Abstract
In the event of a chemical terrorist attack on a transportation hub, post-event remediation and restoration activities necessary to attain unrestricted facility reuse and re-entry could require hours to multiple days. While restoration timeframes are dependent on numerous variables, a primary controlling factor is the level of pre-planning and decision-making completed prior to chemical terrorist release. What follows is the first of a two-part analysis identifying key considerations, critical information, and decision criteria to facilitate post-attack and post-decontamination consequence management activities. A conceptual site model and human health-based exposure guidelines are developed and reported as an aid to site-specific pre-planning in the current absence of U.S. state or Federal values designated as compound-specific remediation or re-entry concentrations, and to safely expedite facility recovery to full operational status. Chemicals of concern include chemical warfare nerve and vesicant agents and the toxic industrial compounds phosgene, hydrogen cyanide, and cyanogen chloride. This work has been performed as a national case study conducted in partnership with the Los Angeles International Airport and The Bradley International Terminal. All recommended guidelines have been selected for consistency with airport scenario release parameters of a one-time, short-duration, finite airborne release from a single source followed by compound-specific decontamination.
Keywords: chemical warfare agents, CWA, TIC, terrorism, clearance guidelines and goals, airport, decision criteria
INTRODUCTION
This analysis describes a specific one-time, short-duration, chemical airborne release attack scenario at a major U.S. airport as a means to demonstrate key assessment considerations and decision criteria that facilitate post-attack and post-decontamination consequence management activities. This work reflects information and lessons learned as part of an ongoing U.S. Department of Homeland Security (DHS) domestic preparedness activity. A primary objective of this analysis is to provide and document information for chemical warfare agents (CWAs) and toxic industrial compounds (TICs) likely to be released in such an incident. Decision-makers can employ this information to ensure that appropriate health protective clearance levels are part of any preplanning remediation activities.
A conceptual site model and human health-based exposure guidelines are developed and reported as an aid to site-specific pre-planning given the current absence of U.S. state or Federal values designated as compound-specific remediation or re-entry concentrations, and to safely expedite facility recovery to full operational status. Exposure pathways analyzed include a variety of routes associated with airborne vapors and potential surface residues such as inhalation and direct ocular vapor, percutaneous vapor, surface contact, and ingestion. Populations considered include transit passengers, various airport personnel, and decontamination personnel.
This work will be presented in two parts: Part I (“Key Assessment Considerations”) characterizes the specific one-time, short-duration, chemical airborne release attack scenario at a major U.S. airport that serves as the basis of the evaluation. Scenario assumptions are described, various post-event phases and timelines are summarized, and a conceptual site model (CSM) is derived to characterize potential health risks of concern. Application of the CSM incorporates characterization of individual threat compounds, identification of population(s) of concern, and determination of likely exposure pathways. Part II (“Decision Criteria for Multipathway Exposure Routes”) presents first-time, open-literature documentation of multi-pathway and health-based remediation exposure guidelines for CWA and TICs (and their degradation products) for application to chemical terrorist pre-planning. Specifically, the chemical characteristics and population exposure pathways demonstrated by the CSM in Part I are evaluated against a variety of established toxicologically based criteria, most of which have been peer-reviewed and identified in established policies. Where specific gaps in established criteria for certain pathways were found, the authors applied accepted procedures to develop technical derivations.
As a risk assessment, this 2-part analysis focuses on developing an objective hazard identification and characterization approach consistent with the release scenario as well as scenario-relevant dose response, exposure estimation and risk characterization. It is understood that risk managers will additionally incorporate political, social, economic, and engineering elements into the clearance decision-making process; these elements are not examined here. The present work has been prepared to aid in the making of transparent health-based determinations supported by a robust foundation of sound toxicological assessment and knowledge of compound-specific characteristics.
BACKGROUND
The DHS has been given responsibility to improve domestic preparedness for potential chemical terrorist release incidents as well as other forms of terrorism at key U.S. transportation nodes. A multi-year research program involving several national laboratories as well as subject matter experts from various state and federal agencies has been initiated to fulfill DHS Chemical and Biological Countermeasures Program core objectives of (1) minimizing loss of life and economic impact from chemical attacks and (2) expediting facility recovery to operational status. The focus of the Chemical Restoration Operational Technology Demonstration Project has been on the consequence management phase (e.g., remediation and restoration) of the response (Table 1); specifically, effort is directed to pre-plan the recovery process, select “best available” methods and technologies for each recovery activity, and address both data and technology gaps critical to the recovery process. Companion work has identified the importance of preplanning and the fact that some currently available technologies need further development to better prepare the Nation (Raber and Kirvel 2008; Raber et al. 2009).
Table 1.
Response and recovery phases to a chemical terrorist attack. Current assessment focus is on developing guidelines to direct Remediation/Cleanup and Restoration/Re-occupancy decisions and activities.a
| Response and recovery | |||||
|---|---|---|---|---|---|
| Remediation/Cleanupbc |
|||||
| Notification | First response | Characterization | Decontamination | Clearance | Restoration/Reoccupancy |
| Receive information on chemical incident Identification of suspect release sites Notification of appropriate agencies |
Initial threat assessment HazMat and emergency actions Forensic investigation Public health actions Initial environmental sampling Determine agent type and concentration Risk communication |
Detailed characterization of CWA or TIC Characterization of affected site Site containment Prompt source reduction Continue risk communication Characterization, environmental sampling and analysis Initial risk assessment Clearance goals |
Decontamination strategy Remediation Action Plan Worker health and safety Site preparation Continued source reduction Waste disposal Decontamination of sites, items, or both Verification of decontamination parameters |
Clearance environmental sampling and analysis Clearance decision |
Renovation Reoccupation decision Potential long-term environmental and public health monitoring |
aDerived from Figure 1-1 in DHS (2009a);
bWithin 24 hours post-release, full operation restored to all 3 Tokyo (Japan) subway lines in which nerve agent GB had been released by chemical terrorists on March 20, 1995; phased operations allowed full-service restoration on 2 subway lines within 10 hours post-release (Komiya and Kamakura 1995; Lillibridge 1995; Tu 2002, 2007; Ember 1995);
cWithin 16 days post-release, full re-occupancy of all homes and businesses previously evacuated following 70-ton chlorine release from overturned train tank car in Graniteville, SC, on January 6, 2005; phased operations allowed re-occupancy of certain residences within 6 days post (Mitchell et al. 2005).
Post-event remediation and restoration activities necessary to attain unrestricted facility re-use and re-entry could require hours to multiple days. The longer the time expended in the remediation and restoration phases, the greater the expected public and economic impacts. While restoration timeframes are dependent on numerous variables, a primary controlling factor is the level of pre-planning and decision-making completed prior to chemical terrorist release. By assisting major transportation nodes and their related agencies to make advance planning decisions in critical areas such as decontamination procedures and clearance goal development, the DHS Science and Technology Directorate is enhancing the likelihood of rapid facility recovery. Minimizing potential economic and public impact from chemical attacks is an effective countermeasure to chemical terrorism.
This analysis develops and reports critical assumptions, a conceptual site model, and human health-based exposure guidelines as an aid to site-specific pre-planning in preparedness for a chemical terrorist incident. To expedite remediation activities should such an incident occur, it is important for state and local stakeholders to become familiar with available knowledge and resources in advance so that informed risk-based decisions can be made. It is imperative that health-based clearance and re-occupancy exposure guidelines be incorporated into decision-making as soon as possible, as such guidance provides the technical basis for design and implementation of characterization and decontamination tasks; incident on-scene commanders request and require such guidelines almost immediately after a release incident.
Airport scenario compounds include the CWA nerve agents tabun (GA), sarin (GB), soman (GD), cyclosarin (GF), and VX as well as the vesicant agent sulfur mustard (HD); and the TIC compounds phosgene (CG), hydrogen cyanide (AC), and cyanogen chloride (CK) (properties are summarized in Table 2a, b). These 2-letter codes were originally developed by the North Atlantic Treaty Organization (NATO) and are internationally recognized as compound-specific identifiers. Threat analysis determined that the selected CWAs and TICs were the most likely compounds to be deployed. Further, their toxicological and chemical properties are considered representative of many CWAs and TICs not specifically mentioned. Exposure guidelines for principal CW agent degradation products of interest (properties summarized in Table 3) are also provided. Exposure routes considered for this assessment include those associated with airborne vapors (direct ocular and inhalation vapor, percutaneous vapor) and potential surface residues (surface contact with skin, hand-to-eye transfer, hand-to-mouth ingestion, resuspension inhalation).
Table 2a.
Chemical and physical properties of CWAs and TICs.
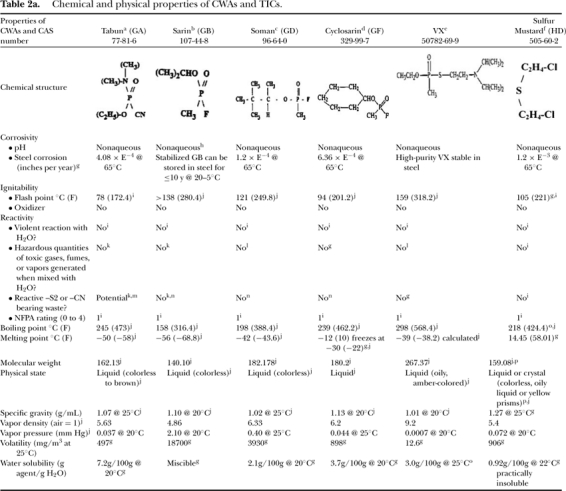 |
Table 2b.
Chemical and physical properties of CWAs and TICs.
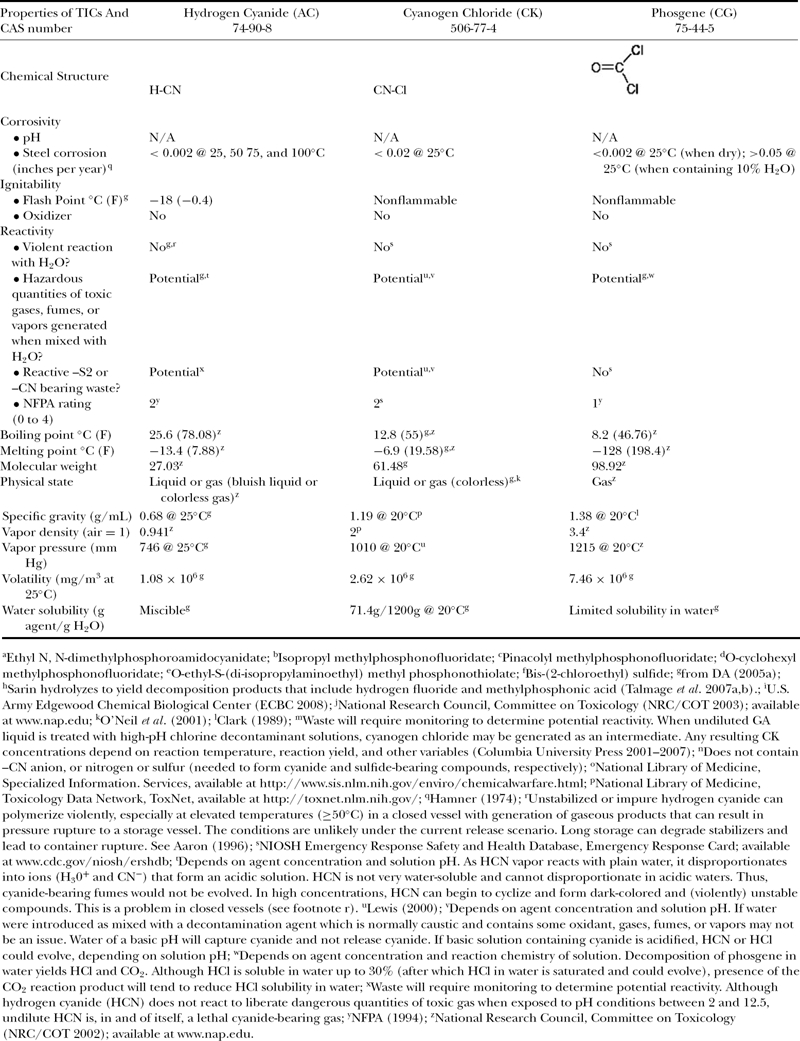 |
Table 3.
Properties of principal CWA degradation products (commercial compounds are provided for comparison).
| Degradation producta | Acute toxicity | Toxicity | Vapor pressured | Water solubilitya | ||
| (formula; CAS number) | Parent CWA | Persistenceb | (oral LD50) (mg/kg) | ratingc | (mm Hg) | (mg/L) |
| MPA (solid) (CH5O3P; | GB, GD, GF, VX | High | 5000 (rat) | Slightly toxic (#2) | 2 × 10−6 | > 1.0 × 106 |
| 993-13-5) | hydrolysis | >5000 (mouse)a | ||||
| EMPA (liquid) | VX hydrolysis | Moderate | Considered similar to | Slightly toxic (#2) | 3.6 × 10−4 | 1.8 × 105 |
| (C3H9PO3; 1832-53-7) | IMPAa | |||||
| EA 2192 (solid) | VX hydrolysis | Moderate | 0.630 (rat)a | Supertoxic (#6) | Not detectable; | Infinitely soluble |
| (C9H22NPO2S; | (pH 7-10) | 5.1 × 10−6 | ||||
| 73207-98-4) | (est.) | |||||
| IMPA (liquid) | GB hydrolysis | High | 6070-7650 (rat) | Slightly toxic (#2) | 1.2 × 10−2 (est.) | 5.0 × 104 |
| (C4H11PO3; | 5620-6550 (mouse)a | |||||
| 1832-54-8) | ||||||
| Thiodiglycol (liquid) | HD hydrolysis | Moderate | 6610 (rat)a | Slightly toxic (#2) | 2 × 10−5 | Miscible |
| (C4H10O2S; 111-48-8) | ||||||
| Aspirin (50-78-2) | Not applicable | — | 50-500e | Very toxic (#4)e | 2.5 × 10−5 (calc.) | 4.6 × 103 |
| Table salt (NaCl; | Not applicable | — | 3750 (rat)e | Moderately toxic | 1.0 at 865°C | 3.6 × 105 |
| 7647-14-5) | (#3)e | |||||
| Saccharin (soluble) | Not applicable | — | 5000-15000e | Slightly toxic | Sublimes in | 4.3 × 103 |
| (128-44-9) | (#2)e | vacuum |
aDegradation products selected on the basis of environmental persistence, toxicity, or both from Talmage et al. (2007a, Table 1, Ch. 4); Munro et al. (1999); Reddy et al. (2005); Capacio et al. (2008, Table 19.2, Ch. 19);
bPersistence ranking based on chemical/physical properties and degradation data/estimates; mod = weeks to months, high = months to years (Talmage et al. 2007a).
cKlaassen et al. (1986, Table 2-2, p. 13);
dMunro et al. (1999); Howard and Meylan (1997); Michel et al. (1962); Rosenblatt et al. (1995); Hazardous Substances Data Bank, U.S. National Library of Medicine, Bethesda, available at www.toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?index.html;
Development and application of these health-based guidelines is integral to fulfilling DHS countermeasures program objectives in determining “How clean is clean enough?” Answers to that question, as well as the sampling and clearance methods by which the answer is determined, are key to establishing effective and successful remediation (Raber and Kirvel 2008; Raber et al. 2009; Hauschild and Lee, 2004; Watson et al. 2006a,b). Specific choices are governed by the chemical, physical, and toxicological properties of the CWA or TIC released; location(s), duration, and distribution of release; extent of any contamination; characterization of potentially exposed populations; and principal exposure routes. These parameters would then become the focus of sampling and assessment during facility remediation activities. All of these topics are under investigation within this DHS effort, which has partnered with the Los Angeles International Airport (LAX; Los Angeles, CA) to generate a national case study.
An overall project objective has been to evaluate existing resources and guidance so as to efficiently leverage known planning and preparedness tools. Therefore, emphasis is placed here on exposure guidelines consistent with parameters of the site-specific and one-time release scenario for which this project is designed. In addition, focus is maintained on guidelines that already exist, are published and accessible to the public, have undergone credible peer and public review, are health-based and protective, are compound-specific, and have demonstrated utility in use and practice. Relevant work is ongoing in many fields that will continue to inform future evolution of clearance guidelines. Nevertheless, if a chemical terrorist incident should occur tomorrow, it is important to have an available set of appropriate and reasonable clearance goals from which to begin. It is understood and acknowledged that each release event will involve site- and incident-specific parameters requiring in-context evaluation regarding guideline applicability. Final decisions by appropriate on-scene personnel are expected to reflect multiple factors as well as subjective considerations of risk acceptance and socioeconomic concerns.
These analyses and evaluations are not intended to be encyclopedic, and the interested reader is referred to the bibliography for additional background and specific documentation of chemical, physical, and toxicological characteristics. Further, note that this article does not address public health responses (i.e., medical treatment) or risk management elements.
PROGRAMMATIC ASSUMPTIONS
Release Scenario
The DHS maintains pre-determined “reference scenarios” for use in homeland security analysis and planning (Homeland Security Exercise and Evaluation Program; https://hseep.dhs.gov/); these reference scenarios have been established to facilitate coordination and standardization among Federal, state, and local organizations during planning and exercise efforts. Elements and parameters of a reference scenario for a plausible indoor chemical attack are the basis for the present evaluation, which is focused on a specific terminal (the Bradley International Terminal, or TBIT) within the Los Angeles International Airport (LAX). This scenario was deliberately designed to illustrate critical decisions and consequences associated with an indoor attack to a large, high-value, public-use facility.
High-value indoor facilities that provide critical regional services and public access to large, dynamic populations are considered plausible terrorist targets. These facilities also represent some of the most complex technical risk assessment and political risk management decisions. Therefore application of this type of scenario for airport preparedness planning is considered broadly beneficial. Further assumptions inherent to this analysis include the high likelihood for agent containment in a confined space, that relatively modest release volumes are necessary to initiate the chemical terrorist event, and that significant regional impact is possible due to facility use denial. Whereas delivery methods may vary, the single-source, acutely toxic, short-term airborne release, and dispersion concept is common to many chemical attack scenarios from other realistic threat assessments (Hauschild and Bratt 2005; Raber and Kirvel 2008), and was well-documented for the 1995 chemical terrorist attack on the Tokyo subway system (nerve agent GB; Lillibridge 1995; Komiya and Kamakura 1995; Tu 2002, 2007; Ember 1995; and others). Volatile airborne releases typically disperse from the source location via advection and turbulence generated by facility air flow. Potential cross-contamination from tracking and contact exposure to any liquid hazard are more commonly associated with a limited area of the facility, especially for chemicals with low vapor pressure (Table 2a, b).
The single indoor release event may be overt and result in immediate signs and symptoms (e.g., hydrogen cyanide vapor) or covert and initially identified only when latent signs are exhibited in exposed populations (e.g., a delayed-onset blister agent). Fast-acting and acutely toxic compounds that induce signs and symptoms within seconds or minutes post-exposure are considered plausible threats by both homeland defense assessments as well as military defense programs (Hauschild and Bratt 2005; DA 2005a; USACHPPM 2007a,b; Raber and Kirvel 2008).
More specific assumptions regarding the airport reference scenario that served as the basis for this assessment are summarized as follows:
An undefined quantity of a single chemical of concern is released as a vapor containing some aerosol from a single clandestine dispersal device in a public access area of The Bradley International Terminal (TBIT); release duration terminates within minutes.
All terminal mechanical ventilation systems were operating as designed at the time of release and during the release event; operating conditions incorporate 20% outside air and 80% internal recirculated air. Primary distribution of the released compound is via the mechanical ventilation system.
Command to shut down the HVAC system was issued approximately 20 min after incident discovery; completing that action required approximately 60 min. The HVAC system has remained off. All items (e.g., luggage, carry-on bags, and food) left by passengers and not considered evidence have been left in place.
The release device was secured, contained, and removed from the TBIT approximately 1 hour after incident discovery. Exterior doors of the terminal were closed by first responders after they completed search and evacuation operations. All subsequent entries during incident investigation were made through a single entry point equipped with a decontamination station appropriately designed and equipped for removal and degradation of the specific toxic compound released.
Subsequent assessment of the release device by appropriate investigative authorities finds that target compound release was terminated during the first-response phase, obviating the possibility of a continuous and ongoing CWA or TIC release inside the TBIT. There is no potential for continuous chemical “replenishment” from an external source into airport atmospheres or onto airport surfaces.
No bulk liquid releases or bulk containers of CWAs or TICs are assumed to be present in the airport terminal, nor is there any assumption of in-terminal “saturation” releases similar to those found on chemical warfare battlefields. There is no assumption of other special conditions such as continuous low temperatures or agent burial belowground that would limit known dissipation processes of volatilization and hydrolysis.
Law enforcement personnel and investigative units have completed their tasks over a period of multiple days, all forensic assessment has been completed, and the chemical release site has been turned over to remediation personnel.
During remediation activities, source absence is frequently verified and validated by real-time site and personnel monitoring.
Before unprotected persons are allowed to re-enter and re-occupy the affected terminal and resume normal activities, all active and passive decontamination actions are completed, and attainment of clearance concentrations in air and on surfaces has been certified by sampling and analysis.
Phases of Response and Recovery
While differing terminologies have been used to describe various response phases to a toxic chemical release, this assessment categorizes post-release actions into six principal phases (Table 1), beginning with identification of an incident (notification phase) and ending with verification that all cleanup and decontamination criteria have been met (restoration phase) (Raber et al. 2002). Re-occupancy or unrestricted re-entry of a site or facility follows completion of final restoration phase activities. A brief summary follows, and clarifies where activities and decisions related to clearance are most relevant during the remediation process (Raber et al. 2009). The terminology used is consistent with DHS and U.S. Environmental Protection Agency (USEPA) documentation previously developed to characterize response and recovery from a biological attack at a major airport (DHS and USEPA 2008), and is applicable to describe comparable elements of chemical response and recovery.
Notification phase. An Emergency Communications Center (such as a police dispatch center) or an Emergency Operations Center (EOC) received knowledge about a threat or a release incident. Information-gathering and dissemination are the main tasks.
First-Response phase. This phase begins with ad hoc response of local emergency personnel such as police and fire units, activation of an Incident Command as well as law enforcement and emergency operations personnel (e.g., security, medical, and hazardous materials teams), and establishment of a Unified Command Structure to provide integration and risk communication. The First-Response Phase continues as long as emergency personnel are present. Central activities are rescue and evacuation, personal decontamination and treatment, mitigation of any conditions that pose an immediate threat to human health such as fire or explosion and exposure to the release, documenting and limiting dispersion of the release (especially any visible liquid), controlling and securing the release area (a likely crime scene), and sampling associated with the release area. First responders facilitate evacuation of the site, search for additional release devices and perform initial sampling to begin identifying the released chemical(s) of concern. The release site may also undergo initial stabilization by containment, source reduction, or both so as to reduce dispersion and material sorption as well as to control potential for secondary distribution or contamination. The first-response phase ends when conditions dangerous to human health are controlled and when law enforcement agencies complete all necessary forensic examination (e.g., control of the scene is returned to facility authorities and remediation personnel).
Characterization phase. This phase encompasses the gathering of information needed for subsequent activities, including obtaining positive confirmation of the chemical(s) of concern with verifiable protocols (if not performed during first response). Characterization sampling determines extent of any contamination as well as identifies areas and materials that may need remediation procedures (such as active decontamination). Chemical and physical characteristics of the released compound (e.g., volatility and solubility) as well as toxicity are evaluated to gauge potential consequences to exposed human populations and the environment as well as to identify appropriate mitigation. It is usual to set initial clearance goals during this phase due to their incorporation into subsequent actions such as sample design and decontamination method(s) selection.
Decontamination phase. During this phase, plans are implemented to decontaminate affected areas and materials so as to achieve clearance goals; additional actions are often taken to further stabilize or contain released source material. Early decontamination actions during the Characterization Phase may commence when such actions can reduce or eliminate area or source contamination that could lead to secondary transfer or surface and material sorption. Options for CWA and TIC decontamination in place include monitored natural attenuation (i.e., weathering), application of surface decontaminants such as bleach, and gas- or vapor-phase decontamination with hot air or a mixture of reactive compounds (Ho et al. 2006; Talmage et al. 2007a,b). Decontamination terminates when treatment solutions and foams, if any, are removed or neutralized and all decontamination activities (including waste disposal) are complete. Note that, if wastes are held exterior to the affected building or facility, the Clearance Phase may begin prior to completion of waste disposal. Verification is performed to determine overall effectiveness of the decontamination technology applied.
Clearance phase. After decontamination is complete, focus is on determining any remaining human health risk associated with reoccupying the facility and reestablishing operations. Appropriate personnel review and evaluate relevant data, such as characterization and clearance sampling results as well as decontamination process parameters and quality assurance/quality control information. Specific clearance criteria are applied to not only judge effectiveness of the decontamination process but to also determine whether unacceptable risks remain in reoccupying the facility. Final decisions on clearance are generally made at the local and state regulatory level with input from appropriate Federal agencies, depending on site-specific jurisdictional authority.
Restoration phase. The focus of this phase is on preparing the facility for re-occupancy. Activities include renovating areas that have been adversely affected by the chemical attack or necessary decontamination (as chlorine bleach decolorization of floor coverings and upholstery), and addressing any potential need for post-clearance monitoring.
In general, the Characterization, Decontamination, and Clearance phases constitute the remediation portion of response to a chemical attack (Table 1). The identification of appropriate pre-planning clearance guidelines is central to restoration of all operations and completion of all remediation in an efficient and timely manner. It is noted that actions associated with the six principal phases do not necessarily occur in strictly sequential order and may be concurrent. Further, various portions of a facility may be in different phases at the same point in time.
Overall durations for completion of each phase vary substantially, are site-specific, and can extend from hours to multiple days. For example, within 24 h post-release, full operation was restored to all three Tokyo subway lines in which nerve agent GB had been released by chemical terrorists on March 20, 1995; concurrent remediation operations allowed full-service restoration on two subway lines within only 10 h post-release (Komiya and Kamakura 1995; Lillibridge 1995; Tu 2002, 2007; Ember 1995; Okumura et al. 1996). Following a 70-ton pressurized liquid chlorine release from an overturned train tank car in Graniteville, SC, on January 6, 2005, full re-occupancy of all previously evacuated homes and business was completed within 16 d. Concurrent remediation operations and an integrated decision-making protocol allowed re-occupancy of certain residences within 6 d post-event (Mitchell et al. 2005).
While each incident is governed by site-specific and compound-specific characteristics, the duration of restoration timelines is often the result of decisions made and actions taken during the Characterization, Decontamination, and Clearance phases. A primary controlling factor is the level of pre-planning and decision-making completed prior to chemical terrorist release. Advance planning decisions in critical areas such as clearance guideline development for use in governing sample design and selection of analytical procedures can notably reduce actual time expended during each of the Consequence Management phases, and greatly enhance the likelihood of rapid facility recovery to full operational status.
The Tokyo and Graniteville, SC, examples illustrate this point. Key contributors to rapid facility recovery and relatively small number of fatalities in both the Tokyo and Graniteville incidents were:
keen situational awareness,
pre-planning development of decision criteria,
advance joint exercises,
involvement and availability of a large, well-trained hazardous materials team integrated across multiple agencies,
passive (e.g., non-explosive, non-aerosolized) characteristic of release,
volatility of chlorine vapor, and
volatility and impurity of nerve agent GB vapor release.
Conceptual Site Model
Standard protocols for developing a conceptual site model (CSM; ASTM 2008) were followed in the development of overall Project remediation guidance (Tucker and Raber 2008). A generic description of the LAX/TBIT airport-specific CSM follows:
In and around the release location, there may be an area of known or assumed compound-specific contamination. In this area, characterization sampling is focused on quickly identifying what types of materials are contaminated, and by which threat compound.
Areas where contamination is considered highly likely, but not confirmed, lie away from the release location. In such areas, sampling is focused on rapidly confirming and defining extent of any contamination.
In the next tier, and at some distance further away, are areas that may or may not be contaminated; the focus here is on rapidly finding any contamination if present, and on developing confidence that areas designated as uncontaminated are clean.
Depending on the size and characteristics of a release and the facility layout and structure, there may be areas relatively distant from the release location that are plausibly not contaminated at all. Here, the focus in these distant areas is on generating technical confidence that such areas are, in fact, not contaminated.
Consequence management strategies outlined in Table 1 are in accordance with the conceptual model above.
Useful, relevant, logic, and compound-specific characterization are also provided in numerous resources available from ASTM (2003) and various U.S. military services, particularly the U.S. Army Center for Health Promotion and Preventive Medicine (USACHPPM 1999, 2004, 2008; DA 2005a,b; DA 2008). Additional materials are provided in the links and cited references contained within these resources.
Potentially Exposed Populations
Three principal populations have been considered during development of CWA and TIC initial clearance goals for the Bradley International Terminal at LAX:
Decontamination personnel-trained personnel specifically tasked with performing decontamination of areas, equipment, and furnishings following a CWA or TIC release. Because of the nature of assigned duties related to specific chemical hazard characteristics, decontamination personnel represent a temporary occupational population. These individuals are expected to enter areas and atmospheres where undiluted CWAs or TICs may be present in unknown or known concentrations. As a consequence, decontamination personnel perform work in protective clothing and equipment (and stay time management) suitable for expected CWA or TIC concentrations encountered, thus reducing their potential exposures to negligible (or nil) levels. These individuals may also work in areas or atmospheres containing spent or reactive decontaminant solutions or vapors and their reaction products, for which appropriate personal protective equipment (PPE) is available. Under field operational conditions, PPE breaches are possible and are addressed in this analysis.
Various airport personnel-a broad category including gate agents, baggage handlers, vendors, restoration and recovery personnel performing refurbishment tasks, and others. Restoration and recovery personnel include individuals that repair, maintain, or service airport components and facilities (e.g., maintain and restore power and water infrastructure, repair or replace carpet and furniture). Such individuals, along with other airport employees and vendors, would begin tasks only after the release device and chemical of concern are removed and/or neutralized, decontamination is complete, and monitoring has characterized atmospheres and surfaces to verify no hazard is present above clearance concentrations. Due to initial source removal as well as active and passive decontamination and continued CWA and TIC degradation via well-characterized reactions such as hydrolysis, assumptions of potential exposure duration for this population are the same as those for the general public transit passengers in the terminal, as earlier proposed by Raber et al. (2001). This exposure assessment is not based solely on the mere presence of employees in a facility and the frequency and duration of time they will be on duty, but rather addresses the question of how much and for how long a chemical hazard would be expected to persist given completion of decontamination verification, completion of sampling operations and consequent destruction and dissipation of a CWA or TIC.
Transit passengers-members of the general public (including individuals of all ages and infirmities) who occupy airport terminals for limited times as they change flights, collect baggage, and perform other activities common to aircraft passengers. The site-specific airport release scenario, source containment, and decontamination procedures to be implemented make it unlikely that any residual CWA or TIC will remain as a source of biologically significant post-decontamination exposure. However, sampling assurances and associated clearance health criteria are necessary to address public concerns. Therefore, an evaluation of estimated dwell times of transit passengers in LAX was conducted and the results employed as reasonable surrogates for protective “exposure” assumptions. Specifically, domestic and international passenger survey data collected in 2005 from key LAX airport terminals found that between 80 and 90% of all passengers spent ≤120 min in the most heavily used LAX terminals (CAM 2005). Although the time needed to undergo airport security and screening procedures has changed in the years since 2005, it is considered reasonable to assume that most passengers spend ≤4 h in terminal transit. Accordingly, two protective assumptions of dwell time and potential exposure duration for transit passengers were employed in developing passenger exposure assessments and to allow flexibility by decision-makers: <8 h, and >8 h but <24 h. Dwell time exposure durations of up to 8 h are considered reasonable to accommodate nearly all passengers; >8 h but <24 h durations represent more extreme cases.
Some concern has been expressed that airport employees, personnel, vendors, and tenants who perform daily duties within an airport facility such as The Bradley International Terminal should not be considered members of the public for the purpose of setting clearance goals. Such concerns center on unsupported assumptions that employees, vendors, and tenants undergo repeated or continuous long-term, low-level CWA or TIC exposure for years in a post-attack airport workspace.
Such assumptions do not consider the finite source term of the one-time release, known physical and chemical properties such as volatility, crisis management activities such as device removal and neutralization, nor decontamination and other remediation measures required before re-entry by these populations. There is no expectation that long-term exposures would occur with successful decontamination of the release, continued CWA and TIC degradation of any residual material over time via well-characterized reactions such as hydrolysis, and removal of materials that cannot be effectively decontaminated.
In the resulting absence of a continuous source, post-decontamination airport atmospheres are not considered comparable to those found in industrial chemical settings involving manufacture or processing of TICs or CWAs in quantity over years. As a consequence, the specific LAX airport terminal chemical attack scenario evaluated does not support a plausible long-term duration hazard (e.g., post-decontamination workshift ≠ exposure duration).
This logic is consistent with existing assessments developed by California state regulatory authorities to guide remediation of facilities where persistent compounds have already been released in toxic quantities (Salocks 2009). Former clandestine methamphetamine production facilities (“labs”) are often characterized by surface residues (and potential off-gas) of methamphetamine at hazardous levels, and thus pose a public health clean-up challenge. Recently completed development of a risk-based cleanup standard for surface methamphetamine residues by the Office of Environmental Health Hazard Assessment (OEHHA; California Environmental Protection Agency) underwent extensive review before finalization in February 2009 (Salocks 2007, 2008, 2009). In a manner similar to the present analysis, Salocks and his colleagues also based their post-remediation exposure assessment on assumptions that former clandestine production facilities have been cleaned to target remediation standards and that no “reservoirs” of unremediated methamphetamine remain after completion of decontamination procedures.
The USEPA (2009a) acknowledges the validity of the California OEHHA health-based remediation standard for methamphetamine and points out this standard's utility for cleanup verification by homeowners, contractors, and state legislators.
Some reviews of Salocks (2009; see reviews incorporated into appendices of same) took issue with the OEHHA assumption that cleanup procedures and processes were effective and appropriately executed. A reviewer of Salocks (2009) expressed concern regarding potential remaining “hot spots,” and that target populations encountering “hot spot” areas could experience elevated exposures. The Salocks (2009) response pointed out that any “consideration of potential contamination ‘hot spots’ is essentially a ‘what if’ proposal” that “would be a consequence of inadequacies in the cleanup process, not a shortcoming of the target cleanup standard.” Further, Salocks (2009) states that the reviewer's “conjecture about potential contamination ‘hot spots’ should more rightly be directed toward the cleanup and verification procedures that are needed to demonstrate that a former clandestine methamphetamine lab meets the proposed target cleanup level and is fit for re-habitation.”
The current analysis concurs, and determines that the exposure assessment logic applied by the California Environmental Protection Agency (OEHHA) for guiding methamphetamine production facility cleanup is also relevant to development of pre-planning clearance goals for the one-time chemical terrorist release scenario being assessed here.
Compound Persistence and Decontamination Considerations
The primary goal of decontamination is to reduce or eliminate residual chemicals that would otherwise persist long enough to pose a health hazard. Since many specific details of the release event (release conditions, dissemination, magnitude and physicochemical characteristics of release, surface composition, etc.) will govern the potential for residual chemical hazard persistence, both compound- and release-event characteristics are acknowledged to directly impact selection and application of the most appropriate decontamination approaches.
While the airport scenario threat compounds are often generally categorized as “nonpersistent” or “persistent,” these categories were originally established to generally reflect whether a chemical in liquid state has the ability to present a hazard for 24 h or more (persistent) or whether the liquid state would volatilize and/or otherwise degrade in minutes to hours (non-persistent) (USACHPPM 2008a). However, it is understood that ambient conditions and surface interactions can modify these general categories on a site-specific basis. For example, at a temperature of 25°C, nerve agent GB has been observed to degrade 1500-fold in less than 10 min; at 0°C, 1500-fold degradation of agent GB has required 4-5 h (Puzderliski 1980; Small 1984) (DA 2002, 2005a; Sage and Howard 1989; MacNaughton and Brewer 1994; Munro et al. 1999; Talmage et al. 2007a,b; Sass et al. 1970; Van Kampen et al. 1969, 1970; Watson and Munro 1990). Under operational conditions, it will be essential to perform direct verification of environmental persistence by chemical analysis to assure that complexities of event-specific conditions are incorporated into the overall restoration effort. Therefore, it is important to distinguish between the general persistence category of a compound and the potential persistence of any residual chemical exposure hazard.
For several compound/release scenario/surface combinations, there is minimal or no potential for a residual chemical exposure hazard. In such cases, decontamination may occur passively with time (e.g., degradation in combination with aeration, often termed “natural attenuation”; Munro et al. 1999), or with application of minimal active decontamination. Compound/release scenario/surface combinations with greater likelihood to result in a persistent residual chemical exposure hazard could require more aggressive active decontamination efforts (e.g., scrubbing with reactive solutions) (Figures 1 and 2). Other important elements incorporated into selection and application of decontamination processes and procedures include stakeholder and public perception of risk, availability and quality of technical information on potentially hazardous levels and consequent risk, and applicable regulations.
Figure 1.
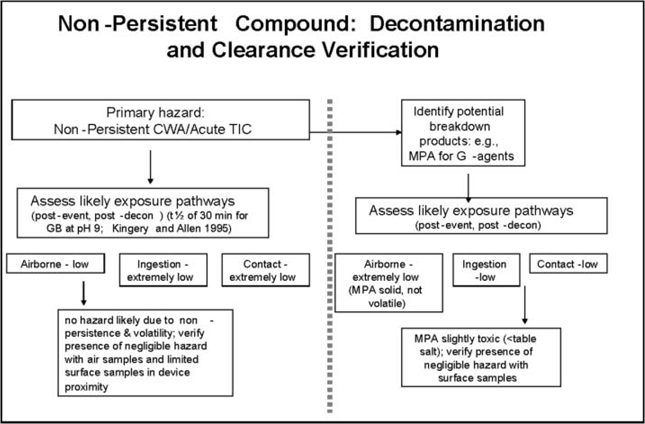
Nonpersistent compound: Decontamination and clearance verification.
Figure 2.
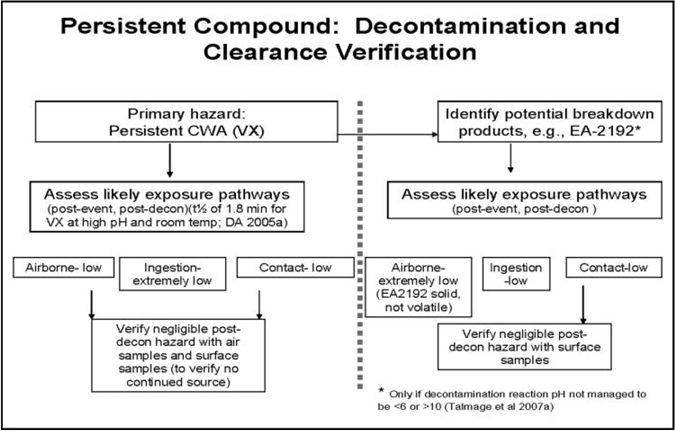
Persistent compound: Decontamination and clearance verification.
As part of this DHS effort, experimental studies of CWA persistence in the absence of active decontamination on four finish materials commonly found in airport interiors were performed in a closed system for liquid droplet spikes (Figures 3-5; Love et al. 2009; Love et al. in review). While experimental details can be found in Love et al. (2009; in review), several findings are pertinent to consideration of persistence and are presented here. Spike treatments were single applications of a 1 μl CWA droplet containing either GB (910 µg/droplet), HD (790 µg/droplet), or VX (990 µg/droplet). This droplet size represents a typical liquid agent droplet that could be generated by the airport scenario release (ASAE 2009). Since the persistence of smaller agent droplets or vapors is expected to be less, experimental findings characterizing persistence of 1 µ1 CWA droplets would represent longer timescales than what is considered likely for smaller droplets and vapors.
Figure 3.
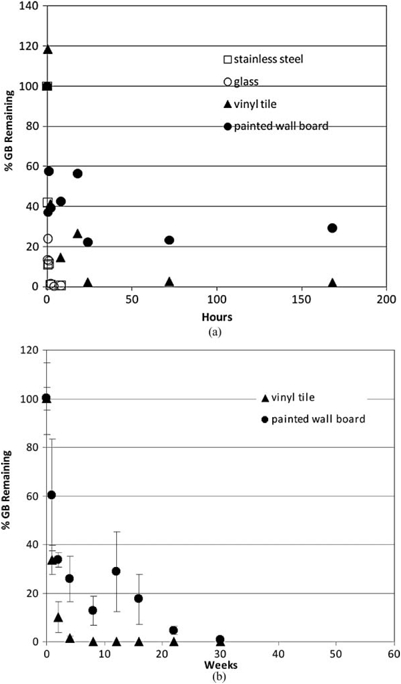
Effects of natural attenuation only (e.g., no active decontamination) on persistence of liquid GB spike on indoor airport surfaces; 1 µl droplet (containing 910 µg GB) (Love et al. 2009; Love et al. in review). The percent of GB remaining is depicted relative to the amount of GB initially applied to the surface. (a) Short-term persistence (within 1 week). (b) Longer-term persistence (within 1 year).
Figure 5.
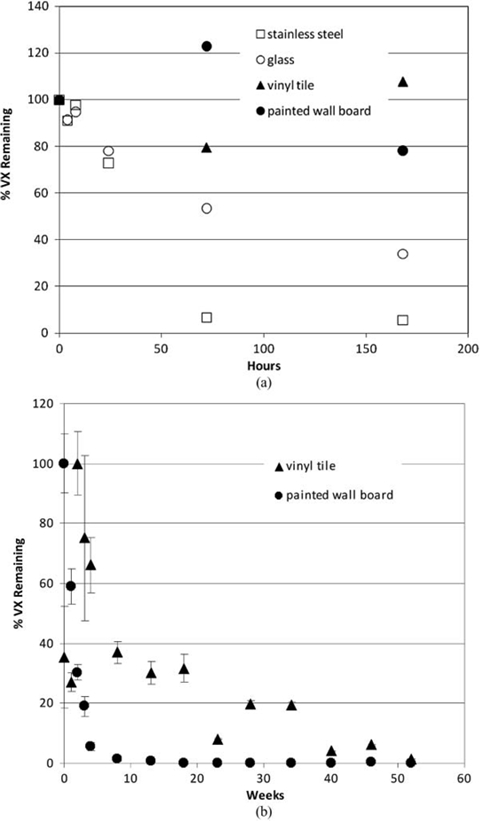
Effects of natural attenuation only (e.g., no active decontamination) on persistence of liquid VX spike on indoor airport surfaces; 1 µl droplet (containing 990 µg VX) (Love et al. 2009; Love et al. in review). The percent of VX remaining is depicted relative to the amount of VX initially applied to the surface. (a) Short-term persistence (within 1 week). (b) Longer-term persistence (within 1 year).
Materials evaluated included stainless steel, glass, latex-painted wall board, and vinyl tile. This persistence study was designed in part to identify materials that should be targeted for application of rigorous, compound-specific active decontamination, and containment or potential removal. For this experiment, no active decontamination was performed on any material/agent combination; all agent diminution observed was due to the action of passive decontamination (natural attenuation) processes.
Extraction of all CWA from each material sample was performed at various times post-spike application to quantify the total maximal amount of CWA present in or on each material. In all cases, agent attenuation was greatest and most rapid from stainless steel and glass, with 0 percent remaining within 12 h for GB and 25 h for HD (Figures 3 and 4). A longer period (between 170-200 h) of natural attenuation was required to attain 0% remaining for the VX spike on stainless steel and glass (Figure 5). Non-decontaminated liquid agent spikes on the porous and permeable materials tested (vinyl tile and latex-painted wall board) displayed a longer interval between spike application and 0% remaining, with precipitous concentration declines by liquid agents GB (to 0-30% remaining) and HD (to 20-50% remaining) within 50 h (Figures 3 and 4). As expected, natural attenuation (only) to 0% remaining liquid agent VX on these porous and permeable materials required a longer interval (weeks; Figure 5). These VX recovery data indicate that, following a liquid VX release, aggressive decontamination activities and materials should be preferentially focused on vinyl tile, latex-painted wall board and similar porous and permeable finish materials during the decontamination phase that precedes clearance.
Figure 4.
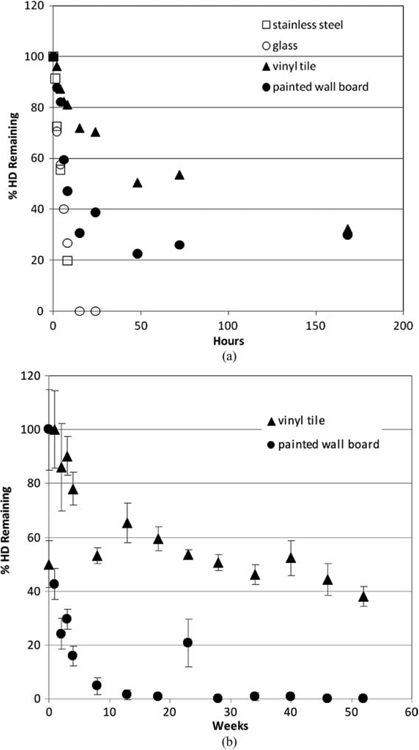
Effects of natural attenuation only (e.g., no active decontamination) on persistence of liquid HD spike on indoor airport surfaces; 1 µl droplet (containing 790 µg HD) (Love et al. 2009; Love et al. in review). The percent of HD remaining is depicted relative to the amount of HD initially applied to the surface. (a) Short-term persistence (within 1 week). (b) Longer-term persistence (within 1 year).
While these studies demonstrate that a wide range of liquid CWA persistence is theoretically possible for various agent/surface combinations in the absence of active decontamination, it is clear that CWA concentrations continue to decline over time in all cases. One critical importance of these data is that, with the exception of VX, the residual chemical exposure hazard posed by liquid droplet (aerosol) release of CWA may be realistically mitigated by natural attenuation within a 24-h period. Thus, consideration of continuous and long-term exposure at some constant source concentration is not supported by these experimental data.
It is also critical to observe that these results illustrate a physicochemical isolation of CWA in porous and polymeric materials that serves to reduce available chemical hazard by physically limiting opportunities for potential primary (e.g., surface contact as well as vapor off-gas inhalation) and secondary exposure while preferentially sequestering agent (Figures 3-5). Sequestered agent concentrations continue to decline over time with natural attenuation; further, sequestration limits potential spread of contamination and enables targeted application of aggressive and active decontamination procedures and processes (Love et al. 2009; Love et al. in review). Indeed, this information is already being incorporated into planning of scheduled infrastructure upgrades at LAX, where impermeable substitutes for vinyl tile, wallboard, and other porous media are to be installed (DHS 2009b).
The G-series nerve agents as well as hydrogen cyanide, cyanogen chloride, and phosgene are generally considered “nonpersistent” due to their rapid volatilization, reactivity, and consequent dispersion (Table 2a, b). Therefore these compounds are unlikely to pose long-term chemical exposure concerns. As a consequence, the recommended health impact focus for these nonpersistent compounds is on acute hazard prevention.
In addition, natural attenuation can effectively contribute to hazard reduction as agent concentrations decline with passage of time via physicochemical mechanisms such as volatilization, advection, and hydrolysis for these nonpersistent compounds (Munro et al 1999; Talmage et al. 2007a,b). Natural attenuation is less time-consuming and resource-intensive than technology-driven decontamination methods, and may be sufficient to reduce or eliminate the potential for exposures of toxicological concern, depending on source strength and composition and characterization of exposed matrices (DA 2002; Ho et al. 2006). Within a risk-based framework, re-entry and resumption of airport operations could be allowed after a suitable elapsed time and verification that necessary hazard reduction has been achieved by means of clearance sampling.
Labor- and resource-intensive decontamination processes should be reserved for persistent chemical hazards. Of the airport scenario threat list compounds, sulfur mustard agent HD and nerve agent VX (Table 2a) are considered persistent; sulfur mustard due to its freezing point and low volatility at ambient temperatures (FP of 13-15°C, volatility of 906 mg/m3 at 25°C) and agent VX due to its low volatility (12.6 mg/m3 at 25°C), especially when compared to nerve agent GB (volatility of 1.9 × 104 mg/m3) (Table 2a). As evidenced in Figures 4b and 5b for HD and VX, and if sufficiently large quantities of liquid chemical agent have been released and deposited on surfaces, reliance on natural attenuation alone for VX and HD may not be sufficient for a rapid (<24 h) restoration. Nevertheless, even for persistent compounds, natural attenuation can serve to reduce overall residual chemical agent load requiring active decontamination, and can help eliminate potential for continuous exposure.
Many remediation technologies are specifically designed to convert persistent compounds to nonpersistent hazards (DA 2002; Ho 2006). Utilization of active and intensive decontamination processes such as application of high-pH solutions accelerates agent degradation (e.g., half-time of nerve agent GB at pH 10 is on the order of minutes; Kingery and Allen 1995; DA 2002); this reaction was used to rapidly return the Tokyo subway infrastructure to service following a chemical terrorist attack with nerve agent GB in March 1995 (Tu 2002, 2007). More generally, compounds that demonstrate persistence under certain conditions that minimize volatility, hydrolysis, and oxidation (e.g., neutral pH or freezing temperatures) may also undergo optimal degradation when physicochemical conditions are altered to enhance decontamination reactions. For example, at alkaline pH, VX hydrolysis half-times range from 2 min to approximately 3 h (Yang et al. 1993, 1994; Yang 1995, 1999; DA 2005a). Peroxy hydrolysis of nerve agent VX is particularly swift, and rapidly cleaves the P-S bond (Yang 1999). Presented in Table 4 are the hydrolysis half-lives of liquid VX experimentally determined in various NaOH solutions and water by Yang et al. (1994) and Yang (1999). As an illustrative example, Yang's half-life determinations are used to estimate the amount of VX remaining on a surface after 48 h hydrolysis degradation from an initial unit concentration of 1.0 mg VX/cm2 for 10 half-lives and 2 d post-deposition. This degradation analysis is provided in Table 4 and plotted in Figure 6.
Table 4.
Half-lives of liquid VX and HD in hydrolysis or oxidation solutions with estimated agent remaining after 48 hour degradation (initial unit concentration of 1.0 mg/cm2).
| CWAs in solution | Half-life (minutes) | Estimated agent remaining after 48 hr degradation for an initial unit concentration (1.0 mg/cm2) |
| VX Hydrolysis | ||
| 1.25M NaOH | 1.8a | <4.23E-304 (mg VX/cm2)b |
| 0.25M NaOH | 10.8a | 5.53E-81 (mg VX/cm2) |
| 0.1M NaOH | 31a | 1.09E-28 (mg VX/cm2) |
| 0.01M NaOH | 198a | 4.19E-05 (mg VX/cm2) |
| 0.001M NaOH | 1248a | 2.02E-01 (mg VX/cm2) |
| Pure Water | 3600a | 5.74E-01 (mg VX/cm2) |
| Unbuffered Water | 4680c | 6.53E-01 (mg VX/cm2) |
| Sulfur mustard (HD) | ||
| Oxidation | ||
| >20% H2O2 | <2d | <4.23E-304 (mg HD/cm2)b |
bThis value represents limit of software computational capability; actual value is less;
Figure 6.
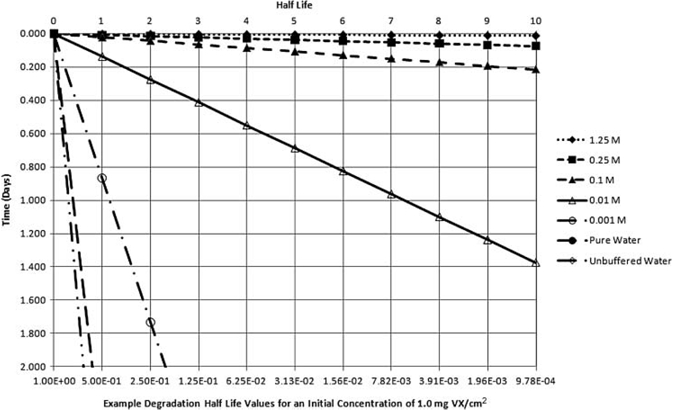
2-day hydrolysis degradation of liquid VX in NaOH solutions and water (half-life determinations from experimental data of Yang et al. 1994 and Yang 1999 applied to a unit concentration).
The persistent sulfur mustard agent HD can be effectively oxidized in minutes (Wagner and Yang 2001, 2002; review by Talmage et al. 2007b). Also presented in Table 4 is the Wagner and Yang (2002) oxidation half-life of HD in a solution of H2O2 as well as the calculated amount of HD remaining on a surface after 48 h oxidation degradation from an initial unit concentration of 1.0 mg HD/cm2.
The information presented in Table 4 and Figure 6 illustrate that multiple degradation half-lives for persistent agents VX and HD are achieved within a very short time following application of appropriate decontamination solutions. It is further illustrated in Figure 6 that NaOH solutions diluted to as low as 0.01 M can result in more than a 10 half-life reduction from initial concentration in less than 2 d. As a consequence, this degradation analysis further asserts that assumptions of long-term chronic exposure in the post-decontamination environment are unfounded.
It is important to distinguish between the persistence of a compound and the persistence of a hazard. By following a systematic logic involving consideration of the
amount released as well as nature and extent of release,
decontamination reaction chemistry,
characterized outcomes of decontamination methods and procedures,
degradation products,
media properties, and
environmental parameters,
an accurate assessment of potential for residual chemical hazard can be obtained.
When a persistent compound can be determined to pose a nonpersistent hazard, the logical and recommended focus for cleanup activities is to minimize acute hazards. This logic has been tailored to the LAX scenario in the present analysis.
Inhalation and Direct Ocular Most Likely Exposure Routes
The National Research Council (NRC), Centers for Disease Control and Prevention (CDC), and numerous investigators familiar with the legacy and battlefield use of CWAs and their manufacture agree that the most likely routes of chemical warfare agent exposure are inhalation of vapor or aerosol and direct vapor eye (ocular; a local exposure) contact (IOM 1993; NRC/COT 2003; DHHS 1988, 2002, 2003, 2004; Watson and Griffin 1992; Watson et al. 2006a; Munro et al. 1994; Saxena et al. 2008; and others). Similar logic is found for phosgene, hydrogen cyanide, and cyanogen chloride (NRC/COT 2002; ACGIH 2003, 2008; AIHA 2007). Thus, the air exposure pathway is a primary focus for developing clearance and re-occupancy goals for the scenario compounds.
Potential Surface Contact Exposure
Given the chemical and physical characteristics of the scenario compounds of concern (Table 2a, b) and the efficacy of compound-specific decontamination materials and methods (Ho et al. 2006; Talmage et al. 2007a,b), it is unlikely that scenario CWA or TIC residues would exist on surfaces in quantities sufficient to pose a toxicological hazard after source removal and decontamination have been performed. Nevertheless, and in the absence of any other compound-specific surface exposure guidelines applicable to the airport release scenario, a contact hazard analysis has been performed. At the request of stakeholder communities, the present analysis has developed protective guidance regarding potential surface residues of the persistent CW agents VX and HD as well as their principal degradation products. The working assumption is that, in the unlikely event that exposed skin comes in contact with any residual compound of concern, the residue could be transferred to hands and subsequently carried to the eyes and mouth (the latter as non-dietary ingestion) multiple times/day. Resuspension inhalation has also been evaluated.
These Surface Removal Contaminant Levels (SRCLs; mg/cm2) are unique to the current assessment and have been calculated to be protective for minimal and reversible threshold effect development. Model documentation and results are presented in the companion paper on multi-pathway decision criteria published in this journal issue.
CWA, TIC, AND DEGRADATION PRODUCT CHARACTERISTICS
Informed decision-making regarding expected exposure routes and other significant elements of consequence management will require not only knowledge of chemical and physical properties and reaction products, but also toxicological properties. Mechanisms of toxicity, experimental data, and species susceptibility for the scenario-specific CWAs and TICs have been thoroughly evaluated elsewhere and in numerous publications. Examples include Sidell (1997), NRC/COT (1999, 2002, 2003), Papirmeister et al. (1991), IOM (1993), Munro et al. (1994), Bast and Glass (2009), Young and Bast (2009), Benton et al. (2006a,b), Dabisch et al. (2008a,b), Watson and Griffin (1992) and Watson et al. (2006a,b). Details are available in these reviews and the many original studies cited therein. Brief summaries follow.
Nerve Agents
The nerve agents considered in this study include the G-series agents (GA, tabun; GB, sarin; GD, soman; and GF, cyclosarin) and nerve agent VX. These compounds are all toxic derivatives of phosphonic acid, containing either a cyanide (GA), fluoride (GB, GD, and GF), or sulfur (VX) substituent. They are commonly termed “nerve agents” due to their anticholinesterase properties and effects on the peripheral and central nervous systems. Anticholinesterase effects of nerve agent exposure can be characterized as muscarinic, nicotinic, or CNS (central nervous system). Muscarinic effects occur in the parasympathetic system and, depending on the amount absorbed, can be expressed as miosis, ciliary spasm, nasal discharge, increased bronchial secretion, bronchoconstriction, emesis, abdominal cramps, sweating, diarrhea, salivation, bradycardia, and hypotension. Nicotinic effects are those that occur in somatic (skeletal-motor) and sympathetic systems, and can be expressed as muscle fasciculations and paralysis. At sufficient exposures, CNS effects may be manifested as confusion, reflex loss, anxiety, slurred speech, irritability, forgetfulness, depression, impaired judgment, fatigue, insomnia, depression of central respiratory control, and death (Somani et al. 1992; Sidell 1992, 1997; Sidell and Groff 1974; Opresko et al. 1998; Bakshi et al. 2000; Watson et al. 2006a,b). Low-exposure effects include miosis, a feeling of “tightness” in the chest, rhinorrhea, and dyspnea (Dunn and Sidell 1989).
The “G” series military nomenclature used by NATO-member nations has historically been considered to be an abbreviation for “German,” with the second letter of the code (A, B, etc.) identifying the order in which the compounds were found and analytically identified by Allied forces investigating materials located in captured German military facilities at the close of World War II (WWII; Sidell 1997). Agent VX was industrially synthesized in the United Kingdom in the early 1950s. The letter “V” is a reported reference to venom (Sidell 1997; Robinson 1967).
The G agents are viscous liquids of varying volatility (vapor density relative to air between 4.86 and 6.33) with faint odors (faintly fruity, spicy, or odor of camphor). Agent VX is an amber-colored liquid with a vapor density of 9.2 and is considered odorless. Nerve agent vapors possess little to no olfactory warning properties (Table 2a).
It is generally considered that the order of decreasing vapor exposure hazard is: GB > GD > GF > GA > > VX; the acute toxicity of these agents is sufficiently high for the vapors to be rapidly lethal at appropriate concentrations. Agents VX and GA are expected to also present a contact hazard. The vapor density of agent GF is between that of agents GA and GD. Agent VX, with a vapor density greater than that of any the G agent under consideration, is approximately 3 orders of magnitude less volatile than nerve agent GB (DA 2005a). As a consequence, agent VX is considered a persistent “terrain-denial” military compound.
All of these nerve agents can be absorbed through the skin. One issue is whether percutaneous absorption of vapor through intact skin might contribute significantly to exposure. For agent VX, approximately 100 times greater percutaneous vapor exposure is necessary to attain the same toxic effect as that achieved from inhalation vapor exposures when mild effects are compared (NRC/COT 1997, 2003; Watson et al. 2003). Such estimates indicate that the vapor inhalation pathway is much more significant for induction of toxic effects than the percutaneous vapor pathway. This general relation holds true for the other nerve agents as well as sulfur mustard (NRC/COT 1997, 2003; Watson et al. 2003).
Most signs and symptoms of toxic levels of exposure to a nerve agent, by any route, usually develop within 60 min post-exposure. However, effects are well known to occur hours after percutaneous exposure (Sidell 1997; Watson et al. 1992) due to skin absorption. In general, the smaller the exposure, the longer the time to onset of symptoms; effects that occur many hours post-exposure are usually nonfatal (Sidell 1997; Watson et al. 1992).
Sulfur Mustard Blister Agent
As a cell poison and alkylating chemical vesicant, sulfur mustard (agent HD) damages and destroys cells of any epithelial tissue with which it comes in contact. Depending on the magnitude of the dose received, erythema, blistering, and other tissue damage can result with increasing dose. Sulfur mustard was developed and used as a CWA by the armed forces of several nations during World War I (WWI) and has been deployed in more recent conflicts (IOM 1993).
At ambient temperatures greater than the sulfur mustard freezing point of 13° to 14°C (55° to 57°F), sulfur mustard is an oily liquid heavier than, and sparingly soluble in, water (relative density of 5.4) (DA 1996; O'Neil et al. 2001; Table 2a). Because of its low aqueous solubility, bulk quantities of sulfur mustard are resistant to degradation in the environment. It is sufficiently volatile [vapor pressure 0.072-mm Hg at 20°C (68°F) and 0.11-mm Hg at 25°C (77°F)] to produce toxic vapors when temperatures are greater than the freezing point. At air temperatures ≥ 32°C (90°F), tissue-damaging concentrations can be small as a consequence of the more rapid development of injury to warm, moist tissues (Watson and Griffin 1992). Sulfur mustard has a garlic-like odor, and reported odor thresholds range from 0.15 to 0.6 mg/m3 (NRC/COT 2003).
The principal mechanism of toxicity for sulfur mustard is attributed to its capacity as an alkylating agent and consequent ability to react with DNA and RNA, resulting in disorganization of normal cell function. As a consequence, sulfur mustard is considered a cell poison. The epithelium is an important target because of the presence of a proliferating cell layer. Relatively high sulfur mustard concentrations are required to cause human mortality; “battlefield” concentrations (perhaps in excess of 1500 mg-min/m3) during the Iran-Iraq conflict of the 1980s resulted in mortality rates of 1 to 3% among exposed military personnel (Blewett 1986; Dunn 1986). Such mortality rates are similar to those observed during WWI following battlefield releases (IOM 1993). For the lethality endpoint, mustard agent is much less potent (by approximately 103) than nerve agents under comparable conditions of exposure (Watson and Griffin 1992).
The alkylating reaction of sulfur mustard with cellular constituents is rapid (i.e., cell injury and death occur quickly). Nevertheless, any clinical effects (e.g., conjunctivitis, eye sensitivity to light, skin burns) do not manifest immediately, but develop over hours post-exposure. Such latent effects are characteristic of sulfur mustard exposures (NRC/COT 2003; IOM 1993; Watson and Griffin 1992; Watson et al. 2006a,b).
Hydrogen Cyanide
At ambient temperature and pressure, hydrogen cyanide (HCN, hydrocyanic acid, prussic acid, AC) is a colorless gas or liquid with a boiling point of 25.7°C (78.3°F) (Table 2b). The bitter-almond odor of HCN is detectable by some, but not all, individuals at 0.90 to 4.94 mg/m3 (0.8 to 4.4 ppm; ATSDR 2006). HCN is currently employed in various industrial applications, including fumigation, the production of certain resin monomers, and mining (NRC/COT 2002; ATSDR 2006); HCN has also been used for gas-chamber executions in several countries (ATSDR 2006).
Toxic effects to HCN vapor exposures develop swiftly. Biological effects of HCN result from its ability to rapidly disrupt cellular respiration via inhibition of the enzyme cytochrome oxidase. A volatile toxin [vapor pressure 630-mm Hg at 20°C (68°F)] with a low vapor density (0.94), HCN will volatilize immediately if released passively from a container or other material. Liquid HCN is extremely unstable, and if introduced into a facility, will either rapidly volatilize or undergo an exothermic reaction upon contact with air and decompose by oxidation (NIOSH 2008a,b; Aaron 1996).
HCN can be absorbed by inhalation, ingestion, or by dermal contact with either the vapor or liquid (ATSDR 2006; NIOSH 2008a,b). However, the volatility of HCN, coupled with the instability of HCN liquid, indicate that inhalation exposure is the most important exposure route for an airport release scenario.
HCN crosses mucous membranes rapidly, and HCN entry into the bloodstream after inhalation exposure is nearly instantaneous. Dermal absorption, or absorption across the epithelia of the gastrointestinal tract, is somewhat slower (ATSDR 2006). Inhalation exposure to HCN at sufficiently high concentrations can lead to death within minutes. An average fatal concentration of 600 mg/m3 has been estimated for a 10-min exposure.
Short-term exposures to lower concentrations (∼2.7 mg/m3) may induce symptoms and signs that include headache, dizziness, confusion, nausea, and vomiting (NIOSH 2008b). The principal targets of acute high-level inhalation exposure are the respiratory, central nervous, and cardiovascular systems.
Although HCN can be dermally absorbed in large quantities, available data rarely distinguish whether exposure was to liquid or vapor HCN. There are reports of men equipped with “excellent” respiratory protection who incurred toxic exposures to HCN vapor via dermal absorption across unprotected skin (ATSDR 2006; Drinker 1932). Very high concentrations of HCN vapor (>343,000 mg/m3) were lethal to experimental animals exposed over 2% of their bodies (AIHA 1994).
Cyanogen Chloride
Cyanogen chloride (CK) is a colorless gas at ambient conditions [vapor pressure of 760-mm Hg at its boiling point of 13.8°C (56.8°F)] with a highly irritating odor and an odor threshold of 1 ppm in air (ATSDR 2006; Table 2b). Cyanogen chloride is used in various industrial processes and was historically deployed as a military chemical warfare agent by several nations (ATSDR 2006). A 10-min exposure to 5 mg/m3 has been characterized as intolerable due to odor irritant properties. Given the physical form of CK, inhalation is the most important route of exposure for an airport release scenario. If formulated into an aqueous solution, CK can be absorbed via ingestion, although there are little toxicological data on the effects of this route of exposure or time to onset of symptoms. Data characterizing dermal irritation or dermal absorption of the vapor are not readily available.
Exposure to cyanogen chloride vapor produces both the effects of cyanide poisoning and symptoms of lung irritation. At low concentrations (∼2.51 to 50.3 mg/m3) over brief exposure durations, eye contact produces tearing with spasm and eyelid closure. Principal effects on the respiratory tract are pulmonary irritation, with pulmonary edema developing at exposures between 50 and 300 mg-min/m3 (AIHA 1998). At greater concentrations, the cyanide (CN−) moiety inhibits cellular respiration, with a concentration of 120.63 mg/m3 reportedly fatal to humans after 30 min, and 399.5 mg/m3 fatal within 10 min (AIHA 1998; Opresko et al. 1998).
Phosgene
Phosgene (CG) is a colorless, reactive gas with a vapor pressure of 1215-mm Hg at 20°C (68°F) (USEPA 1986) and a vapor density of 3.4 (Lipsett 1994; Table 2b). In the United States, phosgene is used to synthesize other chemicals or products (NRC/COT 2002). Phosgene also has a history of military use as a war gas during WWI. Phosgene odor (described as resembling new-mown hay) is generally perceived at concentrations >1.67 mg/m3 and recognized at concentrations >6.25 mg/m3. Eye, nose, throat, and bronchiolar irritation occur at greater than 12.5 mg/m3 (AIHA 2002).
Inhalation of phosgene vapor is the primary exposure route for this agent, and lungs are the principal target organs. Phosgene-related tissue damage is caused primarily by acylation of tissue macromolecules in the alveolar region of the lung. Production of HCl from phosgene hydrolysis may also contribute to its toxicity, particularly when phosgene contacts and dissolves in the aqueous layer of eyes and mucous membranes. Chronic, low-level inhalation exposure to phosgene can cause pneumonitis and progress to pulmonary edema (AIHA 2002). Acute, low-level phosgene exposure (>30 ppm-min, equivalent to >125 mg-min/m3) can damage the lung; acute high-concentration exposures (e.g., >150 ppm-min, equivalent to >625 mg-min/m3) can lead to irreversible pulmonary damage or death (NRC/COT 2002). Between the time of phosgene exposure and development of pulmonary edema there is a latent period, ranging from several hours to 24 h; the length of the latent period is considered inversely proportional to the exposure concentration (Diller 1978; Frosolono and Pawlowski 1977).
CW Agent Degradation Products
CWA degradation has been previously examined in many studies characterizing agent fate, and results are compiled in reviews by Munro et al. (1999) and Talmage et al. (2007a,b), among others. Research is ongoing in this area. Principal degradation products of the nerve agents and sulfur mustard agent HD have been identified on the basis of environmental persistence, toxicity, or both (Talmage et al. 2007a) and are summarized in Table 3 along with comparisons to other commercial compounds for perspective. Previous analyses indicate that degradation of GA (tabun) results in no degradation products of potential concern regarding persistence or toxicity (Talmage et al. 2007a; Munro et al. 1999). Most CWA degradation products are water-soluble but exhibit low vapor pressures and are thus of little consequence as a source of vapor inhalation or ocular exposure. The ingestion of degradation products is an unlikely possibility under the airport release scenario, but such a possibility is nonetheless considered for completeness.
Nerve agent degradation products of particular interest are methyl phosphonic acid (MPA) and S-(diisopropylaminoethyl) methylphosphonothioic acid (EA2192). Methyl phosphonic acid (CH5O3P; a hydrolysis degradation product of nerve agents GB, GD, GF, and VX) is a white solid, is stable under a wide variety of environmental conditions in both soil and water, is relatively non-toxic, and does not pose a vapor hazard (vapor pressure of 2 × 10−6 mm Hg; Table 3). As a function of its environmental stability and low toxicity, MPA possesses considerable forensic value and was used by police authorities as conclusive evidence to identify sites where the Aum Shinrikyo cult had either manufactured or tested sarin prior to the cult's chemical terrorist release of GB in the Tokyo subway system in 1995 (Tu 2002, 2007; Crothers et al. 2008). In addition to its forensic value, MPA can also be used as a well-characterized monitor of the hydrolysis degradation reaction. It is noted that MPA is sold commercially as an analytical reagent and used in the production of lubricant additives and for treating textiles (Lewis et al. 1997; HSDB 2008). Isopropyl methylphosphonic acid (IMPA) and ethyl methylphosphonic acid (EMPA) exhibit oral toxicity rankings similar to that of MPA (Table 3).
The VX hydrolysis product, EA 2192 (C9H22NPO2S), is produced during VX hydrolysis reactions conducted within the pH range of >6 to 10 (Talmage et al. 2007a and b; Munro et al. 1999). EA 2192 is a white solid, possesses low vapor pressure, is stable (Michel et al. 1962; Szafraniec et al. 1990), and is water-soluble (Small 1984). Oral toxicity data for the LD50 endpoint indicates that EA 2192 is approximately 6 times less toxic than the parent agent VX. EA 2192 is not an inhalation hazard and is not absorbed through the skin in aqueous or alcohol solutions (Michel et al. 1962), but is thought to present a potential but remote ingestion exposure concern. Since EA 2192 is not significantly formed at pH <6 or >10, where the hydrolysis reaction produces diisopropyl ethyl mercaptoamine and EMPA, it is highly advised that the pH of the VX decontamination reaction be closely monitored to ensure maintenance at pH <6 or >10. Production of EA 2192 can also be prevented by nucleophilic decontamination of VX with excess H2O2 in mildly basic or basic solutions (Yang 1999). Documented hydrolysis reaction yields of EA 2192 from the parent VX are less than 25% (Michel et al. 1962; Szafraniec et al. 1990; Yang et al. 1993; Yang 1995), thus further reducing the potential for exposure.
Sulfur mustard (HD) hydrolysis leads to the formation of thiodiglycol (C9H10O2S, CAS No. 111-48-8) and HC1 (Small 1984; Rosenblatt et al. 1995; Munro et al. 1994). Thiodiglycol (TDG) exhibits a low vapor pressure (2 × 10−5 mm Hg; Table 3) and does not present an inhalation hazard; it is miscible in water and oral toxicity is considered low (rat oral LD50 of 6610 mg TDG/kg and within the oral LD50 range for saccharin; Table 3). The environmental stability of TDG can allow forensic monitoring to ascertain potential use or previous presence of HD; for example, TDG was present among compounds identified in soil, munition fragments and wool samples associated with a CWA release in Iraq (Talmage et al. 2007; Hay and Roberts 1990; Black et al. 1993). Nevertheless, it is noted that the presence of TDG is not a unique forensic identifier given that thiodiglycol is a high-production volume chemical used commercially in the U.S. textile industry (Talmage et al. 2007).
Incomplete oxidation or incomplete dechlorination during decontamination of sulfur mustard vesicant agent (HD) could lead to the generation of the transient toxic intermediate reaction products mustard sulfone (C4H8SO2Cl2, CAS No. 471-03-4; a product of incomplete oxidation reaction with supertropical bleach) or divinyl sulfone (C4H6SO2; CAS No. 77-77-0; an intermediate product of HD dechlorination) (Small 1984; Munro et al. 1999). These compounds are volatile and are not considered persistent. Photochemical oxidation of divinyl sulfone occurs within hours, and volatilization from potentially contaminated soil or water is significant. Divinyl sulfone is not unique to HD decontamination and is produced commercially and used during processing of cotton textiles (HSDB 2008). Production of, and potential exposure to, these sulfone intermediates under the airport scenario can be prevented by monitoring the oxidation and dechlorination of HD so that decontamination reactions go to completion.
PRECEDENTS AND SOURCES OF EXPOSURE GUIDELINES
Exposure guidelines have been implemented for many domestic sites where CWAs are present. The current analysis explores and evaluates available approaches to develop guidelines suitable for remediation pre-planning under the airport chemical terrorist scenario described above.
Agencies and Authorities
Many agencies have developed multiple exposure guidelines for CWA and TICs. Each agency incorporates its own unique set of exposure assumptions (e.g., exposure duration) and population characteristics, and a working knowledge of these assumptions is critical in selecting appropriate guidelines for facility remediation. In addition, values may sometimes need to be extrapolated when experimental data are lacking or unavailable. Well-informed decision-making includes familiarity with the literature as not all of the published guidelines are suitable for developing clearance goals for the airport chemical terrorist scenario and populations under consideration.
Primary exposure routes of concern for various airport personnel as well as transit passengers are direct vapor exposures to the eyes and tissues surrounding the eyes (ocular exposure) or direct vapor exposures to the respiratory tract (combined as the inhalation and ocular routes). The literature considers absorption of CWA vapors through the skin (percutaneous exposure) to be of secondary importance. For rapidly reactive materials such as nerve agents and sulfur mustard, tissues of the eye and upper respiratory tract would be the first to exhibit effects. The eyes and respiratory tract would also be the first tissues to exhibit the effects of CK and phosgene exposure. Whereas HCN can also affect the respiratory tract, signs and symptoms of HCN effects arise from disruption in oxygen transport to critical tissues such as the brain, and would likely dominate. Designation of ocular and inhalation vapor exposure as the primary exposure routes of concern is protective and consistent with previous emergency preparedness decisions made by the DHHS (1988), CSEPP (2003), NRC/COT (2001, 2002, 2003), DA (2004, 2008), and others. In addition, selection of these primary exposure routes is also based on the chemical and physical properties of the scenario compounds as well as historical data on case exposure.
The CWA health-based risk information used as part of this evaluation was initially developed to facilitate disposal of the U.S. stockpile of CWA munitions, maintain U.S. treaty compliance with the Chemical Weapons Convention, and to support remediation or closure at sites where CWAs were historically processed. Existing CWA guidelines and updates have received new interest for homeland defense applications since the events of September 2001. Among the authorities and agencies that have published guidelines for threat CWAs and TICs considered in evaluation of the airport chemical terrorist scenario are the following:
Agency for Toxic Substances and Disease Registry (ATSDR) of the U.S. Department of Health and Human Services (ATSDR 2003, 2005, 2006)
American Conference of Governmental Industrial Hygienists (ACGIH 2003, 2008)
American Industrial Hygiene Association (AIHA 2007)
Centers for Disease Control and Prevention (CDC) of the U.S. Department of Health and Human Services (DHHS 1988, 2002, 2003, 2004)
Committee on Toxicology of the National Research Council (NRC/COT 2001, 2002, 2003)
National Institute for Occupational Safety and Health (NIOSH) and Centers for Disease Control and Prevention (CDC) (NIOSH/CDC 2008; NIOSH 2008a,b)
Regional Screening Levels (RSLs) for Chemical Contaminants at Superfund sites (USEPA 2008, 2009b)
U.S. Army Center for Health Promotion and Preventive Medicine (USACHPPM) (USACHPPM 1999, 2004, 2008; OASA 1999).
U.S. Environmental Protection Agency (USEPA 1991, 1996b)
U.S. Environmental Protection Agency's Integrated Risk Information System (USEPA/IRIS 2005, 2006a,b)
U.S. Environmental Protection Agency, Regions 9 and 3 (USEPA 1996a; 2005)
Whereas site-, situation-, compound-, and population-specific factors should all be considered when selecting acceptable clearance goals for toxic chemicals, various scientifically defensible concentrations have been applied as decision criteria for several CWAs and TICs. Development of decision criteria for the current assessment includes evaluation of these existing precedents, which are discussed below.
CWAs and the Chemical Stockpile Emergency Preparedness Program (8 Host States)
At present, there are six military sites within the continental United States (Alabama, Arkansas, Colorado, Kentucky, Oregon, and Utah) where domestic stockpiles of obsolete CWA munitions are stored and secured (as of January 2009, the historical sulfur mustard munition stockpile at Aberdeen Proving Ground, MD; as well as the WWII-era nerve agent VX munition stockpile at the Newport Chemical Depot, IN, have been fully and successfully demilitarized) (CMA 2009; Watson et al. 2006a). These stockpiles of nerve and sulfur mustard CWAs were originally developed as a Cold War deterrent, and have been undergoing destructive disposal in compliance with international treaty (the Chemical Weapons Convention; website of Organisation for the Prohibition of Chemical Weapons at www.opcw.org). The U.S. Army Chemical Materials Agency (CMA) oversees disposal of the U.S. stockpile and routinely publishes updates on the status at each site (www.cma.army.mil). One of the areas receiving significant attention during demilitarization development was evaluation of procedures to address a potential accidental agent release during the destruction process; stockpile agent destruction was not permitted by host states until emergency response decision criteria and procedures were considered adequate. The Chemical Stockpile Emergency Preparedness Program (CSEPP) was established during the 1990s to ensure the appropriateness of emergency response procedures and resources (models, policies and guidelines, training, etc.) at each of the stockpile sites. The CSEPP is a joint activity overseen by the Federal Emergency Management Agency (FEMA) and the Department of the Army (DA) (through the CMA) and is integrated with state and local decision-makers and agencies (Carnes 1989).
Worst-case release preparedness
A primary decision-making tool of the CSEPP program is air dispersion model development and implementation, in which hypothetical CWA releases are virtually distributed on the basis of site meteorology and reasonable worst-case site-specific release scenarios. The model runs are used to “test” safety plans and to determine resource needs for host areas adjacent to stockpile locations. Model results are also employed to compare plans and needs at multiple geographic and administrative scales (e.g., town, county, and metropolitan areas.).
Initially, CSEPP chemical warfare agent dispersion plume models incorporated agent toxicity values originally designed as simplistic casualty estimators for battlefield scenarios involving young male soldiers. Various assessments of agent toxicity values since that time demonstrated a need to re-evaluate the acute toxicity of these agents and their effects on a more diverse population, and to address the role of different concentrations and exposure durations (NRC/COT 1997; USACHPPM 2004). It was widely recognized within CSEPP that one of the most relevant programs addressing hazardous material exposure assumptions appropriate for civilian populations was that of the USEPA Office of Prevention, Pesticides and Toxic Substances (OPPTS). The OPPTS has collaborated since 1995 with the National Research Council Committee on Toxicology to develop Acute Exposure Guideline Levels (AEGLs) for numerous hazardous compounds, including most of the chemicals evaluated for the airport chemical terrorist scenario.
To address public concerns regarding previous acute exposure criteria, the CSEPP evaluated AEGLs as decision criteria for CWA emergency preparedness and planning. The transparent AEGL process was a modern and more systematic approach than previous internal military technical reports, and the established National Research Council (NRC) review process ensures appropriate scientific credibility and peer review (NRC/COT 2001). Perhaps most importantly, the availability of CWA-specific AEGLs allows consistency with criteria and procedures used by other Federal, state, local, and industry entities in emergency planning and response to releases of TICs. For example, industrial facilities conduct plume modeling of plausible release scenarios and estimate possible exposures to civilian populations as part of Risk Management Plan (RMP) development.
AEGLs (expressed in mg/m3 or ppm) are exposure limits for the general public that are designed to aid state and local government agencies in developing response plans in the event of accidental or deliberate atmospheric release of extremely hazardous chemical substances. AEGL values for vapors of numerous hazardous compounds (including HCN, phosgene, nerve agents, and vesicant agent HD) have been published by the National Academy Press (NRC/COT 2000, 2002, 2003, 2004, 2007, 2008); others are in various stages of review. For each hazardous compound, guideline levels are developed for continuous vapor exposure durations of 10 and 30 min; 1, 4, and 8 h; as well as for three gradations of toxic effect severity. AEGL-1 concentrations are the mildest effect category, while AEGL-3 concentrations represent the most severe effect category (NRC/COT 2001).
In actual practice, and depending on the degree of conservatism incorporated during review of individual hazardous compounds subjected to the AEGL process, the AEGL concentration set for any given effect level (AEGL-1, 2, or 3) is protective and is often established at a concentration that is much less than the known, experimental concentrations at which such “tier” toxicological effects are observed. Such is the case for the CWAs and TICs evaluated in the present assessment (NRC/COT 2002, 2003; Watson et al. 2006a,b).
The organizations that develop, review, and publish AEGL values take no position on how compound-specific AEGL concentrations are to be applied (or not applied). The Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals developed by the USEPA National Advisory Committee and the Committee on Toxicology of the National Research Council (NRC/COT 2001) states: “Because of the complex nature of chemical accidents … and many other considerations related to a specific event, it is beyond the scope of this [Standing Operating Procedures] document to discuss or speculate on specific actions that should or could be taken at any point in time or to a given level of exposure to a specific chemical. However, emergency responders and planners know that various options are available, depending upon the circumstances, for reducing or even preventing the adverse impacts of chemical releases. … Decisions on these options are important and are best left to local emergency planners and responders to be addressed on a case-by-case basis.”
The utility of AEGL values for CWA emergency preparedness planning was further recognized when FEMA and U.S. Army representatives adopted final nerve and sulfur mustard agent AEGL concentrations to replace pre-2000 agent casualty estimators with updated toxicity criteria for emergency response decision-making in the Chemical Stockpile Emergency Preparedness Program (CSEPP 2003). This adoption is in furtherance of U.S. compliance with international treaty obligations under the Chemical Weapons Convention, which mandates destruction of the U.S. chemical weapons stockpile. Since February 2003, standing CSEPP policy guidance for each of the several communities currently hosting CWA demilitarization facilities in the U.S. recommends application of agent-specific AEGL-2 concentrations as the protective action level for evacuation or shelter-in-place decisions, AEGL-1 concentrations as notification levels with no protective action, and < AEGL-1 concentrations as requiring no protective action or notification (CSEPP 2003). Since publication of final AEGL levels by NRC/COT (2003) and enactment of the above CSEPP Policy Paper (CSEPP 2003), multiple stockpile host states, counties, and municipalities have incorporated the Policy Paper recommendations into their individual community emergency response plans and have employed them in making regulatory decisions permitting CWA munition disposal operations (CSEPP 2006a,b).
The LAX site-specific scenario incorporates many of the same elements as those included in CSEPP emergency preparedness scenarios (e.g., one-time release of limited duration, dispersion and dissipation consistent with known compound-specific chemical and physical characteristics). As a consequence, the LAX scenario supports consideration of short-term vapor exposure duration for pre-planning clearance goal selection. Analyses of short-term as well as routine-exposure scenarios suitable for a CW agent-processing facility are provided below for comparison and completeness.
Agent workers in CWA demilitarization facilities: routine operations and assumed potential daily exposures
The Centers for Disease Control and Prevention (CDC) (DHHS 1988, 2002, 2003, 2004) has published chronic industrial exposure guidelines for assumed daily exposures during multiple years of routine CWA demilitarization facility operations managed under the Chemical Stockpile Emergency Preparedness Program (CW agents GA, GB, VX, and sulfur mustard). Following similar logic, the Department of the Army, Office of the Surgeon General has developed chronic industrial exposure guidelines for nerve agents GD and GF (DA 2004). These “agent worker” exposure limits address exposure-specific scenarios tailored for application to trained and specialized CW agent workers outfitted with agent-protective equipment and employed within CSEPP demilitarization facilities. These agent exposure limits have not been authorized by the CDC for application to any other purpose or population.
Worker Population Limit (WPL)-a time-weighted average for a conventional 8-h workday and a 40-h week. The WPL represents a concentration to which it is believed that virtually all agent demilitarization facility workers may be repeatedly exposed, day after day, without adverse effect (DHHS 2002). The CDC assumed continuous exposure conditions equal to that of a stationary worker at the same duty station for the entire shift/work week/work year for multiple years, with fixed atmospheric stability, no ventilation rate change, and no agent degradation (DHHS 2002, 2003, 2004). The CDC acknowledges that these demilitarization facility personnel exposure assumptions are unrealistic. Current compound-specific WPL concentrations are 3 × 10−5 mg/m3 for GA, GB, GD, and GF (DHHS 2003; DA 2004), 1 × 10−6 mg/m3 for VX (DHHS 2003), and 4 × 10−4 mg/m3 for HD (DHHS 2004).
Short-Term Exposure Limit (STEL)-conventional 8-h workday and 40-h week “exposures greater than the WPL up to the STEL should not be longer than 15 min and should not occur more than 4 times per day, and there should be at least 60 min between successive exposures in this range” (DHHS 2002). The CDC considers agent VX to be a special case and advises that excursions to the VX STEL “should not occur more than once per day” (DHHS 2003). For STEL estimations, the CDC also applies the same continuous and unrealistic exposure assumptions as outlined above for the WPL (DHHS 2002, 2003, 2004). Current compound-specific STEL concentrations are 1 × 10−4 mg/m3 for GA and GB (DHHS 2003), 5 × 10−5 mg/m3 for GD and GF (DA 2004), 1 × 10−5 mg/m3 for VX (DHHS 2003), and 3 × 10−3 mg/m3 for HD (DHHS 2004).
Immediately Dangerous to Life or Health (IDLH)-the averaging time for IDLH concentrations is 30 min (DHHS 2003, 2004) and was established to bound the expected concentration from which chemical warfare agent demilitarization facility workers could safely escape within 30 min and without respiratory protection in the event of an emergency situation. Near-real-time monitoring is recommended. Current compound-specific IDLH concentrations are 0.1 mg/m3 for GA and GB (DHHS 2003), 0.05 mg/m3 for GD and GF (DA 2004), 0.003 mg/m3 for VX (DHHS 2003), and 0.7 mg/m3 for HD (DHHS 2004).
Exposure assumptions employed by the CDC during development of agent-specific WPLs and STELs were deliberately designed to guide routine work processes in chemical warfare agent demilitarization facilities or transport operations where large quantities of military-grade and undiluted CWA and CWA munitions are handled and processed daily. Under these specific occupational settings, the CDC assumed an existing potential for chronic exposures from a continuous source (e.g., the unitary munition stockpile which contains “leakers”) (DHHS 1988, 2002, 2003, 2004). As outlined above, the CDC exposure assumptions include 8 h/d, 5 d/wk, continuous CWA exposure for multiple years (e.g., the life of each agent-specific demilitarization operation), and are thus not realistic or in keeping with the airport remediation scenario under consideration (e.g., one-time only release followed by source removal and decontamination). As a consequence, the CDC WPL and STEL estimates are not recommended for use in evaluating airport site-specific populations such as airport employees and vendors; the post-decontamination airport is not comparable to an industrial chemical setting in which CWA processing takes place over an extended duration. Assumptions embedded in the IDLH derivation are considered appropriate for potential application to airport decontamination personnel.
Host communities for CWA demilitarization facilities: routine operations and assumed potential daily exposures
The CDC (DHHS 1988, 2002, 2003, 2004) has also published CWA chronic general population exposure limits (GPLs) for residents of host communities adjacent to operating CW agent demilitarization facilities. The GPLs are defined as “the maximum concentration to which members of the general population may be continually exposed 24 hr a day, 7 days per week,” for a continuous period of years without adverse health effects (GPL concentrations are 1 × 10−6 mg/m3 for GA, GB, GD, and GF; 6 × 10−7 mg/m3 for VX; and 2 × 10−5 mg/m3 for HD). The CDC further assumes that members of the host community general public are completely stationary for a period of years; that atmospheric stability, wind speed and direction are fixed; and that no agent diminution results from environmental degradation, rainfall dilution, and changes in meteorology (DHHS 2002, 2003, 2004). The CDC acknowledges that these exposure assumptions are unrealistic (DHHS 2002, 2003, 2004), and warns that these limits are not authorized for application to any other purpose or population other than the specific CWA unitary stockpile demilitarization mission. As a consequence, CDC GPL concentrations are not appropriate for application to any members of the airport scenario general public, including transit passengers, airport employees, vendors, and so on and are provided here for completeness.
Spring Valley Formerly Used Defense Site (SVFUDS; Washington, DC)
The Spring Valley Formerly Used Defense Site (SVFUDS) is the location of a WWI-era chemical munitions testing facility. Once remote, the SVFUDS has been surrounded by urban sprawl and is now located within the metropolitan District of Columbia (http://www.nab.usace.army.mil/projects/WashingtonDC/springvalley.htm). The SVFUDS area is surrounded by residential development that contains approximately 1500 homes, numerous embassies and official residences, medical facilities, portions of the American University campus, and commercial shops. The current phase of remediation action for buried WWI-era chemical munitions (largely 75 mm chemical projectiles containing either sulfur mustard agent or arsine) was initiated by the U.S. Army Corps of Engineers in 2007 and is ongoing in the midst of continuing host community life (Drogin 2010; Zongkar 2010). Civilian (USEPA Region III and the Government of the District of Columbia, including D.C. police and fire departments) and military authorities responsible for the Spring Valley site have determined that no protective action is required for exposures to vapor concentrations less than the 10-min AEGL-2 values for sulfur mustard and arsine (U.S. Army Corps of Engineers Baltimore District 2007; PARSONS 2007; DDESB 2008). These authorities consider the toxicity endpoints and uncertainty factors applied during development of arsine and sulfur mustard AEGL-2 concentrations to be protective (NRC/COT 2000, 2003; Watson et al. 2006a).
To bound SVFUDS work zones and develop contingency plans, Maximum Credible Event (MCE) analyses have been performed with the aid of plume dispersion models incorporating worst-case and site-specific meteorology and topography inputs. The Spring Valley MCE has focused on instantaneous release of the entire payload of a chemical projectile (e.g., 1.2 lb arsine or 1.35 lb sulfur mustard), with that entire payload present in the plume (U.S. Army Corps of Engineers Baltimore District 2007). The pace and nature of each day's work are determined by periodic calculations of downwind AEGL-2 distance for the specific agent, MCE, weather and task, and modified accordingly. Maximal downwind hazard distances under worst-case conditions at the Spring Valley site are 742 ft (arsine) and 240 ft (sulfur mustard) (U.S. Army Corps of Engineers Baltimore District 2007). The protective planning decision made by the U.S. Army Corps of Engineers in collaboration with their civilian-authority partners and stakeholders has been to apply the (greater) arsine-specific downwind hazard distance for operations involving either sulfur mustard or arsine munitions.
Interagency Modeling and Atmospheric Assessment Center (IMAAC)
Application of AEGL concentrations is incorporated into Federal dispersion modeling and hazard prediction products developed by the Interagency Modeling and Atmospheric Assessment Center (IMAAC) in generating atmospheric assessments and downwind hazard distance predictions for application during an Incident of National Significance (see links at https://narac.llnl.gov). The AEGL tier concentrations for G-series nerve agents, nerve agent VX and vesicant agent HD as well as several TICs are embedded within multiple atmospheric models maintained by IMAAC as consequence management tools for user training, support to the National Exercise Program (NEP) and specific exercises such as the TOPOFF series, as well as operational incident support for commercial hazard assessment (Dillon et al. 2004; Sugiyama and Nasstrom 2006). In addition, IMAAC tools and products are made available at National Special Security Events (NSSEs).
At present, the National Atmospheric Release Advisory Center (NARAC) at Lawrence Livermore National Laboratory (LLNL, Livermore, CA) is the IMAAC product provider.
National Institute for Occupational Safety and Health (NIOSH) Emergency Response Safety and Health Database (ERSHDB)
Acceptable criteria for “Green Zone” (Level D) declaration includes attainment of less than (compound-specific) AEGL-1 vapor concentrations. Level D is the minimal personal protective equipment (PPE) selection tier established by NIOSH and consists of “coveralls or other work clothes, boots, and gloves.” No respiratory protection is required at Level D, which can be declared “when the contaminant and concentration of the contaminant are known and the concentration is … less than” the AEGL-1 for the stated duration times” (www.cdc.gov/niosh/ershdb; last accessed January 24, 2011).
Military Missions
Other AEGL applications include use as hazard assessment plume modeling criteria for the U.S. Non-Stockpile Chemical Materiel Program and Homeland Defense scenarios, as detection performance goals for advanced equipment acquisition and development, and as a tool for assessing potential exposures during military missions, such as peacekeeping (Watson et al. 2006a,b; USACHPPM 2008b).
CONCLUSIONS
A national case study sponsored by the Department of Homeland Security in partnership with The Bradley International Terminal (TBIT) at the Los Angeles International Airport (LAX) has been performed to develop exposure guidance and pre-planning clearance concentrations for use in the event of a chemical terrorist incident.
The availability of pre-planning clearance concentrations for multiple exposure media is a key component to implementing effective and rapid remediation decision-making that safely minimizes down time and potential economic impact for a major airport. Since minimizing potential public and economic impact from chemical attack is an effective countermeasure to chemical terrorism, publication of these values and their logic for pre-planning application in advance of a release event facilitates such counter-terrorism activity.
Published together for the first time are descriptions of attack scenarios, characterization of potentially exposed populations, timelines of various post-event phases, risk assessment logic, assessment of threat compounds and their principal degradation products, characterization of compound persistence and decontamination, and precedents for utility and application to the attack scenario under consideration. In addition, a conceptual site model (CSM) is derived to characterize potential health risks of concern.
Chemical and physical properties of each threat compound (the CWA G-series nerve agents and nerve agent VX as well as the vesicant agent sulfur mustard; the TIC compounds phosgene, hydrogen cyanide, and cyanogen chloride) as well as principal CWA degradation products are summarized (Tables 2 and 3) to meet stakeholder needs for information access during remediation pre-planning. Toxicological characteristics are summarized in text sections outlining “CWA, TIC, and Degradation Product Characteristics.”
At further stakeholder request, realistic characterization of post-decontamination exposure durations was developed and presented from literature characterizing agent decontamination (Yang et al. 1994; Yang 1999; Wagner and Yang 2002), risk-based cleanup guidelines published by the California Environmental Protection Agency for the persistent compound methamphetamine (Salocks 2009; USEPA 2009a), and evaluation of recent experimental studies characterizing CWA liquid droplet persistence on four finish materials (Love et al. 2009; Love et al. in review) (Table 4; Figures 3-5, Figure 6).
These experimental data were collected in the absence of active decontamination and thus represent a worst case. CWA agent attenuation is greatest and most rapid from stainless steel and glass, while droplet spikes on the porous and permeable materials tested (vinyl tile and latex-painted wall board) displayed a longer interval between spike application and full attenuation. Precipitous concentration declines by GB and HD occurred within 50 h post-spike (Love et al. 2009; Love et al. in review). As expected, natural attenuation of liquid agent VX on these porous and permeable materials required a longer interval. It is clear that CWA concentrations continue to decline over time in all cases, and, with the exception of VX, the residual chemical exposure hazard posed by liquid droplet (aerosol) release of CWA may be realistically mitigated by natural attenuation within a 24-h period. Further, the physicochemical isolation of CWA in porous and polymeric materials serves to reduce available chemical hazard by physically limiting opportunities for potential primary and secondary exposure while preferentially sequestering agent.
It is also clear that many remediation technologies are specifically designed to convert persistent compounds to nonpersistent hazards. For example, hydrolysis half-times of the persistent nerve agent VX at alkaline pH range from 2 min to approximately 3 h; this degradation analysis is provided in Table 4 and plotted in Figure 6. There is no expectation that long-term exposures would occur with successful decontamination of the release, continued CWA and TIC degradation of any residual material over time via well-characterized reactions such as hydrolysis, and site removal of materials that cannot be effectively decontaminated. Thus, consideration of continuous and long-term exposure at some constant source concentration is not supported.
An accurate assessment of potential residual chemical hazard can be determined by application of a systematic logic involving consideration of the amount released as well as nature and extent of release, decontamination reaction chemistry, decontamination methods and procedures, degradation products, media properties and environmental parameters. These findings hold true for chemical warfare agents and toxic industrial compounds. When a persistent compound can be determined to pose a nonpersistent hazard, or a non-persistent compound is the chemical of concern, the logical and recommended focus for cleanup activities is to minimize acute hazards. Further, labor- and resource-intensive decontamination processes should be reserved for persistent chemical hazards only.
This logic is consistent with existing assessments developed by California Environmental Protection Agency to guide remediation of former clandestine methamphetamine production facilities, often characterized by hazardous levels of methamphetamine surface residues (and potential off-gas) posing a public health clean-up challenge (Salocks 2009).
While developed for a specific release scenario at a major airport, the logic and approaches presented for the nine threat compounds evaluated have merit for preplanning clearance goal development when considering other transportation hubs and chemicals of concern.
The following article (Part II in this series; “Developing Health-Based Pre-Planning Clearance Goals for Airport Terminal Remediation Following a Chemical Terrorist Attack: Decision Criteria for Multipathway Exposures”) incorporates the above findings as crucial components in developing specific decision criteria for multiple and likely exposure routes following chemical terrorist attack in a major airport. Models and first-time, open-literature documentation of multi-pathway, health-based remediation exposure guidelines for CWAs and TICs (and their degradation products) are presented for pre-planning applications and to facilitate airport remediation following such an attack.
ACKNOWLEDGMENTS
This work was prepared for the Chemical Restoration Operational Technology Demonstration Project of the U.S. Department of Homeland Security under U.S. Department of Energy (DOE) Interagency Agreement No. 2367-T146-06. The Oak Ridge National Laboratory (ORNL) of Oak Ridge, TN, is managed and operated by UT-Battelle, LLC, for the U.S. Department of Energy under Contract No. DE-AC05-00OR22725. This work has also been performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory, Livermore, CA, under Contract DE-AC52-07NA27344.
The authors and project sponsor thank and acknowledge Los Angeles World Airports (LAWA) for their leadership role in enabling the Los Angeles International Airport (LAX) to serve as a model facility for the Chemical Restoration Operational Technology Demonstration Project.
Participating national laboratories in the Chemical Restoration Operational Technology Demonstration Project include the following: Lawrence Livermore National Laboratory (LLNL; Livermore CA), Los Alamos National Laboratory (LANL; Los Alamos NM), Oak Ridge National Laboratory (ORNL; Oak Ridge TN), Pacific Northwest National Laboratory (PNNL; Richland WA), and Sandia National Laboratory (SNL; Albuquerque NM). Development of preplanning clearance goals for the LAX chemical terrorist release scenario has involved collaborative exchange among colleagues from each of these facilities.
The critical advice and technical review of Robert Young (DABT), Cheryl Bast (DABT), and Tim Borges (DABT) of the Environmental Sciences Division, Oak Ridge National Laboratory, are gratefully acknowledged.
The authors acknowledge the many insights regarding emergency response and preparedness in general, and the Chemical Stockpile Emergency Preparedness Program in particular, provided by John Sorensen and Barry Shumpert of the Environmental Sciences Division, Oak Ridge National Laboratory.
Dan Noble (Project Manager for Munition Recovery, U.S. Army Corps of Engineers, Baltimore District), Bruce Whisenant (U.S. Army Engineering and Support Center, U.S. Army Corps of Engineers, Huntsville, AL), and their supervisors and staff are gratefully acknowledged for providing ready and timely access to results, findings, and images from the Spring Valley Formerly Used Defense Site (SVFUDS) located within the District of Columbia.
The advice and counsel of Dr. Anthony Tu (Professor Emeritus, Department of Biochemistry and Molecular Biology, Colorado State University, Ft. Collins, CO) is thankfully acknowledged.
The authors acknowledge with appreciation the consistent and strong support provided by Ted Medley for developing and publishing compound-specific clearance decision criteria well in advance of chemical warfare agent or TIC release. For many years, Mr. Medley was the Training and Exercise Officer for the Chemical Stockpile Emergency Preparedness Planning Program within the State of Colorado Office of Emergency Management, from which he communicated the high utility of such decision criteria to state and local authorities during remediation pre-planning as well as during post-release decision-making. These papers were prepared in furtherance of the training mission long advocated by Mr. Medley.
AUTHOR-DIRECTED PEER REVIEW
This manuscript was prepared under HERA's author-directed peer review process, wherein a manuscript's authors nominate proposed peer reviewers to HERA's Managing Editor for approval or revision. The following persons reviewed and approved the publication of this manuscript:
Dr. Barbara G. Callahan, Senior Toxicologist, University Research Engineers & Associates, Grantham, NH;
Dr. Martin Clauberg, Dr. Clauberg Consulting, Lenoir City, TN and Solingen, Germany;
Dr. John Doull, Professor Emeritus of Pharmacology and Toxicology, University of Kansas Medical School, Kansas City, KS;
Dr. James L. Regens, Center for Biosecurity Research, University of Oklahoma, Oklahoma City, OK.
ABBREVIATIONS
- AC
hydrogen cyanide; NATO code
- ACGIH
American Conference of Governmental Industrial Hygienists
- AEGLs
Acute Exposure Guideline Levels
- AIHA
American Industrial Hygiene Association
- ASTM
American Society for Testing and Material, International
- ATSDR
Agency for Toxic Substances and Disease Registry of the DHHS
- CAM
Center for Airport Management
- CDC
Centers for Disease Control and Prevention of the DHHS
- CG
phosgene; NATO code
- CK
cyanogen chloride; NATO code
- CMA
U.S. Army Chemical Materials Agency
- CNS
central nervous system
- COPC
Contaminants of Potential Concern
- COT
Committee on Toxicology of the National Research Council
- CSEPP
Chemical Stockpile Emergency Preparedness Program; joint
- FEMA/DA
organization
- CSM
conceptual site model
- CWA
chemical warfare agent
- DA
U.S. Department of the Army
- DABT
Diplomate, American Board of Toxicology
- DDESB
Department of Defense Explosives Safety Board
- DHHS
U.S. Department of Health and Human Services
- DHS
U.S. Department of Homeland Security
- DNA
deoxyribonucleic acid
- DOE
U.S. Department of Energy
- EA2192
S-(diisopropylaminoethyl) methylphosphonothioic acid, C9H22NPO2S
- ECBC
Edgewood Chemical Biological Center
- EMPA
ethyl methylphosphonic acid
- EOC
Emergency Operations Center
- ERSHDB
Emergency Response Safety and health Database of the NIOSH
- FEMA
Federal Emergency Management Agency of the USDHS
- GA
nerve agent tabun; NATO code
- GB
nerve agent sarin; NATO code
- GD
nerve agent soman; NATO code
- GF
nerve agent cyclosarin; NATO code
- GPL
general population limit
- HD
vesicant agent distilled sulfur mustard; NATO code
- HSDB
Hazardous Substances Data Bank
- HVAC
heating, ventilating and air conditioning
- IDLH
immediately dangerous to life or health
- IMAAC
Interagency Modeling and Atmospheric Assessment Center
- IMPA
isopropyl methylphosphonic acid
- IOM
Institute of Medicine of the National Academy of Sciences
- IRIS
Integrated Risk Information System of the USEPA
- LANL
Los Alamos National Laboratory, Los Alamos NM
- LAWA
Los Angeles World Airports
- LAX
Los Angeles International Airport
- LD50
median Lethal Dose
- LLNL
Lawrence Livermore National Laboratory, Livermore CA
- MCE
maximum credible event
- MPA
methyl phosphonic acid; CH5O3P
- NARAC
National Atmospheric Release Advisory Center
- NATO
North Atlantic Treaty Organization
- NEP
National Exercise Program
- NIOSH
National Institute for Occupational Safety and Health
- NRC
National Research Council
- NSSE
National Special Security Events
- OASA
Office of the Assistant Secretary of the Army
- OEHHA
Office of Environmental Health Hazard Assessment of the CalEPA
- OPPTS
Office of Prevention, Pesticides and Toxic Substances of the USEPA
- ORNL
Oak Ridge National Laboratory, Oak Ridge TN
- PNNL
Pacific Northwest National Laboratory, Richland WA
- PPE
Personal Protective Equipment
- RMP
risk management plan
- RNA
ribonucleic acid
- RSLs
Regional Screening Levels of the USEPA
- SC
South Carolina
- SNL
Sandia National Laboratory, Albuquerque NM
- SRCL
surface removal contaminant levels
- STEL
short-term exposure limit
- SVFUDS
Spring Valley Formerly Used Defense Site
- TBIT
The Bradley International Terminal of the Los Angeles International Airport
- TDG
thiodiglycol
- TICs
toxic industrial compounds
- USACHPPM
U.S. Army Center for Health Promotion and Preventive Medicine
- USEPA
U.S. Environmental Protection Agency
- VX
nerve agent; NATO code
- WPL
worker population limit
- WWI
World War I
- WWII
World War II
Footnotes
The views expressed in this article do not necessarily represent official Federal agency position or policy. Mention of trade names or commercial products does not constitute endorsement or recommendation of use.
References
- Aaron HS. Potential Hazards in the Handling of Aged AC and CK Munitions: A Literature Review. 1996. ERDEC-SP-039. Edgewood Research and Development Engineering Center, Aberdeen Proving Ground, MD, USA.
- ACGIH (American Conference of Governmental Industrial Hygienists) TLVs and BEIs, Based on the Documentation of the Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices (Cyanogen Chloride, p 24. 2003. Hydrogen Cyanide, p 35; Phosgene, p 47). American Conference of Governmental Industrial Hygienists, Cincinnati, OH, USA.
- ACGIH. TLVs and BEIs, Based on the Documentation of the Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices. 2008. American Conference of Governmental Industrial Hygienists, Cincinnati, OH, USA.
- AIHA (American Industrial Hygiene Association) Emergency Response Planning Guideline: Hydrogen Cyanide. 2700 Prosperity Avenue, Suite 250, Fairfax, VA, USA: American Industrial Hygiene Association; 1994. [Google Scholar]
- AIHA. Emergency Response Planning Guidelines: Cyanogen Chloride. 2700 Prosperity Avenue, Suite 250, Fairfax, VA, USA: AIHA Press, American Industrial Hygiene Association; 1998. [Google Scholar]
- AIHA; American Industrial Hygiene Association. Emergency Response Planning Guidelines: Phosgene. 2700 Prosperity Avenue, Suite 250, Fairfax, VA, USA: AIHA Press, American Industrial Hygiene Association; 2002. [Google Scholar]
- AIHA. Emergency Response Planning Guidelines and Workplace Environmental Exposure Level Guides (Cyanogen Chloride. 2007. p. 22. Hydrogen Cyanide, p 23; Phosgene, p 24). American Industrial Hygiene Association, 2700 Prosperity Avenue, Suite 250, Fairfax, VA, USA.
- ASAE (American Society of Agricultural Engineers) Spray Nozzle Classification by Droplet Spectra. Standard No. S572.1. 2950 Niles Road, St. Joseph, MI, USA: American Society of Agricultural and Biological Engineers; 2009. [Google Scholar]
- ASTM (American Society for Testing and Materials, International) Standard Guide for Environmental Health Site Assessment Process for Military Deployments. 2003. Standard No. E 2318-03. ASTM Committee E50 on Environmental Assessment, Risk Management and Corrective Action. ASTM International, 100 Barr Harbor Drive, West Conshohocken, PA, USA.
- ASTM. Standard Guide for Developing Conceptual Site Models for Contaminated Sites. 2008. Standard No. E 1689-95 (2008). ASTM Committee E47.05 on Risk Assessment, Communications and Management. ASTM International, 100 Barr Harbor Drive, West Conshohocken, PA, USA.
- ATSDR (Agency for Toxic Substances and Disease Registry) Toxicological Profile for Sulfur Mustard (Update) Atlanta, GA, USA: US Department of Health and Human Services, Public Health Service; 2003. [Google Scholar]
- ATSDR. Medical Management Guidelines (MMGs) for Hydrogen Cyanide (HCN) Atlanta, GA: US Department of Health and Human Services, Public Health Service; 2005. Available at http://www.atsdr.cdc.gov/MHMI/index.asp. [Google Scholar]
- ATSDR. Toxicological Profile for Cyanide (Update) Atlanta, GA, USA: US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry; 2006. [PubMed] [Google Scholar]
- Review of the Army's health risk assessments for oral exposure to six chemical-warfare agents. J Toxicol Environ Health Part A. 2000;59:281–526. Bakshi KS, Pang SNJ, and Snyder R (Eds.) [PubMed] [Google Scholar]
- Bast C, Glass D. Phosgene. In: Gupta R, editor. Handbook of Toxicology of Chemical Warfare Agents. Amsterdam, The Netherlands: Elsevier Publishers, Academic Press; 2009. pp. 321–30. [Google Scholar]
- Benton BJ, Sommerville DR, Anthony S, et al. Low-level effects of VX vapor exposure on pupil size and cholinesterase levels in rats. In: Salem HA, Katz SA, editors. Inhalation Toxicology. 2nd edit. Boca Raton, FL, USA: CRC Press, Taylor and Francis; 2006a. pp. 91–108. [Google Scholar]
- Benton BJ, McGuire JM, Sommerville DR, et al. Effects of whole-body VX vapor exposure on lethality in rats. Inhalation Toxicol. 2006b;18:1091–9. doi: 10.1080/08958370600945598. [DOI] [PubMed] [Google Scholar]
- Black RM, Clarke RJ, Cooper DB, et al. Application of headspace analysis, solvent extraction, thermal desorption and gas chromatography-mass spectrometry to the analysis of chemical warfare samples containing sulphur mustard and related compounds. J Chromatogr. 1993;637:71–80. [Google Scholar]
- Blewett WK. Tactical weapons: Is mustard still king? NBC Defense Technol Int. 1986;1:64–6. [Google Scholar]
- CAM (Center for Airport Management) 2005 On-Airport Intercept Survey. 2005. Management Report prepared for Los Angeles International Airport by The Center for Airport Management in partnership with GC Tech, Inc. Los Angeles, CA, USA (October 26, 2005)
- Capacio BR, Smith JR, Gordon RK, et al. Clinical detection of exposure to chemical warfare agents. In: Romano JA, Lukey BJ, Salem H, editors. Chemical Warfare Agents: Chemistry, Pharmacology, Toxicology and Therapeutics. 2nd edit. Boca Raton, FL, USA: CRC Press; 2008. pp. 501–48. [Google Scholar]
- Carnes SA. Disposing of the chemical weapons: A desired end in search of an acceptable means. Environ Prof. 1989;11:279–90. [Google Scholar]
- Clark DN. Review of Reactions of Chemical Agents in Water. 1989. Report ADA 213287. Final Report to US Army Biomedical Research and Development Laboratory. Defense Technical Information Center, Ft. Belvoir, VA, USA.
- CMA (US Army Chemical Materials Agency) Veolia ceremony marks end of Newport hydrolysate destruction. 2009. CMA News (February 2009). Public Affairs Office, Aberdeen Proving Ground, MD, USA. Available at www.cma.army.mil.
- Columbia University Press. Tabun. The Columbia Encyclopedia. 2001-2007. 6th edit, New York, NY, USA. Available at www.bartleby.com/65/ (accessed February 19, 2009)
- Crothers R, Boyle M, Dickerson T, et al. The AFP Investigation into Japanese Sect Activities in Western Australia. GPO Box 2955, Canberra ACT 2601, Australia: Australian Institute of Criminology; 2008. [Google Scholar]
- CSEPP (Chemical Stockpile Emergency Preparedness Program) Adoption of Acute Exposure Guideline Levels (AEGLs) 2003. Policy Paper No. 20 (revised). Dept. of the Army and Federal Emergency Management Agency, Chemical Stockpile Emergency Preparedness Program. Federal Emergency Management Agency, Dept Homeland Security, Washington, DC, USA (February 2003)
- CSEPP. CSEPP Planning Guidance. 2006a. IEM/TEC05-05. CSEPP Planning Guidance Steering Committee, Dept. of the Army and Department of Homeland Security, Chemical Stockpile Emergency Preparedness Program. Federal Emergency Management Agency, Dept Homeland Security, Washington, DC, USA (March 2006)
- CSEPP. CSEPP Programmatic Guidance. IEM/TEC05-05. 2006b. CSEPP Planning Guidance Steering Committee, Dept. of the Army and Department of Homeland Security, Chemical Stockpile Emergency Preparedness Program. Federal Emergency Management Agency, Dept Homeland Security, Washington, DC, USA (March 2006).
- DA (US Department of the Army) Detailed and General Facts About Chemical Agents. Technical Guide TG 218. MD, USA: U. S. Department of the Army, US Army Center for Health Promotion and Preventive Medicine, Aberdeen Proving Ground; 1996. [Google Scholar]
- DA. NBC Decontamination. Army Field Manual FM 3-5; Marine Corps Warfighting Publication MCWP 3-37.3. Washington, DC, USA: Headquarters, Department of the Army; Commandant, US Marine Corps; 2002. [Google Scholar]
- DA. Nerve Agent Percutaneous Exposure Criteria and Airborne Exposure Levels (AELs) for GD, GF for Use in Interim DA Guidance on Implementation of the New AELs. 5109 Leesburg Pike, Falls Church, VA, USA: Memorandum, Office of the Surgeon General; 2004. (June 29, 2004) [Google Scholar]
- DA. Potential Military Chemical/Biological Agents and Compounds. Field Manual FM 3-11.9. 2005a. Army, Marine Corps, Navy, Air Force Multiservice Tactics, Techniques and Procedures. Commandant, US Army Chemical School, Ft. Leonard Wood, MO, USA (Approved for public release, distribution unlimited).
- DA. Sanitary Control and Surveillance of Field Water Supplies. 2005b. Technical Bulletin TB-MED 577. US Department of the Army Headquarters, Washington, DC, USA.
- DA. Toxic Chemical Agent Safety Standards. 2008. DA Pam 385-61. US Department of the Army, Headquarters, The Pentagon, Washington, DC, USA.
- Dabisch PA, Horsmon MS, Taylor JT, et al. Gender differences in the miotic potency of soman vapor in rats. Cutan Ocul Toxicol. 2008a;27:123–33. doi: 10.1080/15569520802064376. [DOI] [PubMed] [Google Scholar]
- Dabisch PA, Hulet SW, Kristovich R, et al. Inhalation toxicology of nerve agents. In: Romano JA Jr., Lukey BJ, Salem H, editors. Chemical Warfare Agents: Chemistry, Pharmacology, Toxicology and Therapeutics. Boca Raton, FL, USA: CRC Press; 2008b. pp. 233–46. [Google Scholar]
- DDESB (Department of Defense Explosives Safety Board) DDESB Final Approval of Request for Approval, Amendment 2, dated January 4, 2008, Chemical Safety Submission (CSS) for the Spring Valley Formerly Used Defense Site (FUDS) 2008. Washington D.C. Memorandum for Director, US Army Defense Ammunition Center. Signed by Curtis M. Bowling, Chairman, DDESB. Department of Defense Explosives Safety Board, 2461 Eisenhower Ave, Alexandria, VA, USA (January 18, 2008)
- DHHS (US Department of Health and Human Services) Final Recommendations for Protecting the Health and Safety Against Potential Adverse Effects of Long-Term Exposure to Low Doses of Agents GA, GB, VX, Mustard Agent (H, HD, T), and Lewisite (L) Centers for Disease Control and Prevention, Atlanta, GA, USA. Fed Reg. 1988;53:8504–7. [Google Scholar]
- DHHS. Airborne Exposure Limits for Chemical Warfare Agents GA (Tabun), GB (Sarin), and VX. Centers for Disease Control and Prevention, Atlanta, GA, USA. Fed Reg. 2002;67((15)):894–901. [Google Scholar]
- DHHS. Final Recommendations for Protecting Human Health from Potential Adverse Effects of Exposure to Agents GA, GB and VX. Centers for Disease Control and Prevention, Atlanta, GA, USA Fed Reg. 2003;68((196)):58348–51. [Google Scholar]
- DHHS. Interim Recommendations for Airborne Exposure Limits for Chemical Warfare Agents H and HD (Sulfur Mustard) Centers for Disease Control and Prevention, Atlanta, GA, USA. Fed Reg. 2004;69((85)):24164–8. [Google Scholar]
- DHS (US Department of Homeland Security) and EPA (US Environmental Protection Agency) Remediation Guidance for Major Airports after a Bioterrorist Attack. Washington, DC, USA: US Department of Homeland Security; 2008. [Google Scholar]
- DHS. Remediation Guidance for Major Airports after a Chemical Attack. 2009a. LLNL-TR-408173-DRAFT. Prepared by Lawrence Livermore National Laboratory for US Department of Homeland Security, Washington, DC, USA.
- DHS. Proceedings of the Chemical Restoration Operational Technology Demonstration Project CWA Remediation Table-Top Exercise. 2009b. LLNL-PROC-412262. Prepared by Lawrence Livermore National Laboratory for US Department of Homeland Security, Washington, DC, USA Hum. Ecol. Risk Assess. Vol. 17, No. 1, 2011.
- Diller W. Medical phosgene problems and their possible solution. J Occup Med. 1978;20:189–93. doi: 10.1097/00043764-197803000-00007. [DOI] [PubMed] [Google Scholar]
- Dillon MB, Baskett RL, Foster KT, et al. The NARAC Emergency Response Guide to Initial Airborne Hazard Estimates. 2004. UCRL-TM-202990. University of California, Lawrence Livermore National Laboratory, Technical Information Department, Livermore, CA, USA (unlimited distribution).
- Drinker P. Hydrocyanic acid gas poisoning by absorption through the skin. J Ind Hyg. 1932;14:1–2. (as cited in ATSDR 2006) [Google Scholar]
- Drogin B. Digging up chemical weapons in D.C. Los Angeles Times. 2010. May 9, 2010.
- Dunn MA. The chemical war: Iran revisited1986. NBC Defense Technol Int. 1986;1:32–9. [Google Scholar]
- Dunn MA, Sidell FR. Progress in the medical defense against nerve agents. J Amer Med Assoc. 1989;262:649–52. [PubMed] [Google Scholar]
- ECBC (Edgewood Chemical Biological Center) Material Safety Data Sheets (for agents GA, GB, GD, GF, VX and HD) Aberdeen Proving Ground, MD, USA: US Army Edgewood Chemical Biological Center, ATTN: AMSRD-ECB-PI-CB-RR, US Army Research Development and Engineering Command; 2008. Available at http://www.edgewood.army.mil/ps/svcs_safety_surety.htm. [Google Scholar]
- Ember LR. Tokyo subway attack: Chemical weapon possible terrorist tool. 1995. pp. 6–7. Chem &Engr News (March 27):
- Frosolono M, Pawlowski R. Effect of phosgene on rat lungs after single high-level exposure. Arch Environ Health. 1977;32:271–7. doi: 10.1080/00039896.1977.10667294. [DOI] [PubMed] [Google Scholar]
- Gosselin RE, Smith RP, Hodge HC, et al. Clinical Toxicology of Commercial Products. 5th edit. Baltimore MD, USA: Williams and Wilkins; 1984. [Google Scholar]
- Hamner NE. Corrosion Data Survey (Metals Section), 5th edit. 1440 South Creek Drive, Houston, TX, USA: National Assoc. of Corrosion Engineers; 1974. [Google Scholar]
- Hauschild V, Bratt G. Prioritizing industrial chemical hazards. J Toxicol Envir Health (Part A) 2005;68:857–76. doi: 10.1080/15287390590912162. [DOI] [PubMed] [Google Scholar]
- Hauschild V, Lee A. Assessing chemical exposures during military deployments. J Mil Med. 2004;169:142–6. doi: 10.7205/milmed.169.2.142. [DOI] [PubMed] [Google Scholar]
- Hay A, Roberts G. The use of poison gas against the Iraqi Kurds: Analysis of bomb fragments, soil, and wool samples. JAMA. 1990;263:1065. doi: 10.1001/jama.263.8.1065a. [DOI] [PubMed] [Google Scholar]
- Ho P, Tucker MD, Smith W. Decontamination Technologies for Building Restoration. 2006. Sandia Report SAND2006-6580. Sandia National Laboratories, Albuquerque, NM, USA (Official Use Only)
- Howard PH, Meylan WM, editors. Handbook of Physical Properties of Organic Chemicals. Boca Raton, FL, USA: CRC Press, Inc. Lewis Publishers; 1997. [Google Scholar]
- HSDB (Hazardous Substances Data Bank) US National Library of Medicine. 2008. Bethesda, MD, USA Available at www.toxnet.nlm.nih.gov/cgi-bin/sis/search (accessed August 2008).
- IOM (Institute of Medicine) Veterans at Risk: The Health Effects oF Mustard Gas and Lewisite. In: Pechura CM, Rall DP, editors. Committee to Survey the Health Effects of Mustard Gas and Lewisite, Division of Health Promotion and Disease Prevention, Institute of Medicine. Washington, DC, USA: National Academy Press; 1993. [PubMed] [Google Scholar]
- Kingery AF, Allen HE. The environmental fate of organophosphorous nerve agents: A review. Toxicol Environ Chem. 1995;47:155–84. [Google Scholar]
- Klaassen CD, Amdur MO, Doull J, editors. Casarett and Doull's Toxicology: The Basic Science of Poisons, 3rd edit. New York, NY, USA: Macmillan Publishing Company; 1986. [Google Scholar]
- Komiya T, Kamakura H. First Worldwide Conference on Strengthening the Fire and Emergency Response to Terrorism. Berryville, VA, USA: Mt. Weather Emergency Assistance Center; 1995. The rescue operations and EMS activities after the poison gas terror. (November 6-9, 1995), pp 1-12. Federal Emergency Management Agency, US Dept Homeland Security, Washington, DC, USA. [Google Scholar]
- Lewis RJ Sr. Hawley's Condensed Chemical Dictionary. 13th edit. New York, NY, USA: John Wiley and Sons, Inc.; 1997. Methyl phosphonic acid; p. 747. [Google Scholar]
- Lewis RJ Sr. Sax's Dangerous Properties of Industrial Materials, 10th edit. New York, NY, USA: John Wiley and Sons, Inc.; 2000. [Google Scholar]
- Lillibridge S. Trip Report: United States Medical Delegation to Japan. 1995. From: SR Lillibridge, Acting Chief, Disaster Assessment and Epidemiology Section, to RJ Jackson, Director, National Center for Environmental Health, Centers for Disease Control and Protection, Dept. of Health and Human Services, Atlanta, GA, USA (April 25, 1995)
- Lipsett MJ, Shusterman D, Beard R. Phosgene. In: Clayton G, Clayton F, editors. Industrial Hygiene and Toxicology, 4th edit, vol II, Part F. New York, NY, USA: John Wiley and Sons Inc.; 1994. pp. 4557–63. (as cited in NRC/COT 2002) [Google Scholar]
- Love AH, Hanna ML, Koester CJ, et al. Understanding CWA interactions and dynamics with indoor surfaces. 2009. Proceedings of 2009 Annual Chemical and Biological Technologies Conference, January 26-29, 2009, Houston TX. US Department of Homeland Security, Washington, DC, USA.
- Love AH, Hanna ML, Hart BR, et al. Understanding CWA persistence on indoor surfaces. Envir Sci and Technol (in review)
- MacNaughton MG, Brewer JH. Environmental Chemistry and Fate of Chemical Warfare Agents. 1994. Final Report. SWRI Project 01-5864. Southwest Research Institute, San Antonio, TX, USA.
- Michel HO, Epstein J, Plapinger RR, et al. EA 2192: A Novel Anticholinesterase. 1962. CRDLR 3135. US Army Chemical and Research Laboratories, Army Chemical Center, Aberdeen Proving Ground, MD, USA.
- Mitchell J, Edmonds A, Cutter S, et al. Evacuation Behavior in Response to the Graniteville, South Carolina, Chlorine Spill. 2005. Quick Response Research Report 178. Hazards Research Lab, Department of Geography and Department of Journalism and Mass Communication, University of South Carolina, Columbia, SC, USA.
- Munro NB, Ambrose KR, Watson AP. Toxicity of the organophosphate chemical warfare agents GA, GB, and VX: Implications for public protection. Environ Health Perspect. 1994;102:18–38. doi: 10.1289/ehp.9410218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro NB, Talmage SS, Griffin GD, et al. The sources, fate and toxicity of chemical warfare agent degradation products. Environ Health Perspect. 1999;107:933–74. doi: 10.1289/ehp.99107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NFPA (National Fire Protection Association) Hazardous Chemicals Data. NFPA 49. Quincy, MA, USA: National Fire Protection Association; 1994. [Google Scholar]
- NIOSH (National Institute for Occupational Safety and Health) Pocket Guide to Chemical Hazards (Hydrogen Cyanide. 2008a. available at www.cdc.gov/niosh/npg/npgd0333. html; Cyanogen Chloride, available at www.cdc.gov/niosh/npg/npgd0162.html; Phosgene, available at www.cdc.gov/niosh/npg/npgd0504.html). US Department of Health and Human Services, Public Health Service, CDC, NIOSH, Washington, DC, USA.
- NIOSH. NIOSH Emergency Response Pocket Card: Hydrogen Cyanide. 2008b. (Available at http://www.bt.cdc.gov/agent/cyanide/erc74-90-8pr.asp) and Cyanogen chloride (Available at http://www.bt.cdc.gov/agent/cyanide/erc506-77-4pr.asp). US Department of Health and Human Services, Public Health Service, CDC, NIOSH, Washington, DC, USA.
- NIOSH/CDC [National Institute for Occupational Safety and Health (NIOSH) and Centers for Disease Control and Prevention (CDC) ] Emergency Response Safety and Health Database. 2008. Available at www.cdc.gov/NIOSH/ershdb/ (last accessed January 24, 2011).
- NRC/COT (National Research Council, Committee on Toxicology) Review of Acute Human-Toxicity Estimates for Selected Chemical-Warfare Agents. Washington, DC, USA: COT Subcommittee on Toxicity Values for Selected Nerve and Vesicant Agents, Committee on Toxicology. National Academy Press; 1997. Available at www.nap.edu Hum. Ecol. Risk Assess. Vol. 17, No. 1, 2011. [PubMed] [Google Scholar]
- NRC/COT. Review of the U.S. Army's Health Risk Assessment for Oral Exposure to Six Chemical Warfare Agents. Washington, DC, USA: COT Subcommittee on Chronic Reference Dose for Chemical Warfare Agents, Committee on Toxicology. National Academy Press; 1999. Available at www.nap.edu. [Google Scholar]
- NRC/COT. Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol 1. Washington, DC, USA: Subcommittee on Acute Exposure Guideline Levels, Committee on Toxicology. National Academy Press; 2000. Arsine; pp. 65–112. Available at www.nap.edu. [Google Scholar]
- NRC/COT. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC, USA: Subcommittee on Acute Exposure Guideline Levels, Committee on Toxicology. National Academy Press; 2001. Available at www.nap.edu. [PubMed] [Google Scholar]
- NRC/COT. Phosgene; Hydrogen Cyanide; Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol 2; pp. 211–276. respectively Acute Exposure Guideline Levels for Selected Airborne Chemicals 2002 Washington, DC, USA The National Academies Press, 15-70 Available at www.nap.edu. [Google Scholar]
- NRC/COT. Nerve Agents; Sulfur Mustard. Acute Exposure Guideline Levels for Selected Airborne Chemicals. 2003;3:301–383. respectively Washington, DC, USA Subcommittee on Acute Exposure Guideline Levels, Committee on Toxicology. The National Academies Press 15-300 Available at www.nap.edu. [Google Scholar]
- NRC/COT. Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol 4. Washington, DC, USA: Subcommittee on Acute Exposure Guideline Levels, Committee on Toxicology. The National Academies Press; 2004. Available at www.nap.edu. [PubMed] [Google Scholar]
- NRC/COT. Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol Subcommittee on Acute Exposure Guideline Levels. Washington, DC, USA: Committee on Toxicology. The National Academies Press; 2007. Available at www.nap.edu. [Google Scholar]
- NRC/COT. Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol Subcommittee on Acute Exposure Guideline Levels. Washington, DC, USA: Committee on Toxicology. The National Academies Press; 2008. Available at www.nap.edu. [Google Scholar]
- OASA (Office of the Assistant Secretary of the Army) Derivation of Health-Based Environmental Screening Levels (HBESL) for Chemical Warfare Agents. 1999. Memorandum signed by Raymond J. Fatz, Deputy Assistant Secretary of the Army, May 28, 1999. Department of the Army, Office of the Assistant Secretary (Environment, Safety, and Occupational Health), Army Pentagon, Washington, DC, USA.
- Okumura T, Takasu N, Ishimatsu S, et al. Report on 640 victims of the Tokyo sarin attack. Ann Emerg Med. 1996;28:129–35. doi: 10.1016/s0196-0644(96)70052-5. [DOI] [PubMed] [Google Scholar]
- O'Neil MJ, Smith A, Heckelman PR, et al., editors. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th edit. Rahway, NJ, USA: Merck and Co., Inc.; 2001. [Google Scholar]
- Opresko D, Young R, Faust R, et al. Chemical warfare agents: Estimating oral reference doses. Rev Environ Contam Toxicol. 1998;156:1–183. doi: 10.1007/978-1-4612-1722-0_1. [DOI] [PubMed] [Google Scholar]
- Papirmeister B, Feister AJ, Robinson SI, et al. Medical Defense Against Mustard Gas: Toxic Mechanisms and Pharmacological Implications. Boca Raton, FL, USA: CRC Press; 1991. [Google Scholar]
- PARSONS. Site-Specific Work Plan for the Investigation of Burial Pit 3, 4825 Glenbrook Road. 2007. Spring Valley Formerly Used Defense Site (SVFUDS). Prepared for US Army Engineering and Support Center Huntsville and US Army Corps of Engineers Baltimore District. PARSONS, Fairfax, VA, USA.
- Puzderliski A. The persistence of sarin and yperite drops in soil. Naucno-Teh Pregl. 1980;30:47–54. [Google Scholar]
- Raber E, Kirvel RD. Health risk assessment for radiological, chemical, and biological attacks. In: Voeller JG, editor. Wiley Handbook of Science and Technology for Homeland Security. New York, NY, USA: John Wiley and Sons, Inc.; 2008. pp. 1–24. [Google Scholar]
- Raber E, Jin A, Noonan K, et al. Decontamination issues for chemical and biological warfare agents: How clean is clean enough? Int J Env Health Res. 2001;11:128–48. doi: 10.1080/09603120020047519. [DOI] [PubMed] [Google Scholar]
- Raber E, Hirabayashi JM, Mancieri SP, et al. Chemical and biological agent incident response and decision process for civilian and public sector facilities. Risk Analysis. 2002;22:195–202. doi: 10.1111/0272-4332.00026. [DOI] [PubMed] [Google Scholar]
- Raber E, Carlsen T, Kirvel R. Remediation following chemical and biological attacks. In: Maurer SM, editor. WMD Terrorism: Science and Policy Choices. Cambridge, MA, USA: The MIT Press, Massachusetts Institute of Technology; 2009. pp. 365–88. [Google Scholar]
- Reddy G, Major MA, Leach GJ. Toxicity assessment of thiodiglycol. Int J Toxicol. 2005;24:435–42. doi: 10.1080/10915810500368878. [DOI] [PubMed] [Google Scholar]
- Robinson JP. Chemical warfare. Sci J. 1967;4:33–40. [Google Scholar]
- Rosenblatt DH, Small MJ, Kimmell TA, et al. Agent Decontamination Chemistry Technical Report. Phase 1. 1995. Prepared for Environmental Quality Office, US Army Test and Evaluation Command. Argonne National Laboratory, Argonne, IL, USA (June 1995)
- Sage GW, Howard PH. Environmental Fate Assessments of Chemical Agents HD and VX. 1989. CRDEC-CR-034. US Army Chemical Research Development and Engineering Center, Aberdeen Proving Ground, MD, USA.
- Salocks CB. Assessment of Children's Exposure to Surface Methamphetamine Residues in Former Clandestine Methamphetamine Labs, and Identification of a Risk-Based Cleanup Standard for Surface Methamphetamine Contamination. 2007. External Peer Review Draft. Integrated Risk Assessment Branch, Office of Environmental Health Hazard Assessment, California Environmental Protection Agency, Sacramento, CA, USA. (December 2007). Available at http://www.oehha.ca.gov/public_info/public/kids/
- Salocks CB. Assessment of Children's Exposure to Surface Methamphetamine Residues in Former Clandestine Methamphetamine Labs, and Identification of a Risk-Based Cleanup Standard for Surface Methamphetamine Contamination, Revised Draft. 2008. Integrated Risk Assessment Branch, Office of Environmental Health Hazard Assessment, California Environmental Protection Agency, Sacramento, CA, USA (December 2008). Available at http://www.oehha.ca.gov/public_info/public/kids/
- Salocks CB. Assessment of Children's Exposure to Surface Methamphetamine Residues in Former Clandestine Methamphetamine Labs, and Identification of a Risk-Based Cleanup Standard for Surface Methamphetamine Contamination. Sacramento, CA, USA: Integrated Risk Assessment Branch, Office of Environmental Health Hazard Assessment, California Environmental Protection Agency; 2009. (February 2009). Available at http://www.oehha.ca.gov/public_info/public/kids/ [Google Scholar]
- Sass S, Fisher TL, Stutz MH, et al. Analysis of Environmental Samples from Area of Sheep Deaths Near Dugway Proving Ground (U). EASP 100-69. Edgewood Arsenal, MD, USA: US Department of the Army, Chemical Research Laboratory; 1970. [Google Scholar]
- Saxena A, Sun W, Dabisch PA, et al. Efficacy of human serum butyrylcholinesterase against sarin vapor. Chemico-Biological Interactions. 2008;175:267–72. doi: 10.1016/j.cbi.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Sidell FR. Clinical considerations in nerve agent intoxication. In: Somani SM, editor. Chemical Warfare Agents. New York, NY, USA: Academic Press, Inc.; 1992. pp. 155–94. [Google Scholar]
- Sidell FR. Nerve Agents. In: Sidell FR, Takafuji ET, Franz DR, editors. Medical Aspects of Chemical and Biological Warfare. Washington, DC, USA: Office of the Surgeon General, Walter Reed Army Medical Center; 1997. pp. 129–79. [Google Scholar]
- Sidell FR, Groff WA. The reactivatibility of cholinesterase inhibited by VX and sarin in man. Toxicol Appl Pharmacol. 1974;27:241–52. doi: 10.1016/0041-008x(74)90195-1. [DOI] [PubMed] [Google Scholar]
- Small MJ. Compounds Formed from the Chemical Decontamination of HD, GB, and VX and their Environmental Fate. 1984. Technical Report 8304 (AD-A149 515). US Army Medical Bioengineering Research and Development Laboratory, Ft. Detrick, Frederick, MD, USA.
- Somani SM, Solana RP, Dube SN. Toxicodynamics of nerve agents. In: Somani SM, editor. Chemical Warfare Agents. New York, NY, USA: Academic Press, Inc.; 1992. pp. 67–123. [Google Scholar]
- Sugiyama G, Nasstrom JS. Overview of LLNL services and tools for the National Atmospheric Release Advisory Center (NARAC) and Interagency Modeling and Atmospheric Assessment Center (IMAAC). UCRL-PRES-223394-Rev1. Livermore, CA, USA: University of California, Lawrence Livermore National Laboratory; 2006. [Google Scholar]
- Szafraniec LJ, Szafraniec LL, Beaudry WT, et al. On the stoichiometry of phosphonothiolate ester hydrolysis. CRDEC-TR-212, ADA 250773. Aberdeen Proving Ground, MD, USA: US Army Chemical Research Development and Engineering Center; 1990. [Google Scholar]
- Talmage SS, Munro NB, Watson AP, et al. The fate of chemical warfare agents in the environment. In: Marrs TC, Maynard RL, Sidell FR, editors. Chemical Warfare Agents: Toxicology and Treatment. 2nd edit. Chichester, West Sussex, England: John Wiley and Sons, Ltd.; 2007a. pp. 89–125. [Google Scholar]
- Talmage SS, Watson AP, Hauschild V, et al. Chemical warfare agent degradation and decontamination. Current Organic Chemistry. 2007b;11:285–98. [Google Scholar]
- Tu AT. Chemical Terrorism: Horrors in Tokyo Subway and Matsumoto City. Ft. Collins, CO, USA: Alaken Inc.; 2002. [Google Scholar]
- Tu AT. Toxicological and chemical aspects of sarin terrorism in Japan in 1994 and 1995. Toxin Reviews. 2007;26:231–74. [Google Scholar]
- Tucker MD, Raber E. Facility Restoration Following Chemical Contamination Operational Technology Demonstration (OTD) Overview. 2008. Proceedings 2008 Annual Chemical and Biological R&D Technologies Conference, January 28-February 1, 2008, San Antonio, TX. Science and Technology Directorate, US Department of Homeland Security, Washington, DC, USA.
- USACHPPM (US Army Center for Health Promotion and Preventive Medicine) Derivation of Health-Based Environmental Screening Levels for Chemical Warfare Agents: A Technical Evaluation. 1999. Aberdeen Proving Ground, MD, USA.
- USACHPPM. Acute Toxicity Estimation and Operational Risk Management of Chemical Warfare Agent Exposures. 2004. USACHPPM Report No. 47-EM-5863-04. Aberdeen Proving Ground, MD, USA. (May 2004)
- USACHPPM. Deployment Health Guide: Toxic Industrial Chemicals (TIC) Release Response. Brochure. Aberdeen Proving Ground, MD, USA: USACHPPM; 2007a. Available at http://chppm-www.apgea.army.mil/documents/Disaster/TICResponse2.pdf. [Google Scholar]
- USACHPPM. Chlorine Improvised Explosive Devices and Preventive Medicine Actions. 2007b. USACHPPM Fact Sheet No. 36-015-0407. USACHPPM, Aberdeen Proving Ground, MD, USA. Available at http://usachppm.apgea.army.mil/documents/FACT/36-015-0407_Chlorine_IEDs.pdf.
- USACHPPM. Technical Guide 244 (TG 244) Aberdeen Proving Ground, MD, USA: US Army Center for Health Promotion and Preventive Medicine; 2008a. The Medical CBRN Battlebook. (October 2008) [Google Scholar]
- USACHPPM. Health-Based Chemical Vapor Concentration Levels for Future Systems Acquisition and Development. 2008b. USACHPPM Technical Report No. 64-FF-07Z2-07. Aberdeen Proving Ground, MD, USA (July 2008 update)
- U.S. Army Corps of Engineers, Baltimore District. Site-Specific Public Protection Plan for the Investigation of Burial Pit 3 at 4825 Glenbrook Road. Washington, D.C.: Spring Valley FUDS; 2007. US Army Corps of Engineers, Baltimore District, Baltimore, MD, USA. Final (October 2007). Available at www.nab.usace.army.mil/projects/WashingtonDC/springvalley. htm. [Google Scholar]
- USEPA (US Environmental Protection Agency) Health Assessment Document for Phosgene. 1986. EPA/600/8-86/022A. Office of Health and Environmental Assessment, Washington, DC, USA.
- USEPA. Risk Assessment Guidance for Superfund: Volume I-Human Health Evaluation Manual (Part B, Development of Risk-Based Preliminary Remediation Goals) 1991. OSWER Directive 9285.7-01B. Office of Emergency and Remedial Response, Washington, DC, USA.
- USEPA. EPA Region III Risk-Based Concentration Table, Background Information, Development of Risk-Based Concentrations. Philadelphia, PA, USA: Office of Superfund Programs; 1996a. [Google Scholar]
- USEPA. Soil Screening Guidance: Technical Background Document. EPA/540/R-95/128. Washington, DC, USA: Office of Emergency and Remedial Response; 1996b. [Google Scholar]
- USEPA. Region 9 Preliminary Remediation Goals (PRGs), 2004 Table. 2005. Available at http://www.epa.gov/region09/waste/sfund/prg/index.html.
- USEPA. U.S. Environmental Protection Agency Regions 3, 6, and 9. Regional Screening Levels for Chemical Contaminants at Superfund Sites; 2008. Available at http://www.epa.gov/reg3hwmd/risk/human/rb-concentration_table/index.htm. [Google Scholar]
- USEPA. Voluntary Guidelines for Methamphetamine Laboratory Cleanup. EPA-530-R-08-008. Washington, DC, USA: Office of Solid Waste and Emergency Response; 2009a. [Google Scholar]
- USEPA. U.S. Environmental Protection Agency, Regional Screening Levels for Chemical Contaminants at Superfund Sites. User's Guide; 2009b. Available at http://www.epa.gov/reg3hwmd/risk/human/rb-concentration_table/usersguide.htm USEPA/IRIS (US Environmental Protection Agency, Integrated Risk Information System). 2005. Chlorine Cyanide (CASRN 506-77-4). Available at http://www.epa.gov/iris/index. html. [Google Scholar]
- USEPA/IRIS. Integrated Risk Information System (IRIS) Glossary. 2006a. Available at http://www.epa.gov/iris/help_gloss.htm USEPA/IRIS. 2006b. Phosgene (CASRN 75-44-5), Hydrogen Cyanide (CASRN 74-90-8). Available at http://www.epa.gov/iris/index.html.
- Van Kampen KR, James LF, Rasmussen J, et al. Organic phosphate poisoning of sheep in Skull Valley, Utah. J Amer Vet Med Assoc. 1969;154:623–30. [PubMed] [Google Scholar]
- Van Kampen KR, Shupe JL, Johnson AE, et al. Effects of nerve gas poisoning in sheep in Skull Valley, Utah. J Amer Vet Med Assoc. 1970;156:1032–5. [PubMed] [Google Scholar]
- Wagner GW, Yang Y-C. Universal decontaminating solution for chemical warfare agents. 2001. US Patent No. 6, 254, 957. United States Patent and Trademark Office, US Dept. of Commerce, Washington, DC, USA.
- Wagner GW, Yang Y-C. Rapid nucleophilic/oxidative decontamination of chemical warfare agents. Ind Eng Chem Res. 2002;41:1925–8. [Google Scholar]
- Watson AP, Griffin GD. Toxicity of vesicant agents scheduled for destruction by the Chemical Stockpile Disposal Program. Environ Health Perspect. 1992;98:259–80. doi: 10.1289/ehp.9298259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AP, Munro NB. Reentry Planning: The Technical Basis for Offsite Recovery Following Warfare Agent Contamination. ORNL-6628. Oak Ridge, TN, USA: Oak Ridge National Laboratory; 1990. (April 1990) [Google Scholar]
- Watson AP, Sidell FR, Leffingwell SS, et al. General guidelines for medically screening mixed population groups potentially exposed to nerve or vesicant agents. ORNL/TM-12034. Oak Ridge, TN, USA: Oak Ridge National Laboratory; 1992. (January 1992) [Google Scholar]
- Watson A, Opresko DM, Hauschild V. Evaluation of Chemical Warfare Agent Percutaneous Vapor Toxicity: Derivation of Toxicity Guidelines for Assessing Chemical Protective Ensembles. ORNL/TM-2003/180. Oak Ridge, TN, USA: Oak Ridge National Laboratory; 2003. [Google Scholar]
- Watson A, Opresko D, Young R, et al. Development and application of Acute Exposure Guideline Levels (AEGLs) for chemical warfare nerve and sulfur mustard agents. J Toxicol Environ Health B. 2006a;9:173–263. doi: 10.1080/15287390500194441. [DOI] [PubMed] [Google Scholar]
- Watson A, Bakshi K, Opresko D, et al. Cholinesterase inhibitors as chemical warfare agents: Community preparedness guidelines. In: Gupta, RC, editor. Toxicology of Organophosphate and Carbamate Pesticides. New York, NY, USA: Elsevier Publishers/Academic Press; 2006b. pp. 47–68. [Google Scholar]
- Yang Y-C. Chemical reactions for neutralizing chemical warfare agents. Chem Ind. 1995;9:334–7. [Google Scholar]
- Yang Y-C. Chemical detoxification of nerve agent VX. Acc Chem Res. 1999;32:109–15. [Google Scholar]
- Yang Y-C, Szafraniec LL, Beaudry WT. Perhydrolysis of agent VX. J Org Chem. 1993;58:6964–65. [Google Scholar]
- Yang Y-C, Szafraniec LL, Beaudry WT, et al. Hydrolysis of VX: Activation energies and autocatalysis. 1994. In: Proceedings of the 1994 ERDEC Scientific Conference on Chemical Biological Defense Research November 15-18, 1994, 37582 UNCLASSIFIED paper (ADE 479900), ERDEC-SP-036. US Army Edgewood Research Development and Engineering Center, Aberdeen Proving Ground, MD, USA.
- Young Rand. Mustards and vesicants. In: Gupta R, Bast C, editors. Handbook of Toxicology of Chemical Warfare Agents. Amsterdam, The Netherlands: Elsevier Publishers, Academic Press; 2009. pp. 93–108. [Google Scholar]
- Zongkar B. Army Corps finds new WWI chemical site in DC yard. Associated Press; 2010. April 16, 2010. [Google Scholar]


