Abstract
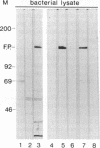
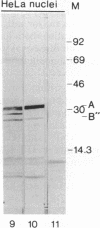
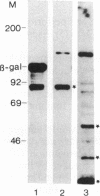
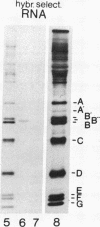
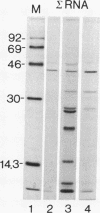
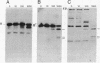
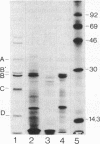
A U2 small nuclear RNA-associated protein, designated B'', was recently identified as the target antigen for autoimmune sera from certain patients with systemic lupus erythematosus and other rheumatic diseases. Such antibodies enabled us to isolate cDNA clone lambda HB''-1 from a phage lambda gt11 expression library. This clone appeared to code for the B'' protein as established by in vitro translation of hybrid-selected mRNA. The identity of clone lambda HB''-1 was further confirmed by partial peptide mapping and analysis of the reactivity of the recombinant antigen with monospecific and monoclonal antibodies. Analysis of the nucleotide sequence of the 1015-base-pair cDNA insert of clone lambda HB''-1 revealed a large open reading frame of 800 nucleotides containing the coding sequence for a polypeptide of 25,457 daltons. In vitro transcription of the lambda HB''-1 cDNA insert and subsequent translation resulted in a protein product with the molecular size of the B'' protein. These data demonstrate that clone lambda HB''-1 contains the complete coding sequence of this antigen. The deduced polypeptide sequence contains three very hydrophilic regions that might constitute RNA binding sites and/or antigenic determinants. These findings might have implications both for the understanding of the pathogenesis of rheumatic diseases as well as for the elucidation of the biological function of autoimmune antigens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adu D., Williams D. G., Quakyi I. A., Voller A., Anim-Addo Y., Bruce-Tagoe A. A., Johnson G. D., Holborow E. J. Anti-ssDNA and antinuclear antibodies in human malaria. Clin Exp Immunol. 1982 Aug;49(2):310–316. [PMC free article] [PubMed] [Google Scholar]
- Billings P. B., Hoch S. O. Characterization of U small nuclear RNA-associated proteins. J Biol Chem. 1984 Oct 25;259(20):12850–12856. [PubMed] [Google Scholar]
- Black D. L., Chabot B., Steitz J. A. U2 as well as U1 small nuclear ribonucleoproteins are involved in premessenger RNA splicing. Cell. 1985 Oct;42(3):737–750. doi: 10.1016/0092-8674(85)90270-3. [DOI] [PubMed] [Google Scholar]
- Boonpucknavig S., Ekapanyakul G. Autoantibodies in sera of Thai patients with Plasmodium falciparum infection. Clin Exp Immunol. 1984 Oct;58(1):77–82. [PMC free article] [PubMed] [Google Scholar]
- Busch H., Reddy R., Rothblum L., Choi Y. C. SnRNAs, SnRNPs, and RNA processing. Annu Rev Biochem. 1982;51:617–654. doi: 10.1146/annurev.bi.51.070182.003153. [DOI] [PubMed] [Google Scholar]
- Chambers J. C., Keene J. D. Isolation and analysis of cDNA clones expressing human lupus La antigen. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2115–2119. doi: 10.1073/pnas.82.7.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Habets W. J., Berden J. H., Hoch S. O., Van Venrooij W. J. Further characterization and subcellular localization of Sm and U1 ribonucleoprotein antigens. Eur J Immunol. 1985 Oct;15(10):992–997. doi: 10.1002/eji.1830151006. [DOI] [PubMed] [Google Scholar]
- Habets W. J., de Rooij D. J., Hoet M. H., van de Putte L. B., van Venrooij W. J. Quantitation of anti-RNP and anti-Sm antibodies in MCTD and SLE patients by immunoblotting. Clin Exp Immunol. 1985 Feb;59(2):457–466. [PMC free article] [PubMed] [Google Scholar]
- Habets W., Hoet M., Bringmann P., Lührmann R., van Venrooij W. Autoantibodies to ribonucleoprotein particles containing U2 small nuclear RNA. EMBO J. 1985 Jun;4(6):1545–1550. doi: 10.1002/j.1460-2075.1985.tb03815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez N., Keller W. Splicing of in vitro synthesized messenger RNA precursors in HeLa cell extracts. Cell. 1983 Nov;35(1):89–99. doi: 10.1016/0092-8674(83)90211-8. [DOI] [PubMed] [Google Scholar]
- Hinterberger M., Pettersson I., Steitz J. A. Isolation of small nuclear ribonucleoproteins containing U1, U2, U4, U5, and U6 RNAs. J Biol Chem. 1983 Feb 25;258(4):2604–2613. [PubMed] [Google Scholar]
- Kinlaw C. S., Dusing-Swartz S. K., Berget S. M. Human U1 and U2 small nuclear ribonucleoproteins contain common and unique polypeptides. Mol Cell Biol. 1982 Oct;2(10):1159–1166. doi: 10.1128/mcb.2.10.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlaw C. S., Robberson B. L., Berget S. M. Fractionation and characterization of human small nuclear ribonucleoproteins containing U1 and U2 RNAs. J Biol Chem. 1983 Jun 10;258(11):7181–7189. [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krainer A. R., Maniatis T. Multiple factors including the small nuclear ribonucleoproteins U1 and U2 are necessary for pre-mRNA splicing in vitro. Cell. 1985 Oct;42(3):725–736. doi: 10.1016/0092-8674(85)90269-7. [DOI] [PubMed] [Google Scholar]
- Krämer A., Keller W., Appel B., Lührmann R. The 5' terminus of the RNA moiety of U1 small nuclear ribonucleoprotein particles is required for the splicing of messenger RNA precursors. Cell. 1984 Aug;38(1):299–307. doi: 10.1016/0092-8674(84)90551-8. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Snurps and scyrps. Cell. 1981 Aug;25(2):298–300. doi: 10.1016/0092-8674(81)90047-7. [DOI] [PubMed] [Google Scholar]
- Liautard J. P., Sri-Widada J., Brunel C., Jeanteur P. Structural organization of ribonucleoproteins containing small nuclear RNAs from HeLa cells. Proteins interact closely with a similar structural domain of U1, U2, U4 and U5 small nuclear RNAs. J Mol Biol. 1982 Dec 15;162(3):623–643. doi: 10.1016/0022-2836(82)90392-8. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W., De Robertis E. M. Nuclear segregation of U2 snRNA requires binding of specific snRNP proteins. Cell. 1985 Jan;40(1):111–118. doi: 10.1016/0092-8674(85)90314-9. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W., Habets W. J., van Venrooij W. J. Monospecific antibodies reveal details of U2 snRNP structure and interaction between U1 and U2 snRNPs. EMBO J. 1986 May;5(5):997–1002. doi: 10.1002/j.1460-2075.1986.tb04314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori T., Hinterberger M., Pettersson I., Steitz J. A. Autoantibodies to the U2 small nuclear ribonucleoprotein in a patient with scleroderma-polymyositis overlap syndrome. J Biol Chem. 1984 Jan 10;259(1):560–565. [PubMed] [Google Scholar]
- Momany F. A., Aguanno J. J., Larrabee A. R. Correlation of degradative rates of proteins with a parameter calculated from amino acid composition and subunit size. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3093–3097. doi: 10.1073/pnas.73.9.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M., Pettersson I., Hinterberger M., Karmas A., Steitz J. A. The U1 small nuclear RNA-protein complex selectively binds a 5' splice site in vitro. Cell. 1983 Jun;33(2):509–518. doi: 10.1016/0092-8674(83)90432-4. [DOI] [PubMed] [Google Scholar]
- Ozaki L. S., Svec P., Nussenzweig R. S., Nussenzweig V., Godson G. N. Structure of the plasmodium knowlesi gene coding for the circumsporozoite protein. Cell. 1983 Oct;34(3):815–822. doi: 10.1016/0092-8674(83)90538-x. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Mount S. M., Steitz J. A., Sharp P. A. Splicing of messenger RNA precursors is inhibited by antisera to small nuclear ribonucleoprotein. Cell. 1983 Nov;35(1):101–107. doi: 10.1016/0092-8674(83)90212-x. [DOI] [PubMed] [Google Scholar]
- Pettersson I., Hinterberger M., Mimori T., Gottlieb E., Steitz J. A. The structure of mammalian small nuclear ribonucleoproteins. Identification of multiple protein components reactive with anti-(U1)ribonucleoprotein and anti-Sm autoantibodies. J Biol Chem. 1984 May 10;259(9):5907–5914. [PubMed] [Google Scholar]
- Quax-Jeuken Y., Janssen C., Quax W., van den Heuvel R., Bloemendal H. Bovine beta-crystallin complementary DNA clones. Alternating proline/alanine sequence of beta B1 subunit originates from a repetitive DNA sequence. J Mol Biol. 1984 Dec 15;180(3):457–472. doi: 10.1016/0022-2836(84)90022-6. [DOI] [PubMed] [Google Scholar]
- Reeves W. H., Fisher D. E., Wisniewolski R., Gottlieb A. B., Chiorazzi N. Psoriasis and Raynaud's phenomenon associated with autoantibodies to U1 and U2 small nuclear ribonucleoproteins. N Engl J Med. 1986 Jul 10;315(2):105–111. doi: 10.1056/NEJM198607103150207. [DOI] [PubMed] [Google Scholar]
- Reuter R., Lehner C. F., Nigg E. A., Lührmann R. A monoclonal antibody specific for snRNPs U1 and U2. FEBS Lett. 1986 May 26;201(1):25–30. doi: 10.1016/0014-5793(86)80564-6. [DOI] [PubMed] [Google Scholar]
- Reuter R., Lührmann R. Immunization of mice with purified U1 small nuclear ribonucleoprotein (RNP) induces a pattern of antibody specificities characteristic of the anti-Sm and anti-RNP autoimmune response of patients with lupus erythematosus, as measured by monoclonal antibodies. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8689–8693. doi: 10.1073/pnas.83.22.8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebroek A. J., Schalken J. A., Bussemakers M. J., van Heerikhuizen H., Onnekink C., Debruyne F. M., Bloemers H. P., Van de Ven W. J. Characterization of human c-fes/fps reveals a new transcription unit (fur) in the immediately upstream region of the proto-oncogene. Mol Biol Rep. 1986;11(2):117–125. doi: 10.1007/BF00364823. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Welling G. W., Weijer W. J., van der Zee R., Welling-Wester S. Prediction of sequential antigenic regions in proteins. FEBS Lett. 1985 Sep 2;188(2):215–218. doi: 10.1016/0014-5793(85)80374-4. [DOI] [PubMed] [Google Scholar]
- Wieben E. D., Rohleder A. M., Nenninger J. M., Pederson T. cDNA cloning of a human autoimmune nuclear ribonucleoprotein antigen. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7914–7918. doi: 10.1073/pnas.82.23.7914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]