BACKGROUND
Opioid-induced constipation is a common and significant problem for patients with advanced cancer. In the U.S., opioid analgesics are prescribed to more than 50% of cancer patients with terminal disease.1 The prevalence of opioid-induced constipation is difficult to estimate because many other factors contribute to the problem in these patients. In one series of studies in cancer patients receiving hospice care, it was estimated that 40% to 63% experienced opioid-induced constipation.2 In another study, up to 95% of patients indicated that opioid-induced constipation had a negative effect on their quality of life.3 Because of its widespread prevalence and its profound impact on well-being, it is an issue requiring the attention of clinicians who treat these patients.
Treatment with traditional laxatives is often successful, but constipation remains refractory in some patients despite aggressive laxative therapy. Methylnaltrexone bromide (Relistor, Pfizer) received FDA approval for the treatment of opioid-induced constipation in April 2008 based on two randomized, placebo-controlled trials.4 In these two studies, significantly more patients experienced a bowel movement within four hours of administration of the study drug compared with patients receiving placebo (48% vs. 16% and 62% vs. 14%).5,6 As a result of its mechanism of action, methylnaltrexone has specific effects on constipation that can be attributed to opioid use. The drug acts as an antagonist of gastrointestinal (GI) mu-opioid receptors, thereby inhibiting opioid-induced delay of GI transit.
In the initial clinical trials, adverse effects were mild, most commonly consisting of abdominal pain, flatulence, nausea, dizziness, and diarrhea. However, postmarketing cases of GI perforation have been reported, particularly in patients with reduced structural integrity of the GI tract.7 These reports prompted the FDA to amend the drug’s prescribing information to include a warning that methylnaltrexone be used with caution in patients with a known or suspected GI lesion, such as cancer, peptic ulcer, or Ogilvie’s syndrome.
The clinical trials that led to the approval of methylnaltrexone were conducted in a specific patient population and led to a narrow indication in the prescribing information. The package insert states that methylnaltrexone “is indicated for the treatment of opioid-induced constipation in patients with advanced illness who are receiving palliative care, when response to laxative therapy has not been sufficient.”8
These details were included as a consequence of the clinical trials, which had been conducted in patients receiving opioid therapy, consisting of a median daily baseline dose of oral morphine 150 mg or its equivalent. The patients with opioid-induced constipation (fewer than three bowel movements in the preceding week or no bowel movement for two days) had incurable cancer or another terminal illness, were receiving only palliative care, and had been following a stable laxative regimen for at least three days before entry into the study.
When methylnaltrexone was added to the formulary of our comprehensive tertiary cancer hospital (M.D. Anderson Cancer Center) in October 2008, discussions focused on whether to restrict its use to the Department of Palliative Care and Rehabilitation Medicine (the palliative care service) in order to avoid off-label uses. However, it was decided to add the drug to the formulary without restriction with a subsequent drug use evaluation (DUE) to determine whether there was adherence to the labeled indication. The importance of conducting a DUE is even more evident now, because of the FDA’s label update indicating potential safety concerns with methylnaltrexone.7
METHODS
We conducted a single-center, retrospective chart review of all patients who received methylnaltrexone at our institution from April 1, 2008, to August 31, 2009. The pharmacy informatics database was used to identify patients receiving methylnaltrexone. Patients were excluded from the study if there was no documentation of methylnaltrexone administration in the medical record.
Demographic data collected included patients’ age, sex, weight, cancer diagnosis, extent of disease, renal function, and inpatient or outpatient hospital location. We collected data pertaining to prescribing, including the methylnaltrexone dose administered, the dosing schedule, the number of doses administered, and the prescribing service.
To determine adherence to the label’s indication, we obtained documentation of opioid use, constipation, days since the last bowel movement, and the number of laxatives used. Historical and safety data included information obtained about bowel obstruction, previous abdominal surgery, prior radiation therapy to the abdomen or pelvis, and the date of the last radiation treatment.
Definitions
We defined constipation as any documentation of constipation with no prespecified number of days since the last bowel movement.
Laxative use was defined as therapy with such agents as stimulants, osmotic laxatives, or stool softeners of any duration before methylnaltrexone administration.
Opioid use was defined as the inpatient or outpatient administration of any opioid agent (e.g., morphine, propoxyphene, or hydromorphone) scheduled or as needed, with no minimum dose or duration.
Extent of disease was categorized as advanced illness (metastatic disease) or non-advanced illness (no metastases and no evidence of disease indicating metastases).
We defined renal dysfunction as an estimated creatinine clearance (CrCl) of 30 mL/minute or less, using the Cockcroft–Gault equation to make our calculations. Bowel obstruction was evaluated via diagnostic imaging (e.g., x-ray and computed tomography scans), which was performed within 72 hours before methylnaltrexone administration.
The potential for compromised bowel integrity was assumed if patients had a diagnosed GI malignancy, had infiltration of an abdominal malignancy into the GI tract, had undergone earlier abdominal surgery involving the GI tract, and/or had previous radiation to the abdomen or pelvis.
Statistical Analysis
We used descriptive statistics to characterize the data collected. All data were captured based on documentation prior to methylnaltrexone treatment. Each physician order was analyzed as an event.
RESULTS
We evaluated 202 physician orders for methylnaltrexone to determine their appropriate use. A total of 282 doses were administered, and 200 patients received the drug during this time period. Patients’ average age was 55 years, with a standard deviation (SD) of ± 12 years. Of the 202 orders, 116 (57%) were written for women, and 149 (74%) were written with the correct dose.
Of the 53 physician orders that were written with the incorrect dose, 48 orders (91%) were inappropriate for the patient’s weight and five orders (9%) were incorrect because of a failure to adjust the dose for renal dysfunction. Of the 48 patients (91%) who received an inappropriate dose, 43 patients (90%) received too low a dose. One hundred eighty-seven orders (93%) were written within the recommended prescribing schedule of once-daily or every-other-day dosing; however, 15 orders (7%) were prescribed for scheduled daily administration. The median number of doses administered per physician order was one (range, one to nine doses).
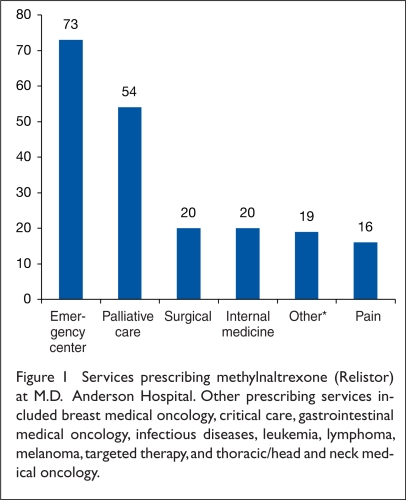
Methylnaltrexone was prescribed by a variety of services (Figure 1). The palliative care service wrote only 54 orders (27%); 120 orders (59%) were written for inpatient administration; however, 82 orders (41%) were for the outpatient setting, primarily in the emergency department.
Figure 1.
Services prescribing methylnaltrexone (Relistor) at M.D. Anderson Hospital. Other prescribing services included breast medical oncology, critical care, gastrointestinal medical oncology, infectious diseases, leukemia, lymphoma, melanoma, targeted therapy, and thoracic/head and neck medical oncology.
Of the 202 orders, 67 (33%) were prescribed for patients with non-advanced illness; five of these patients (2%) had no evidence of disease.
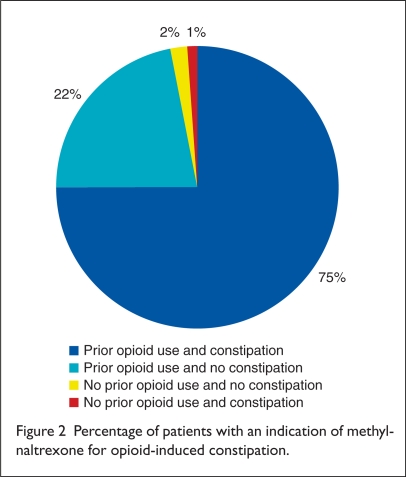
Fifty orders (25%) were written for patients who did not meet criteria for opioid-induced constipation, and five orders (3%) were written for patients without any history of opioid use (Figure 2). Thirty-eight orders (19%) were prescribed for patients without a history of prior laxative use. For patients with a history of prior laxative use, an average of two agents was used (SD, 0.92).
Figure 2.
Percentage of patients with an indication of methylnaltrexone for opioid-induced constipation.
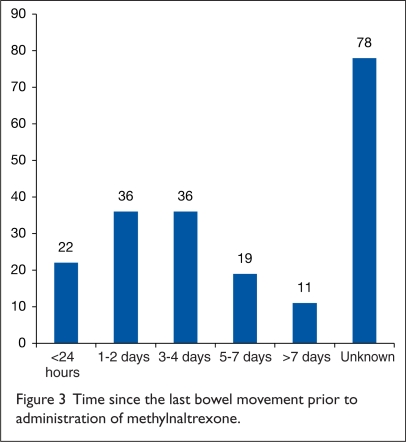
Figure 3 illustrates bowel movement history before therapy was given. For the 124 orders in which documentation was available, 94 of these (76%) were written within four days of the last bowel movement; 22 of these orders (18%) were administered on the same day as the patient’s last bowel movement.
Figure 3.
Time since the last bowel movement prior to administration of methylnaltrexone.
Eleven orders (5%) were written for patients who had a documented bowel obstruction within 72 hours of opioid administration; however, no patients receiving methylnaltrexone experienced significant adverse events related to its use.
Seventy-six orders (38%) were written for patients who had not undergone abdominal imaging to rule out bowel obstruction before administration of methylnaltrexone.
Of the 202 orders written, 70 (35%) were identified in patients at risk for compromised bowel integrity. Forty-four orders (22%) were written for patients who had received radiation therapy to the abdomen or pelvis. Of those patients with a history of radiation therapy, 30 patients (68%) received their last treatment within the previous 12 months. Twelve orders (27%) were written for patients who were undergoing radiation therapy or who had received their last radiation dose within the past month.
Of the 202 orders, 17 (8%) were written for patients whose primary site of disease was the bowel, and nine of these orders (4%) were for patients who had undergone resection of the tumor with creation of a colostomy. In addition, 20 orders (10%) were written for patients with tumor invasion into the bowel, and eight of these orders (4%) were for patients who had bowel resection attributable to disease.
One order was written for a patient who had recently had a colostomy secondary to a rectal obstruction. Of these 38 orders in patients with GI involvement or procedures, 12 patients (32%) also had a history of radiation treatment.
DISCUSSION
Previously published studies and reports have demonstrated the tolerability and efficacy of methylnaltrexone. These studies, conducted in cancer patients with advanced illness, led to the drug’s approval with a limited indication. In our population, which consisted entirely of cancer patients, we found that methylnaltrexone was not being used in accordance with its labeled indication. Multiple services were responsible for generating these orders, and the orders were inappropriate for several reasons.
We found that 26% of the orders for methylnaltrexone were not administered correctly; they were written without an appropriate indication (no documentation of opioid use or constipation). In fact, 18% of the orders were written for patients who had had a bowel movement within the 24 hours before receiving methylnaltrexone.
Laxative use in our population was not consistent with the instructions in the package insert, as many patients had not taken laxatives previously. Further, compared with patients in the clinical trials, many of our patients were not on a stable laxative regimen for three days prior to methylnaltrexone use. We also noted that multiple laxatives had been ordered for many patients simultaneously, and methylnaltrexone was often given first.
In addition to the problem of patients not having opioid-induced constipation or laxative use, one-third of patients did not have advanced illness and 5% had no evidence of disease. Particularly troubling is the fact that the use of methylnaltrexone was contraindicated because of a documented bowel obstruction in 5% of patients who received it.
In the clinical trials on which the FDA based its approval, radiation to the bowel or compromised GI integrity was not evaluated; therefore, the drug’s safety in these populations has not been established.4 In addition to the postmarketing data correlating perforation with GI lesions, it has been reported that an irradiated bowel carries an increased risk of perforation as well.9 Given the potential for perforation of bowel, the risks of using methylnaltrexone may outweigh the potential benefits in these patients. More than one-third of the orders (70 of 202) were written for patients with potentially compromised bowel integrity resulting from the presence of a primary abdominal malignancy, invasion of tumor into the bowel, a history of a surgery involving the GI tract, or treatment with abdominal or pelvic radiation. Although a significant number of patients received the drug outside of its labeled indication, there were no significant treatment-related adverse events.
The off-label use of methylnaltrexone suggests that there might be situations, other than those indicated, in which the drug might be suitable. Further studies are needed to determine whether this medication can be used safely and effectively in those scenarios.
STUDY LIMITATIONS
Several limitations to our DUE originated from the retrospective methodology used. We found it necessary to apply definitions for opioid and laxative use, constipation, and advanced illness that were not consistent with those used in clinical trials of methylnaltrexone. We applied these definitions because many of the patients received the drug in the emergency department or shortly after admission; thus, documentation of events that occurred outside of the hospital was often lacking. Given this challenge, we developed liberal definitions that probably underestimated the improper use of methylnaltrexone and that might have provided the best-case scenario for drug utilization. A further limitation was that our study was conducted at a single institution with a homogeneous population.
CONCLUSION
Our DUE confirmed the original concern, expressed during the formulary approval process, that methylnaltrexone (Relistor) might not be used appropriately. The drug was added to our formulary with no restrictions, and our findings indicate that this step led to a high rate of inappropriate use at our institution. Most of the misuse was probably related to a lack of familiarity with methylnaltrexone and to an inadequate understanding about which patients met the limited indication. This explanation was supported by the fact that 131 orders (65%) were generated by services other than palliative care.
Several methods may be considered to control misuse of this drug, including more instruction for hospital staff, restriction of the drug to specific hospital services, and order-set implementation. Although increasing education among prescribers might temporarily improve proper utilization of a drug, other methods can create a safe environment more effectively.10 Staff education is flawed, because its effects are often of limited duration. Restricting medication use to a specific service has been tried, but this is a difficult proposition at many institutions, including ours, because of ingrained behaviors and attitudes of prescribers.
Through our institution’s P&T committee, a mandatory order set was developed and implemented in which prescribers were required to document opioid use, constipation, and prior laxative use on the order form. There is also a prompt for practitioners to obtain diagnostic imaging if bowel obstruction is suspected. Order sets have been beneficial in a number of situations, including glycemic control, infection, sepsis, and pain control.11–13 Given their proven success, we feel that this order set will promote the safe and proper use of methylnaltrexone. An additional benefit is that future evaluation of drug use will be enhanced as a result of the required documentation.
Our DUE and the subsequent development of the order set are representative of the challenges facing modern health care systems.14 The regulation of drug use for an agent with a narrow indication is likely to increase. Even though various methods of controlling drug utilization exist, it is our hope that requiring an order set will alter the way in which health care practitioners prescribe methylnaltrexone and, ultimately, improve patient safety.
Figure 4.
Methylnaltrexone order set. (Courtesy of M.D. Anderson Cancer Center, Copyright 2010.)
Footnotes
Disclosure: The authors report no financial or commercial relationships in regard to this article. This manuscript was supported in part by the National Institutes of Health through M.D. Anderson’s Cancer Center Support Grant, CA 016672.
REFERENCES
- 1.Choi YS, Billings JA. Opioid antagonists: A review of their role in palliative care, focusing on use in opioid-related constipation. J Pain Symptom Manage. 2002;24:71–90. doi: 10.1016/s0885-3924(02)00424-4. [DOI] [PubMed] [Google Scholar]
- 2.McMillan SC. Assessing and managing opiate-induced constipation in adults with cancer. Cancer Control. 2004;11:3–9. doi: 10.1177/10732748040110S302. [DOI] [PubMed] [Google Scholar]
- 3.Panchal SJ, Müller-Schwefe P, Wurzelmann JI. Opioid-induced bowel dysfunction: Prevalence, pathophysiology and burden. Int J Clin Pract. 2007;61(7):1181–1187. doi: 10.1111/j.1742-1241.2007.01415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.FDA. Drug approval package: Relistor (methylnaltrexone bromide) subcutaneous injection. Jun 3, 2008. [Accessed June 30, 2010]. Available at: http://tinyurl.com/2uby2ufand www.fda.gov.
- 5.Thomas J, Karver S, Cooney GA, et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med. 2008;358(22):2332–2343. doi: 10.1056/NEJMoa0707377. [DOI] [PubMed] [Google Scholar]
- 6.Slatkin N, Thomas J, Lipman AG, et al. Methylnaltrexone for treatment of opioid-induced constipation in advanced illness patients. J Support Oncol. 2009;7(1):39–46. [PubMed] [Google Scholar]
- 7.FDA safety information. Relistor (methylnaltrexone bromide) subcutaneous injections. Aug 25, 2010. [Accessed September 29, 2010]. Available at: www.fda.gov/Safety/MedWatch/SafetyInformation/ucm221639.htm.
- 8.Relistor (methylnaltrexone bromide), prescribing information. Philadelphia: Wyeth; Tarrytown, N.Y: Progenics; Updated May 2010; revised September 2010. [Google Scholar]
- 9.Novak JM, Collins JT, Donowitz M, et al. Effects of radiation on the human gastrointestinal tract. J Clin Gastroenterol. 1979;1(1):9–39. doi: 10.1097/00004836-197903000-00003. [DOI] [PubMed] [Google Scholar]
- 10.How to prevent prescribing errors. Lancet. 2009;374(9706):1945. doi: 10.1016/S0140-6736(09)62104-8. [DOI] [PubMed] [Google Scholar]
- 11.Riddle E, Bush J, Tittle M, Dilkhush D. Alcohol withdrawal: Development of a standing order set. Crit Care Nurs. 2010;30:38–47. doi: 10.4037/ccn2010862. [DOI] [PubMed] [Google Scholar]
- 12.Weber LM, Ghafoor VL, Phelps P. Implementation of standard order sets for patient-controlled analgesia. Am J Health Syst Pharm. 2008;65:1184–1191. doi: 10.2146/ajhp060416. [DOI] [PubMed] [Google Scholar]
- 13.Thiel SW, Asghar MF, Micek ST, et al. Hospital-wide impact of a standardized order set for the management of bacteremic severe sepsis. Crit Care Med. 2009;37:819–824. doi: 10.1097/CCM.0b013e318196206b. [DOI] [PubMed] [Google Scholar]
- 14.Gillick MR. Controlling off-label medication use. Ann Intern Med. 2009;150:344–347. doi: 10.7326/0003-4819-150-5-200903030-00108. [DOI] [PubMed] [Google Scholar]