The epigenetic origin of aneuploidy. Curr Genomics 9:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott AM, Mathews MB 2007 Novel rapidly evolving hominid RNAs bind nuclear factor 90 and display tissue-restricted distribution. Nucleic Acids Res 35:6249–6258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf JB, Hager R 2009 Selective abortion and the evolution of genomic imprinting. J Evol Biol 22:2519–2523 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.