The dermatologic management of organ transplant recipients is challenging. Chronic use of immunosuppressive medications in this population results in a 65 to 100 times greater risk of nonmelanoma skin cancer (NMSC), particularly squamous cell carcinoma (SCC).1,2 These cancers are more aggressive and more difficult to treat and have higher rates of metastatic spread than SCCs that arise in immunocompetent individuals.3 Dermatologic surgeons play a critical role in managing these tumors and in recognizing additional risk factors for carcinogenesis in the transplant population.
Given their degree of immunosuppression, organ transplant recipients are prescribed a variety of prophylactic antimicrobial agents, including antibacterial, antifungal, and antiviral medications. In 2002, the U.S. Food and Drug Administration approved voriconazole (Vfend, Pfizer, New York, NY) for the treatment of serious fungal infections. It is a second-generation triazole broad-spectrum antifungal that inhibits P450-dependent ergosterol synthesis, disrupting cell membrane lipid formation. Its ease of administration and efficacy against Aspergillus, zygomycete species, and other rarer molds, including Fusarium and Scedosporium, has made voriconazole a widely used antifungal agent for prophylaxis and treatment of fungal infections in lung transplant recipients.
Recently, voriconazole-associated phototoxicity has been linked to the development of aggressive SCC in immunocompromised patients.4-8 We report here two lung transplant recipients who developed fatal SCC in the setting of voriconazole-induced photodysplasia.
Case 1
The first patient was a 56-year-old woman who underwent combined heart and lung transplant in 1988 at age 34 for primary pulmonary hypertension. Her immunosuppressive regimen initially included prednisone, cyclosporine, and azathioprine, but over the course of several years, she was transitioned to sirolimus, mycophenolate mofetil, and prednisone. She developed her first NMSC 12 years after the transplant and, over the next 9 years, developed at least 20 additional SCCs that were managed using standard excision, curettage and desiccation, or Mohs micrographic surgery (MMS).
The patient was initiated on voriconazole at 200 mg administered orally twice daily in early 2004 for Aspergillus colonization and was increased to 300 mg administered orally twice daily in 2008. Over the course of her dermatologic treatment, she was repeatedly noted to have extensive photodamage, with actinic keratoses and lentigines in sun-exposed areas (Figure 1). In June 2009, the patient developed a poorly differentiated SCC of the left forearm (Figure 2A). The cancer was extirpated with clear margins using MMS.
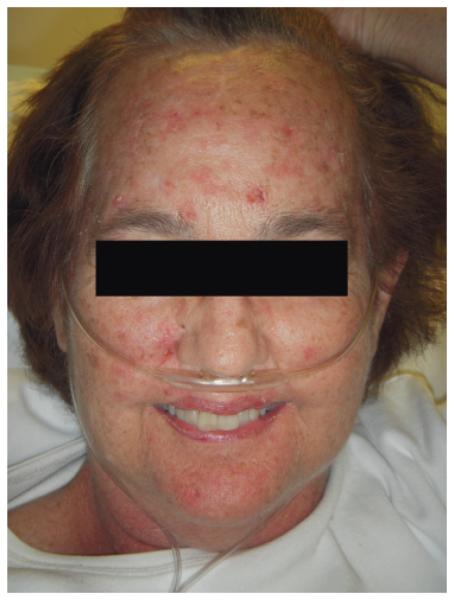
Figure 1.
Photograph of Patient 1 demonstrating diffuse photodamage of the face, including rhytides, lentigines, and actinic keratoses.
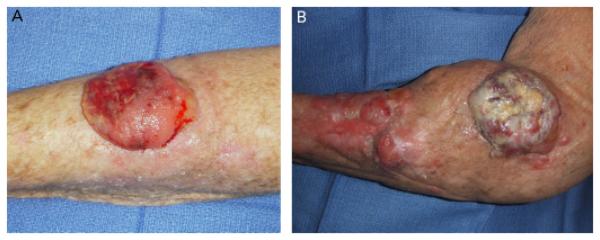
Figure 2.
(A) Initial presentation of poorly differentiated squamous cell carcinoma (SCC) of the forearm of Patient 1. (B) Recurrent, multifocal poorly differentiated SCC approximately 1 month after Mohs micrographic surgery resection with clear margins of tumor shown in A.
Soon after surgery, the patient sustained a traumatic fracture to the left elbow, requiring open reduction and internal fixation with casting of the arm. Upon removal of the cast 1 month later, the patient was noted to have new, rapidly growing nontender cutaneous nodules adjacent to the previous Mohs site on the left forearm (Figure 2B). Biopsy of the largest of these lesions was consistent with recurrent, poorly differentiated SCC. Magnetic resonance imaging (MRI) and computed tomography (CT) scans of the left upper extremity revealed multiple soft tissue nodules and axillary adenopathy. A positron emission tomography (PET)/CT scan showed two mildly fludeoxyglucose (FDG)-avid pulmonary nodules in the left upper lobe. The consulting medical oncologist and transplant pulmonologist felt that a platinum- or taxane-based chemotherapy would further decrease her immune function and that cetuximab was contraindicated given her poor baseline respiratory function and prior reports of fatal diffuse alveolar damage in lung transplant patients treated with this medication.9 After declining amputation, radiation therapy was initiated in the left upper arm. She received 1,250 cGy over five fractions before rapidly decompensating. Capecitabine was offered as a final treatment, but the patient elected to pursue hospice care and died several weeks later.
Case 2
The second patient was a 54-year-old woman who underwent bilateral lung transplantation in 2003 at the age of 49 for a pretransplant diagnosis of lymphangioleiomyomatosis. After surgery, her diagnosis was changed to eosinophilic granulomatosis based on detailed pathologic review of the explanted lungs. Her initial immunosuppressive regimen included azathioprine, cyclosporine, and prednisone. Azathioprine was transitioned to tacrolimus and subsequently to sirolimus. She had a history of a single basal cell carcinoma before her transplant and developed her second NMSC 2 years after the transplant. Over the following 5 years, she developed 11 NMSCs.
As part of standard post-transplant antifungal prophylaxis, the patient was initiated on voriconazole at 200 mg administered orally twice daily on postoperative day 1. She subsequently noted the rapid onset of lesions consistent with actinic keratoses and photodamage in sun-exposed areas.
The patient initially presented to the University of California at San Francisco Department of Dermatology for evaluation and management of two large SCCs of the left wrist and frontoparietal scalp (Figure 3). She reported undergoing previous surgical excision of both sites, with rapid recurrence. She had been treated with external beam radiation therapy to a total dose of 6,000 cGy delivered over 30 fractions to the scalp and wrist. Both tumors persisted despite radiation, and additional nodules appeared within both radiation fields during the course of treatment. She reported severe pain in the left forearm and numbness and weakness of the left fingers. MRI of the left upper extremity revealed tumor involvement of the lateral antebrachial cutaneous nerve and the superficial branch of the radial nerve. A PET/CT scan demonstrated extensive cutaneous disease but no lymph node or visceral metastases. The patient declined limb amputation and scalp resection. For reasons similar to Patient 1, she was deemed a poor candidate for chemotherapy and opted for palliative care, including resection of the left superficial radial, lateral cutaneous, and posterior cutaneous nerves. The patient succumbed to metastatic disease shortly thereafter.
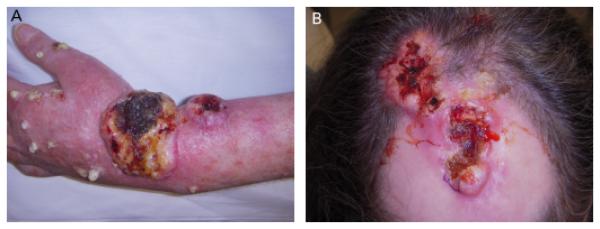
Figure 3.
Poorly differentiated squamous cell carcinoma in Patient 2: (A) wrist, (B) scalp.
Discussion
The evidence linking voriconazole use with aggressive SCC in the setting of chronic immunosuppression is mounting. The association was first reported in late 2007, with further cases being reported in bone marrow and solid organ transplant recipients, as well as those with the human immunodeficiency virus.4-8,10 In patients using voriconazole, the field dysplasia and diffuse lentigines described have been compared with those seen in patients treated with psoralen plus ultraviolet light, and a xeroderma pigmentosum-like phenotype seen in pediatric bone marrow transplant cases. These reports provide troubling evidence of a carcinogenic effect of voriconazole beyond that attributable to immunosuppression alone. In four cases reported by Epau-lard and colleagues, the actinic damage and evidence of phototoxicity rapidly improved upon discontinuation of voriconazole and replacement with posaconazole or itraconazole.8
The immunosuppressed patients presented here and in previous reports were at high risk for developing SCC regardless of voriconazole treatment, but in data presented at the American Thoracic Society International Conference in 2009, Feist and colleagues reported an incidence of NMSC in a population of lung transplant recipients who received voriconazole that was approximately 4 times as great as in those who did not, despite a similar immunosuppressive regimen.10 These cancers occurred earlier and demonstrated higher aggressiveness than tumors occurring in patients not taking voriconazole. Furthermore, three of 12 patients with suspected voriconazole-associated SCC died of metastatic disease, whereas no metastases occurred in the group not taking voriconazole. Additional epidemiologic and pharmacogenomic studies are needed to better characterize patients at risk for this devastating outcome.
Dermatologic surgeons serve a major role in the diagnosis and treatment of skin cancer in organ transplant recipients. It is of increasing importance for our community as well as the transplant community to be aware of the association between voriconazole and the development of advanced, life-threatening SCC in the immunocompromised population. Although all immunocompromised patients should practice diligent sun protection and have close dermatologic follow-up, special care must be taken with patients on voriconazole, because there appears to be a synergistic effect with immunosuppressive medications in the development of aggressive SCC. The appearance of phototoxicity, lentigines, and actinic keratoses should prompt communication with the transplant physician to discuss withdrawing this agent in favor of alternative antifungal medications. Further work to substantiate a causal relationship between voriconazole and SCC in the setting of immunosuppression is warranted.
Footnotes
The authors have indicated no significant interest with commercial supporters.
References
- 1.Jensen P, Møller B, Hansen S. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol. 1999;40:177–86. doi: 10.1016/s0190-9622(99)70185-4. [DOI] [PubMed] [Google Scholar]
- 2.Lindelöf B, Sigurgeirsson B, Gäbel H, Stern RS. Incidence of skin cancer in 5,356 patients following organ transplantation. Br J Dermatol. 2000;143:513–9. [PubMed] [Google Scholar]
- 3.Kanitakis J, Euvrard S, Claudy A. Skin cancers in organ transplant recipients. J Am Acad Dermatol. 2006;54:1115. doi: 10.1016/j.jaad.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 4.Cowen EW, Nguyen JC, Miller DD, et al. Chronic phototoxicity and aggressive squamous cell carcinoma of the skin in children and adults during treatment with voriconazole. J Am Acad Dermatol. 2010;62:31–7. doi: 10.1016/j.jaad.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCarthy KL, Playford EG, Looke DFM, Whitby M. Severe photosensitivity causing multifocal squamous cell carcinomas secondary to prolonged voriconazole therapy. Clin Infect Dis. 2007;44:e55–6. doi: 10.1086/511685. [DOI] [PubMed] [Google Scholar]
- 6.Vanacker A, Fabré G, Van Dorpe J, et al. Aggressive cutaneous squamous cell carcinoma associated with prolonged voriconazole therapy in a renal transplant patient. Am J Transplant. 2008;8:877–80. doi: 10.1111/j.1600-6143.2007.02140.x. [DOI] [PubMed] [Google Scholar]
- 7.Brunel AS, Fraisse T, Lechiche C, et al. Multifocal squamous cell carcinomas in an HIV-infected patient with a long-term voriconazole therapy. AIDS. 2008;22:905–6. doi: 10.1097/QAD.0b013e3282f706a9. [DOI] [PubMed] [Google Scholar]
- 8.Epaulard O, Saint-Raymond C, Villier C, et al. Multiple aggressive squamous cell carcinomas associated with prolonged voriconazole therapy in four immunocompromised patients. Clin Microbiol Infect. 2009 Nov 20; doi: 10.1111/j.1469-0691.2009.03124.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Leard LE, Cho BK, Jones KD, et al. Fatal diffuse alveolar damage in two lung transplant patients treated with cetuximab. J. Heart Lung Transplant. 2007;26:1340–4. doi: 10.1016/j.healun.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Feist A, Osborne SL, Thistlethwaite PA. Voriconazole use increases the incidence of skin cancer in lung transplant recipients [abstract] J Heart Lung Transplant. 2009;28:S5242. [Google Scholar]