Abstract
In the event of a chemical terrorist attack on a transportation hub, post-event remediation and restoration activities necessary to attain unrestricted facility re-use and re-entry could require hours to multiple days. While timeframes are dependent on numerous variables, a primary controlling factor is the level of pre-planning and decision-making completed prior to chemical release. What follows is the second of a two-part analysis identifying key considerations, critical information and decision criteria to facilitate post-attack and post-decontamination consequence management activities. Decision criteria analysis presented here provides first-time, open-literature documentation of multi-pathway, health-based remediation exposure guidelines for selected toxic industrial compounds, chemical warfare agents, and agent degradation products for pre-planning application in anticipation of a chemical terrorist attack. Guideline values are provided for inhalation and direct ocular vapor exposure routes as well as percutaneous vapor, surface contact, and ingestion. Target populations include various employees as well as transit passengers. This work has been performed as a national case study conducted in partnership with the Los Angeles International Airport and The Bradley International Terminal. All recommended guidelines have been selected for consistency with airport scenario release parameters of a one-time, short-duration, finite airborne release from a single source followed by compound-specific decontamination.
Keywords: chemical warfare agents, CWA, TIC, terrorism, clearance guidelines and goals, risk assessment, decision criteria
INTRODUCTION
This analysis describes a specific one-time, short-duration, chemical airborne release attack scenario at a major U.S. airport as a mechanism to demonstrate key considerations and decision criteria that can facilitate post-attack and post-decontamination consequence management activities. This work reflects information and lessons learned as part of an ongoing U.S. Department of Homeland Security (DHS) domestic preparedness activity, the Chemical Restoration Operational Technology Demonstration Project. A primary objective of this analysis is to provide and document information for chemical warfare agents (CWAs) and toxic industrial compounds (TICs) appropriate and useful to airport facility remediation should such an incident occur.
This work is presented in two parts: Part I (“Key assessment considerations”) (Watson et al. 2011, this issue) characterized the airborne release chemical attack scenario at a major U.S. airport that serves as the basis of the evaluation. Scenario assumptions were described, chemicals and populations of concern were characterized, various post-event phases and timelines were summarized, a conceptual site model (CSM) to characterize potential health risks of concern was derived, and precedents and resources were evaluated. The current analysis, which represents Part II (“Decision criteria for multipathway exposure routes”), provides first-time, open-literature documentation of multi-pathway and health-based pre-planning remediation exposure guidelines for CWA and TICs, as well as degradation products, for application in anticipation of a chemical terrorist attack.
BACKGROUND
Detailed project background was provided in Part I of this analysis (Watson et al. 2011, this issue) and is only briefly summarized here. The DHS, and specifically the DHS Chemical and Biological Countermeasures Program, has been given responsibility to improve domestic preparedness for potential chemical terrorist release incidents at key U.S. transportation nodes. Focus of this Project has been on the Consequence Management phase (e.g., restoration and recovery) of the response; specifically, effort is directed to pre-plan the recovery process, select “best available” methods and technologies for each recovery activity, and address both data and technology gaps critical to the recovery process. It is recognized that minimizing potential health and economic impacts from chemical attacks is an effective countermeasure to chemical terrorism.
As part of a multi-year research program involving several national laboratories as well as subject matter experts from various state and Federal agencies, the current analysis presents and documents human health-based exposure guidelines for use as an aid to site-specific pre-planning and preparedness for a chemical terrorist incident. This subject area has been under investigation and development during the current work, in which project participants partnered with the Los Angeles International Airport (LAX; Los Angeles, CA) to generate a national case study. While the following exposure guidelines analysis is necessarily site-specific for a single terminal at LAX, many factors common to any restoration operation have been evaluated; results are thus also applicable to other transportation nodes considered vulnerable to chemical terrorist attack.
Airport scenario compounds evaluated include the chemical warfare agent (CWA) nerve agents tabun (GA), sarin (GB), soman (GD), cyclosarin (GF), and VX as well as the vesicant agent sulfur mustard (HD); and the toxic industrial compounds (TICs) phosgene (CG), hydrogen cyanide (AC), and cyanogen chloride (CK). Exposure pathways analyzed include a variety of routes associated with airborne vapors (inhalation and direct ocular vapor, percutaneous vapor) and potential surface residues (surface contact with skin, hand-to-eye transfers, hand-to-mouth ingestion, resuspension inhalation). Populations considered include specifically trained decontamination personnel, transit passengers, and various airport personnel including restoration and recovery personnel performing refurbishment tasks, vendors, gate agents, baggage handlers, and others. Population characteristics, elements of the consequence management analysis, and specific properties for chemicals of concern and their degradation products are detailed in Watson et al. (2011, this issue).
An overall project objective has been to leverage existing resources and guidance. As a consequence, emphasis has been placed on exposure guidelines consistent with parameters of the site-specific and one-time release scenario for which this project is designed. In addition, programmatic focus is maintained on guidelines that already exist, are published and accessible to the public, have undergone credible peer and public review, are health-based and protective, are compound-specific, and have demonstrated utility in use and practice. Airport stakeholders have made it clear that, if a chemical terrorist incident should occur tomorrow, it is important to have an available set of appropriate and reasonable clearance goals from which to begin. It is understood and acknowledged that each release event will involve site- and incident-specific parameters requiring in-context evaluation regarding guideline applicability.
This article does not address public health responses (i.e., medical treatment) or risk management elements.
CURRENT EXPOSURE AND EQUIPMENT GUIDELINES FOR DECONTAMINATION AND EMERGENCY RESPONSE PERSONNEL
Personal protective equipment and clothing (PPE) selection requirements and related information for emergency response and decontamination personnel (the latter entering the facility to collect characterization or clearance samples, install decontamination equipment, and perform related tasks) are summarized by the National Institute for Occupational Safety and Health (NIOSH) (NIOSH/CDC 2008; see: www.cdc.gov/NIOSH/ershdb/ for links to Emergency Response Cards for individual compounds of concern and compound categories). This information is provided as the NIOSH Emergency Response Safety and Health Database (ERSHDB; www.cdc.gov/NIOSH/ershdb/about.html). Categories of PPE protection (e.g., Level A-D, with A [Red Zone] providing the greatest level of protection and D [Green Zone] providing the least) are defined on the basis of whether or not identity of the hazardous material is known, as well as atmospheric concentrations relative to exposure limits such as Acute Exposure Guideline Level (AEGL) concentrations. As the NIOSH guidelines are subject to change, the user is advised to confirm the PPE and exposure guideline values posted on the NIOSH sites provided above before field application.
The DHS has also adopted science and technology standards developed by NIOSH or the National Fire Protection Association (NFPA) for personal protective gear for first responders (DHS 2006). These chemical response standards specify technical requirements for respirators and clothing.
In addition, the NIOSH Pocket Guide to Chemical Hazards and NIOSH emergency response cards (NIOSH 2005a,b; NIOSH 2008a,b; www.cdc.gov/niosh/npg/) provide respirator and skin protection recommendations for hydrogen cyanide, cyanogen chloride, and phosgene developed either by NIOSH alone or collaboratively with the Occupational Safety and Health Administration (OSHA). More specific recommendations for nonrespiratory chemical protective clothing (boots, gloves, and suits) are available via a link from the NIOSH website (Mansdorf 1998; www.cdc.gov/niosh/ncpc/ncpcl.html). Whereas the NIOSH Pocket Guide recommendations have been developed for application to industrial workplaces where personnel can be routinely exposed during manufacture and processing and related industrial activities, many consider these occupational values to be important when considering exposure guidance for decontamination personnel potentially exposed to hydrogen cyanide, cyanogen chloride, or phosgene in the course of their specialized duties. The immediately dangerous to life and health (IDLH) concentrations are particularly relevant.
PERCUTANEOUS VAPOR EXPOSURE TO CWAs
In the event of damage or breach to skin-protective clothing, toxicologically significant vapor contact exposure to skin could be possible in high-vapor concentration situations potentially encountered during emergency response and decontamination activities associated with a CWA attack. Percutaneous vapor concentrations necessary to produce adverse effects similar to those induced following inhalation or ocular vapor exposure to these same compounds are often greater by several orders of magnitude due to skin barrier effects (NRC/COT 2003). The U.S. Army Office of the Surgeon General (OTSG) has established emergency military guidelines for percutaneous vapor exposure to allow safe exit from a CWA atmosphere in the event of damage or breach to skin-protective clothing worn by specialized personnel with CWA expertise and under military management, such as personnel employed in military munition demilitarization facilities (DA 2004, 2005a) (Table 1). It is noted that application of the guidelines shown in Table 1 assumes that respiratory protection is in place and fully functional throughout CWA exposures and that individuals are under monitoring surveillance.
Table 1.
Percutaneous vapor exposure guidelines for (military) CWA personnel escape from selected CWA and TIC atmospheres, assuming respiratory protection.a
| CWA or TIC | Example CWA personnel escape guidelines (mg/m3) for 30-minute percutaneous exposure (with respiratory protection only, or a breach in skin-protective clothing) |
| Tabun (GA) | 11.1ab |
| Sarin (GB) | 6.0a |
| Soman (GD) and Cyclosarin (GF) | 1.5ab |
| VX | 0.13ab |
| Sulfur mustard (H/HD) | 0.1ab |
| Hydrogen cyanide (AC) | Not determined by NIOSHc |
| Cyanogen chloride (CK) | Not determined by NIOSHc |
| Phosgene (CG) | Not determined by NIOSHc |
bMilitary guideline established by the Army Office of the Surgeon General (see footnote a). Percutaneous vapor exposure would be a route of concern if personnel wear respiratory protection but experience a rip or tear in the protective suit, or if personnel are in mask-only protective ensemble. Agent vapor concentrations of concern for skin-only exposure are greater than those for vapor exposure to personnel with no respiratory protection (where fast-acting inhalation and ocular exposure would occur).
Watson et al. (2003) observed that dose effects arising from cumulative percutaneous exposure to the listed CWAs are likely to remain constant for exposures of approximately 30 min to 2 h. Thus, 30 min was selected as a reasonable duration for specialized personnel with CWA expertise to undergo percutaneous exposure to the concentrations provided in Table 1. Furthermore, 30 min is considered sufficient to allow specialized personnel with fully functional respiratory protection to perform multiple data collection cycles and to then safely exit from a CWA atmosphere.
Under appropriate PPE and administrative controls, the available U.S. Army Office of the Surgeon General guidelines for percutaneous vapor exposure (Table 1) could be reasonably considered for application in safeguarding civilian decontamination personnel.
The relative toxicity of percutaneous vapor exposure versus direct inhalation/ocular exposure has not been clearly established for the TIC compounds of concern. Neither cyanogen chloride nor phosgene vapors are known to be dermally absorbed. Existing reports of toxicity for the scenario TICs focus exclusively on inhalation toxicity since any percutaneous vapor absorption is widely considered to be a less significant source of exposure than vapor inhalation. Although percutaneous absorption of HCN (agent AC) vapor occurs (the NIOSH short-term exposure limit [STEL] for HCN has a skin notation; NIOSH 2003, 2005a), the relative importance of skin exposure for HCN is not well characterized. Anecdotal human data (ATSDR 2006) for HCN and experimental animal data reviewed by the American Industrial Hygiene Association (AIHA 2007) indicate that markedly higher (greater than 40-fold) concentrations may be required to induce lethality when HCN vapor exposure occurs percutaneously rather than by inhalation. NIOSH has not developed percutaneous vapor exposure guidelines for cyanogen chloride or phosgene.
INHALATION/OCULAR EXPOSURE GOALS SUITABLE FOR GENERAL PUBLIC UNDER LAX AIRPORT SCENARIO
As previously documented, the air exposure pathway is a key focus for developing clearance and re-occupancy goals for the scenario compounds; in addition, the release scenario hypothesized in this evaluation assumes that a CWA or TIC source is removed, neutralized or otherwise terminated during the first-response phase and that the absence of further agent release is frequently verified and validated under standard protocols for monitoring air and personnel at the site. Because the average passenger dwell time in the terminal is much less than 8 h (CAM 2005), the short-term exposure durations assumed here reflect actual and measured dwell times by airline passengers in modern, commercial airport terminals.
For similar reasons, any assumptions of continuous exposure to the chemicals of concern are rejected as incompatible with expected jobsite conditions for various airport personnel performing tasks associated with repair, maintenance, replacement or servicing of airport components and facilities; as well as airport employees (e.g., gate agents, baggage handlers) and vendors. In other words, this description encompasses all airport personnel performing tasks other than decontamination of CWAs or TICs. Remediation protocols (Watson et al. 2011, this issue) require that all of these tasks and services be performed only after obtaining confirmation that (1) the agent source is removed and/or neutralized, (2) all forensic assessment is completed, (3) any decontamination processes are completed and verified, and (4) clearance sampling has characterized atmospheres. The airport response protocol recommends that any subsequent detection of compounds of concern at clearance concentrations would result in the prompt exclusion of personnel until additional remediation occurs. As a result of the protection afforded by source removal, decontamination, and clearance sampling before initiating restoration and recovery tasks, protective assumptions developed for transit passenger populations are also considered appropriate for all airport personnel performing tasks other than decontamination of CWAs or TIC, and vendors. All of the above populations are considered to be members of the general public.
Of the many options considered, those short-term exposure concentrations developed by the National Research Council Committee on Toxicology (NRC/COT) in collaboration with the U.S. Environmental Protection Agency's (USEPA's) National Advisory Committee for Acute Exposure Guideline Levels (AEGLs) for Hazardous Substances offer several advantages. AEGLs are available for several tiers (1, 2, 3) of gradually increasing toxic effects and multiple exposure durations, have undergone rigorous peer review (including publication in the Federal Register for public comment), and have employed a modern and systematic approach to data analysis in a manner transparent to the public and other stakeholders (NRC/COT 2001, 2002, 2003). The established review process ensures appropriate scientific credibility and peer review. In addition, AEGL values are available for the specific compounds of interest and are currently being implemented by local, state, and Federal agencies in preparation for accidental or intentional releases [Watson et al. (2011, this issue) discussion of “Precedents and Sources”). Use of these values allows for identification of an initial and credible starting point and, depending on the incident- and site-specific parameters, can be adjusted accordingly.
Because of their recognized scientific credibility and expanding uses in domestic preparedness planning by affected communities as well as Federal and state authorities, this analysis considers the AEGL concentrations as protective, appropriate and useful for making site-access re-entry determinations for the traveling public, airport personnel, and vendors. This analysis further considers application of the mildest effect tier (AEGL-1) (NRC/COT 2001) to be appropriate, and application of the 8-h AEGL-1 concentrations (the lowest values) as reasonable criteria for all transit passenger stay times ≤8 h as well as for airport personnel and vendors. Selecting the minimal exposure concentrations developed for assumed 8-h exposure durations is a highly protective assumption for LAX transit passengers given the one-time only release scenario and the fact that most passengers spend ≤4 h in the most heavily used LAX terminals (CAM 2005). It is acknowledged that use of any given dwell (“stay”) time assumption may be airport-specific; nevertheless, the 8-h continuous-exposure durations evaluated above are considered protective for post-decontamination applications.
The values presented in Table 2 provide compound-specific and protective air concentrations for clearance screening decision-making. The “reasonable maximum clearance goals” compound-specific concentrations are for an assumed, continuous-exposure duration of 8 h derived under the AEGL derivation protocol of NRC/COT (2001). Therefore, this concentration is considered protective not only for exposure durations of 8 h, but also for shorter-term exposure durations of < 8 h. This approach is precautionary given data documenting that the passenger transit time at LAX is much less than 8 h (CAM 2005). Clearance goal selection of a vapor concentration for the unlikely continuous CWA or TIC exposure duration of 8 h is thus considered highly protective. To address those rare situations where public dwell times may be >8 h but <24 h, alternative clearance goals are also provided in Table 2. These clearance goals are derived in the current analysis from standard time-extrapolation protocols (NRC/COT 2001), and provide additional flexibility to decision-makers. The range provided in Table 2 represents both reasonable maximum and alternative re-entry screening guidelines.
Table 2.
Recommended airborne (inhalation, ocular) pre-planning clearance goals for transit passengers as well as various airport employees and personnel for selected CWAs and TICs.a
| Reasonable maximum clearance goals (mg/m3) |
Alternative clearance goals (mg/m3) |
|
| CWA or TIC | <8-h durationb exposure | >8 but <24-h duration exposure |
| Tabun (GA) | < 0.0010c | < 0.0003e |
| Sarin (GB) | < 0.0010c | < 0.0003e |
| Soman (GD) and Cyclosarin (GF) | < 0.00050c | < 0.0002e |
| VX | < 0.000071c | < 0.000024e |
| Sulfur mustard (H/HD) | < 0.008c | < 0.003e |
| Hydrogen cyanide (AC) | < 1.1d | < 0.37e |
| Cyanogen chloride (CK) | < 0.25f | < 0.08f |
| Phosgene (CG) | < 0.08g | < 0.03g |
aChemical formulae: tabun, C5H11N2O2P (NATO code GA); sarin, C4H10FO2P (NATO code GB); soman, C7H16FO2P (NATO code GD); cyclosarin, C7H14FO2P (NATO code GF); VX, C11H26NO2PS (NATO code VX); sulfur mustard, C4H8Cl2S (NATO code HD); hydrogen cyanide, HCN (NATO code AC); cyanogen chloride, CNCl (NATO code CK); phosgene, COCl2 (NATO code CG).
bMay also be used as appropriately protective and health-based vapor screening criteria for releasing items, equipment, and facilities that have not been exposed to liquid or heavy aerosol forms of the chemical of concern; these screening criteria would also be useful for evaluating non-porous items and surfaces that have undergone decontamination. If these vapor screening criteria are attained, such decontaminated items would not be expected to pose a vapor exposure hazard (DA 2008, Table C-5).
eProposed >8 but ≤24-h protective estimates presented for use in this analysis are derived by straight-line extrapolation from the 8-h AEGL-1 values and follow standard protocols (NRC/COT 2001).
fIn the absence of a previously derived CK exposure guideline comparable to AEGL-1, these estimates were calculated for use in the current assessment (see text for details of derivation. and Wood 1997). 8-h estimate is derived from experimental data with straight-line extrapolation to 24 h. This derivation is considered a reasonable approach given the absence of published AEGL-1 values for cyanogen chloride.
gIn the absence of a previously derived phosgene (agent CG) exposure guideline comparable to the AEGL-1, these estimates were calculated for use in the current assessment (see text for details of derivation). 8-h estimate is derived as 50% of 8-h AEGL-2 for phosgene (NRC/COT 2002), with straight-line extrapolation to 24 h. This derivation is considered a reasonable approach given the absence of published AEGL-1 values for phosgene.
Limitations in toxicity data for cyanogen chloride (Cohrssen 2001) and phosgene have restricted development of short-term exposure concentration guidelines, and result in absence of some or all final AEGL or Emergency Response Planning Guideline (ERPG) values for these two compounds. Accordingly, this evaluation developed protective estimates for phosgene and cyanogen chloride specifically designed for application to the LAX scenario. Because the estimates are unique to the present analysis, a comprehensive explanation of the derivation is provided below and in footnotes of Table 2. Comparable detailed guideline explanations are not provided for the remaining CWAs and TICs, given that this material has already been widely published and is incorporated by reference in this analysis (e.g., NRC/COT 2002, 2003; Watson et al. 2006a,b; Bast and Glass 2009; Young and Bast 2009; others).
Related Considerations
Inhalation minimal risk levels (MRLs) were also considered in this analysis, but are only available for sulfur mustard agent HD (the MRL for an “acute-duration” exposure of ≤14 days is 0.0007 mg HD/m3; MRL for “intermediate-duration” exposure of 15 to 364 days is 0.00002 mg/m3) (ATSDR 2003). The acute MRL for agent HD differs from the minimal re-entry screening guideline for HD in Table 2 by an approximate factor of 4. Given that the Agency for Toxic Substances and Disease Registry (ATSDR) assumptions of multiple-day and continuous exposure duration are not supported by the release and decontamination scenario governing development of this airport remediation analysis, the extended-release inhalation MRLs for HD are not further considered as clearance guidance for the airport scenario.
The Standing Operating Procedure for an ongoing effort by USEPA/National Homeland Security Research Center to develop emergency reference levels has been recently published (Young et al. 2009). These levels (Provisional Advisory Levels; PALs) are being derived for assumed continuous 24-h, 30-d, 90-d, and 2-yr exposure durations for both inhalation and oral exposure routes and 3 tiers of effect, with tier 1 being least severe and tier 3 being most severe. As provisional values, PALs are considered “temporary values that will neither be promulgated, nor be formally issued as regulatory guidance” (Adeshina et al. 2009). Rather their intent is to “assist in emergency planning and decision-making,” and for use at the discretion of risk managers.
The >8 h but <24 h protective vapor concentration estimates for the CWAs and TICs in Table 2 have been compared with compound-specific PALs (Watson et al. 2007; Glass et al. 2008, 2009; see also www.epa.gov/NHSRC/news/news121208.html). PAL values for phosgene were recently published in Glass et al. (2009); PAL values for HCN, the G-series nerve agents, nerve agent VX and sulfur mustard agent HD are within USEPA review at this writing and are not available for examination unless specifically requested (www.epa.gov/NHSRC/news/news121208.html).
For the lowest effect level (PAL 1) and 24 h exposure duration to the public, the PAL 1 vapor concentrations for the G-agents are similar to the >8 h but < 24 h protective estimates in Table 2. The draft nerve agent PAL vapor estimates have undergone extensive review and have been approved as scientifically sound by the PAL Advisory Panel for internal USEPA use, although they have not yet been released for public access or use as of August 2010. Internal analysis during the current evaluation has noted close similarity between compound-specific LAX preplanning clearance goals (Table 2) for >8 h but <24 h, and both G-agent and nerve agent VX 24-h PAL 1 values for inhalation/ocular exposure. The draft 24-h PAL 1 values for sulfur mustard, phosgene, and hydrogen cyanide differ slightly from the compound-specific LAX remediation guidance protective estimate for >8 h but <24 h (Table 2). At present (August 2010), no 24-h PAL 1 vapor exposure estimates are available for cyanogen chloride due to sparse data. When PAL values are made broadly available in a manner that facilitates transparent examination by state and local regulatory authorities and members of the general public, they can be more readily provided to decision-makers for further consideration.
Derivation of Airport Inhalation/Ocular Exposure Guidelines
Guideline level basis for chemical warfare agents
The NRC/COT (2001) describes and documents selection protocols for critical effects and studies, AEGL derivation, time scaling, use and selection of uncertainty and modifying factors, and a description of the lengthy and deliberative review process employed. This material is also available in recent papers by Krewski et al. (2004) and Watson et al. (2006a,b). Because exposure-response data are usually not available for each AEGL-specific exposure duration (for any hazardous chemical and not just CWAs; NRC/COT 2001), temporal extrapolation is employed in the development of AEGL values for some AEGL-specific time periods. The concentration-exposure time relation for many systemically acting vapors and gases may be described by Cn × t = k, where the exponent n ranges from 0.8 to 3.5 (C is concentration in mg/m3 and t is exposure duration time; Ten Berge et al. 1986). Haber's rule (C × t = k) is a special case of this principle, and occurs when n = 1; Haber's rule has often been used for short-term exposure predictions involving a small set of highly toxic gases (NRC/COT 2001). For larger sets, and structurally heterogeneous compounds, the more general relation of Cn × t = k applies (Ten Berge et al. 1986; NRC/COT 2001).
In general, toxicity data from human studies are preferred for AEGL development over those obtained from animal studies (NRC/COT 2001). Furthermore, vapor exposure data are preferred over those available from alternate exposure pathways (NRC/COT 2001). Human studies evaluated in the AEGL process must meet rigorous ethical criteria for acceptance of human subject data in that (1) subjects should provide informed consent, and (2) there is evidence that human studies were performed under appropriate clinical supervision (NRC/COT, 2001). Final AEGL values for nerve agents and sulfur mustard are published in NRC/COT (2003), while final values for phosgene and hydrogen cyanide are published in NRC/COT (2002). AEGL values for cyanogen chloride have never been finalized due to scarcity of data.
When available, AEGL-1 values for the 8 h continuous exposure duration (the longest duration considered in the AEGL process, and consequently associated with the smallest AEGL exposure concentration) are incorporated into the LAX remediation guidance as reasonable maximum re-entry screening guidelines for all the reasons outlined earlier. Application of standard time-extrapolation protocols (NRC/COT 2001) provides additional flexibility in estimating an alternative re-entry screening guideline for rare instances when potential exposure durations could be >8 h but <24 h. A summary of compound-specific derivation follows.
AEGL-1 derivation for sarin and other nerve agents. The toxicological database for agent GB is robust and includes sufficient human and animal data for derivation of AEGL-1 and AEGL-2 estimates as well as ample laboratory animal lethality data for directly deriving AEGL-3 values. AEGL-1 values for agent GB were derived from a well-conducted study on adult female Sprague-Dawley (SD) rats subjected to whole-body exposures in a dynamic airflow chamber to a range of GB vapor concentrations (0.01 to 0.48 mg/m3) for exposure durations of 10, 60, or 240 min (Mioduszewski et al. 2002a,b). The endpoint of interest and point of departure was the well-defined median effective concentration (EC50) for reversible and transient miosis in a susceptible gender (female; Dabisch et al. 2008a,b); this degree of miosis is not considered adverse in humans (NRC/COT 2003) and is a direct, local, and transient effect to the ocular iris.
Of the 283 GB-exposed rats, 142 were female and 141 were male. With the inclusion of range-finding experiments and controls, a total of 423 rats was tested in the well-conducted study of Mioduszewski et al. (2002a,b), which employed highly credible protocols for GB vapor generation and measurement. Analysis of pre- and post-exposure rat pupil diameters allowed determination of EC50 values for miosis (defined by Mioduszewski and his colleagues as a post-exposure pupil diameter of 50% or less of the pre-exposure diameter in 50% of the exposed population). Blood samples collected from tail vein and heart at 60 min and 7 d post-exposure indicated no significant change from pre-exposure baseline in monitored blood RBC-ChE (red blood cell cholinesterase), BuChE (butylcholinesterase), or carboxylesterase activity. No other clinical signs were evident throughout the duration of the study. Thus, the potential for systemic absorption was evaluated, and did not occur. These results further document the fact that miosis alone, and in the absence of signs such as cholinesterase or carboxylesterase activity inhibition, is a local effect reflecting an exposure much less than that required to generate a systemic clinical effect. Gender differences (females more susceptible) were statistically significant. The EC50 for miosis in female SD rats is thus a well-defined transient, reversible, and nondisabling animal endpoint in a susceptible gender.
An EC50 for miosis is widely considered the first measurable change (and first noticeable effect, or FNE) by modern and reproducible techniques in the continuum of response to anticholinesterase compounds, and is comparable to the pupil size reduction observed in human subjects who enter bright sunlight after time spent in a dimly lit room. During the (1995) Tokyo Subway Incident of GB release by domestic terrorists, persons experiencing much greater than 50% reduction in pupil diameter self-rescued and rendered aid to others (Watson et al. 2006a).
Since miosis in the absence of other clinical signs occurs as a direct and local vapor exposure effect prior to systemic effects such as whole blood acetylcholinsterase (AChE) activity depression, and is the FNE associated with exposure to low-level nerve agent vapor concentrations (Dabisch et al. 2008a,b), the NRC/COT (2003) considered this endpoint to be a highly protective point of departure for prevention of systemic effects and estimating acceptable inhalation vapor exposure concentrations. The authors agree with this assessment.
When compared to the human data, NRC/COT (2003) determined that miosis data derived from the Mioduszewski et al. (2002a,b) study on rats are a more reliable dataset because of the contemporary and multiple analytical techniques employed for quantifying exposures and measuring miosis, and the experimental protocol incorporating sufficiently large test and control populations. With the additional knowledge that the EC50 for miosis exhibited by rats in the study of Mioduszewski et al. (2002a,b) is transient and reversible, the EC50 for miosis in female (susceptible gender) SD rats is well supported as an appropriate and protective point of departure for estimating AEGL-1 values. Further, the NRC/COT (2003) ascertained that the miotogenic response of mammal eyes to GB vapor exposure is similar across multiple mammal species, including standard laboratory species (rats, rabbits, and guinea pigs; Mioduszewski et al., 2002a,b; Callaway and Dirnhuber 1971; Van Helden et al. 2001, 2002), nonhuman primates (marmosets; Van Helden et al. 2001, 2002, 2003, 2004), and humans (Harvey 1952; Johns 1952). As a consequence, the interspecies uncertainty factor (UFA) for the critical AEGL-1 endpoint of EC50 miosis is equal to 1.
To accommodate known variation in human cholinesterase and carboxylesterase activity that may make some individuals susceptible to the effects of cholinesterase inhibitors, such as nerve agents, the intraspecies uncertainty factor (UFH) was set to 10. A modifying factor was not considered applicable. Thus, the total uncertainty factor for estimating AEGL-1 values for agent GB is 10 (NRC/COT 2003; Watson et al. 2006a).
In accordance with standard techniques and procedures (NRC/COT 2001; Ten Berge et al. 1986), the temporal extrapolation used in estimating AEGL values for GB is based in part on a log-log linear regression of female SD rat miosis data following GB vapor exposures (Mioduszewski et al. 2002a,b). Regression analysis of the miosis data yields an n value of 2.00 (NRC/COT 2003). The experimentally derived n = 2 from the Mioduszewski et al. (2000, 2001, 2002a,b) rat miosis dataset has been used as the scaling function for nerve agent AEGL derivations.
AEGL-1 values for other G-agents and agent VX were derived from those of agent GB by a relative potency protocol (NRC/COT 2003; Mioduszewski et al. 1998; Watson et al. 2006a,b). This is considered a toxicologically acceptable approach given that all mammalian toxicity end points observed in the nerve agent dataset represent different points on the response continuum for anticholinesterase effects and that the principal mechanism of mammalian toxicity for the G-agents and agent VX is cholinesterase activity inhibition. As a consequence, target organ effects are considered identical, but different in magnitude. Furthermore, NRC/COT determined that there are no uncertainties for these nerve agents regarding toxic endpoints such as reproductive or developmental effects or carcinogenicity.
Recently published experimental data for GB vapor exposure in nonhuman primates, G-agents in swine, and rats exposed to VX further document the highly protective nature of these recommended clearance values (Genovese et al. 2008, 2009; Dabisch et al. 2008a,b; Whalley et al. 2004, 2007).
AEGL-1 derivation for sulfur mustard. Effects observed at low vapor concentrations of sulfur mustard are minimal, primarily ocular; and include conjunctivitis, photophobia, and ocular irritation; these effects are transient, nondisabling, and reversible upon cessation of exposure. The eye is considered the most sensitive and rapidly responding target tissue to sulfur mustard vapor, and the conjunctival endpoint is a direct and local vapor exposure effect to the ocular tissues. As such, and given that this mild ocular tissue response represents first noticeable effects (FNE), this endpoint is considered by NRC/COT (2003) as a protective point of departure for estimating acceptable respiratory exposure concentrations and AEGL-1 values. This ocular FNE develops prior to manifestation of any respiratory or further systemic effects resulting from inhalation exposure to sulfur mustard vapor (NRC/COT 2003; Young and Bast 2009).
Available ocular studies include controlled exposures to human volunteers as well as characterizations of war casualties and occupational exposures. Of these, only the controlled laboratory studies are suitable for use in deriving AEGL-1 values. The human-subject studies by Reed (1918), Reed et al. (1918), Walker et al. (1928), Guild et al. (1941), and Anderson (1942) provide useful information for assessing minimal adverse effect levels, and are described below.
The human subject data of Anderson (1942) characterize ocular effects under hot weather conditions when various parameters (high skin temperature and presence of moisture leading to an elevated sulfur mustard reaction rate) combine to enhance the alkylating action of sulfur mustard vapor to exposed epithelial tissues. The Anderson (1942) dataset thus represents a “worst-case” exposure scenario. Under temperate weather conditions, ocular effects following vapor exposure would occur at higher sulfur mustard vapor concentrations. Thus, derivation of AEGL estimates for sulfur mustard from the Anderson (1942) data provides a protective estimate for exposures that may occur under typical ambient conditions.
Anderson (1942) reported no effects at a sulfur mustard cumulative exposure Ct of 12 mg-min/m3, whereas 30 mg-min/m3 represented the upper range for mild ocular effects of conjunctival swelling and minor discomfort with no functional decrement. Slightly higher cumulative exposures (e.g., Cts of 34 to 38.1 mg-min/m3) were also without appreciable ocular effects. Thus, the Anderson (1942) human data indicate that the ocular response to 30mg-min/m3 is consistent with AEGL-1 effects. Analysis of the exposure-effect values from these human studies further indicates that the lower, 12mg-min/m3 value represents a defensible estimate of the no-effects threshold for ocular effects, and is thus a protective point of departure for estimating an AEGL-1 value.
Use of human data results in an interspecies UF = 1; furthermore, the use of human-subject data allows application of an intraspecies UF of 3 for protection of sensitive individuals. The adjustment is considered appropriate for acute exposures to chemicals whose mechanism of action primarily involves surface contact irritation of ocular and/or respiratory tract tissue rather than systemic activity that involves absorption and distribution of the parent chemical or a biotransformation product to a target tissue. Anderson (1942) noted that there was little variability in the ocular responses among individual participants.
Analysis of data characterizing similar ocular effects, as reported by Reed (1918), Reed et al. (1918), Guild et al. (1941), and Anderson (1942), indicate that for exposure periods up to several hours, the concentration-exposure time relation is a near-linear function (i.e., Haber's Law where n = 1 for Cn × t = k) as shown by n values of 0.96 and 1.11 for various datasets consistent with AEGL-1 effects. Therefore, an empirically derived, chemical-specific estimate of n = 1 was employed, rather than a default value, in the derivation. Derivation of the exponent (n) utilized human response data where 75 to 100% of responders showed a mild response that would be consistent with the definition of AEGL-1 effects.
Because human subject data collected under hot-weather conditions (resulting in enhanced toxicity) were employed as the critical effects and points of departure for sulfur mustard AEGL-1 derivation, the resulting AEGL estimates are considered highly protective and incorporate small uncertainty (interspecies UF = 1; intraspecies UF = 3; composite UF = 3) (NRC/COT 2003; Watson et al. 2006a).
Protective nature of CWA guidelines. The selection of direct local effects to the eye is a protective approach to critical effect determination, especially when the ocular effects observed occur in the absence of systemic toxicity signs. Additional protection is conferred by the knowledge that these endpoints (EC50 for miosis in the case of nerve agents; mild ocular irritation and conjunctivitis in the case of sulfur mustard) are transient and reversible (NRC/COT 2003; Watson et al. 2006a). The NRC does not consider the EC50 for miosis as an adverse effect in humans, and NRC notes that the level of ocular irritation expressed at the sulfur mustard critical effect endpoint occurs in the absence of functional decrement (NRC/COT 2003). Incorporation of various uncertainty factors inherent to the AEGL process results in an 8-h AEGL-1 concentration that is at least one, and often several, orders of magnitude below those concentrations known to generate miosis (nerve agents) or ocular irritation (sulfur mustard) in humans (Watson et al. 2006a; and Figures 5, 9, and 13 therein).
When further compared against longer-duration data, these endpoints continue to remain protective. For example, experimental rats exposed to 0.001 mg GB/m3 (equivalent to the reasonable maximum clearance goal for 8-h exposure duration as presented in Table 2) for 6 h/d, 5 times/wk over 24 consecutive weeks exhibited no miosis, no RBC-ChE activity inhibition; and no signs of neuromuscular, GI, respiratory, or behavioral effects (Weimer et al. 1979). In the case of sulfur mustard, dogs exposed to a time-weighted average concentration of 0.03 mg HD/m3 (with peaks at 0.1 mg HD/m3 for 6.5 h/d), 5 d/wk for 8 consecutive weeks exhibited no ocular or any other agent-related effects (McNamara et al. 1975). The experimental time-weighted concentration of 0.03 mg HD/m3 from McNamara et al. (1975) is approximately 4 times greater than the reasonable maximum clearance goal presented in Table 2 (e.g., 0.008 mg HD/m3), and an order of magnitude greater than the alternate clearance goal (e.g., 0.003 mg HD/m3) for HD exposure durations >8 but< 24 h (Table 2).
Guideline level basis for selected toxic industrial compounds (TICs)
Of all TICs evaluated in this assessment, hydrogen cyanide (CAS # 74-90-8) is the most completely characterized; available values include AEGL 1, 2, and 3 concentrations for all AEGL exposure durations (NRC/COT 2002); Emergency Response Planning Guidelines (ERPGs) (AIHA 2007); Integrated Risk Information System (IRIS) reference concentration (RfC) and reference dose (RfD) estimates (USEPA/IRIS 1993); NIOSH and OSHA industrial workplace exposure guidelines (www.cdc.gov/niosh/npg; www.cdc.gov/niosh/ershdb), and others. The HCN dataset is rich and robust, and includes well-conducted studies of human and nonhuman primate subjects as well as standard laboratory species (NRC/COT 2002; ATSDR 2006). Pre-planning clearance goals for the LAX chemical terrorism release scenario could thus be developed in a manner similar to that for the CW agents; for example, selection of the 8 h AEGL 1 concentration (1.1 mg HCN/m3 derived from lowest NOAEL for mild headache in humans; NRC/COT 2002) as a reasonable maximum, and estimation of an extrapolated alternative value (0.37 mg HCN/m3) for application in uncommon situations when a more lengthy dwell time (>8 h but <24 h) is possible (Table 2 and footnotes d and e).
Available data from which to estimate exposure guidelines for low-concentration exposures to cyanogen chloride and phosgene are less robust than those available for the CW agents. As a consequence, certain reasonable assumptions were required to develop exposure guidelines for these TICs, and are documented below.
As outlined earlier for the CW agents, the airport scenario considers that various airport employees and personnel would be allowed access to, and occupancy of, airport facilities only after decontamination for these volatile TICs is complete and monitoring has characterized workspace atmospheres. Ongoing monitoring will ensure that exposures (if any) to compounds of concern would be extremely limited given that detection of cyanogen chloride, phosgene or hydrogen cyanide would result in the prompt exclusion of such personnel until additional remediation occurs. As a result of the protection afforded by monitoring and the time limits such monitoring imposes on potential exposures, protective estimates developed for transit passenger populations are also considered appropriate for airport employees and personnel.
Cyanogen chloride. Cyanogen chloride (CK) is the least characterized of all compounds considered under the airport scenario.
Existing exposure estimates for CK. Literature analysis identified no ERPG-1, AEGL-1, or reference concentration (RfC; USEPA Integrated Risk Information System, IRIS) for CK. The AIHA (1998) does not recommend an ERPG-1 concentration for CK because “serious health effects-including marked irritation-may occur below the odor thresholds” of 0.8 to 1.0 ppm CK (2.01 to 2.51 mg CK/m3), and CK odor thresholds are greater than the ERPG-2 concentration of 0.4 ppm (1.0 mg/m3). The National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC) has considered CK and commissioned development of a draft technical support document for estimating AEGL values for this compound (Wood 1997). At present, the NAC has declined to propose or finalize AEGL values for CK because of data limitations and minimal documentation characterizing dose response and experimental protocols.
As a result, the present assessment considers the logic and protocols for RfC and AEGL derivation as a way of evaluating information critical to development of protective airborne estimates for the transient traveling public, a principal population of concern.
Data and options for CK. In the absence of suitable CK-specific toxicity data, Opresko et al. (1998) estimated two oral reference doses (RfDe) for CK on the basis of cyanide toxicity. One estimate (0.035 mg/kg-d) is based on reproductive toxicity in cyanide (CN−)-exposed rats (NTP 1993), in which both a lowest adverse effect level (LOAEL) and a no observable adverse effect level (NOAEL) were identified. The second RfD estimate (RfDe = 0.026 mg CK/kg-d) from Opresko et al. (1998) was developed from human data reported by the Ministry of Health in Mozambique (1984a,b) and based on effects associated with ingestion of CN− found naturally in cassava. Opresko et al. (1998) selected the estimated value of 0.03 mg CK/kg-d as the best RfD estimate. An RfCe of 0.035 mg CK/m3 can be derived from this RfDe by adjusting for adult body weight (70 kg), adult inhalation rate (20 m3/d), and applying an uncertainty factor of 3 to account for extrapolation between ingestion and inhalation exposure routes. If no adjustment is made for route-to-route extrapolation, the RfCe would be 0.11 mg CK/m3.
The USEPA/IRIS (2005) calculated an RfD for cyanogen chloride of 0.05 mg CK/kg-d based on data from a 1955 chronic dietary study in which rats were fed food fumigated with HCN (Howard and Hanzel 1955). A NOAEL was identified for CN− and converted to an equivalent dose of cyanogen chloride. The RfD was calculated by applying uncertainty factors of 10 each for extrapolation between species and for sensitive subpopulations, and a modifying factor of 5 to account for tolerance to CN− that may develop when exposure is via the diet rather than by gavage or ingestion in drinking water. This value can be directly extrapolated to an RfC estimate (0.18 mg CK/m3) by assuming that the uncertainty factor for extrapolation between exposure routes is 1, and adjusting for adult body weight (70 kg) and inhalation volume (20 m3/d). Alternatively, applying an uncertainty factor of 3 to adjust for route-to-route extrapolation gives an RfC estimate of 0.058 mg CK/m3.
Prior to 2008, Region 9 of the USEPA developed an air preliminary remediation goal (PRG) for cyanogen chloride as an exposure guideline for chronic lifetime inhalation exposure. [PRGs are risk-based concentrations intended to be used as guidelines by risk assessors in initial screening-level evaluations of environmental measurements (www.epa.gov/cgi-bin/)]. Region 9 extrapolated directly from the USEPA IRIS oral RfD of 0.05 mg CK/kg-d to an inhalation RfD of 0.05 mg CK/kg-d. By applying several default exposure assumptions (USEPA 2004a,b), USEPA Region 9 calculated an ambient air PRG of 0.18 mg CK/m3. This value was withdrawn in 2008 when Region 9 recommended the use of Regional Screening Levels (RSLs) to replace region-specific PRGs. No new chronic ambient air concentration for CK is proposed because current derivation protocols do not support extrapolation from oral RfDs. Estimated RfC values for cyanogen chloride are shown in Table 3.
Table 3.
Summary of estimated RfC values for CK.
| Derived from information in these sources | Estimated RfC (mg/m3) | Uncertainty factor for route-to-route extrapolation |
| Opresko et al. 1998 | 0.11 | 1 |
| 0.035 | 3 | |
| USEPA IRIS | 0.18 | 1 |
| 0.058 | 3 | |
| USEPA Region 9a | 0.18a | 1a |
aWithdrawn in 2008.
Wood (1997) examined human case reports of CK exposures, epidemiological studies conducted at CK-manufacturing facilities and experimental animal data for the NAC. The concentration of 1 ppm (2.51 mg/m3) is generally accepted as the lowest irritant concentration for a 10-min exposure (Hartung 1994), but this concentration has also been reported to result in copious tearing of the eyes in some persons (Prentiss 1937; Jacobs 1942). Human exposure concentrations of 2 and 20 ppm (5.02 and 50.2 mg/m3) are reported to be intolerable after 10- and 1-min exposures, respectively (Hartung 1994; Flury and Zernick 1931). Increased exposure duration to lower concentrations in humans (no duration or concentration specified) can result in inflammation of the conjunctiva and hoarseness (Flury and Zernick 1931). A minimum detectable odor of 0.6 ppm (1.5 mg/m3) has been reported (Jacobs 1942). For both lethal and nonlethal effects of cyanogen chloride, humans appear to be the most sensitive species. Although irritation and pulmonary edema have been observed in animal studies, these signs occur at concentrations greater than the approximate human irritation threshold of 1 ppm (2.51 mg/m3). Nonlethal studies of CK-exposed experimental animals are limited. Dogs recovered from exposure to either 20 ppm (50 mg/m3) for 20 min or 120 ppm (302 mg/m3) for 8 min, but not without severe injury (Hartung 1994; Flury and Zernick 1931). These concentrations are more than one order of magnitude greater than the concentrations known to elicit signs and symptoms in humans.
Wood (1997) developed a draft technical support document that estimated an AEGL-1 for CK on the basis of the reported minimum irritating concentration in humans from Hartung (1994) [i.e., 1 ppm (2.51 mg/m3)]. Although a NOAEL is not identified in the available human data, this minimum irritation concentration of 1 ppm is not reported to be intolerable or debilitating. Application of an uncertainty factor of 3 (for intraspecies variability) was considered appropriate because the mechanism of action (and resulting physiological response) for an irritant vapor is not expected to vary greatly among individuals in the population. The NAC considered in 1997 that irritation described for CK is not likely to depend on exposure duration for durations between 10 min and 8 h. As a consequence, the NAC decided that it was not necessary to time-extrapolate between the estimated 10-min AEGL-1 [0.33 ppm (0.83 mg/m3)] and the estimated 8-h AEGL-1 [also 0.33 ppm (0.83 mg/m3)] (Wood 1997).
Given the incompleteness of toxicity data for CK, the present analysis concludes that it is appropriate to apply an additional uncertainty factor of 3 to the minimum irritant concentration of 1 ppm in humans (Hartung 1994). Application of the resulting composite uncertainty factor of 10 (3 for intraspecies variability and 3 for database incompleteness) yields a concentration of 0.1 ppm (0.25 mg/m3) as a protective estimate that is applicable to exposure durations less than 8 h. This value is 3-fold lower than the draft AEGL-1, and is also less than the TLV© of 0.75 mg/m3 (ACGIH 2003, 2008). Under maximal continuous exposure durations assumed in the current analysis (greater than 8 h but less than 24 h), a protective assumption of possible cumulative irritant effects is made. Thus, straight-line extrapolation from 8 h to 24 h provides a protective estimate of 0.03 ppm (0.08 mg/m3).
Developing protective CK estimates for airport scenario. In the absence of contemporary experimental data, an ERPG-1 or a finalized AEGL-1, this analysis recommends the following health-protective estimates as pre-planning clearance goals for remediation (Table 2) under the LAX airport scenario parameters governing this remediation assessment:
Exposure durations of <8 h: 0.1 ppm CK (0.25 mg CK/m3).
Exposure durations of >8 h but <24 h: 0.03 ppm CK (0.08 mg CK/m3).
Phosgene. Low-level exposure data for phosgene are sparse. The focus of this evaluation is to identify those data adequate to support estimating a protective exposure concentration of phosgene, as an AEGL-1 has never been developed for this compound (NRC/COT 2002).
Existing exposure estimates for phosgene. Examination of the AEGL database, as well as contact with staff and technical contractors managing the database, found general concurrence that toxicity data on phosgene are inadequate to meet standard protocols (NRC/COT 2001) for deriving an AEGL-1 for any exposure duration. For similar reasons, no ERPG-1 has been developed for phosgene by the AIHA. Available reports and data considered for remediation guidance development include Cucinell (1974), Currie et al. (1987a,b), Diller et al. (1985), Kaerkes (1992), Kodavanti et al. (1997), NRC/COT (2002), USEPA (1986), and Selgrade et al. (1995).
Data and options for phosgene. From an examination of data from Cucinell (1974), Currie et al. (1987a,b), Diller et al. (1985), and their evaluation by NRC/COT (2002), the present analysis draws the following conclusions. At the low exposures tested (0.125 to 1 ppm, or 0.5 to 4 mg/m3),
there is no indication that effects such as decrease in lung ATP (adenosine triphosphate) concentrations as well as increased bronchiolar alveolar lavage fluid protein in the laboratory rat are clinically significant.
there is no indication that the experimentally observed low-level effects noted above are likely to progress to a clinically significant level.
In other experiments, Selgrade et al. (1995) and Kodavanti et al. (1997) used identical exposure protocols in which rats were exposed to 0.1 to 1 ppm (0.4 to 4 mg/m3) phosgene 6 h/d for 4 or 12 wk, followed by a 4-wk recovery period. In Selgrade et al. (1995), the statistically significant effects of 0.1 ppm (0.4 mg/m3) phosgene exposure included (1) Impaired pulmonary clearance of bacteria (animals were challenged with a supra-physiological dose of airborne bacteria after subchronic exposure to phosgene), (2) increased polymorphonuclear leukocytes in bronchoalveolar lavage (BAL) fluid, and (3) decreased number of natural killer cells in BAL fluid.
In Kodavanti et al. (1997), the statistically significant effects of 0.1 ppm (0.4 mg/m3) phosgene exposure included increased lung-to-body-weight ratio, increased lung displacement volume, thickening of terminal bronchioles accompanied by an increased number of inflammatory cells, and increased level of pulmonary hydroxyproline. Collagen staining of the lungs also increased with phosgene exposure, but was not evaluated for statistical significance.
The USEPA (USEPA/IRIS, 2006) utilized data from Kodavanti et al. (1997) as the principal study for deriving a reference concentration for phosgene. That analysis concluded that collagen staining and elevated pulmonary hydroxyproline levels documented by Kodavanti et al. (1997) represented pathological changes indicative of fibrosis. Although acknowledging that the reversibility of these effects is not actually known, the USEPA assumed that the changes did, in fact, reflect irreversible lung fibrosis, identified a NOAEL of 0.1 ppm (0. 4 mg/m3), and derived an RfC of 3 × 10−4 mg/m3.
With the exception of the Kodavanti et al. (1997) data used for RfC derivation, the documented effects of low-level phosgene exposure appear to be reversible and represent indicators (biomarkers) of exposure only. They are not indicative of a functionally or clinically significant effect.
One issue raised in the reviewed papers is whether concentration (C) alone, or C and time (Ct), are key determinants of observed effects for phosgene. Examination of this issue by the NCR/COT (Subcommittee on AEGLs) determined that both C and t are important in characterizing the toxicity of phosgene, and that the data do not allow discrimination between C and t as a key determinant. As a consequence, n = 1 for the Cn × t = k relation, within limits for exposure duration (Haber 1924; Ten Berge et al. 1986; NRC/COT 2001). The current analysis has found no data that would support a value of n ≠ 1. NRC/COT (2002) consider the use of n = 1 to be valid for time extrapolation to 8 h. It is noteworthy that Haber's Law (C × t = k; for n = 1) was derived from phosgene data (Haber 1924).
Developing protective phosgene estimates for airport scenario. Comparison with human and animal data in the AEGL category plots for phosgene illustrates the protective nature of phosgene AEGL derivations (NRC/COT 2002). Data points associated with the (calculated) phosgene AEGL-2 value line at 4-h exposure are those of Currie et al. (1987a,b), and are considered biomarkers of exposure (decrease in lung ATP concentration in the rat; increased bronchiolar alveolar lavage fluid protein in the rat) with no known clinical significance. Thus, the published AEGL-2 values are already considered operationally protective. For comparison, if the 8-h AEGL-2 concentration [0.04 ppm (0.16 mg/m3)] is further down-adjusted by a factor of 2 (i.e., 50%), the resulting concentrations [0.02 ppm (0.08 mg/m3) for 8 h; 0.007 ppm (0.03 mg/m3) for 24 h] fall below the concentrations at which Kodavanti et al. (1997) and Selgrade et al. (1995) observed no clinically significant effects in rats, even when exposure durations extended over weeks [i.e., 0.1-ppm (0.4-mg/m3) phosgene, 6 h/d for 4 or 12 wk].
In the absence of exposure guidelines for phosgene comparable to the AEGL-1, the present evaluation considered existing analyses published and judged scientifically credible by the NRC/COT; evaluated pertinent literature for extended exposure durations; and obtained professional counsel from recognized pulmonary toxicologists and investigators in the field. The following concentrations of phosgene in air as derived from the toxicological investigation of NRC/COT (2002) can be considered health-protective estimates for use as remediation guidelines (Table 2) under the scenario parameters governing this assessment:
Exposure durations of <8 h: 0.02 ppm CG (0.08 mg CG/m3).
Exposure durations of >8 h but <24 h: 0.007 ppm CG (0.03 mg CG/m3).
Comparison with human occupational (Kaerkes 1992) and laboratory animal (Diller 1985; Currie et al. 1987a,b; Kodavanti et al. 1997; Selgrade et al. 1995) exposure data documents the protective nature of the above recommended exposure guidelines. Either no effects or minimal reversible effects were observed at greater concentrations (e.g., 0.1 and 0.35 ppm phosgene). In the case of Kaerkes (1992), workforce monitoring of approximately 200 phosgene processing workers over a period of 10 yr (at an average of 34 phosgene exposures/yr) determined that no signs or symptoms of phosgene toxicity were observed at repeated phosgene exposures at concentrations at least 5 times greater than the reasonable maximum value of 0.02 ppm CG (0.08 mg CG/m3) estimated above.
AGENT INGESTION GUIDELINES
As indicated in the plots of liquid CWA persistence on airport interior media presented in the companion paper by Watson et al. (2011, this issue), it is highly unlikely that non-persistent CWAs will remain on surfaces after decontamination has been performed and verified. However, in cases where natural attenuation or degradation can be anticipated, consideration of surface values becomes extremely important. Post-release concerns could arise regarding the potential for persistent agents such as sulfur mustard or VX to present a continued source of exposure if liquid or aerosols droplets have been released. To meet potential waste-management determinations and landfill agreements with state and Federal agencies as well as stakeholder concerns regarding disposition of porous media as well as soils associated with horticultural plantings located within The Bradley International Terminal, reference dose (RfD) input to standard USEPA exposure models [such as the USEPA Regional Screening Levels for Chemical Contaminants at Superfund Sites (RSLs) and Preliminary Remediation Goals (PRGs)] was evaluated. These same models are often used to estimate protective concentration goals in water and food. Available concentration goals developed for chemicals of concern are provided here to assist pre-planning.
To facilitate such determinations when necessary, summarized in Table 4 are available RfDs and reference dose estimates (RfDe) (in units of mg/kg/day) that have been previously developed and published (Opresko et al. 1998, 2001; USEPA/IRIS 1993, 2005) for the airport compounds of concern. Principal CWA degradation products of interest were previously identified in Watson et al. (2011, this issue); available RfDs and RfDe for the principal CWA degradation products are summarized in Table 5.
Table 4.
Reference dose (RfD) and reference dose estimates (RfDe) for CWAs and TICs.a
| CWA or TIC | Chronic Reference dose (RfD or RfDe) (mg/kg/day) Ingestion: estimate of daily exposure level for general population; chronic exposure duration (7 yr to lifetime) |
| Tabunb (GA) | 4 × 10−5 |
| Sarinb (GB) | 2 × 10−5 |
| Somanb (GD) and Cyclosarinb (GF) | 4 × 10−6 |
| VXb | 6 × 10−7 |
| Sulfur mustardb (H/HD) | 7 × 10−6 |
| Hydrogen cyanidec (AC) | 0.02 |
| Cyanogen chlorided (CK) | 0.05 |
| Phosgenee (CG) | No estimate availablee |
aAdapted from Munro et al. (1999) and Talmage et al. (2007a).
bOpresko et al. (2001). Values for nerve and sulfur mustard agents are estimates (RfDe) and considered scientifically valid by the National Research Council (NRC/COT 1999; Bakshi et al. 2000); they have not been formally reviewed by IRIS. The value for GF is also an RfDe and has been developed by analogy to GD.
dUSEPA/IRIS (2005). A comprehensive review of toxicological studies was completed in May, 2005; USEPA determined that results of review did not warrant a change in RfD at that time (http://www.epa.gov/ncea/iris/subst). CK also known as chlorine cyanide.
eIRIS (http://www.epa.gov/iris/subst) states that phosgene oral RfD was under discussion as of January 31, 2006. There has been no change in this determination as of August 2010.
Table 5.
Principal CWA degradation products and their estimated RfDe.a
| Agent Parent (CAS number) | Key degradation product | Degradation Product CAS number | Chronic toxicity valueb (estimated RfDe, in mg/kg/day) |
| Tabun (GA) (77-81-6) | None of potential concern | - | - |
| Sarin (GB) (107-44-8) | Methyl phosphonic acid (MPA) | 993-13-5 | 0.02 |
| Isopropyl methylphosphonic acid (IMPA) | 1832-54-8 | 0.10 | |
| Soman (GD) (96-64-0) | Methyl phosphonic acid (MPA) | 993-13-5 | 0.02 |
| Cyclosarin (GF) (329-99-7) | |||
| VX (50782-69-9) | S-(2-diisopropylaminoethyl) methylphosphonothioic acid (EA 2192) | 73207-98-4 | 6 × 10−7 |
| Ethyl methylphosphonic acid (EMPA) | 1832-53-7 | 0.028 | |
| Methyl phosphonic acid (MPA) | 993-13-5 | 0.02 | |
| Sulfur mustard (H, HD) (505-60-2) | Thiodiglycol (TDG) | 111-48-8 | 0.400c |
aDegradation products selected on basis of environmental persistence and/or toxicity; Talmage et al. (2007a,b), Munro et al. (1999), Reddy et al. (2005), and Capacio et al. (2008).
bUnless otherwise noted, values were estimated by Bausum et al. (1999).
The CWA RfD estimates summarized in Table 4 have been previously incorporated into standard USEPA models developed by Region 9 (PRG) and Region 3 [Risk-Based Concentration (RBC) model] (USEPA 1996a,b, 2001) to develop agent-specific and health-based environmental screening levels (USACHPPM 1999; Watson and Dolislager, 2007). Resulting RBC and PRG values for airport scenario compounds are summarized in Table 6. For the purpose of the present analysis, Regional Screening Levels (RSLs) have also been estimated by application of the RSL calculator available at USEPA (2009a; http://www.epa.gov/reg3hwmd/risk/human/rb-concentration_table/usersguide.htm). Because PRG, RBC and RSL models incorporate assumptions of continuous release and multi-year exposure durations, the health-based screening levels in Table 6 are highly protective under the one-time, single-release airport scenario, which incorporates subsequent source removal and decontamination. PRG and RSL estimates incorporate consideration of chronic incidental soil ingestion as well as dermal contact with soil, and inhalation of particulates and vapors emitted from soil (USEPA 1991, 1996a,b, 2001). Calculation of risk-based concentrations (RBCs) from the RBC model does not consider inhalation or dermal contact components, which are important to overall risk management analysis and final determination of acceptable clearance goals. As a consequence, RBC values are provided only for completeness.
Table 6.
Health-based environmental screening levels for CWAs and TICs in residential and industrial soils.a
| Residential soilb |
Industrial soil |
||||
| CWA or TIC | Preliminary Remediation Goal (PRG) (mg/kg soil) | Risk-Based Concentration (RBC) (est.) (mg/kg soil)a | Regional Screening Level (RSL) (est.) (mg/kg/soil)e | Preliminary Remediation Goal (PRG) (mg/kg soil) | Risk-Based Concentration (RBC) (est.) (mg/kg soil)a |
| Tabun (GA) | 2.8 (est.)a | 3.1 | 3.1 | 68 (est.)a | 82 |
| Sarin (GB) | 1.3 (est.)a | 1.6 | 1.6 | 32 (est.)a | 41 |
| Soman (GD) | 0.22 (est.)a | 0.31 | 0.31 | 5.2 (est.)a | 8.2 |
| VX | 0.042 (est.)a | 0.047 | 0.047 | 1.1 (est.)a | 1.2 |
| Sulfur mustard (HD) | 0.01 (est.)ac | 0.55 | 0.55 | 0.3 (est.)ac | 14 |
| Hydrogen cyanide(AC) | 1.6 × 103d | NA | 1.6 × 103 | 2.0 × 104d | NA |
| Cyanogen chloride (CK) | 3.9 × 103d | NA | 3.9 × 103 | 5.1 × 104d | NA |
| Phosgene (CG) | NA | NA | NA | NA | NA |
aDerived and presented in USACHPPM (1999) and OASA (1999) using EPA Region 3 model for RBC calculation, USEPA Region 9 model for PRG calculation, and agent-specific RfD estimates. Recent verification confirmed the protective nature of USACHPPM (1999) derivations (Watson and Dolislager 2007).
bMay be used alone or in conjuction with vapor exposure criteria to assess possible existence of residual agent in semi-porous or porous media and to demonstrate potential for chemical agent being present in/on item or material at levels of public health concern. Useful for facilities and areas potentially exposed to extended high vapor or liquid concentrations (DA 2008, Table C-5).
cCancer-based; calculated from a target excess risk level of 10−5 for residential and 10−4 for industrial [(USACHPPM 1999; See also Watson and Dolislager (2007)].
dEPA Regional Screening Levels for Chemical Contaminants (USEPA 2008) and EPA Region 9 PRG; values to be used as a screening goal or initial cleanup level. Considered protective for humans (including sensitive groups) over a lifetime. Guideline only, not regulation. EPA Region 9 “Regional Screening Levels for Chemical Contaminant at Superfund Sites, May 20, 2004” assumes soil ingestion only (USEPA 2004b, 2005; Available at http://www.epa.gov/region09/waste/sfund/prg/index.html). See “Regional Screening Levels for Chemical Contaminants …” (USEPA 2008; Available at http://epa-prgs.ornl.gov/chemicals/).
eFor the purpose of the present analysis, Regional Screening Levels (RSLs) have also been estimated by use of the RSL calculator available at: USEPA (2009; see http://www.epa.gov/reg3hwmd/risk/human/rb-concentration_table/usersguide.htm). The resulting RSL estimates are identical to the above RBC (residential soil) values for nerve agents and sulfur mustard, and are identical to the above PRG (residential soil) values for hydrogen cyanide and cyanogen chloride. There are still no comparable values for phosgene.
The Regional Screening Level (RSL) application was released by the USEPA in late 2008; the RSL user guide disclaimer states that the RSL guidance is not mandatory, does not provide binding rules, and points out that alternative approaches for risk assessment may be more appropriate on a site-specific basis (USEPA 2009a, http://www.epa.gov/reg3hwmd/risk/human/rb-concentration_table/usersguide.htm). While originally developed by Regions 3, 6, and 9, the RSLs are presently (August 2010) accepted by many other USEPA regions. It is known that Preliminary Remediation Goals (PRGs) other than RSLs are in current use within all ten USEPA Regions, and reference to PRG use continues to be valid at this writing.
In 2004, USEPA Region 9 had developed PRG values for HCN and cyanogen chloride ingestion based on long-term (chronic) and direct ingestion of contaminated soil (USEPA 2005). The same PRG values are listed in the most recent regional screening level update (USEPA 2008) identified as an update of the USEPA Region 3 RBC Table, Region 6 HHMSSL (Human Health Medium-Specific Screening Levels) Table and the Region 9 PRG Table.
As of this writing, Region 9 maintains a link to the 2004 PRGs as well as providing RSLs. Analogous values have not been developed for phosgene. Separate PRGs that address either residential- or industrial-use scenarios (Table 6) are available from USEPA (2005, 2008) for hydrogen cyanide and cyanogen chloride.
RfD information for degradation products of interest has also been incorporated into PRG models to develop compound-specific degradation product concentration estimates for soil (Table 7). This approach could be employed to develop site-specific degradation product estimates for water and food in accordance with site-specific risk management criteria.
Table 7.
Health-based environmental screening levels for principal CWA degradation products in residential and industrial soils and employing EPA PRG parameters.
| Residential soil |
Industrial soil |
||
| Degradation Product of CW Agent | Preliminary Remediation Goal (PRG) (mg/kg soil)ab (estimates) | Outdoor Worker Preliminary Remediation Goal (PRG) (mg/kg soil)a (estimates) | Indoor Worker Preliminary Remediation Goal (PRG) (mg/kg soil)a (estimates) |
| TDG (thiodyglycol) | 2.4 × 104 | 6.0 × 104 | 8.2 × 105 |
| EMPA (ethyl methylphosphonic acid) | 1.7 × 103 | 4.2 × 103 | 5.7 × 104 |
| MPA (methyl phosphonic acid) | 1.2 × 103 | 3.0 × 103 | 4.1 × 104 |
| EA 2192 | 4.7 × 10−2 | 6.8 × 10-1 | 1.2 |
aDerived and presented in Watson and Dolislager (2007). PRG values to be used as a screening goal or initial cleanup level, and are considered protective for humans (including sensitive groups) over a lifetime. Guideline only, not regulation.
bMay be used alone or in conjuction with vapor exposure criteria to assess possible existence of residual agent in semi-porous or porous media and to demonstrate potential for chemical agent being present in/on item or material at levels of public health concern. Useful for facilities and areas potentially exposed to extended high-concentration vapor or liquid concentrations (DA 2008, Table C-5).
Drinking water concentrations considered protective estimates for the chemicals of concern and summarized as compound-specific concentrations in Table 8 are developed from assumptions of a 5 or 15 liter/day drinking water consumption rate (DA 2005b, 2010). Because most U.S. domestic drinking water consumption rate estimates for adults are ≤2 liter/day (USEPA 1989), the low drinking water concentrations resulting from an assumed 5 or 15-liter/day consumption rate are thus highly protective (e.g., lower concentration associated with high-volume consumption) for application as general public decision criteria for water ingestion.
Table 8.
Drinking water concentrations considered protective estimates for site decontamination and verification.
| 5 liter/day consumptiona |
15 liter/day consumptiona |
|
| CWA or TIC | Drinking water concentration (μg/L) | |
| Tabun (GA) | <140 | <46 |
| Sarin (GB) | <28 | <9.3 |
| Soman (GD) | <12 | <4 |
| VX | <15 | <5 |
| Generic nerve agent | <12 | <4b |
| Sulfur mustard (HD) | <140 | <47 |
| Hydrogen cyanide (HCN; agent AC) | <6 | <2 |
aDOD Tri-Service standard for adult consumption rate of 5 and 15 liter/day, respectively, for exposure duration <7 days (DA 2005b). The most recent update to these Tri-Service standards (DA 2010) makes no reference to the daily volume of water ingested and establishes the same concentrations presented above for 15 liter/day consumption as standard (e.g., no concentrations specific to 5 liters/day consumption). DA (2010) further recommends application of the “Generic nerve agent” concentration of 4 μg/L for all nerve agents.
b“Generic nerve agent” water concentration standard equivalent to lowest concentration estimated for all nerve agents evaluated.
It is noted that subchronic RfDs for some persistent (illicit drug) compounds such as methamphetamine are being used by the California Environmental Protection Agency (CalEPA) Office of Environmental Health Hazard Assessment (OEHHA) (Salocks 2007, 2008, 2009) in determination of protective re-entry criteria to buildings formerly used as clandestine manufacturing facilities and that have undergone decontamination. This host-state example documents that subchronic RED estimates are recognized as useful and appropriate for specific screening level assessment applications and thus appropriate for the LAX scenario CWAs and TICs; USEPA (2009b) recognizes the utility of this approach by California regulatory authorities in developing a health-based target remediation standard for methamphetamine.
Oral Minimal Risk Levels (MRLs) are presently available only for sulfur mustard agent HD (MRL for acute-duration exposure of ≤14 d is 0.0005 mg HD/kg/d; MRL for intermediate-duration exposure of 15 to 364 d is 0.00007 mg/kg/d) (ATSDR 2003), and may also be useful for specific screening assessments.
GUIDELINES FOR IN-SITU ITEMS
Following a terrorist attack involving the release of a CWA or TIC, it may be necessary to decontaminate, handle, or otherwise manage large in-place items, such as X-ray scanners. It will also be necessary to manage numerous objects such as furniture and potentially contaminated samples. General guidance for sample handling is summarized on a compound-specific basis in recently published Quick Reference Guides for CWAs (NRT 2009; available at www.nrt.org). The National Response Team cautions that sample packaging or shipping protocols should be closely coordinated with the receiving analytical laboratory.
The previously derived “Reasonable maximum re-entry screening guidelines” (Table 2; equivalent to the 8-h AEGL 1), may be used as appropriately protective and health-based vapor screening criteria for releasing items, equipment and facilities that have not been contaminated by liquid or aerosol forms of the chemicals of concern (DA 2008, Table C-5). Further, these same screening criteria would also be useful to evaluate decontaminated non-porous items and surfaces, providing that vapor and surface screening criteria are attained (DA 2008).
For solid matrices, existing Health Based Environmental Screening Levels (mg/kg; HBESLs) as documented in Table 6 for nerve and sulfur mustard agents are considered highly protective exposure criteria for lifetime (24-h) daily ingestion exposure by the general population (based on NOAEL; USACHPPM 1999; Watson and Dolislager 2007). These criteria could be used alone or in parallel with vapor exposure criteria (see above) to assess potential existence of residual nerve or sulfur mustard agents within porous or semi-porous media at a level of public health insignificance. Such solid matrix decision criteria could be reasonably applied in the event of an extended high-concentration vapor or liquid exposure.
During World War I (WWI), the U.S. Government established an experimental facility (American University Experiment Station) in Spring Valley (in what was then rural Maryland) for testing and preparing chemical warfare compounds, incendiaries, smokes, and signal materials for use in battlefields (Zongkar 2010). Protective clothing and equipment to counter use of such materials were also developed at the Spring Valley facility. The facility was closed at the end of the War, and agent-containing materials underwent disposal in various pits and trenches on-site. CWAs and TICs tested at the facility included vesicant agents sulfur mustard and Lewisite (an organic arsenical not included in the airport threat scenario), phosgene, cyanogen chloride, arsine, and chloropicrin. Nerve agents were not formulated until decades after WWI and are not an issue at Spring Valley (PARSONS 2007).
As a result of urban growth since the close of WWI, the former American University Experiment Station is now located within the District of Columbia. Protocols have recently been developed to guide sample and site clearance of soils, scrap, PPE, and bulk items recovered from the Spring Valley site in metropolitan Washington, D.C. (PARSONS 2007).
Criteria for the Spring Valley site and materials release involve warming a sample (15 min equilibration at 90 ± 10°F for soil; 4 h equilibration at 70 ± 10°F for scrap, PPE and bulk items); analyzing headspace offgas concentrations of HD, phosgene, and CK; then comparing offgas concentrations with agent-specific exposure limits (see below for specific values; Appendix J of PARSONS 2007). Sample clearance determinations at Spring Valley are as follows (Appendix J of PARSONS 2007):
If offgas concentrations are <0.25 of the short term exposure limit (STEL) (i.e., equal to 0.00075 mg/m3 for sulfur mustard, 0.10 mg/m3 for phosgene, and 0.15 mg/m3 for CK), the sample is cleared for disposal.
If offgas concentrations are >0.25 of the STEL (i.e., equal to 0.00075mg/m3 for sulfur mustard, 0.10 mg/m3 for phosgene, 0.15 mg/m3 for CK), then samples are reheated and allowed to equilibrate at the media-specific temperatures identified above, and re-sampled.
Application of the above guidelines would be useful in making clearance determinations involving problematic in-place items otherwise difficult (or destructive) to treat with aqueous solutions, foams, oxidizing vapors, and so on.
GUIDELINES FOR SURFACE CONTACT WITH POTENTIAL CWA AND DEGRADATION PRODUCT RESIDUES
Given the chemical and physical characteristics of the scenario compounds of concern [Watson et al. (2011, this issue), Table 2] and the efficacy of compound-specific decontamination materials and methods (Ho et al. 2006), it is unlikely that scenario CWA or TIC residues would exist on surfaces in quantities sufficient to pose a toxicological hazard after source removal and decontamination have been performed. Nevertheless, a contact hazard analysis that focuses on the persistent nerve agent VX and the persistent vesicant agent sulfur mustard (HD) has been performed to address stakeholder community requests for protective guidance regarding potential surface residues. The working assumption is that, in the unlikely event that exposed skin comes in contact with any residual compound of concern, the residue could be transferred to hands and subsequently carried to the eyes and mouth multiple times/day. Resuspension inhalation of particulates has also been considered. Exposure estimates, termed Surface Removal Contaminant Levels (SRCLs), have been developed and calculated so as to prevent development of a minimal and reversible effect threshold; SRCLs represent an estimated surface concentration in mass per unit area (mg/cm2). Specific parameters and assumptions are identified and characterized below.
For VX, the SRCL estimates are designed to prevent non-adverse levels of miosis (the first noticeable effect) in the absence of any other signs or measureable inhibition of blood cholinesterase activity. For HD, the SRCL estimates are designed to prevent mild reversible ocular irritation (the most sensitive endpoint and tissue).
Concepts and protocols considered by the USEPA dermal risk-assessment guidance (USEPA 2004a) have been evaluated for application to the airport chemical terrorist release scenario. In addition, information from the World Trade Center (WTC) Indoor Assessment (COPC 2003) as well as the U.S. Army Man-in-Simulant Test (MIST) Program (NRC/BAST 1997) to calculate risk-based and site-specific concentrations for building interiors is also incorporated. Other sources contributing to the evaluation of acceptable residues on surfaces include agent-specific percutaneous toxicity data, concepts of acceptable residues and re-entry intervals developed by various state agencies and the USEPA for pesticide-treated crops, and exposure models under development by USEPA's National Exposure Research Laboratory (Stallings et al. 2008; Zartarian et al. 2008; Cohen Hubal et al. 2000, among others). Concepts underlying risk-based criteria developed by CalEPA for use in assessing child potential exposure to surface methamphetamine residues in former clandestine laboratories (Salocks 2007, 2008, 2009) also contribute to the analyses. The equations used in this assessment are also based on the USEPA Risk Assessment Guidance for Superfund (USEPA 1991). The stochastic model approach developed by CalEPA was used for verification purposes in the present assessment. As data allow, and as recommended by CalEPA, compound-specific subchronic reference doses have been estimated for the scenario compounds and incorporated into the present calculation of SRCLs.
The same analytical procedure has been applied to previously identified, stable, agent-specific degradation products of toxicological concern, such as methyl phosphonic acid (MPA) and EA 2192 (potential hydrolysis product of agent VX, which occurs when pH is not adequately controlled during VX decontamination) (Michel et al. 1962; also Watson et al. 2011, this issue, Table 3).
Estimated SRCLs for persistent CWAs and key degradation products are unique to the present analysis and are developed here as reasonable maximum exposure (RME) concentrations derived from standardized equations that combine exposure information and available compound-specific toxicity information. SRCLs are presented for child transit passengers (the most susceptible transit passenger subpopulation with expected maximal exposure to surface residues due to frequent object mouthing behavior and potential for large-area skin contact with airport terminal surfaces) as well as various airport employees and personnel. SRCLs presented here are protective risk-based screening values for use in evaluating surfaces that have (or may have) been in contact with HD or VX released during a chemical terrorist event, or degradation products of interest. SRCL analysis has been performed as a screening level assessment to protect against the unlikely event that airport surfaces have been incompletely decontaminated and inadequately sampled and monitored. It is suggested that SRCL results be used during post-decontamination monitoring to ascertain that surface contact hazards to chemical residues do not remain. Exceeding a SRCL usually suggests that further evaluation of the potential chemical risks is warranted, while attainment of SRCL levels would indicate that potential chemical risk is so low as to allow unprotected access to the terminal by the general public as well as various airport employees and personnel. In addition to utility as screening levels, SRCLs could also be used to trigger further investigation or serve as initial clearance goals for decision-makers.
Assumptions Regarding Percutaneous Contact with Residual Liquids or Aerosols
The more volatile compounds on the scenario threat list (e.g., the nonpersistent G-series nerve agents and the TICs phosgene, hydrogen cyanide, and cyanogen chloride) dissipate readily and pose much less risk of percutaneous contact or non-dietary ingestion exposure to deposited liquid or aerosol than the persistent CWA compounds HD and VX. Nevertheless, equations developed to evaluate persistent compounds can also be applied to provide highly protective estimates for the more volatile compounds.
It is understood that agents HD and VX will volatilize and degrade by known processes such as hydrolysis during the interval between the time of release and initiation of decontamination as well as during the interval between completion of decontamination and finalization of a clearance decision [Watson et al. (2011, this issue), Tables 2 through 4; and Talmage et al. 2007a,b)]. To ensure protectiveness, known rates of volatilization and degradation are not factored into the SRCL equations.
The instability of liquid HCN [NIOSH 2005b; Aaron 1996; ATSDR 2005, 2006; Watson et al. (2011, this issue), Table 2] indicates that HCN deposited as a liquid, or present as a residual aerosol, will not persist for more than a few minutes at ambient temperature. The possibility of direct percutaneous exposure to liquid or aerosolized HCN is also considered extremely low.
Assumed opportunities for exposure to a toddler child transit passenger would be from contacting hard surfaces (vinyl floors and chairs, desks, counters, walls, etc.) and soft surfaces (carpet, upholstered chairs, drapes, etc) with hands and other exposed skin. A fraction of any compound transferred to hands is assumed to be subsequently transferred to eyes and mouth. To develop a protective estimate, exposure assumptions for a toddler child transit passenger (the most susceptible population) have been selected as representative of the entire transit passenger population. Further, any potential adult airport employee/personnel exposure is assumed to occur during contact with hard or soft surfaces such as carpets, counters, drapes, seating, walls, and ceilings during post-decontamination activities.
Because the eye is extremely sensitive to the effects of HD and VX vapor and liquid (NRC/COT 2003), contact hazard equations focus on consideration of eye exposure to estimate the most protective SRCL. This is a unique aspect of the current assessment, as direct eye exposure is not commonly evaluated in surface contact assessments. Agent-specific eye contact (ocular) toxicity values were not available in the literature; as a consequence, dermal toxicity values for skin were substituted, and 100% transfer from hand to eye was assumed; this approach is considered protective.
Media that can be sampled for comparison to the SRCLs could range from agentladen dust to any surface films transferable to a sample wipe or surface probe.
Surface Exposure Parameters
Exposure parameters, variables, and values incorporated into the SRCL model equations for agents HD and VX, as well as HD and VX degradation products, are identified and characterized in Tables 9 and 10. To account for variability in likely exposure scenarios, estimates are presented for the average and maximum anticipated exposures. Such an analysis replicates many of the assumptions employed in the WTC assessment for indoor environments (COPC 2003). A notable deviation from the WTC assessment is the fact that the present SRCL equations do not include a dissipation factor for surface residues. Instead, the present analysis incorporates agent degradation rates previously summarized in Watson et al. (2011, this issue) to evaluate SRCL-specific exposure durations and frequencies. Calculations in Watson et al. (2011, this issue; Table 4 and Figure 6) illustrate that multiple degradation half-lives for VX and HD can be achieved in less than 2 d following application of appropriate decontamination solutions. In the case of the present analysis, selection of values for ED and EF are based on well-characterized and rapid degradation generated during the preceding decontamination phase. Hence, the SRCL equations do not include a decay/dissipation rate. While traditional exposure assessments have been based on consideration of human activity patterns and not the presence/absence of contamination (USEPA 1991), rapid agent degradation generated during the decontamination phase directs the selection of ED and EF values in the present analysis.
Table 9.
Exposure parameter assumptions for HD and VX incorporated into SRCL assessment.a
| Parameter | Estimated airport average | Estimated airport maximum | Units |
| ABSHD | 0.084 | 0.084 | Unitless |
| ABSVX | 0.033 | 0.033 | Unitless |
| BWW | 70 | 70 | Kg |
| BWC | 15 | 15 | Kg |
| CF1 | 3.65E+02 | 3.65E+02 | days/year |
| CF2 | 0.041667 | 0.041667 | days/hour |
| CF3 | 10000 | 10000 | cm2/m2 |
| DF work HD | 285 | 427.5 | cm2/day |
| DF work VX | 8.075 | 12.1125 | cm2/day |
| DF trans child HD | 70.0 | 420 | cm2/day |
| DF trans child VX | 1.98 | 11.9 | cm2/day |
| ED work | 1 | 1 | Years |
| ED child | 1 | 1 | Years |
| EF work | 2 | 2 | days/year |
| EF trans (child) | 2 | 2 | day/year |
| ETh child | 2 | 12 | hours/day |
| ETh work | 4 | 6 | hours/day |
| ETs work | 4 | 6 | hours/day |
| ETs child | 2 | 12 | hours/day |
| FQc | 9.5 | 9.5 | events/hour |
| FQeye | 10 | 10 | event/day |
| FQwork | 1 | 1 | event/hour |
| FTSHSh HD | 0.5 | 0.5 | Unitless |
| FTSHSh VX | 0.0018 | 0.0018 | Unitless |
| FTSHSs HD | 0.1 | 0.1 | Unitless |
| FTSHSs VX | 0.0152 | 0.0152 | Unitless |
| FTSBSh HD | 0.25 | 0.25 | Unitless |
| FTSBSh VX | 0.0009 | 0.0009 | Unitless |
| FTSBSs HD | 0.05 | 0.05 | Unitless |
| FTSBSs VX | 0.0076 | 0.0076 | Unitless |
| GIABS HD&VX | 1 | 1 | Unitless |
| IF work HD | 54 | 81 | cm2/day |
| IF work VX | 1.53 | 2.295 | cm2/day |
| IF trans HD | 85.5 | 513 | cm2/day |
| IF trans VX | 2.4225 | 14.535 | cm2/day |
| OF work HD | 33.4 | 50.1 | cm2/day |
| OF work VX | 0.9463 | 1.4195 | cm2/day |
| OF trans HD | 16.7 | 100.2 | cm2/day |
| OF trans VX | 0.47 | 2.839 | cm2/day |
| RF | 1.00E-06 | 1.00E-06 | m-1 |
| RfD est. subchron HD | 7.00 E-05 | 7.00 E-05 | mg HD/kg/d |
| RfD est. subchron VX | 2.00 E-06 | 2.00 E-06 | mg VX/kg/d |
| SA eyes | 33.4 | 33.4 | cm2/event |
| SA trans exposed skin | 2800 | 2800 | cm2/day |
| SA trans hand | 15 | 15 | cm2/event |
| SA work exposed skin | 5700 | 5700 | cm2/day |
| SA work hand | 45 | 45 | cm2/event |
| SE | 0.5 | 0.5 | Unitless |
| THQ | 1 | 1 | Unitless |
| AT nc | 365∗ED | 365∗ED | Days |
aVariables: dermal absorption factor (ABS), averaging time (AT), body weight (BW), conversion factor (CF1-3), dermal factor (DF), exposure duration (ED), exposure frequency (EF), exposure time (ET), frequency of hand-to-mouth for child (FQc), frequency of hand-to-eye (FQeye), fraction transferred from surface to body skin (FTSBS), fraction transferred from surface to hand skin (FTSHS), gastrointestinal absorption factor (GIABS), ingestion factor (IF), ocular rubbing factor (OF), resuspension factor (RF), reference dose (RfD), hand surface area (SAhand), exposed skin surface area (SAtrans and SAwork), eye surface area (SAeye), saliva extraction factor (SE), target hazard quotient (THQ); “c” and “w” modifers refer to child transit passenger and various airport employees and personnel (worker), respectively; “s” and “h” modifiers refer to soft and hard surfaces, respectively; “nc” refers to non-cancer. See text for definitions and assumptions.
Table 10.
Exposure parameter assumptions for significant HD and VX breakdown products incorporated into SRCL assessment.
| Parameter | Estimated airport average | Estimated airport maximum | Units |
| ABSTDG | 0.009 | 0.009 | Unitless |
| ABSEMPA | 0.084 | 0.084 | Unitless |
| ABSMPA | 0.084 | 0.084 | Unitless |
| ABSEA2192 | 0.0 | 0.0 | Unitless |
| DF trans child TDG | 70 | 420 | cm2/day |
| DF trans child EMPA | 70 | 420 | cm2/day |
| DF trans child MPA | 70 | 420 | cm2/day |
| DF trans child EA2192 | 1.98 | 11.9 | cm2/day |
| FTSHSh TDG | 0.5 | 0.5 | Unitless |
| FTSHSh EMPA | 0.5 | 0.5 | Unitless |
| FTSHSh MPA | 0.5 | 0.5 | Unitless |
| FTSHSh EA2192 | 0.0018 | 0.0018 | Unitless |
| FTSHSs TDG | 0.1 | 0.1 | Unitless |
| FTSHSs EMPA | 0.1 | 0.1 | Unitless |
| FTSHSs MPA | 0.1 | 0.1 | Unitless |
| FTSHSs EA2192 | 0.0152 | 0.0152 | Unitless |
| FTSBSh TDG | 0.25 | 0.25 | Unitless |
| FTSBSh EMPA | 0.25 | 0.25 | Unitless |
| FTSBSh MPA | 0.25 | 0.25 | Unitless |
| FTSBSh EA2192 | 0.0009 | 0.0009 | Unitless |
| FTSBSs TDG | 0.05 | 0.05 | Unitless |
| FTSBSs EMPA | 0.05 | 0.05 | Unitless |
| FTSBSs MPA | 0.05 | 0.05 | Unitless |
| FTSBSs EA2192 | 0.0076 | 0.0076 | Unitless |
| IF trans TDG | 85.5 | 513 | cm2/day |
| IF trans EMPA | 85.5 | 513 | cm2/day |
| IF trans MPA | 85.5 | 513 | cm2/day |
| IF trans EA2192 | 2.4225 | 14.5 | cm2/day |
| OF trans TDG | 16.7 | 100.2 | cm2/day |
| OF trans EMPA | 16.7 | 100.2 | cm2/day |
| OF trans MPA | 16.7 | 100.2 | cm2/day |
| OF trans EA2192 | 0.47 | 2.839 | cm2/day |
| RfD est. subchron TDG | 4.00E+00 | 4.00E+00 | mg TDG/kg/d |
| RfD est. subchron EMPA | 2.80E-01 | 2.80E-01 | mg EMPA/kg/d |
| RfD est. subchron MPA | 2.00E-01 | 2.00E-01 | mg MPA/kg/d |
| RfD est. subchron EA2192 | 2.00E-06 | 2.00E-06 | mg EA2192/kg/d |
| RfC est. subchron TDG | 4.70E+00 | 4.70E+00 | mg TDG/m3 |
| RfC est. subchron EMPA | 3.40E-01 | 3.40E-01 | mg EMPA/m3 |
| RfC est. subchron MPA | 2.40E-01 | 2.40E-01 | mg MPA/m3 |
| RfC est. subchron EA2192 | 0.00E+00 | 0.00E+00 | mg EA2192/m3 |
Variables: See footnote to Table 9.
Illustrated in Figure 1 are the SRCL equation and calculation for estimating VX effects for toddler child (sensitive receptor) transit passengers; in Figure 2 are presented the comparable VX SRCL model equation and calculation for airport employees and personnel. While nerve agent VX was chosen as the example calculation for Figures 1 and 2, the user can substitute HD- or degradation product-specific parameters as needed. Presented in Figure 3 is a conceptual site model of potential exposure pathways evaluated for surfaces (as modeled in Figures 1 and 2) as well as direct and offgas vapor exposure.
Figure 1.
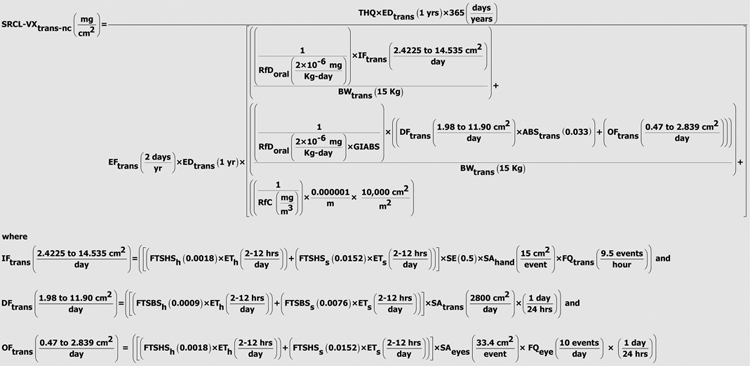
Airport transit passenger toddler SRCL estimate for VX (see Table 9 for CWA-specific parameters).
Figure 2.
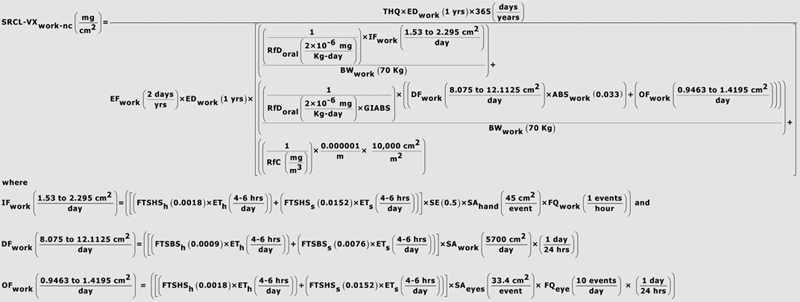
Airport employee and personnel SRCL for VX (see Table 9 for CWA-specific parameters).
Figure 3.
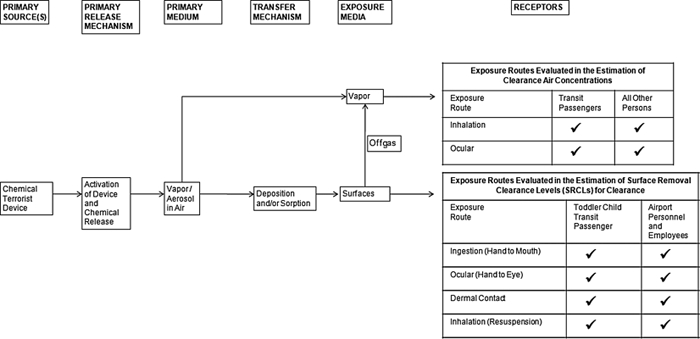
Conceptual site model of potential exposure pathways evaluated.
Exposure time (ET)
Exposure time (ET) for this surface analysis represents the estimated hours per day that a receptor spends in contact with potentially contaminated media. In general, exposure times vary by exposure scenario, age of the receptor, and whether the source is located on a hard or soft surface. Most ET data and USEPA default values for children [COPC (2003) and the USEPA Office of Pesticide Programs (OPP)] have been experimentally determined from USEPA observational studies characterizing the time spent by child residents per day in the kitchen and bathroom. Estimates of carpet exposure time are based on remaining indoor time, excluding sleeping. The World Trade Center assessment (COPC 2003) judged these data to be representative of many children under age 6 who spend most of their time at home. It is acknowledged that potential exposures at an airport would not be representative of at-home exposures in terms of activity and duration, and that data characterizing time spent on hard and soft surfaces at airports are not available.
To more closely represent the non-resident characteristics of the airport chemical terrorist scenario, it was necessary to depart from use of generic default exposure parameters (e.g., child resident defaults are 8 h/day for carpets and 4 h/day for hard surfaces) (COPC 2003). As a consequence, this analysis divided the exposure to hard and soft surfaces equally for a full 24 h (e.g., 12 h for hard and 12 h for soft). The duration that a passenger spends in a terminal is designated as the “dwell time” or stay time (CAM 2005). The Center for Airport Management (CAM 2005) data revealed that the total dwell time for most passengers is ≤4 h. Because the time spent in a terminal in one day can vary greatly, an average, minimum total ET to hard and soft surfaces is estimated to be 4 h (e.g., 2 h for soft surface exposure plus 2 h for hard surface exposure), and the maximum is estimated to be 24 h (to accommodate long layovers and delays) (e.g., 12 h each for soft and hard surface exposures).
COPC (2003) does not provide information on WTC generic employee exposures to hard and soft surfaces. Because of the high variability of potential surface contact for airport employees and personnel, surface exposure time is also divided equally between exposure to hard and soft surfaces in the current analysis. Furthermore, because the workday length can vary, an average total workday ET to hard and soft surfaces was estimated to be 8 h (regular work shift), and the maximum is estimated to be 12 h. The same work shift assumptions are used by the CDC (DHHS 2003, 2004) and USEPA (1997).
Exposure duration (ED)
The equations in Figures 1 and 2 were developed for this analysis and for non-carcinogenic effects. These equations are consistent with examination of compound-specific toxicological data that indicate diminishing toxic response and effects recovery following cessation of exposure. In non-cancer effect PRG equations, the exposure duration (ED) in the numerator multiplied by 365 days per year represents the time over which exposure is averaged. The ED in the denominator multiplied by the exposure frequency represents the time of exposure. Dividing the numerator by the denominator “pro-rates” the exposure (USEPA 1989).
Exposure durations between 2 weeks and less than 7 years are considered subchronic, while exposure durations greater than 7 years are considered chronic (USEPA 1989). For the transit passenger receptor as well as airport employee/personnel receptors, an ED of one year is selected for the present analysis. This ED is based on the degradation analysis performed in the first paper of this series (Watson et al. 2011, this issue; Table 4 and Figure 6), which illustrates that multiple degradation half-lives for persistent agents VX and HD can be achieved in less than 2 days following application of appropriate decontamination solutions. Note that an exposure duration of 1 year is the smallest exposure duration presented in USEPA (1989) for standard risk equations.
It is recognized that most exposure parameters have been traditionally based on human activity patterns and not the presence/absence of contamination (USEPA 1991). In the case of a transit passenger, it is possible that, during a single round trip, a passenger would pass through the same terminal during a single year. Also, it is acknowledged that, without appropriate consideration of the decontamination phase and well-characterized degradation reactions previously discussed (Watson et al. 2011, this issue; Talmage et al. 2007a,b; Yang et al. 1994; Yang 1999; many others), some investigators may unknowingly consider setting the ED for an airport employee as high as the EPA-recommended default of 25 years. Previous characterization of the agent release and response scenario (Watson et al. 2011, this issue) precludes the necessity of relying on default values. With appropriate decontamination and treatment, a persistent compound from the airport scenario threat list can pose a nonpersistent hazard in that any residual contamination can be quickly degraded. No chronic exposure duration [e.g., > 7 years as per (USEPA 1991)] is assumed.
Exposure frequency (EF)
Exposure frequency (EF) is the number of days within the ED that exposure is assumed to occur. Based on human activity, it is reasoned that a single round-trip transit passenger may be exposed to potentially contaminated surfaces in the same terminal for a maximum of 2 days during a single round trip (the first day for trip departure, and the second day for the trip return). Additionally, and based on the ED discussion above and calculations in Watson et al. (2011, this issue; Table 4 and Figure 6) illustrating that multiple degradation half-lives for VX and HD are achieved in less than 2 days following application of appropriate decontamination solutions, an EF of 2 days/year was selected for the transit passenger. No chronic exposure is assumed
The EF assumptions for airport employees and personnel mirror those of the transit passengers (e.g., no repeat exposure cycles). An EF of 2 days/year was selected for various airport employees and personnel based on the degradation analysis above and in Watson et al. (2011, this issue).
Fraction transferred from surface to hand skin (FTSHS)
The FTSHS is the fraction of residue on a surface that could be transferred to the skin of the hand. Hand-press experiments with house-dust surrogate particles were conducted to measure surface transfer to dry skin (Rodes et al. 2001). Based on Rodes et al. (2001), the WTC report (COPC 2003) recommended use of default transfer fractions of 10% for carpets and 50% for hard surfaces (such as vinyl). In addition, COPC (2003) references an USEPA Malathion® exposure study that reported Malathion® transfer-to-hand results of 0.0018 (0.18%) and 0.0152 (1.52%) for vinyl flooring and carpets, respectively. Malathion® (C10H19O6PS2) is an organophosphate insecticide that is physically similar to nerve agent VX (C11H26NO2PS) in many respects (vapor pressures of VX and malathion are 7 × 10−4 and 1.78 × 10−4 mm Hg, respectively, while Henry's Law constants for VX and malathion are 3.5 × 10−9 and 4.9 × 10−9 atm-m3/mole, respectively) (NRC/COT 2003; Daubert and Danner 1989; Fendinger and Glotfelty 1990). It is thus reasonable to apply the Malathion®-specific FTSHS fractions provided in COPC (2003) for use in estimating a value for VX. Due to the unavailability of HD-specific transfer rates, the assumed FTSHS fractions for HD are the generic defaults recommended by COPC (2003) of 10% for soft and 50% for hard surfaces.
Fraction transferred from surface to body skin (FTSBS)
In terms of the fraction transferred from surface to body skin (FTSBS) for dermal contact, it is important to consider that, in comparison to hands, much less transfer will occur to body regions such as the arms, legs, and face that have typically less intensive surface contact. In accordance with assumptions made in the WTC report (COPC 2003), fractions for surface-to-hand transfer were reduced by half to represent an area-weighted transfer to all exposed body skin.
Hand surface area (SAhand)
Hand surface area (SA hand) is the skin area contacted during a mouthing event. The WTC report (COPC 2003) assumes that the finger area used for hand-to-mouth transfer varies linearly from 15 cm2 for a 2-year-old child, to 45 cm2 at age 17, and remains constant thereafter throughout adulthood.
Exposed skin surface area (SAtrans and SAwork)
For dermal contact, it is important to consider that much less transfer will occur to body parts such as the arms, legs, and face that undergo less intensive surface contact than hands. This analysis follows Superfund exposure assessment guidance, which has established exposed skin surface areas (SAtrans and SAwork) as 2800 cm2 for clothed children and 5700 cm2 for clothed adult workers (USEPA 2004a).
Frequency of hand-to-mouth contact (FQ)
Based on observations at day-care centers and the literature (Michaud et al. 1994), the WTC report (COPC 2003) groups the age cohort-specific hand-to-mouth contact frequency (FQ) as follows: toddler child (1 to 6 yr; FQc) = 9.5 times/h; adult (≥18 yr; FQa) = 1 time/h.
Saliva extraction factor (SE)
The fraction transferred from skin to mouth depends on the contaminant, mouthing duration, and other behavioral patterns. The WTC assessment used the USEPA Office of Pesticide Programs (OPP) default of 50%, based on pesticide studies (COPC 2003). Michaud et al. (1994) assumed that all of the residues deposited on the fingertips would be transferred to the mouth, twice per day. In the Binghamton State Office Building post-fire re-entry guideline derivation, a range of factors was used: 0.05, 0.1, and 0.25, representing the fraction of residue on the hand that is transferred to the mouth (Kim and Hawley 1985). For purposes of the airport evaluation, the OPP default of 50% used in the WTC assessment was selected as the saliva extraction factor (SE) for all ages.
Ingestion factor (IF)
To account for variability in exposure to hard and soft surfaces, the WTC assessment developed a dust ingestion factor (IF) equation (COPC 2003). This equation averages the differences in exposure to hard and soft surfaces by the exposure times. The following generic equation is presented below without scenario-specific inputs (Table 9 for variable identification and characterization). This equation applies to toddler child transit passengers as well as various airport employees and personnel:
Dermal factor (DF)
To account for variability in exposure to hard and soft surfaces, the WTC assessment developed a dermal absorption factor (DF) equation (COPC 2003). This equation averages the differences in exposure to hard and soft surfaces by the exposure durations (Table 9 for variable identification and characterization). As for the ingestion factor, the following generic equation is presented without scenario-specific inputs and is applied to toddler child transit passengers as well as various airport employees and personnel:
Frequency of hand-to-eye (FQeye)
Because the eye is the most sensitive body part for exposure to HD and VX (NRC/COT 2003), this evaluation follows the WTC model concept of hand-to-mouth contact by developing and adding a hand-to-eye exposure route. Consideration of hand-to-eye exposure is a unique feature of the SRCL model approach. A literature search was performed to determine hand-to-eye contact frequency (FQeye).
Sensitive populations, such as contact lens wearers, allergy sufferers, and people with the medical condition keratoconus, were not excluded. McMonnies and Boneham (2003) report that subjects without contacts and without any eye abnormality usually lightly rub their eyes for a short time with the pads of their fingers at a frequency of less than once per day. Subjects with kerataconus use their knuckles and aggressively grind the eye socket with multiple, prolonged episodes each day.
Yusel et al. (2001) noted that, out of 64 subjects with vernal keratoconjunctivitis, 66% rubbed their eyes frequently (>10 times daily), 24% rubbed their eyes occasionally (5 to 10 times daily), and 10% rubbed their eyes rarely (<5 times daily). Cameron et al. (1989) questioned 44 patients who reported either frequent (>10 times/day), occasional (5 to 10 times/day) or rare (1 to 4 times/day) eye rubbing. Korb et al. (1991) found that prolonged, forceful, multiple-knuckle eye rubbing occurs 3 to 10 times per day in patients with kerataconus, whereas control group frequency is less than once per day and is manifest by application of finger tips and a brief, light rubbing. For the purposes of the present evaluation, the threshold for “frequent” (e.g., 10 times/day) hand-to-eye rubbing reported in these studies was used. It is also assumed that hand-to-eye contact could also occur without a rubbing episode. Because of the deep pressure applied to the eye during a kerataconus eye-rubbing episode and the normal moisture present around the eye, it was assumed for the purpose of the present assessment that 100% of a contaminant on the fingers and hand would be transferred to the eye during a rubbing episode.
Eye surface area (SAeye)
The surface area of the eye (SAeye) is needed to calculate this exposure route. The studies reported in the earlier assessment of hand-to-eye contact frequency (FQeye) report that both eyes are often rubbed at the same time. Data from literature (Bozkir et al. 2003) allowed calculation of the combined surface area of both eyes (33.4 cm2). The exposed eye surface between the upper and lower eyelids is elliptical, and the standard formula for elliptical area is employed; the long axis estimate is 32.6 mm (palpebral fissure length); the short axis estimate is 16.3 mm (palpebral fissure height of 10.3 mm added to 3 mm each for upper and lower eyelids) (Bozkir et al. 2003).
Ocular rubbing factor (OF)
To account for variability in hand exposure to hard and soft surfaces, an ocular rubbing factor (OF) equation was developed for the current analysis. The following equation (see Table 9 for variable identification and characterization) averages differences in hand exposure to hard and soft surfaces, and consequent differences in potential contaminant exposure to the eye:
Resuspension factor (RF)
It is possible that agent may be resuspended in dust from a contaminated surface by the action of foot traffic and cleaning activities (Gomes et al. 2007). Reports of the U.S. Nuclear Regulatory Commission (NUREG 1992, 2002) present resuspension factors (relating air concentration to surface concentration) ranging from 6 × 10−8 to 7 × 10−4 m−1 for multiple activities. Based on the NUREG (2002) recommendations, an intermediate resuspension factor (RF) of 1 × 10−6 m−1 was selected as a representative default RF for the airport scenario analysis.
Dermal absorption factor (ABS)
Dermal absorption factor (ABS) represents the unitless fraction of potential contaminant that could be transferred through the skin for systemic absorption from contaminated media. Dermal absorption factor values of 0.056 for HD and 0.022 for VX have been used for CW agent demilitarization facility personnel estimates, while ABS values of 0.084 for HD and 0.033 for VX have been used in calculating residential estimates (USACHPPM 1999). The residential ABS values from USACHPPM (1999) were incorporated into the present analysis. For nerve agent VX, the ABS value of 0.033 compares well with results of in vitro experimental absorption on human cadaver skin samples exposed to 14C parathion, an anticholinesterase insecticide considered to be a reasonable surrogate for nerve agent VX (Wester et al. 2000). Following a single exposure and a 96-h absorption period, 1.78 ± 0.41% (e.g., 0.018) of the labeled parathion was taken up by unclothed, dry cadaver skin samples (Wester et al. 2000).
The present analysis compared SRCL values resulting from inclusion of the ABS value of 0.033 for a residential scenario (a protective assumption for the airport assessment; USACHPPM 1999) versus the ABS estimate of 0.018 taken from Wester et al. (2000). The SRCL results from both calculations equal 3.1 × 10−4 mg/cm2 for the 24-h exposure duration (Tables 11 and 12).
Table 11.
Estimated Sulfur Mustard and Agent VX Surface Removal Contaminant Levels (SRCLs) for child transit passengers.a
| CWA | Transit Passenger SRCL4bc Protective estimate (mg/cm2 surface, continuous exposure) | Transit Passenger SRCL24bd Protective estimate (mg/cm2 surface, continuous exposure) |
| Sulfur mustard (HD) | 1.77 × 10−3 | 2.95 × 10−4 |
| VX | 1.85 × 10−3 | 3.08 × 10−4 |
aChemical formulae: HD, C4H8Cl2S; VX, C11H26NO2PS.
bThe “child passenger” estimate employs toddler child-specific information regarding object mouthing behavior and dermal contact with surfaces, and is thus a protective estimate for adult transit passengers. Passenger SRCL estimates incorporate contaminant transfer from hard and soft surfaces to the eye, skin, and mouth (incidental ingestion) as well as potential particle inhalation arising from resuspension.
cSRCL4 assumes average exposure dwell time of 4 continuous h (incorporates inhalation toxicity value for exposure durations ≤8 h).
dSRCL24 assumes maximal exposure dwell time of 24 continuous h (incorporates inhalation toxicity value for exposure durations >8 h but ≤24 h).
Table 12.
Estimated Sulfur Mustard and Agent VX Surface Removal Contaminant Levels (SRCLs) for adult airport employees and personnel.a
| CWA | Airport Employee/Personnel SRCL8bc Protective estimate (mg/cm2 surface, CWA continuous exposure) | Airport Employee/Personnel SRCL12bd Protective estimate (mg/cm2 surface, continuous exposure) |
| Sulfur mustard (HD) | 8.65 × 10−3 | 5.77 × 10−3 |
| VX | 9.56 × 10−3 | 6.33 × 10−3 |
aChemical formulae: HD, C4H8Cl2S; VX, C11H26NO2PS.
bAirport employee estimate includes various airport employees and personnel (all of whom are restrained from entering the facility until after the agent source is removed or neutralized, any decontamination process is completed, and monitoring has characterized workspace atmospheres as suitable for reoccupancy). Airport employee and personnel SRCL estimates incorporate contaminant transfer from hard and soft surfaces to the eye, skin, and mouth (incidental ingestion) as well as potential particle inhalation due to resuspension.
cSRCL8 assumes average exposure time (shift) of 8 continuous h (incorporates inhalation toxicity value for exposure durations ≤8 h).
dSRCL12 assumes maximal exposure time (shift) of 12 continuous h (incorporates inhalation toxicity value for exposure durations >8 h but ≤24 h).
Body weight (BW)
Body weights (BWs) of 70 kg and 15 kg were assumed for adults and children, respectively (USEPA 2004a).
Gastrointestinal absorption factor (GIABS)
Gastrointestinal absorption factors (GIABSs) are commonly used to convert oral toxicity values into dermal toxicity values because dermal toxicity values are seldom available; such conversions are considered protective. The GIABS is the unitless fraction of contaminant absorbed in the gastrointestinal tract. When applied to dermal toxicity estimates, use of the GIABS maximizes the dermal absorption estimate by assuming that GI tract and skin absorption are equal for a given compound. The following equation from USEPA (2004a) illustrates how to convert an oral RfD to a dermal RfD. GIABS values of 1 were used for both HD and VX because chemical-specific values were not available; this is a highly protective assumption.
SRCL Results for Agents HD and VX
Summarized in Tables 11 and 12 are HD and VX results for the assumed average and maximum (24-h) exposure times for a child transit passenger and adult airport employees and personnel, respectively. For any given airport population and dwell time evaluated, there is little nominal difference between the estimated SRCLs for agents HD and VX, despite the large differences in toxicity values included in the calculation (Tables 11 and 12). This “flattening” of compound-specific differences is a result of the choice of values selected to characterize the fraction transferred from surfaces to skin of the hand or body in the equations employed. Values selected for nerve agent VX estimation are those for the organophosphate insecticide Malathion®, being considered here as a surrogate for nerve agent VX due to previously identified similarities in the physical characteristics of vapor pressure and Henry's Law Constants (see earlier characterization of FTSHS) (NRC/COT 2003; Daubert and Danner 1989; Fendinger and Glotfelty 1990). It is thus reasonable to apply Malathion®-specific fractions (transfer-to-hand fraction of 0.0018 and 0.0152 for vinyl flooring and carpets, respectively; COPC 2003) for use in VX characterization during the current evaluation.
In contrast, those skin transfer values employed for sulfur mustard are standard defaults originally developed for particle transfer (10% for carpets, and 50% for hard surfaces such as vinyl) as recommended by COPC (2003), and from hand-press experiments by Rodes et al. (2001). The use of default skin transfer values for HD is due to the unavailability of HD-specific (or reasonable surrogate-specific) hard- and soft-surface skin-transfer data for this vesicant agent; this is a significant data gap. The chemical and physical properties of sulfur mustard agent HD [Watson et al. (2011, this issue), Table 2] indicate that actual skin transfer values from hard and soft surfaces would be less than the default values necessarily employed in the present estimation. As a consequence, the SRCL estimates presented in Tables 11 and 12 for HD are considered highly protective.
This analysis indicates that the most protective approach for all examined exposure durations and populations would be to select the compound-specific 24-h SRCL estimates for the transit passenger child receptor for application as compound-specific screening criteria for persistent CWAs.
SRCL Results for Degradation Products of HD and VX
Compound-specific parameters and toxicity values were applied in a similar manner to estimate SRCL values for degradation products of HD and VX. Results are summarized in Table 13 for the child transit passenger, which represents the most susceptible subpopulation evaluated in the surface contact assessment.
Table 13.
Estimated HD and VX Degradation Product Surface Removal Contaminant Levels (SRCLs) for child transit passengers.a
| CWA Degradation Product | Transit Passenger SRCL4bc Protective estimate (mg/cm2 surface, continuous exposure) | Transit Passenger SRCL24bd Protective estimate (mg/cm2 surface, continuous exposure) |
| TDG (thiodiglycol) | 1.06 × 102 | 1.77 × 101 |
| EMPA (ethyl methylphosphonic acid) | 7.08 | 1.18 |
| MPA (methyl phosphonic acid) | 5.06 | 8.44 × 10−1 |
| EA2192 | 1.89 × 10−3 | 3.15 × 10−4 |
aChemical formulae: TDG, C4H10O2S; EMPA, C3H9O3P; MPA, CH5O3P; EA2192, C9H22NO2PS.
bThe “child passenger” estimate employs child-specific information regarding object mouthing behavior and dermal contact with surfaces, and is thus a protective estimate for adult transit passengers. Passenger SRCL estimates incorporate contaminant transfer from hard and soft surfaces to the eye, skin, and mouth (incidental ingestion), as well as potential particle inhalation due to resuspension from surfaces.
cSRCL4 assumes average exposure dwell time of 4 continuous h (incorporates inhalation toxicity value for exposure durations ≤8 h).
dSRCL24 assumes maximal exposure dwell time of 24 continuous h (incorporates inhalation toxicity value for exposure durations >8 h but ≤24 h).
This analysis indicates that the most protective approach for all examined exposure durations and populations would be to select the compound-specific 24-h SRCL estimates for the transit passenger child receptor for application as compound-specific screening criteria for these key degradation products of persistent CWAs.
Exposure Time Fraction and Route-Specific Relative Contribution
The World Trade Center (WTC) Indoor Assessment (COPC 2003) model incorporates an exposure time fraction for the dermal exposure route, and those portions of the WTC model were followed for the current analysis. The WTC model mathematically assumes that, once contact with a contaminated surface is terminated, exposure also terminates. Other USEPA models of dermal exposure assume that a contaminant resides in a medium such as soil, with adherence of the medium to the skin throughout the day with continuing exposure even when the receptor is not at the original exposure site. USEPA (2004a) currently does not address more refined approaches for indoor surfaces [(as used in the WTC report of COPC (2003) and the present analysis]. The airport scenario considers direct contact with a surface residue and does not assume the presence of a delivery medium.
The relative contribution of each exposure route (e.g., ingestion, ocular, dermal skin, and resuspension inhalation) to the total SRCL has been characterized by calculating each route-specific component of the SRCL equation. The estimated route-specific relative contributions for transit passengers and various airport employees and personnel potentially exposed to HD or VX are summarized in Tables 14 and 15. The relative contribution for HD and VX degradation products for the child transit passenger population is summarized in Table 16. For verification purposes, the present analysis incorporates a stochastic model approach employed by CalEPA (Salocks 2007, 2008, 2009).
Table 14.
Estimated contribution of each exposure route to Sulfur Mustard and Agent VX SRCLs for child transit passengers.a
| CWA | Transit passenger 4-h dwell time (%) | Transit passenger 24-h dwell time (%) |
| Sulfur mustard (HD) | Ingestion: 79.1 | Ingestion: 79.1 |
| Eye: 15.5 | Eye: 15.5 | |
| Dermal skin: 5.4 | Dermal skin: 5.4 | |
| Resuspension inhalation: <1 | Resuspension inhalation: <1 | |
| VX | Ingestion: 81.7 | Ingestion: 81.8 |
| Eye: 16.0 | Eye: 16.0 | |
| Dermal skin: 2.2 | Dermal skin: 2.2 | |
| Resuspension inhalation: 0.14 | Resuspension inhalation: 0.07 |
aChemical formulae: HD, C4H8Cl2S; VX, C11H26NdO2PS.
Table 15.
Estimated contribution of each exposure route to Sulfur Mustard and Agent VX SRCLs for adult airport employees and personnel.a
| CWA | Airport employee/personnel 8-h workshift (%) | Airport employee/personnel 12-h workshift (%) |
| Sulfur mustard (HD) | Ingestion: 52.2 | Ingestion: 52.3 |
| Eye: 32.3 | Eye: 32.3 | |
| Dermal skin: 15.5 | Dermal skin: 15.4 | |
| Resuspension inhalation: <1 | Resuspension inhalation: <1 | |
| VX | Ingestion: 57.2 | Ingestion: 56.9 |
| Eye: 35.4 | Eye: 35.1 | |
| Dermal skin: 6.6 | Dermal skin: 6.6 | |
| Resuspension inhalation: 0.74 | Resuspension inhalation: 1.4 |
aChemical formulae: HD, C4H8Cl2S; VX, C11H26NO2PS.
Table 16.
Estimated contribution of each exposure route to Degradation Product SRCLs (mg/cm2 of surface) for child transit passengers.a
| CWA Degradation Product | Transit Passenger, 4-h dwell time (%) | Transit Passenger, 24-h dwell time (%) |
| TDG (thiodiglycol) | Ingestion: 83.0 | Ingestion: 83.1 |
| Eye: 16.2 | Eye: 16.2 | |
| Dermal skin: 0.6 | Dermal skin: 0.6 | |
| Resuspension inhalation: <1 | Resuspension inhalation: <1 | |
| EMPA (ethyl | Ingestion: 79.0 | Ingestion: 79.1 |
| methylphosphonic | Eye: 15.4 | Eye: 15.4 |
| acid) | Dermal skin: 5.43 | Dermal skin: 5.44 |
| Resuspension inhalation: <1 | Resuspension inhalation: <1 | |
| MPA (methyl | Ingestion: 79.0 | Ingestion: 79.1 |
| phosphonic acid) | Eye: 15.4 | Eye: 15.4 |
| Dermal skin: 5.43 | Dermal skin: 5.44 | |
| Resuspension inhalation: <1 | Resuspension inhalation: <1 | |
| EA2192 | Ingestion: 83.7 | Ingestion: 83.7 |
| Eye: 16.3 | Eye: 16.3 | |
| Dermal skin: NAb | Dermal skin: NAb | |
| Resuspension inhalation: NAb | Resuspension inhalation: NAb |
aChemical formulae: TDG, C4H10O2S; EMPA, C3H9O3P; MPA, CH5O3P; EA2192, C9H22NO2PS.
bEA 2192 is a solid, is not an inhalation hazard, and is not absorbed through the skin in aqueous or alcohol solutions (Michel et al. 1962).
For child transit passengers, the largest estimated component of the composite transit passenger average and maximum VX (and HD) SRCL is that provided by indirect (incidental, nondietary) ingestion (approximately 80%) due to object mouthing behavior. Direct eye exposure accounts for 15 to 16%, with the bulk of the remainder provided by direct dermal. The contribution of resuspension inhalation is much less than 1% for this population.
For VX, the largest estimated component of the composite airport personnel and employee average and maximum VX SRCL is that provided by indirect (incidental, nondietary) ingestion (57%), whereas direct eye exposure accounts for 35%; the remainder is comprised of direct dermal (skin) exposure (6.6%) and resuspension inhalation (approximately 1%). Estimates for HD are similar to those of VX for reasons outlined above.
In general, estimated relative contributions for HD and VX degradation products mirror those for the parent compound, except for EA 2192, which is not absorbed through the skin and is not an inhalation hazard (Michel et al. 1962). The resuspension inhalation component for EA 2192 is minimal.
SRCL Evaluation
Although the logic and assumptions of COPC (2003) were largely employed in the present analysis, a number of refinements were added to more closely simulate conditions expected in a transportation hub that has been the target of a deliberate CWA or TIC release. These refinements include:
Exposure duration (ED) and exposure frequency (EF) have been developed for transit passengers and airport personnel/employees based on agent degradation analysis from Watson et al. (2011, this issue) characterizing surfaces in the post-decontamination environment. This method more accurately reflects the expected, acute nature of potential post-decontamination exposures from residual CWAs or TICs in the specific airport-release scenario under consideration (e.g., one-time only release with passenger and airport personnel re-entry only after release device is removed and/or neutralized, decontamination is complete, and monitoring has characterized atmospheres and surfaces as suitable for re-occupancy). Traditional exposure assessments are based on human activity patterns and not the likely presence of contamination (USEPA 1991). However, there is precedence to include dissipation terms in risk calculations for dust on surfaces, as in the WTC assessment (COPC 2003), and evaluations of radioactive decay. In the case of the present analysis, ED and EF are based on the rapid and well-characterized degradation reactions taking place during the decontamination phase (Watson et al. 2011, this issue; Yang et al. 1994; Yang 1999; Talmage et al. 2007a,b; others). Hence, the SRCL equations do not include a decay rate variable.
Addition of hand-to-eye exposure estimates because the eye is considered the most susceptible target organ for sulfur mustard or nerve agent exposures.
Addition of a resuspension factor for dust inhalation arising from foot traffic or cleaning activities.
Development of compound-specific, subchronic oral RfD estimates (7 × 10−5 mg HD/kg/day; 2 × 10−6 mg VX/kg/day) by deletion of the subchronic-to-chronic uncertainty factor previously incorporated into chronic RfD estimation (Opresko et al. 1998).
Use of inhalation toxicity values equivalent to estimated airborne re-entry screening guidelines (Table 2).
In addition, information characterizing transit passenger dwell time in airport terminals and based on recent traveler interviews conducted at LAX, was incorporated into the transit passenger estimates (Table 11). The airport employee and personnel estimates incorporate standard work shifts of 8 and 12 h (Table 12).
This evaluation considers that the most protective estimation for screening surfaces prior to re-entry by the public (including various airport employees and personnel) is to apply SRCL estimations developed for the toddler child transit passenger. This recommendation is based on the fact that a toddler represents the population group for which hand-to-mouth and body-skin exposure is maximized because of frequent object mouthing activities and skin contact with surfaces. Furthermore, because the most reliable values for the FTSHS parameter (fraction transferred from surface to hand skin) (and by extension, the FTSBS body skin parameter) is available for VX, the SRCL estimates for VX are more reliable than those for HD, and are thus preferable. Additional protection is provided by selecting the smallest SRCL estimate for VX-that estimated for 24-h continuous exposure duration (Table 11).
Surface Removal Contaminant Levels (SRCLs) can be calculated or modified quickly following the equations presented in this analysis and use of spreadsheets or hand calculations. This simplicity offers great utility in the event of a terrorist attack. However, to test the validity of the deterministic approach, comparison to a model requiring greater computing power was desired. For verification purposes, a stochastic model approach employed by CalEPA (Salocks 2007, 2008, 2009) was compared to the present analysis. The Stochastic Human Exposure and Dose Simulation Model for Multimedia, Multipathway Chemicals (SHEDS; ver 3) was used by CalEPA to assess children's exposure to methamphetamine residues on surfaces (Stallings et al. 2008; Zartarian et al. 2008); version 3 has been the most current edition available for investigative use at the time of the present analysis.
The SHEDS probabilistic model was developed by the USEPA National Exposure Research Laboratory and estimates a total absorbed dose (mg/kg-day) for a known surface concentration by incorporating inputs from non-dietary ingestion (hand-to-mouth, object-to-mouth), inhalation and dermal contact time-series exposures for specified cohorts. For the present analysis, the California methamphetamine estimates (Salocks 2007, 2008, 2009) were repeated using the SHEDS model. Once the model had been verified, the present analysis evaluated LAX-specific exposure parameters (see Tables 9 and 10, and accompanying text) as well as compound-specific chemical and physical parameters. For nerve agent VX, the SHEDS model estimated a surface concentration guideline of 0.1 ug VX/cm2 for the child transit passenger and 24-h contact exposure duration; this result was within a factor of 3 of the guideline value for the same receptor and contact exposure duration (0.3 ug VX/cm2, Table 11) estimated by means of the deterministic SRCL approach used in this present analysis. Note that the SRCL approach represents an expansion from SHEDs in that SRCL also considers input from ocular (hand-to-eye) exposure. The authors believe that the deterministic approach to calculating SRCLs compares favorably with results obtained from application of the SHEDS probabilistic model.
According to the SHEDS website (http://www.epa.gov/heasd/products/sheds_multimedia/sheds_mm.html), development of SHEDS-Multimedia version 4 is ongoing at this writing (August 2010); when ready, Version 4 will allow for cumulative (multi-chemical) or aggregate (single chemical) assessments and combine residential and dietary modules. A USEPA Science Advisory Panel (SAP) review of SHEDS-Multimedia version 4 occurred in July 2010; at present, the Version 4 model release date has not been announced.
An evaluation of the protective nature of the VX SRCL can be performed by comparisons with published estimates of VX dermal toxicity (human LD50 of 4 μg VX/cm2 when applied as undiluted agent directly to skin and without considering transfer losses that occur during skin contact with surfaces; Wester et al. 2000) and calculation of the amount of VX potentially transferred to the skin after contact with a surface contaminated by the 24-h toddler transit passenger SRCL of 3.08 × 10−4mg VX/cm2 (0.3 μg VX/cm2). Application of the estimated FTSBS for VX (0.0009 for vinyl and 0.0076 for carpet; Table 9) results in an estimated VX transfer to body skin of between 2.8 × 10−4 μg VX/cm2 to 2.3 × 10−3 μg VX/cm2. This range represents a difference of approximately 103 to 104 less than the published human dermal LD50 of 4 μg VX/cm2 for VX (Wester et al. 2000). It is noted that estimates of transfer to the skin of the hand are incorporated into the above FTSBS consideration.
These ratios are protective when compared with other toxicological information for VX, such as the difference between rat threshold lethality (1-h threshold lethality in female rats is 0.50 mg VX/m3, or 500 μg VX/cm3) and reversible EC50 for miosis (considered a nonadverse effect) (1-h miosis EC50 in female SD rats is 0.002 mg VX/m3, or 2 μg VX/cm3; Benton et al. 2005, 2006a,b, 2007). The difference between no-adverse effect (EC50 miosis) and threshold lethality in SD rats is a factor of 102; thus, the greater difference of 103 to 104 exhibited by the difference between the toddler transit passenger SRCL and estimated human dermal LD50 is an indication of the protective nature of the SRCL estimate for VX. Although the Benton et al. (2005, 2006a,b, 2007) data are for VX vapor exposure, relative ratios between lethality and no adverse effect are considered comparable given the same mechanism of toxicity; for example, anticholinesterase activity, is the source of toxic response in both cases.
CONCLUSIONS
If a chemical terrorist incident should occur tomorrow, it is important to have an immediate set of appropriate and reasonable clearance goals from which to begin recovery. Exposure guidance and screening levels for use in chemical terrorist remediation decision-making have been developed in a national case study sponsored by the Department of Homeland Security in partnership with the Bradley International Terminal (TBIT) at the Los Angeles International Airport (LAX; Los Angeles, CA). Part II of the assessment (presented here) documents development of human-health based multipathway decision criteria. Exposure routes evaluated include direct ocular vapor and vapor inhalation, percutaneous vapor, surface contact, hand-to-eye, hand-to-mouth, non-dietary and dietary ingestion and resuspension inhalation (Figure 3 and Tables 1-16). The scenario compounds under consideration are the chemical warfare agent (CWA) nerve agents tabun (GA), sarin (GB), soman (GD), cyclosarin (GF), and VX as well as the vesicant agent sulfur mustard (HD); and the toxic industrial compounds (TIC) phosgene (CG), hydrogen cyanide (AC), and cyanogen chloride (CK). Exposure guidelines for CW agent degradation products of interest are also developed and provided.
This article presents first-time, open-literature documentation of multi-pathway and health-based remediation exposure guidelines for all nine of these chemical terrorist threat compounds and their degradation products. These guidelines are suitable for application to various civilian populations, and are both health-protective and reasonable for use as clearance decision criteria during remediation of a chemical terrorist event within a major domestic transportation hub. Personal protective equipment guidelines for decontamination personnel are also identified.
Emphasis is placed on compound-specific exposure guidelines that already exist, are published and accessible to the public, have undergone credible peer and public review, are health-based and protective, and have demonstrated utility in use and practice. Selection of compound-specific critical effects used as point(s) of departure for this analysis focuses on reversible, non-adverse (often local) effects that exhibit well-defined dose response, such as EC50 for miosis in a susceptible gender following nerve agent vapor exposure. With application of uncertainty factors, the resulting exposure guidance concentrations are protective for all segments of the general population, including susceptible subpopulations. When published low-level exposure guidelines were not available or suitable (e.g., for phosgene, hydrogen cyanide, cyanogen chloride) application of standard risk assessment methods resulted in appropriate guidance values.
Because of their recognized scientific credibility and expanding uses in domestic preparedness planning by affected communities as well as Federal and state authorities, the Acute Exposure Guideline Level (AEGL) concentrations published by the Committee on Toxicology of the National Research Council are considered by this analysis as protective, appropriate and useful for making site-access re-entry determinations for the traveling public, airport personnel, and vendors. This analysis further considers application of the mildest effect tier (AEGL-1) to be appropriate, and application of the 8-h AEGL-1 concentrations (the lowest values) as reasonable criteria for all transit passenger stay times ≤8 h as well as for airport personnel and vendors (Table 2). Selecting the minimal exposure concentrations developed for assumed 8-h exposure durations is a highly protective assumption for LAX transit passengers given the one-time only release scenario and the fact that most passengers spend ≤4 h in the most heavily used LAX terminals. It is acknowledged that use of any given dwell (“stay”) time assumption may be airport-specific; nevertheless, the 8-h continuous-exposure duration is considered protective for post-decontamination applications. To address those rare situations where public dwell times may be >8 h but <24 h, alternative clearance goals are derived from standard time-extrapolation protocols, and provide additional flexibility to decision-makers. The vapor exposure guidance range provided in Table 2 represents both reasonable maximum and alternative re-entry screening guidelines.
Given previous minimal guidance for surface contact exposures to the airport threat compounds, this analysis develops a novel and deterministic approach to estimate non-adverse surface residue concentrations for the persistent nerve agent VX, the persistent vesicant agent HD and degradation products of concern. Unlike other surface assessments, this analysis considers hand-to-eye exposure estimates as well as a resuspension factor for dust inhalation arising from foot traffic or cleaning activities. Population exposure assessment determined that toddlers are the most susceptible subpopulation for surface contact exposure due to object-mouthing and other age-specific behaviors favoring transfer from surfaces; the resulting Surface Removal Contaminant Levels (SRCLs; in mg/cm2 for CWAs) (see Tables 11-13) are thus protective for adults. Results compare well with those of the SHEDS probability model developed by the USEPA National Exposure Research Laboratory for contaminated surfaces, and are consistent with logic employed by CalEPA in assessing children's exposure to methamphetamine residues on surfaces of buildings used as clandestine production facilities. Compound-specific SRCLs can be calculated or modified quickly following application of the equations (Figures 1 and 2) and input parameters (Tables 9-10) presented in this analysis, with use of spreadsheets or hand calculations. This simplicity offers great utility in the event of a terrorist attack.
Some assumptions required to characterize SRCL exposure parameters would be improved by means of compound-specific testing; the most significant are those characterizing the fraction transferred from surface to skin of the hand (FTSHS) or body (FTSBS). Since the authors could locate no HD-specific skin transfer data from “hard” or “soft” surfaces, FTSHS and FTSBS values utilized for sulfur mustard are necessarily standard USEPA defaults for hard and soft surfaces. Examination of the chemical and physical properties of HD indicates that such default transfer values are over-estimates.
It is acknowledged that relevant work is ongoing in many fields that will continue to inform future evolution of clearance guidelines. Nevertheless, Tables 17-19 summarize protective clearance goals for the nine threat compounds and their principal degradation products as developed during the current analyses and recommended for application to the airport remediation scenario. These tables are intended to serve as an aid to decision-makers for pre-planning, and for actual use should a similar incident occur in the future. By providing rationale for reasonable and scientifically supported procedures and health-based criteria, this analysis provides decision-makers with an efficient and effective approach as well as flexibility by which to weigh numerous judgments (decontamination personnel safety, public health, time, funds, resources, public perception, and other concerns) required to establish clearance guidelines for remediating airport facilities in which hazardous chemical release has occurred.
Table 17.
Recommended clearance exposure guidelines for transit passengers as well as various airport employees and personnel for selected CWAs and TICs in air and on surfaces (airborne for inhalation/ocular exposure; percutaneous vapor for protective clothing breach with full respiratory protection; and surface residual for “toddler” transit passenger SRCLs). For use during clearance decision-making following chemical terrorist release of selected CWA or TIC compounds under the LAX remediation protocol.
| Reasonable Maximum Re-entry Screening Guidelines (mg/m3) |
Alternative Re-entry Screening Guidelines (mg/m3) |
CWA Personnel Escape Guidelines (mg/m3) for 30-min percutaneous exposure | Transit Passenger SRCL4hr (mg/cm2 surface, calculated; 4 h continuous exposure duration) | Transit Passenger SRCL24hr (mg/cm2 surface, calculated; 24 h continuous exposure duration) | |
| CWA or TIC | <8-h duration exposurea | >8 but <24-h duration exposureb | |||
| Tabun (GA) | < 0.0010 | < 0.0003 | 11.1 | c | c |
| Sarin (GB) | < 0.0010 | < 0.0003 | 6.0 | c | c |
| Soman (GD) and Cyclosarin (GF) | < 0.00050 | < 0.0002 | 1.5 | c | c |
| VX | < 0.000071 | < 0.000024 | 0.13 | <1.85 × 10−3 | <3.08 × 10−4 |
| Sulfur mustard (H/HD) | < 0.008 | < 0.003 | 0.1 | <1.77 × 10−3 | <2.95 × 10−4 |
| Hydrogen cyanide (AC) | < 1.1 | <0.37 | d | c | C |
| Cyanogen chloride (CK) | <0.25 | <0.08 | d | c | c |
| Phosgene (CG) | <0.08 | <0.03 | d | c | c |
aEquivalent to the 8-h AEGL-1 for nerve agents and sulfur mustard (NRC/COT 2003), and hydrogen cyanide (NRC/COT 2002). Derivation of screening guidelines for phosgene and cyanogen chloride is described in this article.
bDerived by applying standard protocols and straight-line extrapolation from the 8-h AEGL-1 concentration (NRC/COT 2001). The same protocols were used to derive the alternative re-entry screening guidelines for phosgene and cyanogen chloride.
cBecause of the volatility of G-series nerve agents, HCN, phosgene, and cyanogen chloride, these volatile compounds are not considered persistent; there are thus insufficient data from which to estimate surface residues. If there is interest in surface sampling for these volatile compounds, the SRCL concentrations estimated for nerve agent VX can be employed. Doing so is a protective approach given the SRCL assumptions of VX persistence and toxicity, as well as the assumed frequency of child object mouthing behavior and dermal contact with surfaces.
dNot determined by NIOSH.
Table 19.
Summary of clearance exposure guidelines for principal CWA degradation products on surfaces (“toddler” transit passenger SRCL), and via incidental soil ingestion (both residential and industrial).a
| Residential soil |
Industrial soil |
|||||
| CWA Degradation Product | Transit Passenger SRCL4hr Protective estimate (mg/cm2 surface; 4 h continuous exposure) | Transit Passenger SRCL24hrProtective estimate (mg/cm2 surface; 24 h continuous exposure) | Preliminary Remediation Goal (PRG) (mg/kg soil) | Outdoor Worker Preliminary Remediation Goal (PRG) (mg/kg soil) | Indoor Worker Preliminary Remediation Goal (PRG) (mg/kg soil) | |
| TDG (thiodiglycol) | 1.06 × 102 | 1.77 × 101 | 2.4 × 104 | 6.0 × 104 | 8.2 × 105 | |
| EMPA (ethyl methylphosphonic acid) | 7.08 | 1.18 | 1.7 × 103 | 4.2 × 103 | 5.7 × 104 | |
| MPA (methyl phosphonic acid) | 5.06 | 8.44 × 10−1 | 1.2 × 103 | 3.0 × 103 | 4.1 × 104 | |
| EA2192 | 1.89 × 10−3 | 3.15 × 10−4 | 4.7 × 10−2 | 6.8 × 10−1 | 1.2 | |
aRegional Screening Level (RSL) user guide disclaimer states that the RSL guidance is not mandatory, does not provide binding rules, and points out that alternative approaches for risk assessment may be more appropriate on a site-specific basis (USEPA 2009, see http://www.epa.gov/reg3hwmd/risk/human/rb-concentration_table/usersguide.htm). Use of the RSLs has been accepted only by Regions 3, 6, and 9; other EPA regions have not officially approved the application of RSLs. Thus, Preliminary Remediation Goals (PRGs) other than RSLs are still in use within the remaining seven EPA Regions, and reference to PRG use is valid. Also Table 6.
This study's findings are not only crucial to the development of preplanning goals for remediation and release of airport terminals in the event of chemical terrorist attack, but are also applicable to other transportation hubs such as bus, train, ferry, and metro terminals.
Table 18.
Recommended clearance exposure guidelines for incidental soil and drinking water ingestion of selected CWAs and TICs.a
| Residential soil (lifetime) |
Industrial soil (lifetime) |
15 liter/day consumption (<7 day) |
|||
| CWA or TIC | Preliminary Remediation Goal (PRG) (mg/kg) | Risk-Based Concentration (RBC) (est.) (mg/kg) | Preliminary Remediation Goal (PRG) (mg/kg) | Risk-Based Concentration (RBC) (est.) (mg/kg) | Drinking water concentration (μg/liter) |
| Tabun (GA) | <2.8 (est.) | <3.1 | <68 (est.) | <82 | <46 |
| Sarin (GB) | <1.3 (est.) | <1.6 | <32 (est.) | <41 | <9.3 |
| Soman (GD) and | <0.22 (est.) | <0.31 | <5.2 (est.) | <8.2 | <4 (GD only; also |
| Cyclosarin (GF) | applicable for generic nerve agent) | ||||
| VX | <0.042 (est.) | <0.047 | <1.1 (est.) | <1.2 | <5 |
| Sulfur mustard | <0.01 (est.) | <0.55 | <0.3 (est.) | <14 | <47 |
| (H/HD) | |||||
| Hydrogen cyanide | <1.6 × 103 | Unavailable | <2.0 × 104 | Unavailable | <2 |
| (AC) | |||||
| Cyanogen chloride | <3.9 × 103 | Unavailable | <5.1 × 104 | Unavailable | Unavailable |
| (CK) | |||||
| Phosgene (CG) | Unavailable | Unavailable | Unavailable | Unavailable | Unavailable |
aCalculated Regional Screening Levels (RSLs) for residential soil (See Table 6 and footnote d) are duplicative of above PRG and RBC estimates for residential soil.
ACKNOWLEDGMENTS
This work was prepared for the U.S. Department of Homeland Security under U.S. Department of Energy Interagency Agreement No. 2367-T146-06. The Oak Ridge National Laboratory (ORNL) of Oak Ridge, TN is managed and operated by UT-Battelle, LLC, for the U.S. Department of Energy under Contract No. DE-AC05-00OR22725. This work has also been performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory, Livermore, CA, under Contract DE-AC52-07NA27344.
The authors and project sponsor thank and acknowledge Los Angeles World Airports (LAWA) for their leadership role in enabling the Los Angeles International Airport (LAX) to serve as a model facility for the Chemical Restoration Operational Technology Demonstration Project.
Participating laboratories in the Chemical Restoration Operational Technology Demonstration Project include Lawrence Livermore National Laboratory (LLNL; Livermore CA), Los Alamos National Laboratory (LANL; Los Alamos NM), Oak Ridge National Laboratory (ORNL; Oak Ridge TN), Pacific Northwest National Laboratory (PNNL; Richland WA), and Sandia National Laboratory (SNL; Albuquerque NM). Development of preplanning clearance goals for the LAX chemical terrorist release scenario has involved collaborative exchange among colleagues from each of these facilities.
Technical advice and review of Robert Young (DABT), Cheryl Bast (DABT) and Tim Borges (DABT) of the Environmental Sciences Division, Oak Ridge National Laboratory, are gratefully acknowledged.
The authors thank Charles Salocks of the Office of Environmental Health Hazard Assessment, California Environmental Protection Agency, for his thoughtful responses to our technical queries regarding CALMETH task reports as well as his many helpful suggestions regarding the Stochastic Human Exposure and Dose Simulation (SHEDS) model.
Critical information characterizing human eye structure and dimensions was swiftly provided and interpreted for application to the current analysis by Nancy Davis, LDO, ORNL Health Services Division.
The authors acknowledge the many insights regarding emergency response and preparedness in general, and the Chemical Stockpile Emergency Preparedness Program in particular, provided by John Sorensen and Barry Shumpert of the Environmental Sciences Division, Oak Ridge National Laboratory.
Dan Noble (Project Manager for Munition Recovery, U.S. Army Corps of Engineers, Baltimore District), Bruce Whisenant (U.S. Army Engineering and Support Center, U.S. Army Corps of Engineers, Huntsville, AL) and their supervisors and staff are gratefully acknowledged for providing ready and timely access to results, findings and images from the Spring Valley Formerly Used Defense Site (SVFUDS) located within the District of Columbia.
The authors acknowledge with appreciation the consistent and strong support provided by Ted Medley for developing and publishing compound-specific clearance decision criteria well in advance of CWA or TIC release. For many years, Mr. Medley was the Training and Exercise Officer for the Chemical Stockpile Emergency Preparedness Planning Program within the State of Colorado Office of Emergency Management, from which he communicated the high utility of such decision criteria to state and local authorities during remediation pre-planning as well as during post-release decision-making. These papers were prepared in furtherance of the training mission long advocated by Mr. Medley.
AUTHOR-DIRECTED PEER REVIEW
This manuscript was prepared under HERA's author-directed peer review process, wherein a manuscript's authors nominate proposed peer reviewers to HERA's Managing Editor for approval or revision. The following persons reviewed and approved the publication of this manuscript:
Dr. Barbara G. Callahan, Senior Toxicologist, University Research Engineers & Associates, Grantham, NH;
Dr. Martin Clauberg, Dr. Clauberg Consulting, Lenoir City, TN and Solingen, Germany;
Dr. John Doull, Professor Emeritus of Pharmacology and Toxicology, University of Kansas Medical School, Kansas City, KS;
Dr. James L. Regens, Center for Biosecurity Research, University of Oklahoma, Oklahoma City, OK.
ABBREVIATIONS
- ABS
dermal absorption factor
- AC
hydrogen cyanide; NATO code
- ACGIH
American Conference of Governmental Industrial Hygienists
- AChE
Acetylcholinesterase
- AEGLs
Acute Exposure Guideline Levels
- AIHA
American Industrial Hygiene Association
- ATP
adenosine triphosphate
- ATSDR
Agency for Toxic Substances and Disease Registry
- BAL
bronchioalveolar lavage
- BAST
Board on Army Science and Technology (of the National Research Council)
- BuChE
butyrylcholinesterase
- BW
body weight
- CalEPA
California Environmental Protection Agency
- CAM
Center for Airport Management
- CDC
Centers for Disease Control and Prevention of the DHHS
- CF
conversion factor
- CG
phosgene; NATO code
- CK
cyanogen chloride; NATO code
- COT
Committee on Toxicology of the National Research Council
- COPC
Contaminants of Potential Concern
- CSEPP
Chemical Stockpile Emergency Preparedness Program; joint FEMA/DA organization
- CSM
conceptual site model
- CW
chemical warfare
- CWA
chemical warfare agent
- DA
U.S. Department of the Army
- DABT
Diplomate, American Board of Toxicology
- DF
dermal factor
- DHHS
U.S. Department of Health and Human Services
- DHS
U.S. Department of Homeland Security
- DOD
U.S. Department of Defense
- DOE
U.S. Department of Energy
- EA2192
S-(diisopropylaminoethyl) methylphosphonothioic acid, C9H22NPO2S
- EC50
median Effective Concentration
- ED
exposure duration
- EF
exposure frequency
- EMPA
ethyl methylphosphonic acid
- ERPG
Emergency Response Planning Guidance
- ERSH-DB
Emergency Response Safety and Health Database of the NIOSH
- ET
exposure time
- FTSBS
fraction transferred from surface to body skin
- FTSHS
fraction transferred from surface to hand skin
- FNE
first noticeable effect
- FQ
frequency of hand-to-mouth contact
- FQeye
frequency of hand-to-eye contact
- GA
nerve agent tabun; NATO code
- GB
nerve agent sarin; NATO code
- GD
nerve agent soman; NATO code
- GF
nerve agent cyclosarin; NATO code
- GI
gastrointestinal
- GIABS
gastrointestinal absorption factor
- HBESL
Health-Based Environmental Screening Levels
- HD
vesicant agent distilled sulfur mustard; NATO code
- HHMSSL
Human Health Medium-Specific Screening Levels
- IDLH
immediately dangerous to life or health
- IF
ingestion factor
- IMPA
isopropyl methylphosphonic acid
- IRIS
Integrated Risk Information System of the USEPA
- LANL
Los Alamos National Laboratory, Los Alamos NM
- LAWA
Los Angeles World Airports
- LAX
os Angeles International Airport
- LD50
median Lethal Dose (50%)
- LLNL
Lawrence Livermore National Laboratory, Livermore CA
- LOAEL
lowest adverse effect level
- MIST
Man-In-Simulant Test Program (U.S. Dept of Army)
- MPA
methyl phosphonic acid; CH5O3P
- MRL
minimal risk level
- NAC
National Advisory Committee for Acute Exposure Guideline Levels (USEPA)
- NATO
North Atlantic Treaty Organization
- NFPA
National Fire Protection Association
- NIOSH
National Institute for Occupational Safety and Health
- NOAEL
no observable effect level
- NRC
National Research Council
- NTP
National Toxicology Program
- NRT
National Response Team
- NUREG
U.S. Nuclear Regulatory Commission
- OASA
Office of the Assistant Secretary of the Army
- OEHHA
Office of Environmental Health Hazard Assessment of the CalEPA
- OF
ocular rubbing factor
- OPP
Office of Pesticide Programs (USEPA)
- ORNL
Oak Ridge National Laboratory, Oak Ridge TN
- OSHA
Occupational Safety and Health Administration
- OTSG
Office of the Surgeon General (U.S. Dept of the Army)
- PAL
Provisional Advisory Levels (USEPA)
- PNNL
Pacific Northwest National Laboratory, Richland WA
- PPE
personal protective equipment
- PRG
Preliminary Remediation Goal (USEPA)
- RBC
Risk-Based Concentration (USEPA)
- RBC-ChE
red blood cell cholinesterase
- RME
reasonable maximum exposure
- RF
resuspension factor
- RfC
reference concentration
- RfD
oral reference dose
- RfDe
estimated oral reference dose
- RSLs
Regional Screening Levels of the USEPA
- SAeye
surface area of eyes
- SAhand
surface area of hand
- SAtrans
exposed skin surface area of clothed children
- SAwork
exposed skin surface area of clothed airport personnel
- SAP
Science Advisory Panel (USEPA)
- SD
Sprague-Dawley (rat strain)
- SE
saliva extraction factor
- SHEDS
Stochastic Human Exposure and Dose Simulation model (USEPA)
- SNL
Sandia National Laboratory, Albuquerque NM
- SRCL
surface removal contaminant levels
- STEL
short-term exposure limit
- SVFUDS
Spring Valley Formerly Used Defense Site
- TBIT
The Bradley International Terminal of the LA International Airport
- TDG
thiodiglycol
- THQ
toxic hazard quotient
- TIC
toxic industrial compounds
- UFA
interspecies uncertainty factor
- UFH
intraspecies uncertainty factor
- USACHPPM
U.S. Army Center for Health Promotion and Preventive Medicine
- USEPA
U.S. Environmental Protection Agency
- VX
nerve agent; NATO code
- WTC
World Trade Center
- WWI
World War I
Footnotes
The views expressed in this article do not necessarily represent official Federal agency position or policy. Mention of trade names or commercial products does not constitute endorsement or recommendation of use.
References
- Aaron HS. Potential Hazards in the Handling of Aged AC and CK Munitions: A Literature Review. ERDEC-SP-039. Aberdeen Proving Ground, MD, USA: Edgewood Research and Development Engineering Center; 1996. [Google Scholar]
- ACGIH (American Conference of Governmental Industrial Hygienists) TLVs and BEIs, Based on the Documentation of the Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices (Cyanogen Chloride, p 24; Hydrogen Cyanide, p 35; Phosgene, p 47) 2003. American Conference of Governmental Industrial Hygienists, Cincinnati, OH, USA.
- ACGIH. TLVs and BEIs, Based on the Documentation of the Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices. 2008. American Conference of Governmental Industrial Hygienists, Cincinnati, OH, USA.
- Adeshina F, Sonich-Mullin C, Ross RH, et al. Health-based Provisional Advisory Levels (PALs) for homeland security. Inhal Toxicol. 2009;21((S3)):12–6. doi: 10.3109/08958370903202788. [DOI] [PubMed] [Google Scholar]
- AIHA (American Industrial Hygiene Association) Emergency Response Planning Guidelines: Cyanogen Chloride. Fairfax, VA, USA: AIHA Press; 1998. [Google Scholar]
- AIHA. Emergency Response Planning Guidelines and Workplace Environmental Exposure Level Guides (Cyanogen Chloride, p 22 Hydrogen Cyanide, p 23; Phosgene, p 24) 2007. Fairfax, VA, USA.
- Anderson JS. Chemical Defense Research Establishment (CDRE) Report No. 241. Rawalpindi, India: Chemical Defense Research Establishment; 1942. The Effect of Mustard Gas Vapour on Eyes Under Indian Hot Weather Conditions. [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry) Toxicological Profile for Sulfur Mustard (Update) Atlanta, GA, USA: US Department of Health and Human Services, Public Health Service; 2003. [Google Scholar]
- ATSDR. Medical Management Guidelines (MMGs) for Hydrogen Cyanide (HCN) Atlanta, GA, USA: US Department of Health and Human Services, Public Health Service; 2005. Available at http://www.atsdr.cdc.gov/MHMI/index.asp. [Google Scholar]
- ATSDR. Toxicological Profile for Cyanide (Update) Atlanta, GA, USA: US Department of Health and Human Services, Public Health Service; 2006. [Google Scholar]
- Review of the Army's health risk assessments for oral exposure to six chemical-warfare agents. J Toxicol Environ Health Part A. 2000;59:281–526. Bakshi KS, Pang SNJ, and Snyder R (eds). [PubMed] [Google Scholar]
- Bast C, Glass D. Phosgene. In: Gupta R, editor. Handbook of Toxicology of Chemical Warfare Agents. Amsterdam, The Netherlands: Elsevier Publishers, Academic Press; 2009. pp. 321–30. [Google Scholar]
- Bausum H, Reddy G, Leach G. Derivation of Health-Based Environmental Screening Levels for Chemical Warfare Agents: A Technical Evaluation, Appendix F. Aberdeen Proving Ground, MD, USA: US Army Center for Health Promotion and Preventive Medicine; 1999. Primary breakdown products of chemical agents; pp. F-1–F-19. [Google Scholar]
- Benton BJ, Sommerville DR, Scotto J, et al. Low-Level Effects of VX Vapor Exposure on Pupil Size and Cholinesterase Levels in Rats. Technical Report ECBC TR-428. Aberdeen Proving Ground, MD, USA: U.S. Army Edgewood Chemical Biological Center; 2005. [Google Scholar]
- Benton BJ, Sommerville DR, Anthony S, et al. Low-level effects of VX vapor exposure on pupil size and cholinesterase levels in rats. In: Salem HA, Katz SA, editors. Inhalation Toxicology. 2nd edit. Boca Raton, FL, USA: CRC Press, Taylor and Francis; 2006a. pp. 91–108. [Google Scholar]
- Benton BJ, McGuire JM, Sommerville DR, et al. Effects of whole-body VX vapor exposure on lethality in rats. Inhalation Toxicol. 2006b;18:1091–9. doi: 10.1080/08958370600945598. [DOI] [PubMed] [Google Scholar]
- Benton BJ, McGuire JM, Sommerville DR, et al. Effects of Whole-Body VX Vapor Exposure on Lethality in Rats. Technical Report ECBC TR-525. Aberdeen Proving Ground, MD, USA: U.S. Army Edgewood Chemical Biological Center; 2007. [DOI] [PubMed] [Google Scholar]
- Bozkir MG, Karakas P, Oguz Ö. Measurements of soft tissue orbits in Turkish young adults. Surgical Radiol Anat. 2003;25:54–7. doi: 10.1007/s00276-002-0092-8. [DOI] [PubMed] [Google Scholar]
- Callaway S, Dirnhuber P. Estimation of the Concentration of Nerve Agent Vapour Required to Produce Measured Degrees of Miosis in Rabbit and Human Eyes. Porton Down, Salisbury, Wiltshire, UK: Chemical Defence Research Establishment; 1971. Technical Paper No. 64. [Google Scholar]
- CAM (Center for Airport Management) 2005 On-Airport Intercept Survey. Los Angeles, CA, USA: Management Report prepared for Los Angeles International Airport by The Center for Airport Management in partnership with GC Tech, Inc.; 2005. October 26, 2005. [Google Scholar]
- Cameron JA, Al-Rajhi A, Badr IA. Corneal ectasia in vernal keratoconjunctivitis. Opthalmology. 1989;96:1615–23. doi: 10.1016/s0161-6420(89)32677-7. [DOI] [PubMed] [Google Scholar]
- Capacio BR, Smith JR, Gordon RK, et al. Clinical detection of exposure to chemical warfare agents. In: Romano JA, Lukey BJ, Salem H, editors. Chemical Warfare Agents: Chemistry, Pharmacology, Toxicology and Therapeutics. 2nd edit. Boca Raton, FL, USA: CRC Press; 2008. pp. 501–48. [Google Scholar]
- Cohen Hubal EA, Sheldon LS, Burke JM, et al. Children's exposure assessment: A review of factors influencing children's exposure, and the data available to characterize and assess that exposure. Environ Health Perspect. 2000;108((6)):475–86. doi: 10.1289/ehp.108-1638158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohrssen B. Cyanides and nitriles. In: Bingham E, Cohrssen B, Powell CH, editors. Patty's Toxicology. 5th edit. Vol. 4. New York, NY, USA: John Wiley and Sons, Inc.; 2001. pp. 1373–456. [Google Scholar]
- COPC (Contaminants of Potential Concern) Committee of the Word Trade Center Indoor Air Task Force Working Group, World Trade Center Indoor Assessment: Selecting Contaminants of Potential Concern and Setting Health-Based Benchmarks. New York, NY, USA: US Environmental Protection Agency, Region 2; 2003. Available at www.epa.gov/WTC. [Google Scholar]
- Cucinell SA. Review of the toxicity of long-term phosgene exposure. Arch Environ Health. 1974;28:272–5. doi: 10.1080/00039896.1974.10666485. [DOI] [PubMed] [Google Scholar]
- Currie WD, Hatch GE, Frosolone MF. Pulmonary alterations in rats due to acute phosgene inhalation. Fund Appl Toxicol. 1987a;8:107–14. doi: 10.1016/0272-0590(87)90106-0. [DOI] [PubMed] [Google Scholar]
- Currie WD, Hatch GE, Frosolone MF. Changes in lung ATP concentration in the rat after low-level phosgene exposure. J Biol Toxicol. 1987b;2:105–14. doi: 10.1002/jbt.2570020204. [DOI] [PubMed] [Google Scholar]
- DA (US Department of the Army) Nerve Agent Percutaneous Exposure Criteria and Airborne Exposure Levels (AELs) for GD, GF for Use in Interim DA Guidance on Implementation of the New AELs. 2004. Memorandum. Office of the Surgeon General, Falls Church, VA, USA. June 29, 2004.
- DA. Potential Military Chemical/Biological Agents and Compounds. 2005a. Field Manual FM 3-11.9. Army, Marine Corps, Navy, Air Force Multiservice Tactics, Techniques and Procedures. Commandant, US Army Chemical School, Ft. Leonard Wood, MO, USA. (Approved for public release, distribution unlimited)
- DA. Sanitary Control and Surveillance of Field Water Supplies. Technical Bulletin TB-MED 577. Washington, DC, USA: US Department of the Army Headquarters; 2005b. [Google Scholar]
- DA. Toxic Chemical Agent Safety Standards. DA Pam 385-61. The Pentagon, Washington, DC, USA: US Department of the Army, Headquarters; 2008. [Google Scholar]
- DA. Sanitary Control and Surveillance of Field Water Supplies. 2010. Technical Bulletin TB-MED 577/NAVMED P-5010-10/AFMAN 48-138_IP. Headquarters, Departments of the Army, Navy and Air Force, Washington, DC, USA.
- Dabisch PA, Horsmon MS, Taylor JT, et al. Gender differences in the miotic potency of soman vapor in rats. Cutan Ocul Toxicol. 2008a;27:123–33. doi: 10.1080/15569520802064376. [DOI] [PubMed] [Google Scholar]
- Dabisch PA, Hulet SW, Kristovich R, et al. Inhalation toxicology of nerve agents. In: Romano JA Jr., Lukey BJ, Salem H, editors. Chemical Warfare Agents: Chemistry, Pharmacology, Toxicology and Therapeutics. Boca Raton, FL, USA: CRC Press; 2008b. pp. 233–46. [Google Scholar]
- Daubert TE, Danner RP. Physical and Thermodynamic Properties of Pure Chemicals; Data Compilation (p 630) Washington, DC, USA: Taylor and Francis; 1989. [Google Scholar]
- DHHS (US Department of Health and Human Services) Final Recommendations for Protecting Human Health from Potential Adverse Effects of Exposure to Agents GA, GB and VX. Centers for Disease Control and Prevention, Atlanta, GA, USA. Fed Reg. 2003;68((196)):58348–51. [Google Scholar]
- DHHS. Interim Recommendations for Airborne Exposure Limits for Chemical Warfare Agents H and HD (Sulfur Mustard) Centers for Disease Control and Prevention, Atlanta, GA, USA. Fed Reg. 2004;69((85)):24164–8. [Google Scholar]
- DHS (Department of Homeland Security) Emergencies and Disasters, First Responders, Science and Technology Standards, Standards for Personal Protective Gear for First Responders. 2006. Available at http://www.dhs.gov/files/programs/gc_1218226975457.shtm.
- Diller WF. Pathogenesis of phosgene poisoning. Toxicol Ind Health. 1985;1:7–15. doi: 10.1177/074823378500100202. [DOI] [PubMed] [Google Scholar]
- Diller WF, Bruch J, Dehnen W. Pulmonary changes in the rat following low phosgene exposure. Arch Toxicol. 1985;57:184–90. doi: 10.1007/BF00290885. [DOI] [PubMed] [Google Scholar]
- Fendinger NJ, Glotfelty DE. Henry's Law Constants for selected pesticides, PAHs and PCBs. Environ Tox Chem. 1990;9:731–5. [Google Scholar]
- Flury F, Zernik F. Noxious Gases, Vapors, Mists, Smokes and Dust Particles. Berlin, Germany: Springer; 1931. pp. 350–4. [Google Scholar]
- Genovese RF, Benton BJ, Oubre JL, et al. Determination of miosis threshold from whole-body vapor exposure to sarin in African green monkeys. Toxicology. 2008;244:123–32. doi: 10.1016/j.tox.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Genovese RF, Benton BJ, Johnson CC, et al. Assessment of low-level whole-body soman vapor exposure in rats. Neurotoxicol Teratol. 2009;31:110–8. doi: 10.1016/j.ntt.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Glass D, Koller L, McClanahan M, et al. Provisional Advisory Levels (PALS) development for phosgene. The Toxicologist. 2008;102((1)):191. [Google Scholar]
- Glass D, McClanahan M, Koller L, et al. Provisional Advisory Levels (PALS) for phosgene (CG) Inhal Toxicol. 2009;21((S3)):73–94. doi: 10.3109/08958370903202820. [DOI] [PubMed] [Google Scholar]
- Gomes C, Freihaut J, Bahnfleth W. Resuspension of allergen-containing particles under mechanical and aerodynamic disturbances from human walking. Atmospheric Env. 2007;41:5257–70. [Google Scholar]
- Guild WJ, Harrison KP, Fairly A, et al. The Effect of Mustard Gas Vapour on the Eyes. Porton Report No. 2297, Serial No. 12 (8 November 1941) Salisbury, Wiltshire, UK: Chemical Defence Research Establishment; 1941. [Google Scholar]
- Haber FR. Fuenf Vortraege aus den Jahren 1920-23 (Five Lectures from the Years 1920-1923) Berlin, Germany: Verlag von Julius Springer; 1924. Zur Geschichte des Gaskrieges (On the History of the Gas War) pp. 76–92. [Google Scholar]
- Hartung R. Cyanides and nitriles. In: Clayton GD, Clayton FE, editors. Patty's Industrial Hygiene and Toxicology. 4th edit. New York, NY, USA: John Wiley and Sons, Inc.; 1994. pp. 3119–72. [Google Scholar]
- Harvey JC. Clinical Observations on Volunteers Exposed to Concentrations of GB. 1952. Medical Laboratories Research Report No. 114, Publication Control No. 5030-114,
- MLCR 114 (CMLRE-ML-52). US Army Chemical Center, Aberdeen Proving Ground, MD, USA.
- Ho P, Tucker MD, Smith W. Decontamination Technologies for Building Restoration. 2006. Sandia Report SAND2006-6580. Sandia National Laboratories, Albuquerque, NM, USA (Official Use Only).
- Howard J, Hanzel R. Chronic toxicity for rats of food treated with hydrogen cyanide. Agric Food Chem. 1955;3((4)):325–9. [Google Scholar]
- Jacobs MB. War Gases; Their Identification and Decontamination. New York, NY, USA: Interscience Publishers, Inc.; 1942. [Google Scholar]
- Johns RJ. The Effect of Low Concentrations of GB on the Human Eye. 1952. Chemical Corps Medical Laboratories Research Report No. 100, Publication Control 5030-100 (CMLRE-ML-52). US Army Chemical Center, Aberdeen Proving Ground, MD, USA.
- Kaerkes B. Experiences with a Phosgene Indicator Badge During an Eleven-Year Period. 1992. M.D. Dissertation. Heinrich-Heine University, Duesseldorf, Germany.
- Kim NK, Hawley J. Re-Entry Guidelines: Binghamton State Office Building. 1985. Document 0549P. New York State Department of Health, Bureau of Toxic Substances Assessment, Division of Health Risk Control, Albany, NY, USA.
- Kodavanti UP, Costa DL, Giri SN, et al. Pulmonary structural and extracellular matrix alterations in Fischer 344 Rats following subchronic phosgene exposure. Fund Appl Toxicol. 1997;37:54–63. doi: 10.1006/faat.1997.2298. [DOI] [PubMed] [Google Scholar]
- Korb DR, Leahy CD, Greiner JV. Prevalence and characteristics of eye-rubbing for keratoconic and non-keratonic subjects. Investig Opthalmol Vis Sci. 1991;32:884. [Google Scholar]
- Krewski D, Bakshi K, Garrett R, et al. Development of Acute Exposure Guideline Levels for airborne exposures to hazardous substances. Regul Toxicol Pharmacol. 2004;39:184–201. doi: 10.1016/j.yrtph.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Mansdorf S. Recommendations for Chemical Protective Clothing, A Companion Guide to the NIOSH Pocket Guide to Chemical Hazards, NTIS PB98-137730. 1998. Available at http: //www.cdc.gov/niosh/ncpc/ncpc1.html.
- McMonnies CW, Boneham GC. Kerataconus, allergy, itch, eye-rubbing and hand dominance. Clin Exp Optom. 2003;86:376–84. doi: 10.1111/j.1444-0938.2003.tb03082.x. [DOI] [PubMed] [Google Scholar]
- McNamara BP, Owens EJ, Christensen MK, et al. Toxicological Basis for Controlling Levels of Mustard in the Environment. 1975. EASP EBSP 74030. Biomedical Laboratory, Edgewood Arsenal, Aberdeen Proving Ground, MD, USA.
- Michaud JM, Huntley SL, Sherer RA, et al. PCB and dioxin re-entry criteria for building surfaces and air. J Expos Anal Environ Epidem. 1994;4:197–227. [PubMed] [Google Scholar]
- Michel HO, Epstein J, Plapinger RR, et al. EA 2192: A Novel Anticholinesterase. CRDLR 3135. Aberdeen Proving Ground, MD, USA: US Army Chemical and Research Laboratories, Army Chemical Center; 1962. [Google Scholar]
- Ministry of Health, Mozambique. Mantakassa: An epidemic of spastic parapesis associated with chronic cyanide intoxication in a cassava staple area of Mozambique; 1. Epidemiology and clinical and laboratory findings in patients. Bull WHO. 1984a;62:477–84. [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health, Mozambique. Mantakassa: An epidemic of spastic parapesis associated with chronic cyanide intoxication in a cassava staple area of Mozambique; 2. Nutritional factors and hydrocyanic acid content in cassava. Bull WHO. 1984b;62:485–92. [PMC free article] [PubMed] [Google Scholar]
- Mioduszewski RJ, Reutter SA, Miller LL, et al. Evaluation of Airborne Exposure Limits for G-Agents: Occupational and General Population Exposure Criteria. ERDEC-TR-489. Aberdeen Proving Ground, MD, USA: Edgewood Research Development and Engineering Center; 1998. [Google Scholar]
- Mioduszewski RJ, Manthei J, Way R, et al. Estimating the probability of sarin vapor toxicity in rats as a function of exposure concentration and duration. 2000. In: Proceedings of the International Chemical Weapons Demilitarization Conference, CWD-2000, The Hague, The Netherlands.
- Mioduszewski RJ, Manthei J, Way R, et al. ECBC Low Level Operational Toxicology Program: Phase I (Inhalation Toxicity of Sarin Vapor in Rats as a Function of Exposure Concentration and Duration. ECBC-TR-183. Aberdeen Proving Ground, MD, USA: Edgewood Research Development and Engineering Center; 2001. [Google Scholar]
- Mioduszewski RJ, Manthei J, Way R, et al. Low-level Sarin Vapor Exposure in Rats: Effect of Exposure Concentration and Duration on Pupil Size. ECBC-TR-235. Aberdeen Proving Ground, MD, USA: Edgewood Chemical Biological Center, US Army Soldier and Biological Chemical Command; 2002a. [Google Scholar]
- Mioduszewski RJ, Manthei J, Way R, et al. Interaction of exposure concentration and duration in determining acute toxic effects of sarin vapor in rats. Toxicol Sci. 2002b;66:176–84. doi: 10.1093/toxsci/66.2.176. [DOI] [PubMed] [Google Scholar]
- Munro NB, Talmage SS, Griffin GD, et al. The sources, fate and toxicity of chemical warfare agent degradation products. Environ Health Perspect. 1999;107:933–74. doi: 10.1289/ehp.99107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIOSH (National Institute for Occupational Safety and Health) Pocket Guide to Chemical Hazards (Hydrogen Cyanide, p 168; Cyanogen Chloride, p 82; Phosgene, p 252) Washington, DC, USA: US Department of Health and Human Services, Public Health Service, CDC, NIOSH, US Government Printing Office; 2003. [Google Scholar]
- NIOSH. Pocket Guide to Chemical Hazards. Washington, DC, USA: US Department of Health and Human Services, Public Health Service, CDC, NIOSH, US Government Printing Office; 2005a. Available at http://www.cdc/gov/niosh/npg/ [Google Scholar]
- NIOSH. NIOSH Emergency Response Card, Hydrogen Cyanide. 2005b. Available at http: /www.bt.cdc.gov/agent/cyanide/erc74-90-8.asp, accessed August 4, 2005.
- NIOSH. Pocket Guide to Chemical Hazards (Hydrogen Cyanide. 2008a. Available at www.cdc.gov/niosh/npg/npgd0333.html; Cyanogen Chloride, Available at www.cdc.gov/niosh/npg/npgd0162.html; Phosgene. Available at: www.cdc.gov/niosh/npg/npgd0504.html). U.S. Department of Health and Human Services, Public Health Service, CDC, NIOSH, Washington, DC, USA.
- NIOSH. NIOSH Emergency Response Pocket Card: Hydrogen Cyanide. 2008b. (Available at http://www.bt.cdc.gov/agent/cyanide/erc74-90-8pr.asp) and Cyanogen chloride (Available at http://www.bt.cdc.gov/agent/cyanide/erc506-77-4pr.asp). US Department of Health and Human Services, Public Health Service, CDC, NIOSH, Washington, DC, USA.
- NIOSH/CDC [National Institute for Occupational Safety and Health (NIOSH) and Centers for Disease Control and Prevention (CDC)] Emergency Response Safety and Health Database. 2008. Available at www.cdc.gov/NIOSH/ershdb/
- NRC/BAST (National Research Council, Board on Army Science and Technology) Technical Assessment of the Man-in-Simulant Test (MIST) Program. Washington, DC, USA: National Academy Press; 1997. Available at www.nap.edu. [Google Scholar]
- NRC/COT (National Research Council, Committee on Toxicology) Review of the U.S. Army's Health Risk Assessment for Oral Exposure to Six Chemical Warfare Agents. Washington, DC, USA: COT Subcommittee on Chronic Reference Dose for Chemical Warfare Agents, Committee on Toxicology. National Academy Press; 1999. Available at www.nap.edu. [Google Scholar]
- NRC/COT. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC, USA: Subcommittee on Acute Exposure Guideline Levels, Committee on Toxicology. National Academy Press; 2001. Available at www.nap.edu. [PubMed] [Google Scholar]
- NRC/COT. Phosgene; Hydrogen Cyanide. Acute Exposure Guideline Levels for Selected Airborne Chemicals. 2:211–76. respectively Subcommittee on Acute Exposure Guideline Levels, Committee on Toxicology 2002 Washington, DC, USA The National Academies Press 15-70 Available at www.nap.edu. [Google Scholar]
- NRC/COT. Nerve Agents; Sulfur Mustard. Acute Exposure Guideline Levels for Selected Airborne Chemicals. 3:301–83. respectively Subcommittee on Acute Exposure Guideline Levels, Committee on Toxicology 2003 Washington, DC, USA The National Academies Press 15-300 Available at www.nap.edu. [Google Scholar]
- NRT (National Response Team) NRT Quick Reference Guides, US National Response Team. 2009. Available at www.nrt.org.
- NTP (National Toxicology Program) Sodium Cyanide (CAS No. 143-33-9) Administered in Drinking Water to F344/N Rats and B6C3F1 Mice. 1993. NTP toxicity report series no. 37, NIH Publication 94-3386. US Department of Health and Human Services, National Toxicology Program, Washington, DC, USA.
- NUREG (US Nuclear Regulatory Commission) Residual Radioactive Contamination from Decommissioning: Technical Basis for Translating Contamination Levels to Annual Total Effective Dose Equivalent. NUREG/CR-5512 PNL-7994. Vol. 1. Washington, DC, USA: Nuclear Regulatory Commission; 1992. [Google Scholar]
- NUREG. Munition Demilitarization Facility Re-evaluation of the Indoor Resuspension Factor for the Screening Analysis of the Building Occupancy Scenario for NRC's License Termination Rule. NUREG-1720. Washington, DC, USA: Division of Waste Management, Office of Nuclear Material Safety and Safeguards; 2002. [Google Scholar]
- Fatz J., editor. OASA (Office of the Assistant Secretary of the Army) Derivation of Health-Based Environmental Screening Levels (HBESL) for Chemical Warfare Agents. Washington, DC, USA: Deputy Assistant Secretary of the Army 1999 Department of the Army, Office of the Assistant Secretary (Environment, Safety, and Occupational Health), Army Pentagon; 1999 May 28. Memorandum signed by Raymond. [Google Scholar]
- Opresko D, Young R, Faust R, et al. Chemical warfare agents: Estimating oral reference doses. Rev Environ Contam Toxicol. 1998;156:1–183. doi: 10.1007/978-1-4612-1722-0_1. [DOI] [PubMed] [Google Scholar]
- Opresko DM, Young RA, Watson AP, et al. Chemical warfare agents: Current status of oral reference doses. Rev Environ Contam Toxicol. 2001;172:65–85. doi: 10.1007/978-1-4612-1722-0_1. [DOI] [PubMed] [Google Scholar]
- PARSONS. Site-Specific Work Plan for the Investigation of Burial Pit 3, 4825 Glenbrook Road, Spring Valley Formerly Used Defense Site (SVFUDS) 2007. Prepared for US Army Engineering and Support Center Huntsville and US Army Corps of Engineers Baltimore District. PARSONS, Fairfax, VA, USA.
- Prentiss AM. Chemicals in War: A Treatise on Chemical Warfare. 1st edit. New York, NY, USA: McGraw-Hill Book Co., Inc.; 1937. [Google Scholar]
- Reddy G, Major MA, Leach GJ. Toxicity assessment of thiodiglycol. Int J Toxicol. 2005;24:435–42. doi: 10.1080/10915810500368878. [DOI] [PubMed] [Google Scholar]
- Reed CI. The Minimum Concentration of Mustard Gas Effective for Man. 1918. Preliminary Report. Report No. 318 (October 26, 1918). War Department, Medical Division, Chemical Warfare Service, Pharmacological Research Section, American University Experiment Station, Washington, DC, USA.
- Reed CI, Hopkins EF, Weyand CF. The Minimum Concentration of Mustard Gas Effective for Man. 1918. Final Report. Report No. 329 (December 2, 1918). War Department, Medical Division, Chemical Warfare Service, Pharmacological Research Section, American University Experiment Station, Washington, DC, USA.
- Rodes C, Newsome R, Vanderpool R, et al. Experimental methodologies and preliminary transfer factor data for estimation of dermal exposure to particles. J Expos Anal and Envir Epidemiol. 2001;11:123–39. doi: 10.1038/sj.jea.7500150. [DOI] [PubMed] [Google Scholar]
- Salocks CB. Assessment of Children's Exposure to Surface Methamphetamine Residues in Former Clandestine Methamphetamine Labs, and Identification of a Risk-Based Cleanup Standard for Surface Methamphetamine Contamination. External Peer Review Draft. Sacramento, CA, USA: Integrated Risk Assessment Branch, Office of Environmental Health Hazard Assessment, California Environmental Protection Agency; 2007. December 2007. Available at http://www.oehha.ca.gov/public_info/public/kids/ [Google Scholar]
- Salocks CB. Assessment of Children's Exposure to Surface Methamphetamine Residues in Former Clandestine Methamphetamine Labs, and Identification of a Risk-Based Cleanup Standard for Surface Methamphetamine Contamination. Revised Draft. Sacramento, CA, USA: Integrated Risk Assessment Branch, Office of Environmental Health Hazard Assessment, California Environmental Protection Agency; 2008. December 2008. Available at http://www.oehha.ca.gov/public_info/public/kids/ [Google Scholar]
- Salocks CB. Assessment of Children's Exposure to Surface Methamphetamine Residues in Former Clandestine Methamphetamine Labs, and Identification of a Risk-Based Cleanup Standard for Surface Methamphetamine Contamination. Sacramento, CA, USA: Integrated Risk Assessment Branch, Office of Environmental Health Hazard Assessment, California Environmental Protection Agency; 2009. February 2009. Available at http://www.oehha.ca.gov/public_info/public/kids/ [Google Scholar]
- Selgrade MK, Gilmour MI, Yang YG, et al. Pulmonary host defenses and resistance to infection following subchronic exposure to phosgene. Inhal Toxicol. 1995;7:1257–68. [Google Scholar]
- Stallings C, Zartarian V, Glen G. Stochastic Human Exposure and Dose Simulation Model for Multimedia, Multipathway Chemicals: SHEDS-Multimedia Model Version 3 Users’ Guide. EPA/600/R-08/118. Washington, DC, USA: USEPA National Exposure Research Laboratory, Office of Research and Development; 2008. Available at http://www.epa.gov/heasd/products/sheds_multimedia/sheds_mm.html. [Google Scholar]
- Talmage SS, Munro NB, Watson AP, et al. The fate of chemical warfare agents in the environment. In: Marrs TC, Maynard RL, Sidell FR, editors. Chemical Warfare Agents: Toxicology and Treatment. 2nd edit. Chichester, West Sussex, England: John Wiley and Sons Ltd.; 2007a. pp. 89–125. [Google Scholar]
- Talmage SS, Watson AP, Hauschild V, et al. Chemical warfare agent degradation and decontamination. Current Organic Chemistry. 2007b;11:285–98. [Google Scholar]
- Ten Berge WF, Zwart A, Appelman LM. Concentration-time mortality response relationship of irritant and systematically acting vapours and gases. J Hazard Materials. 1986;13:302–9. [Google Scholar]
- USACHPPM (U.S. Army Center for Health Promotion and Preventive Medicine) Derivation of Health-Based Environmental Screening Levels for Chemical Warfare Agents: A Technical Evaluation. Aberdeen Proving Ground, MD, USA: US Army Center for Health Promotion and Preventive Medicine; 1999. [Google Scholar]
- USEPA (US Environmental Protection Agency) Health Assessment Document for Phosgene. 1986. EPA/600/8-86/022A. Office of Health and Environmental Assessment, Washington, DC, USA.
- USEPA. Risk Assessment Guidance for Superfund: Volume IHuman Health Evaluation Manual (Part A) 1989. OSWER Directive EPA/540/1-89/002. Office of Emergency and Remedial Response, Washington, DC, USA.
- USEPA. Risk Assessment Guidance for Superfund: Volume IHuman Health Evaluation Manual (Part B, Development of Risk-Based Preliminary Remediation Goals) 1991. OSWER Directive 9285.7-01B. Office of Emergency and Remedial Response, Washington, DC, USA.
- USEPA. Development of Risk-Based Concentrations. Philadelphia, PA, USA: Office of Superfund Programs; 1996a. EPA Region III Risk-Based Concentration Table, Background Information. [Google Scholar]
- USEPA. Soil Screening Guidance: Technical Background Document. EPA/540/R-95/128. Washington, DC, USA: Office of Emergency and Remedial Response; 1996b. [Google Scholar]
- USEPA. General Factors Exposure Handbook, vol I, Update to Exposure Factors Handbook EPA/600/8-89/043 (May 1989). EPA/600/P-95/002Fa. Washington, DC, USA: Office of Research and Development; 1997. [Google Scholar]
- USEPA. Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites. 2001. Interim Guidance, OSWER 9355.4-24. Washington, DC, USA.
- USEPA. Risk Assessment Guidance for Superfund, Volume I, Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment, Final) 2004a. EPA/540/R/99/005, OSWER/9285.7-02EP, PB 99-963312. Office of Superfund Remediation and Technology Innovation, Washington, DC, USA.
- USEPA. Users’ Guide and Background Technical Document for USEPA Region 9's Preliminary Remediation Goals (PRG) Table. 2004b. Available at http://www.epa.gov/reg3hwmd/risk/human/rb-concentration_table/usersguide.htm.
- USEPA. Region 9 Preliminary Remediation Goals (PRGs), 2004 Table. 2005. Available at http://www.epa.gov/region09/waste/sfund/prg/index.html.
- USEPA. U.S. Environmental Protection Agency Regions 3, 6, and 9. 2008. Regional Screening Levels for Chemical Contaminants at Superfund Sites. Available at http://www.epa.gov/reg3hwmd/risk/human/rb-concentration_table/index.htm.
- USEPA. U.S. Environmental Protection Agency, Regional Screening Levels for Chemical Contaminants at Superfund Sites, User's Guide. 2009a. Available at http://www.epa.gov/reg3hwmd/risk/human/rb-concentration_table/usersguide.htm.
- USEPA. Voluntary Guidelines for Methamphetamine Laboratory Cleanup. EPA-530-R-08-008. Washington, DC, USA: Office of Solid Waste and Emergency Response; 2009b. [Google Scholar]
- USEPA/IRIS (US Environmental Protection Agency, Integrated Risk Information System) Hydrogen Cyanide (CASRN 74-90-8) 1993. Available at http://www.epa.gov/iris/index.html.
- USEPA/IRIS. Chlorine Cyanide (CASRN 506-77-4) 2005. Available at http://www.epa.gov/iris/index.html.
- USEPA/IRIS. Phosgene (CASRN 75-44-5), Hydrogen Cyanide (CASRN 74-90-8) 2006. Available at http://www.epa.gov/iris/index.html.
- Van Helden HPM, Langenberg JP, Benschop HP. Low Level Exposure to GB Vapor in Air: Diagnosis/Dosimetry, Lowest Observable Effect Levels, and Performance Incapacitation. 2001. Award No. DAMD17-97-1-7360. Final Report, Prepared by TNO Prins Maurits Laboratory, Rijswijk, The Netherlands, for the US Army Medical Research and Materiel Command, Fort Detrick, MD, USA.
- Van Helden HPM, Trap HC, Kuijpers WC, et al. Low level exposure to GB vapor in air: Diagnosis/dosimetry, lowest observable effect level, and performance incapacitation. 2002. In: Operational Medical Issues in Chemical and Biological Defense, Proceedings of the 75th Meeting of the Research and Technology Organisation (RTO) held in Estoril, Portugal, May 14-17, 2001. RTO-MP-075, AC/323 (HFM-060) TP/37. North Atlantic Treaty Organisation, Research and Technology Organisation, Neuilly-sur-Seine CEDEX, France.
- Van Helden HPM, Trap HC, Oostdijk JP, et al. Long-term, low-level exposure of guinea pigs and marmosets to sarin vapor in air: Lowest-observable-adverse-effect level. Toxicol Appl Pharmacol. 2003;189:170–9. doi: 10.1016/s0041-008x(03)00131-5. [DOI] [PubMed] [Google Scholar]
- Van Helden HPM, Trap HC, Kuipers WC, et al. Low-level exposure of guinea pigs and marmosets to sarin vapour in air: Lowest-observable-adverse-effect Level (LOAEL) for miosis. J App Toxicol. 2004;24:59–68. doi: 10.1002/jat.948. [DOI] [PubMed] [Google Scholar]
- Walker HW, Smith BF, Blake A. Summary and Discussion of Available Mustard Gas Data from Field Tests Conducted Prior to 1928. Report No. 462. Edgewood Arsenal, Edgewood, MD, USA: Chemical Warfare Service; 1928. [Google Scholar]
- Watson A, Dolislager F. Reevaluation of 1999 Health-Based Environmental Screening Levels (HBESLs) for Chemical Warfare Agents. ORNL/TM-2007/080. Oak Ridge, TN, USA: Oak Ridge National Laboratory; 2007. [Google Scholar]
- Watson A, Opresko DM, Hauschild V. Evaluation of Chemical Warfare Agent Percutaneous Vapor Toxicity: Derivation of Toxicity Guidelines for Assessing Chemical Protective Ensembles. ORNL/TM-2003/180. Oak Ridge, TN, USA: Oak Ridge National Laboratory; 2003. [Google Scholar]
- Watson A, Opresko D, Young R, et al. Development and application of Acute Exposure Guideline Levels (AEGLs) for chemical warfare nerve and sulfur mustard agents. J Toxicol Environ Health B. 2006a;9:173–263. doi: 10.1080/15287390500194441. [DOI] [PubMed] [Google Scholar]
- Watson A, Bakshi K, Opresko D, et al. Cholinesterase inhibitors as chemical warfare agents: Community preparedness guidelines. In: Gupta RC, editor. Toxicology of Organophosphate and Carbamate Pesticides. New York, NY, USA: Elsevier Publishers/Academic Press; 2006b. pp. 47–68. [Google Scholar]
- Watson A, Adeshina F, Opresko D, et al. Provisional Advisory Levels (PALS) development for G-series nerve agents. The Toxicologist. 2007;96((1):319 (March 2007)) [Google Scholar]
- Watson A, Hall L, Raber E, et al. Developing health-based pre-planning clearance goals for airport remediation following chemical terrorist attack: Introduction and key assessment considerations. Hum Ecol Risk Assess. 2011;17:2–56. doi: 10.1080/10807039.2010.534721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer JT, McNamara BP, Owens EJ, et al. Proposed Revision of Limits for Human Exposure to GB Vapor in Nonmilitary Operations Based on One-Year Exposures of Laboratory Animals to Low Airborne Concentrations. ARCSL-TR-78056. Aberdeen Proving Ground, MD, USA: US Army Armament Research and Development Command, Chemical Systems Laboratory; 1979. [Google Scholar]
- Wester RM, Tanojo H, Maibach HI, et al. Predicted chemical warfare agent VX toxicity to uniformed soldier using Parathion in vitro human skin exposure and absorption. Toxicol Appl Pharmacol. 2000;168:149–52. doi: 10.1006/taap.2000.9028. [DOI] [PubMed] [Google Scholar]
- Whalley CE, Benton BJ, Manthei JH, et al. Low-level Cyclosarin (GF) Vapor Exposure in Rats: Effect of Exposure Concentration and Duration on Pupil Size. ECBC-TR-407S (081004) Aberdeen Proving Ground, MD, USA: US Army Edgewood Chemical Biological Center; 2004. [Google Scholar]
- Whalley CE, McGuire JM, Miller DB, et al. Kinetics of sarin (GB) following a single sublethal inhalation exposure in the guinea pig. Inhal Toxicol. 2007;19:667–81. doi: 10.1080/08958370701353296. [DOI] [PubMed] [Google Scholar]
- Wood C. Acute Exposure Guideline Levels (AEGLs) for Cyanogen Chloride (CNCl), draft of June 1997. Washington, DC, USA: National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances, USEPA; 1997. [Google Scholar]
- Yang Y-C. Chemical detoxification of nerve agent VX. Acc Chem Res. 1999;32:109–15. [Google Scholar]
- Yang Y-C, Szafraniec LL, Beaudry WT, et al. Hydrolysis of VX: Activation energies and autocatalysis. 1994. In: Proceedings of the 1994 ERDEC Scientific Conference on Chemical Biological Defense Research November 15-18, 1994 375-82 UNCLASSIFIED paper (ADE 479900), ERDEC-SP-036. US Army Edgewood Research Development and Engineering Center, Aberdeen Proving Ground, MD, USA.
- Young Rand. Mustards and vesicants. In: Gupta R, Bast C, editors. Handbook of Toxicology of Chemical Warfare Agents. Amsterdam, The Netherlands: Elsevier Publishers, Academic Press; 2009. pp. 93–108. [Google Scholar]
- Young RA, Bast CB, Wood CS, et al. Overview of the Standing Operating Procedure (SOP) for development of Provisional Advisory Levels (PALS) Inhal Toxicol. 2009;21((S3)):1–11. doi: 10.3109/08958370903202747. [DOI] [PubMed] [Google Scholar]
- Yusel T, Hepsen I, Cekic O, et al. Incidence of keratoconus in subjects with vernal keratoconjuctivitis: A videokeratographic Study. Opthalmology. 2001;108:824–7. doi: 10.1016/s0161-6420(00)00664-3. [DOI] [PubMed] [Google Scholar]
- Zartarian V, Glen G, Smith L, et al. Stochastic Human Exposure and Dose Simulation model for Multimedia, Multipathway Chemicals: SHEDS Multimedia Model Version 3 Technical Manual. EPA 600/R-08/118. Washington, DC: National Exposure Research Laboratory, USEPA, Office of Research and Development; 2008. [Google Scholar]
- Zongkar B. Army Corps finds new WWI chemical site in DC yard. Associated Press; 2010. April 16, 2010. [Google Scholar]


