Summary
Basement membrane plays important roles in hair growth. We characterized changes in laminin isoform expression during hair cycling. At the mRNA level, laminin-511 (10) expression underwent a steady increase during anagen stages. In contrast, laminin-332 (5) expression was initially upregulated in outer root sheath (ORS) keratinocytes at anagen II and then transiently downregulated. Laminin-332 significantly increased coincident with the signal in inner root sheath and hair matrix cells after anagen IV. Levels of laminin-332 proteins were also upregulated at late anagen I–III but dropped after anagen IV. This decrease coincided with increased levels of mRNA encoding the two proteases, membrane type 1 metalloproteinase and bone morphogenetic protein 1, involved in laminin-332 processing. Immunohistochemistry demonstrated that laminin-332 and α6β4 integrin were well colocalized, but their signals were remarkably decreased in the lower half of follicles after anagen VI. Consistent with these data, ultrastructurally mature hemidesmosomes were seen in ORS keratinocytes at anagen II, whereas at anagen VI, only fragmental hemidesmosomes were present. In hair follicle culture, laminin-511 (10)/521 (11)-rich human placental laminin enhanced hair growth, whereas recombinant laminin-332 antagonized hair growth induced by laminin-511. Our results indicate a positive role for laminin-511 and a negative role for laminin-332 on hair growth.
Keywords: laminin, hemidesmosome, integrin, protease, hair cycle
The hair follicle is an important complex organ that has morphogenetic functions and also acts as a protective barrier (Stenn and Paus 2001). Histologically, the hair follicle can be divided into two major zones: the upper region comprising the follicular infundibulum and isthmus and the lower region comprising the hair bulb (de Berker et al. 1986). The upper follicle is a relatively constant structure, whereas the lower follicle undergoes repeated episodes of regression and regeneration throughout the hair cycle (de Berker et al. 1986). During this cycle (anagen, catagen, and telogen), the epithelium and mesenchyme are believed to regulate hair growth and regression cooperatively by a distinct set of molecular signals that are unique for each phase of the hair cycle (Botchkarev and Kishimoto 2003).
The epithelium of the hair follicle is separated from the mesenchyme by the basement membrane zone (BMZ). However, little is known about the role of BMZ components in regulating hair development and cycling. In this regard, laminins are major structural elements of all basement membranes. They have a profound influence not only on tissue morphogenesis but also on the induction and maintenance of cell polarity, establishment of barriers between tissue compartments, organization of cells into tissues, and protection of adherent cells from detachment-induced cell death, e.g., anoikis (Miner and Yurchenco 2004). Each laminin is a glycoprotein heterotrimer composed of α, β, and γ subunits; thus far, five laminin αs, four βs, and three γs have been identified (Miner and Yurchenco 2004). Mammals possess at least 15 laminin isoforms assembled by various combinations of α, β, and γ subunits (Miner and Yurchenco 2004). Among them, laminin-5 (α3β3γ2; new nomenclature laminin-332) plays an important role in the adhesion of epithelial tissue to the basement membrane through an interaction with two receptors: α6β4 or α3β1 integrin (Jones et al. 1998; Frank and Carter 2004; Aumailley et al. 2005). Integrin α6β4 binds laminin-332 at the site of the hemidesmosome that stably affixes epidermal cells to the BMZ (Jones et al. 1998; Borradori and Sonnenberg 1999). This integrin also regulates signal transduction pathway and controls cell proliferation, differentiation, and migration (Rabinovitz and Mercurio 1997; Mercurio et al. 2001a; Mercurio and Rabinovitz 2001b; Nikolopoulos et al. 2005). The interaction of integrin α3β1 with laminin-332 occurs at cell–matrix adhesion sites termed focal contacts (Carter et al. 1991). Like α6β4 integrin, ligated α3β1 integrin likely regulates the proliferation and differentiation of keratinocytes, with its role in epidermal cell migration being well established (DiPersio et al. 1997). Moreover, α3β1 integrin is involved in BMZ formation and degradation because it regulates the production of matrix proteins or activities of metalloproteinases (MMPs) and other enzymes (DiPersio et al. 1997).
The role that laminin-332 plays in hair development and cycling is controversial. Although some patients with a mutation in the β3 subunit of laminin-332 (LAMB3) show patchy alopecia or sparse secondary sexual hair (McGrath et al. 1995; Mellerio et al. 1998; Takizawa et al. 2000), others with mutations in the α3, β3, or γ2 subunits of laminin-332 exhibit normal hair follicle development (Skoven and Drzewiecki 1979). Indeed, laminin-332 has been expressed to only a limited extent during hair follicle development in the human embryo (Nanba et al. 2000). Thus, one might assume that laminin-332 is not important for hair development. In contrast, recent data suggest that laminin-10 (α5β1γ1; new nomenclature laminin-511) is crucial for embryonal hair morphogenesis (Li et al. 2003). Moreover, like laminin-332, integrin α3β1 binds laminin-511 (Kikkawa et al. 1998,2000), whereas laminin-511 is localized to the BMZ of all epithelial tissues including those of the epidermis and hair follicle (Ekblom et al. 1998; Määtä et al. 2001; Yurchenco and Wadsworth 2004).
Interaction between integrins and laminins in the epithelial BMZ around the hair follicle is incompletely characterized. Furthermore, little is known about the expression of laminin-332 and -511 during hair cycling after birth. We have therefore investigated expression patterns of laminin-332 and -511 during the active proliferation phase of the hair cycle (anagen) by using murine hair cycle models. We have also studied the impact of these matrix molecules on hair growth by using an in vitro human hair follicle culture model.
Materials and Methods
Antibodies
A rabbit polyclonal antiserum termed J18 generated against all three subunits of rat laminin-332, a rabbit polyclonal antibody prepared against mouse recombinant α5 laminin, and a rabbit polyclonal antibody against mouse α3 integrin were used (Langhofer et al. 1993; DiPersio et al. 1995; Miner et al. 1997). Rat monoclonal antibodies (MAbs) directed against mouse α6 and β1 integrin and horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG antibody were obtained from Chemicon (Temecula, CA). Rat anti-mouse β4 integrin MAb was obtained from BD Pharmingen (San Diego, CA). FITC-conjugated goat anti-rat IgG and HRP-conjugated goat anti-rabbit IgG were obtained from Zymed Laboratories (South San Francisco, CA). Goat anti-rabbit IgG Alexa Fluor 594 was obtained from Molecular Probes (Eugene, OR).
Mouse Hair Cycling Model
We used 8-week-old C57BL/6 mice to induce the hair growth and regression cycle by plucking hairs along the skin of the back (Chase et al. 1951; Stenn and Paus 2001). Skin samples were obtained every day for 19 days from initial depilation. We used at least five animals for each time point. Tissue samples were cut out, divided into four pieces, and then processed for paraffin embedment, cryosectioning, protein analyses, or real-time quantitative RT-PCR. Distinct hair cycle stages were determined as previously described (Müller-Röver et al. 2001). All animal studies were approved by the Osaka City University Medical School Committee on Research Animal Care and were conducted according to the principles of the Declaration of Helsinki.
Semiquantitative RT-PCR
Skin specimens were homogenized in ISOGEN (Nippon Gene; Tokyo, Japan), which included phenol and guanidine thiocyanate. Addition of chloroform to these lysates enabled RNA-rich aliquots to be extracted. Following precipitation with isopropyl alcohol and 70% ethanol, RNA samples were isolated. Levels of mRNAs encoding the subunits of laminin-332 (α3, β3, γ2), those of laminin-511 (α5, β1, γ1), MMP-2, membrane type 1 (MT1)-MMP, bone morphogenetic protein 1 (BMP-1), tissue plasminogen activator (t-PA), keratin 14, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in each of our samples were measured by semiquantitative RT-PCR by using an ABI PRISM 7700 sequence detector (PE Applied BioSystems; Foster City, CA). PCR conditions were as follows: 42C for 20 min; 95C for 5 min; 40 cycles of 95C for 15 sec, 60C for 1 min. Taqman reaction reagents were obtained from TOYOBO (Osaka, Japan). Taqman probes and primers were purchased from Sigma-Genosys (Hokkaido, Japan). mRNA levels were all normalized to GAPDH mRNA. Data were analyzed for statistical significance by using Fisher's Protected Least Significant Difference in a modified ANOVA test.
Nucleotide sequences for primers were as follows: laminin-α3A subunit forward: 5′ CAA TGT GGA CCG AAT CCG AG 3′, reverse: TTC TAG GTC ATT CGG CAG TCG; laminin-β3 subunit forward: AGA GCG CTG CGA CCT TTG, reverse: TCT TTC CGG GCA CCT AAG ATA C; laminin-γ2 subunit forward: CCT GCC AAA TTC CTC GGT AAC, reverse: ACA TCG TAG GCA GAC GGC TG; laminin-α5 subunit forward: GCT GGA GAG GCT ACG CAC AC, reverse: ACG CTG GTT GAT CCA CGG T; laminin-β1 subunit forward: GTG TGC TTT GAG AAG GGA ATG AA, reverse: GAG TCG ATG AAC GTG TAA GGG C; laminin-γ1 subunit forward: CTG TGA AAA GTG CCT GCC TTT, reverse: GGG ATC GGC CAT TGC A; MMP-2 forward: CCC CAT GAA GCC TTG TTT ACC, reverse: TTG TAG GAG GTG CCC TGG AA; MT1-MMP forward: ACC TGC GTA CCC ACA CAC AA, reverse: CAA ATC AGC CTT GCC TGT CA; MT1-MMP forward: ACC TGC GTA CCC ACA CAC AA, reverse: CAA ATC AGC CTT GCC TGT CA; BMP-1 forward: CCG TAT CTC CCT GCA ATT TGA, reverse: GCT TAG AGT CCG CCG TGA GT; t-PA forward: CTG CAG AAA CCC AGA CCG AG, reverse: TTC CCT TAG GGC AAG CTG GT; keratin 14 forward: GGT GGG TGG AGA CGT CAA TG, reverse: CTC AGC ATC CTT GCG GTT CT; GAPDH: forward: TGC ACC ACC AAC TGC TTA G, reverse: GGA TGC AGG GAT GAT GTT C.
Nucleotide sequences for probes in the Taqman reaction were as follows: laminin-α3A subunit: CAA ACA AGG TTG CAA TTC CCA TGA GGT T; laminin-β3 subunit: TGG CTT CAC CGG GCT CAC CTT C; laminin-γ2 subunit: AGA GCC TGT CTT TTG ACT ACC GCG TGG A; laminin-α5 subunit: TCC AGC CCA GGA TTG TGG CCA; laminin-β1 subunit: CTG CCC CAG TAT ACG GCA TCG GG; laminin-γ1 subunit: CGT GGA GGA GGG CGA CTG CTG; MMP-2: GAA TGC TGA TGG ACA GCC CTG CA; MT1-MMP: CAC CCC AGT CAC TCT CAG CTG CCA; BMP-1: TTC TTC GAG ACT GAG GGC AAT GAT GTG TG; t-PA: GCC CTG GTG CTA TGT CTT TAA GGC AGG GA; keratin 14: GAC CTG AGC CGC ATC CTG AAC GAG AT; GAPDH: CAG AAG ACT GTG GAT GGC CCC TC.
In Situ Hybridization
Sense and antisense probes for the laminin-α3 subunit, α5 subunit, and 28s ribosome RNA were purchased from Bex Co. (Tokyo, Japan). Nucleotide sequences for probes were as follows: laminin-α3A sense probe: TTA TTA GGC AGC TCT GGG CCA GTG CCT GGG GTA TGG CTC AGA GCA GAT TAT TAT T; laminin-α3A antisense probe: TTA TTA CTG CTC TGA GCC ATA CCC CAG GCA CTG GCC CAG AGC TGC CAT TAT TAT T; laminin-α5 sense probe: TTA TTA TGG GCG CTG TGT CTG TCA TGG CCA CGC AGA TGT CTG TGA CAT TAT TAT T; laminin-α5 antisense probe: TTA TTA GTC ACA GAC ATC TGC GTG GCC ATG ACA GAC ACA GCG CCC AAT TAT TAT T; 28s rRNA antisense probe: TTA TTA TGC TAC TAC CAC CAA GAT CTG CAC CTG CGG CGG CAT TAT TAT T; probe with scrambled sequence: TTA TTA ACA GCT GGC CGC CCG GAT CCT GGA GGC GCA CCA GAA TTA TTA TT. Probes were dimerized at the thymine–thymine (T-T) dimer sequence by using an ultraviolet lamp at 12,000 J/m2. For in situ hybridization, fresh skin specimens were fixed in 4% paraformaldehyde in PBS at room temperature overnight, washed with distilled water, dehydrated in a graded ethanol series, and embedded in paraffin. Sections were cut, placed on glass slides, dewaxed, rehydrated, and then immersed in methanol for 15 min. After a 20-min incubation in 0.2 M HCl, they were treated with 10 μg/ml proteinase K in PBS for 15 min, rinsed in PBS, and refixed with 4% paraformaldehyde in PBS for 5 min. Sections were rinsed in 2 mg/ml glycine in PBS, prehybridized with 4× standard sodium citrate (SSC) containing 40% deionized formamide for 30 min, hybridized with 10 mM Tris–HCl, pH 7.4, 600 mM NaCl, 1 mM EDTA, pH 7.4, 1× Denhart's medium, 0.25 mg/ml yeast tRNA, 0.125 mg/ml salmon sperm DNA, 2 μg/ml T-T dimerized probe in Tris–EDTA containing 40% deionized formamide at 37C overnight, washed five times with 2× SSC/50% formamide at 37C for 1 hr and twice with 2× SSC at room temperature for 15 min, blocked with 500 μg/ml normal mouse IgG (Sigma-Aldrich, St Louis, MO), 5% bovine serum albumin (BSA), 100 μg/ml salmon sperm DNA, 100 μg/ml yeast tRNA at room temperature for 1 hr, and then covered with anti-T-T dimer antibody, diluted 80× (Kyowa Medic; Tokyo, Japan), 5% BSA, 100 μg/ml salmon sperm DNA, and 100 μg/ml yeast tRNA at 37C overnight. Unreacted antibodies were washed off the sections by four rinses with 0.075% Brij 35 in PBS at room temperature for 15 min and a further wash with PBS. Bound antibodies were visualized by treatment with 0.5 mg/ml DAB (Dojindo; Kumamoto, Japan), 0.025% cobalt chloride, 0.02% ammonium nickel (II) sulfate hexahydrate, and 0.01% hydrogen peroxide in 0.1 M phosphate buffer, pH 7.2, for 10 min.
Western Blotting Analyses
Proteins extracted from fresh skin specimens were reduced and separated on a 7.5% SDS–polyacrylamide gel. Separated proteins were transferred to nitrocellulose membranes (Protran BA 85 nitrocellulose; Schleicher and Schuell, Dassel, Germany). These membranes were processed with J18 rabbit polyclonal laminin-332 antiserum or anti-β actin antibody (Ambion; Austin, TX), washed, and probed with HRP-conjugated goat anti-rabbit (for J18) or mouse antibody (for β-actin). Signals were detected with an ECL Western blotting detection reagent (Amersham Pharmacia Biotech; Little Chalfont, UK).
Histology and Immunohistochemistry (IHC)
For histochemical examination, skin specimens were fixed in 4% paraformaldehyde in PBS, dehydrated, and embedded in paraffin. Five-μm-thick sections were cut and stained with hematoxylin and eosin (H&E). For IHC, fresh skin specimens were embedded in optimal cutting temperature compound (OCT; Sakura Fine Chemical, Tokyo, Japan) and frozen in liquid nitrogen. Cryosections were cut and air dried. For laminin-332 and integrin β4 staining, tissue sections were treated with 1% Triton X-100 in PBS for 10 min and then fixed in 2% formaldehyde in PBS for 20 min at room temperature. For α3 integrin and laminin-511 staining, sections were treated with 1% Triton X-100 in PBS for 10 min and fixed in acetone at 4C for 2 min. For β1 integrin staining, sections were fixed in 3.7% formaldehyde in PBS for 5 min and treated with 1% Triton X-100 in PBS for 10 min. For α6 integrin staining, tissue sections were unfixed. Sections were incubated with normal goat serum at room temperature for 15 min, primary antibodies at 37C for 1 hr, and washed in PBS. Secondary antibodies in PBS at 37C were then added for an additional 1 hr. The following secondary antibodies were used: goat anti-rabbit IgG Alexa Fluor 594 for the laminin-332 serum J18, laminin-511, and α3 integrin and FITC-conjugated goat anti-rat IgG for α6, β4, and β1 integrin. Sections were washed in PBS, mounted, and examined by laser confocal microscopy (LSM510; Carl Zeiss AG, Oberkochen, Germany).
Electron Microscopy
Skin specimens were fixed in 0.1 M cacodylate buffer containing 2.5% glutaraldehyde and 2% paraformaldehyde and were then immersed in 1% osmium tetroxide in the same buffer. The tissue was dehydrated in a gradient series of ethanol, immersed in propylene oxide, and embedded in plastic resin. Thin and thick sections were generated on a Leica Ultracut UCT (Leica; Vienna, Austria). Thin sections were stained with uranyl acetate and lead citrate and observed with an electron microscope (JEM-1200EXII; JEOL, Tokyo, Japan).
Purification of Recombinant Human Laminin-332
In all, 293 cells were sequentially transfected with pcDNA3.1 plasmids expressing the complete coding sequences of each of the three subunits of laminin-332. The β3 subunit sequence contained a C-terminal 6-His tag. Each plasmid also expressed a different drug-resistance gene to permit selection. From these 293 cells, a triple-transfected clone that expressed high levels of all three subunits was clonally isolated, grown to confluence, and then switched to serum-free medium. Two-day conditioned medium was harvested, loaded onto a His-Bind column (EMD Biosciences; Madison, WI), eluted with 60 mM imidazole, dialyzed against 10 mM Tris–HCl (pH 7.5), and concentrated by using Vivaspin 15R protein concentrators (Sartorius AG; Goettingen, Germany). All three laminin-332 subunits appeared to be present in similar concentrations by Coomassie Blue staining, and all were detectable by Western blotting (data not shown).
Isolation and Culture of Human Hair Follicles
After obtaining informed consent, human scalp skin specimens including normal hair follicles from five individuals (four males and one female) between 30 and 50 years of age were obtained from surgically removed scalp skin around a benign skin tumor. These samples were at least 1 cm distant to the tumor. Of intact hair follicles, 94 were isolated by a method previously reported by Philpott et al. (1990). The length of hairs was uniformly 3 mm. The follicles were cultured in either 1 ml Williams E medium containing 100 U penicillin, 10 μg streptomycin, 2.5 μg amphotericin B, 10 μg insulin, 10 ng hydrocortisone, 10 μg transferrin, and 10 ng sodium selenite (Soma et al. 1998) alone or supplemented with 5 μg/ml per week of recombinant human laminin-332, as mentioned above, and/or 5 μg/ml per week of human placental laminin (Chemicon), which is reported to contain mainly laminins-10 (511)/11 (α5β2γ1; new nomenclature laminin-521) (Ferletta and Ekblom 1999), at 37C in 5% CO2/95% air. The amount of hair growth (length after 7 days minus the length at isolation) was determined. Data were analyzed for statistical significance by using Fisher's Protected Least Significant Difference in a modified ANOVA test.
Results
Skin Samples and Histology
C57BL/6 mice, whose back skin was depilated, showed cyclical hair growth with gradual skin pigmentation and skin thickening throughout the time course, as previously reported (Müller-Röver et al. 2001). H&E-stained sections showed that the staging of anagen hairs in our animals was as follows: days 0–2: anagen I (Figure 1A), day 3: anagen II (Figure 1B), day 4: anagen III, days 5–6 (Figure 1C): anagen IV, day 7: anagen V, days 8–17: anagen VI (Figure 1D).
Figure 1.

Gradual elongation of hair follicles and an increase of melanin were associated with anagen progression in C57BL/6 mice after depilation. Sections of back skin stained with hematoxylin and eosin at (A) anagen I, (B) anagen II, (C) anagen IV, and (D) anagen VI after depilation. Bar = 120 μm.
Semiquantitative RT-PCR Analyses
To analyze expression of the mRNAs encoding the subunits of laminin-332 and -511 at distinct anagen stages, we performed semiquantitative RT-PCR by using Taqman probes specific for the subunits of laminin-332 (α3, β3, γ2) and -511 (α5, β1, γ1). The laminin-α3 subunit mRNA was transiently upregulated at anagen II, reduced to the level of anagen I at anagen III, and then gradually increased through anagen IV–VI (Figure 2A). The same results are obtained for mRNAs for the laminin-β3 and -γ2 subunits (Figures 2B and 2C). In contrast, the laminin-α5 subunit mRNA gradually increased during the progression from one anagen stage to the next (Figure 2D), with mRNA for the laminin-β1 and γ1 subunits following the same pattern (Figures 2E and 2F). To exclude the possibility that such an upregulation of both laminin-332 and -511 was caused by an increase in the number of keratinocytes in our assay, we performed semiquantitative RT-PCR for keratin 14. The results indicated that this was not the case (Figure 2G).
Figure 2.

Upregulation of laminin-332 and -511 mRNA as quantified by semiquantitative RT-PCR. mRNAs encoding laminin-α3 (A), -β3 (B), and -γ2 (C) subunits were transiently upregulated at anagen II (arrows) and then returned to their levels at anagen I. They then increased with anagen stage progression. Laminin-α5 (D), -β1 (E), and -γ1 (F) subunit mRNA levels gradually increased with progression of anagen stage. Keratin 14 (G) mRNA levels were stable throughout anagen stage. Stages of anagen are indicated by Arabic numbers along the x-axis. Data are expressed in arbitrary units (y-axis) from 13 individual experiments. Black bars ± SEM. *p<0.05. LN, laminin; K14, keratin 14.
In Situ Hybridization Analyses
To investigate the localization of mRNAs for the laminin-α3 and -α5 subunits at each day during anagen, we performed in situ hybridization with T-T dimerized oligonucleotide probes. Positive staining of the antisense probe for the laminin-α3 subunit was observed in both epidermal and outer root sheath (ORS) keratinocytes at anagen II. Staining decreased at anagen III–IV but increased once more at anagen VI (Figures 3A, 3E, 3I, and 3M). Moreover, inner root sheath (IRS) keratinocytes and hair matrix cells also showed positive staining (Figure 3M). In contrast, the antisense probe for laminin-α5 was strongly positive in epidermal, IRS, ORS, and hair matrix cells after anagen II (Figures 3C, 3G, 3K, and 3O). As a control, an antisense probe for 28s ribosome RNA also showed positive staining in all cells discernible in the specimen (Figure 3Q), whereas sense probes were negative at all times (Figures 3B, 3D, 3F, 3H, 3J, 3L, 3N, and 3P). In addition, positive staining was diminished when we pretreated tissue sections with 100 μg/ml RNase A (Sigma-Aldrich), and when we treated specimens with an excess of T-T undimerized antisense probes (data not shown). Positive staining was not diminished when we pretreated tissue sections with an excess of T-T undimerized probes with scrambled sequence (data not shown).
Figure 3.

Differential expression of laminin-α3A or -α5 mRNA was observed by in situ hybridization. Laminin-α3A subunit mRNA was detected throughout the epidermal and outer root sheath (ORS) keratinocytes at anagen II and anagen VI. The same staining was detected in the inner root sheath (IRS) and hair matrix cells at anagen VI. (A) Anagen I, antisense probe. (B) Anagen I, sense probe. (E) Anagen II, antisense probe. (F) Anagen II, sense probe. (I) Anagen IV, antisense probe. (J) Anagen IV, sense probe. (M) Anagen VI, antisense probe. (N) anagen VI, sense probe. Laminin-α5 subunit mRNA showed positive reactivity in keratinocytes of the epidermis, IRS, ORS, and hair matrix after anagen II. (C) Anagen I, antisense probe. (D) Anagen I, sense probe. (G) Anagen II, antisense probe. (H) Anagen II, sense probe. (K) Anagen IV, antisense probe. (L) Anagen IV, sense probe. (O) Anagen VI, antisense probe. (P) Anagen VI, sense probe. Arrows indicate positive staining in the epidermal and ORS keratinocytes, and arrowheads indicate melanin granules. (Q) Anagen IV, 28s rRNA antisense probe. Bar = 100 μm.
Western Immunoblotting of Laminin-332
To investigate expression levels of laminin-332 protein at each day during anagen, we performed Western immunoblot analyses with a polyclonal antiserum (J18) that recognized all three laminin-332 subunits (Langhofer et al. 1993). Results with J18 indicated that laminin-332 proteins were transiently upregulated at late anagen I–anagen III but rapidly decreased after anagen IV (Figure 4).
Figure 4.

Immunoblot detection revealed transient upregulation and then a loss of laminin-332 after anagen IV. During late anagen I–anagen III (Lanes 2–5), a transient upregulation was seen in laminin-332 levels. After anagen IV (Lanes 7 and 8), laminin-332 levels decreased. Lower panel shows the expression of β-actin as a loading control. Left: size marker.
Semiquantitative RT-PCR Analyses of MMP-2, MT1-MMP, and BMP-1 During Anagen
We next determined whether changes in protease expression were correlated with a loss of laminin-332. This might reflect a role for proteolysis of laminin-332 subunits during the mid- to late anagen stages. A number of proteases are known to cleave laminin-332. These include matrix MMP-2, MT1-MMP, BMP-1, and plasmin (Giannelli et al. 1997; Goldfinger et al. 1998; Amano et al. 2000; Veitch et al. 2003). We therefore performed semiquantitative RT-PCR with specific Taqman probes to MMP-2, MT1-MMP, BMP-1, and t-PA, which converts plasminogen to plasmin (Goldfinger et al. 1998). These mRNA signals were all normalized to GAPDH mRNA. Expression levels of MT1-MMP (Figure 5) and BMP-1 (Figure 5) increased significantly as anagen progressed. In contrast, MMP-2 mRNA levels increased at mid-anagen and decreased at late anagen (Figure 5). t-PA mRNA levels were constant throughout anagen (data not shown).
Figure 5.

(A–C) MMP-2 (A), MT1-MMP (B), and BMP-1 (C) mRNA expression levels changed during anagen as assessed by semiquantitative RT-PCR. (A) MMP-2 mRNA was upregulated at mid-anagen and downregulated at late anagen. Upregulation of MT1-MMP (B) and BMP-1 (C) occurred as anagen progressed. Arabic numbers along the x-axis indicate anagen stages. Numbers along the y-axis are arbitrary units from eight individual experiments. Black bars ± SEM. *p<0.05.
IHC of Laminin-332 and -511
We extended the above immunoblotting studies with an analysis of the localization of laminin-332 and -511 subunits during anagen by using IHC. In the upper part of the hair follicles and epidermis, laminin-332 staining with J18 antibody was positive at the BMZ through all anagen stages (Figures 6A–6D), whereas in the lower part of hair follicles (hair bulbs) and the junction between dermal papilla (DP) and hair bulb matrix, positive staining of laminin-332 was only transiently detected in anagen II–III (Figure 6B). The latter staining decreased at anagen IV (Figure 6C) and was totally lost at anagen VI (Figure 6D). In contrast, laminin-α5 antibody staining was positive in the BMZ beneath the basal keratinocytes and around the entire hair follicles, including the junction between the DP and hair bulb matrix, at all anagen stages (Figures 6E–6H).
Figure 6.

Immunofluorescence analyses of laminin-332 revealed downregulation at late anagen, whereas no change occurred in laminin-511 protein staining. Laminin-332 staining was observed in the basement membrane zone (BMZ) at the epidermal–dermal junction and around the ORS in the upper part of hair follicles throughout all anagen stages: (A) anagen I, (B) anagen II, (C) anagen IV, (D) anagen VI. Positive reactivity for laminin-332 antibodies was transient at anagen II (B), decreased at anagen IV (C), and had diminished (arrow in D) at anagen VI (D) in the BMZ of the hair bulb and the junction between the dermal papilla and hair bulb matrix. Laminin-511 antibodies generated intense staining along the BMZ of the epidermis, including around the hair follicles throughout all anagen stages: (E) anagen I, (F) anagen II, (G) anagen IV, (H) anagen VI. Bar = 200 μm.
IHC of α6β4 Integrin and α3β1 Integrin
Next we performed IHC staining with the anti-α6, β4, α3, and β1 integrin antibodies. The β4 (Figures 7A–7D) integrin antibody gave positive staining in the upper part of hair follicles and epidermis throughout all stages of anagen. In anagen II, we detected transient positive staining for β4 integrin in the lower part of hair bulbs and the junction between the DP and hair bulb matrix (Figure 7B). However, expression of integrin β4 decreased at anagen IV (Figure 7C) and was further diminished at anagen VI (Figure 7D). The same expression pattern was obtained when we used α6 integrin antibody (data not shown). Double IHC with anti-β4 integrin antibody (green) and laminin-332 (red) antibody showed that these proteins were completely colocalized throughout the epidermal and upper ORS (Figure 7E). In contrast, integrin α3 (Figures 7F–7I) antibody showed intense staining in the BMZ beneath basal keratinocytes and around the entire hair follicles, including the junction between the DP and hair bulb matrix at all anagen stages (Figures 7F–7I). A similar expression pattern was detected when we used the β1 integrin antibody (data not shown). The above results therefore indicated that the expression patterns of integrin α3β1 and laminin-511 were similar. We performed mirror-image staining because the antibodies that we used against α3 and laminin-511 were both raised from same animal. It would have been easier to make visual comparisons of adjacent serial sections; however, this would have involved a distance of at least 3 μm between adjacent sections and the probable loss of the second image of a potentially costained cell. In mirror images, we could take advantage of the possible detection of two different staining patterns in exactly the same cell within the tissue. This technique revealed the colocalization of the α3 integrin signal with that for laminin-511 (Figures 7J and 7K).
Figure 7.
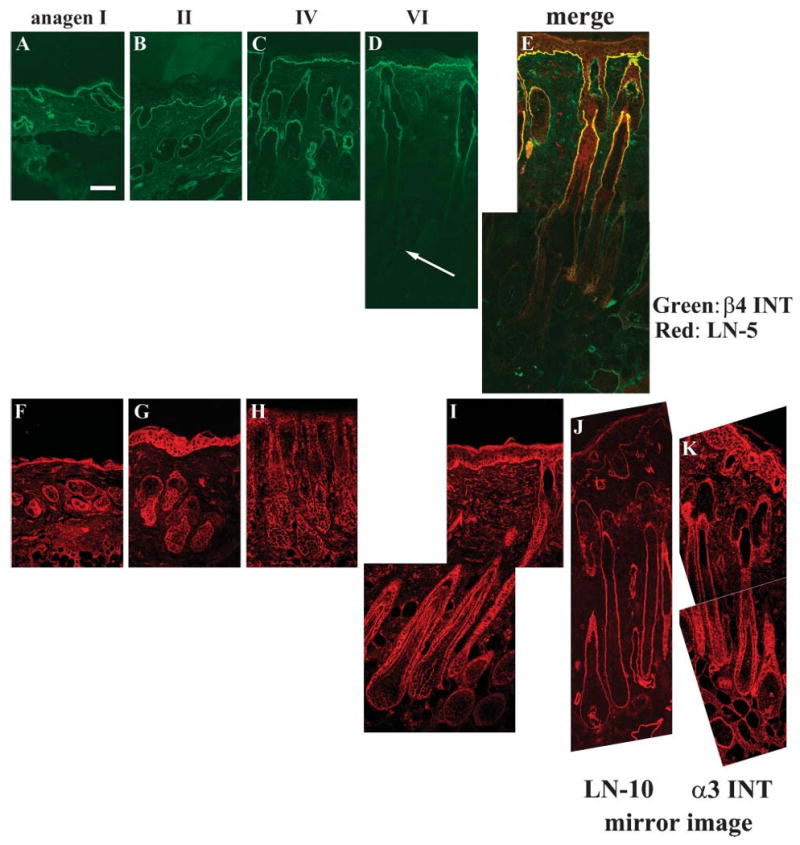
Immunofluorescence analyses of integrin β4 showed that localization corresponded well with that of laminin-332, whereas that of integrin α3 was colocalized with laminin-511. (A–D) Integrin β4. (A) Anagen I. (B) Anagen II. (C) Anagen IV. (D) Anagen VI. β4 integrin was localized at the BMZ of the epidermis and around hair follicles at anagen II (B). Its expression around hair follicles decreased at anagen IV (C) and was further diminished at anagen VI (arrow in D). Double staining of laminin-332 (red) and β4 integrin (green) revealed that they were colocalized at anagen VI (E). (F–I) Integrin α3. (F) Anagen I. (G) Anagen II. (H) Anagen IV. (I) Anagen VI. Expression of α3 integrin was detected at the BMZ of the dermoepidermal junction and around hair follicles throughout anagen. Mirror images of laminin-511 (J) and integrin α3 (K) showed colocalization on BMZ at anagen VI. LN, laminin; INT, integrin. Bar = 200 μm.
Ultratructural Analyses of Anagen II and VI
As mentioned previously, we observed a transient upregulation of laminin-332 in the BMZ of the hair bulb and the junction between DP and hair bulb matrix at anagen II. However, laminin-332 expression and its receptor integrin α6β4 were dramatically downregulated after anagen IV. Because laminin-332 and α6β4 are components of hemidesmosomes, we next wondered whether changes in their localization would be manifest in changes in hemidesmosome assembly. We therefore processed hair at anagen stages II and VI for electron microscopy. At anagen II, abundant normal-appearing hemidesmosomes were seen along with the BM of both epidermal (Figure 8A) and ORS keratinocytes (Figure 8B). In contrast, although normal hemidesmosomes were seen along with the BM of epidermal keratinocytes (Figure 8C), only immature or incomplete hemidesmosome structures were observed along with that of the ORS keratinocytes at anagen VI (Figure 8D). These findings were consistent in three individual experiments.
Figure 8.

Hemidesmosomes on the ORS keratinocytes were diminished at late anagen. At anagen II (A,B), numerous mature hemidesmosomes were located along the BMZ. (A) Epidermal keratinocytes. (B) ORS keratinocytes. (C) Mature hemidesmosomes were found on the epidermal keratinocytes along the basement membrane. (D) No mature hemidesmosomes were observed on the ORS keratinocytes at anagen VI. (A–C) Arrows indicate hemidesmosomes. Bar = 600 nm.
Effect of External Application of Laminin-332 and -511/521 on Hair Growth
To investigate the potential functional roles played by laminin-332 and -511 during hair growth, we isolated human hair follicles from scalp and cultured them for 1 week following the method of Philpott et al. (1990) in the presence of recombinant human laminin-332 and commercially available human placental laminin, which is rich in laminins-511/521 (Ferletta and Ekblom 1999). Isolated human hair follicles were incubated in (1) basal medium alone, (2) recombinant laminin-332, (3) human placental laminin, and (4) a combination of laminin-332 and human placental laminin. After 1 week of incubation, hair elongation in cultures incubated in media supplemented with human placental laminin was greater than that in cultures incubated in basal media alone (Figures 9A and 9B). On the contrary, hair elongation in cultures of hair follicles incubated with laminin-332 was comparable with the elongation of hair in cultures maintained in basal media alone. Laminin-332 dramatically antagonized the hair elongation induced by human placental laminin in vitro (Figures 9A and 9B).
Figure 9.

Human placental laminin, rich in laminin-511 but not in laminin-332, enhanced hair growth in cultured hair follicles. Cultured human hair follicles were placed in culture and maintained in basal media alone, the same medium supplemented with laminin-332, the same medium supplemented with human placental laminin, or the same medium supplemented with human placental laminin rich and laminin-332. (A) Representative images of hair follicles during preculture (upper panel) and postculture (lower panel). (B) Quantification of hair elongation. Hair growth was evaluated at 1 week. Recombinant proteins added with basal media are indicated along the x-axis. Values are means ± SEM obtained from 10 independent experiments. Bar = 3 mm. *p<0.05.
Discussion
We report here five major findings. (1) Laminin-332 is transiently upregulated in early anagen at both mRNA and protein levels. This finding is confirmed by the appearance of hemidesmosomes at the basement membrane around hair bulbs. (2) After anagen IV, a loss of laminin-332 occurs in the lower part of hair follicles. (3) Laminin-511 mRNA is upregulated in the epidermal, IRS, ORS, and hair matrix cells throughout all anagen stages. (4) mRNA levels of certain laminin-332-degrading proteases, e.g., MT1-MMP and BMP-1, are upregulated at the same anagen stages at which the loss of lamnin-332 occurs. (5) Most importantly, results from our functional assays suggest that the application of laminin-511/521 to in vitro cultured human anagen hair by the method of Philpott et al. (1990) enhances hair growth and that this enhancement is antagonized by laminin-332. This latter assay suggests that laminin-332 and -511 have a functional impact. However, we have not been able to determine whether these exogenous molecules are precisely incorporated into the extracellular matrix of the follicles. Further study is required to clarify this point.
We have observed loss of laminin-332 at mid- to late anagen by Western immunoblot analyses. Loss of laminin-332 is probably consistent with increases of MT1-MMP and BMP-1, which have previously been reported to cleave laminin-332 (Amano et al. 2000; Koshikawa et al. 2000; Gilles et al. 2001; Koshikawa et al. 2005). However, we cannot rule out the possibility of translational control of the expression of the laminin-332 subunit. This aspect requires further investigation.
We also suggest that the loss of laminin-332 during anagen is correlated with the disassembly of hemidesmosomes. Hemidesmosomes are significantly diminished or appear fragmentary on the basement membranes of the hair bulb after anagen IV. A decrease in laminin-332 along the basement membrane of the lower part of the hair follicles has been noted by others (Nanba et al. 2000; Joubeh et al. 2003). Moreover, Nanba et al. (2000) state that the hemidesmosome proteins integrin β4, laminin-332, bullous pemphigoid antigen 230 (BP230), and HD1 are also downregulated during early hair morphogenesis events and have speculated that a change of adhesiveness of hair cells is required for early hair development. Joubeh et al. (2003) have shown that the expression of laminin-332 is strong along the basement membrane in the upper part of hair follicles but weak in the lower part of the basement membrane in hair follicles from human scalp during anagen. In addition, they suggest that the loss of hemidesmosome is correlated with the downregulation of laminin-332 (Joubeh et al. 2003). Neither Nanba et al. (2000) nor Joubeh et al. (2003) discusses the possibility of the proteolytic cleavage of laminin-332 during hair growth, as we suggest here, nor do they propose that a relationship exists between hemidesmosome assembly and laminin-332 expression during hair cycling.
How does the loss of laminin-332 play a role in hair cycling? One possible explanation could be that the loss of hemidesmosomes allows the proliferating population of epidermal cells to grow down into connective tissue, facilitating elongation of hair. On the other hand, laminin-511 acts in an opposite manner to laminin-332 because our experiments suggest that laminin-511/521 stimulates the elongation of hair. This is consistent with other studies of laminin-511 showing that laminin-511 is essential for hair morphogenesis (Li et al. 2003).
Our data also implicate receptors in the regulation of the effects of laminins in hair development. Dual staining for laminin-332 and β4 integrin and the mirror images of laminin-511 and α3 integrin have effectively shown the colocalization of these molecules. In this system, we suggest that the α3β1 integrin–laminin-511 interaction leads to anagen hair growth, and that α6β4 integrin–laminin-332 interaction inhibits anagen hair growth. Previous reports that mice deficient in α3 integrin or β1 integrin exhibit abnormalities in hair follicle morphogenesis (Brakebusch et al. 2000; Raghavan et al. 2000; Conti et al. 2003) support this suggestion. Furthermore, Li et al. (2003) have shown that hair growth in human scalp xenografted onto nude mice is inhibited by treatment with an antibody that blocks β1 integrin function. Based on our data, one might assume that mice deficient in α6 integrin, β4 integrin, and laminin-332 would show an enhancement in hair growth, but unfortunately the mice do not live long enough to assess whether this is indeed the case (Dowling et al. 1996; Georges-Labouesse et al. 1996; van der Neut et al. 1996; Ryan et al. 1999). Recently, Raymond et al. (2005) constructed conditional β4 knockout mice by the use of the Cre-mediated recombination system but have not yet reported any abnormalities or enhancement of hair growth. We aim to use these conditional knockout mice to clarify this problem.
In conclusion, our findings suggest that the spatial and temporal regulation of laminin-332 and laminin-511 are in turn important regulators of hair growth. Moreover, proteolytic cleavage of laminin-332 may lead to the stimulation of hair growth driven by laminin-511.
Acknowledgments
We thank Drs. Jeffrey Miner, Peter Marinkovich, and Mike DiPersio for generous gifts of antibody. We are grateful for the technical assistance of Dr. Chika Hirata.
Literature Cited
- Amano S, Scott IC, Takahara K, Koch M, Champliaud MF, Gerecke DR, Keene DR, et al. Bone morphogenetic protein 1 is an extracellular processing enzyme of the laminin 5 γ2 chain. J Biol Chem. 2000;275:22728–22735. doi: 10.1074/jbc.M002345200. [DOI] [PubMed] [Google Scholar]
- Aumailley M, Bruckner-Tudermann L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, et al. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: more than simple adhesion complexes. J Invest Dermatol. 1999;112:411–418. doi: 10.1046/j.1523-1747.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Kishimoto J. Molecular control of epithelial-mesenchymal interactions during hair follicle cycling. J Investig Dermatol Symp Proc. 2003;8:46–55. doi: 10.1046/j.1523-1747.2003.12171.x. [DOI] [PubMed] [Google Scholar]
- Brakebusch C, Grose R, Quondamatteo F, Ramirez A, Jorcano JL, Pirro A, Svensson M, et al. Skin and hair follicle integrity is crucially dependent on β1 integrin expression on keratinocytes. EMBO J. 2000;19:3990–4003. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter WG, Ryan MC, Gahr PJ. Epiligrin, a new cell adhesion ligand for integrin α3β1 in epithelial basement membranes. Cell. 1991;65:599–610. doi: 10.1016/0092-8674(91)90092-d. [DOI] [PubMed] [Google Scholar]
- Chase HB, Rauch H, Smith VW. Critical stages of hair development and pigmentation in the mouse. Physiol Zool. 1951;24:1–8. doi: 10.1086/physzool.24.1.30152098. [DOI] [PubMed] [Google Scholar]
- Conti FJA, Rudling RJ, Robson A, Hodivala-Dilke KM. α3β1-integrin regulates hair follicle but not interfollicular morphogenesis in adult epidermis. J Cell Sci. 2003;116:2737–2747. doi: 10.1242/jcs.00475. [DOI] [PubMed] [Google Scholar]
- de Berker DAR, Messenger AG, Sinclair RD. Disorders of the hair. In: Burns T, Breathnach S, editors. Rook's Textbook of Dermatology. 7th. Vol. 4. Oxford: Blackwell Scientific Publishers; 1986. pp. 1–15. [Google Scholar]
- DiPersio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg JA, Hynes RO. α3β1 integrin is required for normal development of the epidermal basement membrane. J Cell Biol. 1997;137:729–742. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio CM, Shah S, Hynes RO. α3Aβ1 integrin localizes to focal contacts in response to diverse extracellular matrix proteins. J Cell Sci. 1995;108:2321–2336. doi: 10.1242/jcs.108.6.2321. [DOI] [PubMed] [Google Scholar]
- Dowling J, Yu QC, Fuchs E. β4 integin is required for hemidesmosomal formation, cell adhesion and cell survival. J Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom M, Falk M, Salmivirta K, Durbeej M, Ekblom P. Laminin isoforms and epithelial development. Ann NY Acad Sci. 1998;857:194–211. doi: 10.1111/j.1749-6632.1998.tb10117.x. [DOI] [PubMed] [Google Scholar]
- Ferletta M, Ekblom P. Identification of laminin-10/11 as a strong cell adhesive complex for a normal and a malignant human epithelial cell line. J Cell Sci. 1999;112:1–10. doi: 10.1242/jcs.112.1.1. [DOI] [PubMed] [Google Scholar]
- Frank DE, Carter WG. Laminin-5 deposition regulates keratinocyte polarization and persistent migration. J Cell Sci. 2004;117:1351–1363. doi: 10.1242/jcs.01003. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of integrin α6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- Gilles C, Polette M, Coraux C, Tournier JM, Meneguzzi G, Munaut C, Volders L, et al. Contribution of MT1-MMP and of human laminin-5 γ2 chain degradation to mammary epithelial cell migration. J Cell Sci. 2001;114:2967–2976. doi: 10.1242/jcs.114.16.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfinger LE, Stack MS, Jones JC. Processing of laminin-5 and its functional consequences: role of plasmin and tissue-type plasminogen activator. J Cell Biol. 1998;141:255–265. doi: 10.1083/jcb.141.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JCR, Hopkinson SB, Goldfinger LE. Structure and assembly of hemidesmosomes. Bioessays. 1998;20:488–494. doi: 10.1002/(SICI)1521-1878(199806)20:6<488::AID-BIES7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Joubeh S, Mori O, Owaribe K, Hashimoto T. Immunofluorescence analysis of the basement membrane zone components in human anagen hair follicles. Exp Dermatol. 2003;12:365–370. doi: 10.1034/j.1600-0625.2002.120402.x. [DOI] [PubMed] [Google Scholar]
- Kikkawa Y, Sanzen N, Fujiwara H, Sonnenberg A, Sekiguchi K. Integrin binding specificity of laminin-10/11: laminin-10/11 are recognized by α3β1, α6β1 and α6β4 integrins. J Cell Sci. 2000;113:869–876. doi: 10.1242/jcs.113.5.869. [DOI] [PubMed] [Google Scholar]
- Kikkawa Y, Sanzen N, Sekiguchi K. Isolation and characterization of laminin-10/11 secreted by human lung carcinoma cells. J Biol Chem. 1998;273:15854–15859. doi: 10.1074/jbc.273.25.15854. [DOI] [PubMed] [Google Scholar]
- Koshikawa N, Gianneli G, Cirulli V, Miyazaki K, Quaranta V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J Cell Biol. 2000;148:615–624. doi: 10.1083/jcb.148.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshikawa N, Minegishi T, Sharabi A, Quaranta V, Seiki M. Membrane-type matrix metalloproteinase-1 (MT1-MMP) is a processing enzyme for human laminin γ2 chain. J Biol Chem. 2005;280:88–93. doi: 10.1074/jbc.M411824200. [DOI] [PubMed] [Google Scholar]
- Langhofer M, Hopkinson SB, Jones JCR. The matrix secreted by 804G cells contains laminin-related components that participate in hemidesmosome assembly in vitro. J Cell Sci. 1993;105:753–764. doi: 10.1242/jcs.105.3.753. [DOI] [PubMed] [Google Scholar]
- Li J, Tzu J, Chen Y, Zhang YP, Nguyen NT, Gao J, Bradley M, et al. Laminin-10 is crucial for hair morphogenesis. EMBO J. 2003;22:2400–2410. doi: 10.1093/emboj/cdg239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Määtä M, Virtanen I, Burgeson R, Auto-Harmainen H. Comparative analysis of the distribution of laminin chains in the basement membranes in some malignant epithelial tumors. J Histochem Cytochem. 2001;49:711–726. doi: 10.1177/002215540104900605. [DOI] [PubMed] [Google Scholar]
- McGrath JA, Pulkkinen L, Christiano AM, Leigh IM, Eady RAJ, Uitto J. Altered laminin 5 expression due to mutations in the gene encoding the β3 chain (LAMB3) in generalized atrophic benign epidermolysis bullosa. J Invest Dermatol. 1995;104:467–474. doi: 10.1111/1523-1747.ep12605904. [DOI] [PubMed] [Google Scholar]
- Mellerio JE, Eady RAJ, Atherton DJ, Lake BD, McGrath JA. E210K mutation in the gene encoding the β3 chain of laminin-5 (LAMB3) is predictive of a phenotype of generalized atrophic benign epidermolysis bullosa. Br J Dermatol. 1998;139:325–331. doi: 10.1046/j.1365-2133.1998.02377.x. [DOI] [PubMed] [Google Scholar]
- Mercurio AM, Rabinovitz I. Towards a mechanistic understanding of tumor invasion—lessons from the α6β4 integrin. Semin Cancer Biol. 2001b;11:129–141. doi: 10.1006/scbi.2000.0364. [DOI] [PubMed] [Google Scholar]
- Mercurio AM, Rabinovitz I, Shaw LM. The α6β4 integrin and epithelial cell migration. Curr Opin Cell Biol. 2001a;13:541–545. doi: 10.1016/s0955-0674(00)00249-0. [DOI] [PubMed] [Google Scholar]
- Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, Copeland NG, et al. The laminin alpha chains: expression, developmental transitions, and chromosomal locations of alpha1–5 identification of heterotrimeric laminins 8–11 and cloning of a novel alpha3 isoform. J Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- Müller-Röver S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, Stenn KS, et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- Nanba D, Hieda Y, Nakanishi Y. Remodeling of desmosomal and hemidesmosomal adhesion systems during early morphogenesis of mouse pelage hair follicles. J Invest Dermatol. 2000;114:171–177. doi: 10.1046/j.1523-1747.2000.00842.x. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, Puri C, Tacchetti C, Giancotti FG. Targeted deletion of the integrin beta4 signaling domain suppresses laminin-5-dependent nuclear entry of mitogen-activated protein kinases and NF-kappaB, causing defects in epidermal growth and migration. Mol Cell Biol. 2005;25:6090–6102. doi: 10.1128/MCB.25.14.6090-6102.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott MP, Green MR, Kealey T. Human hair growth in vitro. J Cell Sci. 1990;97:463–471. doi: 10.1242/jcs.97.3.463. [DOI] [PubMed] [Google Scholar]
- Rabinovitz I, Mercurio AM. The integrin α6β4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J Cell Biol. 1997;139:1873–1884. doi: 10.1083/jcb.139.7.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. Conditional ablation of β1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol. 2000;150:1149–1160. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond K, Kreft M, Janssen H, Calafat J, Sonnenberg A. Keratinocytes display normal proliferation, survival and differentiation in conditional β4-integrin knockout mice. J Cell Sci. 2005;118:1045–1060. doi: 10.1242/jcs.01689. [DOI] [PubMed] [Google Scholar]
- Ryan MC, Lee K, Miyashita Y, Carter WG. Targeted disruption of the LAMA3 gene in mice reveals abnormalities in survival and late stage differentiation of epithelial cells. J Cell Biol. 1999;145:1309–1323. doi: 10.1083/jcb.145.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoven I, Drzewiecki KT. Congenital localized skin defect and epidermolysis bullosa hereditaria letalis. Acta Derm Venereol. 1979;59:533–537. [PubMed] [Google Scholar]
- Soma T, Ogo M, Suzuki J, Takahashi T, Hibino T. Analysis of apoptotic cell death in human hair follicles in vivo and in vitro. J Invest Dermatol. 1998;111:948–954. doi: 10.1046/j.1523-1747.1998.00408.x. [DOI] [PubMed] [Google Scholar]
- Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- Takizawa Y, Hiraoka Y, Takahashi H, Ishiko A, Yasuraoka I, Hashimoto I, Aiso S, et al. Compound heterozygosity for a point mutation and a deletion located at splice acceptor sites in the LAMB3 gene leads to generalized atrophic benign epidermolysis bullosa. J Invest Dermatol. 2000;115:312–316. doi: 10.1046/j.1523-1747.2000.00051.x. [DOI] [PubMed] [Google Scholar]
- van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin β4 null mice. Nat Genet. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- Veitch DP, Nokelainen P, McGowan KA, Nguyen TT, Nguyen NE, Stephenson R, Pappano WN, et al. Mammalian tolloid metalloproteinase, and not matrix metalloprotease 2 or membrane type 1 metalloprotease, processes laminin-5 in keratinocytes and skin. J Biol Chem. 2003;278:15661–15668. doi: 10.1074/jbc.M210588200. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Wadsworth WG. Assembly and tissue functions of early embryonic laminins and netrins. Curr Opin Cell Biol. 2004;16:572–579. doi: 10.1016/j.ceb.2004.07.013. [DOI] [PubMed] [Google Scholar]


