Abstract
In this issue of the JCI, Wu et al. and Marin et al. describe two new mouse models of inherited disorders of the RAS/MAPK signal transduction pathway that display hypertrophic cardiomyopathy (HCM); the model from the former paper was from a gain-of-function Raf1 mutation, and the model from the latter paper was from a protein tyrosine phosphatase, non-receptor type 11 (Ptpn11) mutated allele encoding Shp2 with impaired catalytic function. The two groups show that HCM arises from increased signaling through Erk1/2 and the mTor complex 1, respectively, and that those cardiac issues can be prevented or reversed with small-molecule therapies inhibiting the appropriate pathway. Aside from being the first studies of treatment for Noonan syndrome and related disorders in a mammalian system, these papers provide important insights into the role of RAS signaling in cardiac hypertrophy and suggest the complexity in developing meaningful therapy for individuals with these RASopathies.
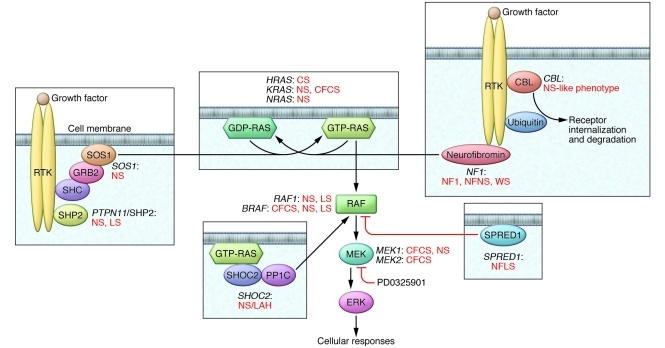
Signaling initiated by extracellular ligands, such as growth factors and cytokines, that is transduced through RAS proteins to multiple effectors, including the MAPKs, is central to cell proliferation, survival, differentiation, metabolism, and migration. The paradigmatic RAS/MAPK pathway (Figure 1) involves RAF proteins, particularly RAF1 and BRAF, which are MAPK kinase kinases that are activated after binding to RAS-GTP and then activate MAPK kinases, MEK1 and MEK2, which, in turn, activate the MAPKs, ERK1 and ERK2. Since its discovery nearly 30 years ago (1), the enormous role of perturbed RAS/MAPK signaling in cancer biology has become evident. Specifically, more than 30% of human cancers include mutations in genes encoding proteins in this pathway, particularly RAS proteins and BRAF. The vast majority of these genetic defects are acquired and result in increased activation of ERK1/2, often through gain-of-function alterations of the mutant proteins.
Figure 1. The RAS/MAPK signal transduction pathway.
Schematic diagram showing the RAS/MAPK cascade and affected disease genes in the RASopathies. The genes for which mutations are known and their associated phenotypes are indicated near the cartoon representation of the proteins they encode. Positive regulatory interactions are indicated with black arrows, and the negative regulatory interaction is indicated with a red blunted arrow. PD0325901 is an ATP-uncompetitive inhibitor of MEK (discussed in the text) and is also indicated with a blunted red arrow. CFCS, CFC syndrome; CS, Costello syndrome; NF1, neurofibromatosis type 1; NFLS, neurofibromatosis type 1–like syndrome (also termed Legius syndrome); NFNS, neurofibromatosis-NS; NS/LAH, Noonan-like syndrome with loose anagen hair; RTK, receptor tyrosine kinase; WS, Watson syndrome.
The importance of increased RAS/MAPK signaling in cancer spurred efforts to develop novel therapies that can reduce it (2). One strategy is to inhibit the mutant protein specifically. The best example of that approach is the ongoing work with PLX4032 (also known as RO5185426), which targets the BRAFV600E, the most common oncoprotein implicated in melanoma (3). Successful phase I and II clinical trials with malignant melanoma have been completed, and a phase III trial is ongoing (4). However, the more common approach has been to develop small molecules that inhibit RAS/MAPK signaling broadly. Products of this track have included the farnesyl transferase inhibitors, which are intended to reduce RAS translocation to the cell membrane, a necessary step for signaling, and inhibitors of RAF and MEK activities. To date, these efforts have been less successful (5). Since RAS/MAPK signaling is present in a wide array of normal cells and RAS proteins control multiple cellular processes and have several downstream effectors, titrating the right level of inhibition to provide therapeutic efficacy without incurring intolerable side effects is challenging. Indeed, clinical trials with PD325901, which is a highly specific MEK inhibitor, were terminated early due to ophthalmologic and neurologic toxicity, despite the fact that MEK1/2 are downstream in the RAS pathway and are only known to activate ERK1/2 (6).
After the discovery of the RAS/MAPK pathway, studies with model organisms like Drosophila melanogaster and Caenorhabditis elegans elaborated its central role in organismal development (7, 8). Subsequent experiments with loss-of-function alleles and cancer-related gain-of-function mutations, particularly in mouse models, generally produced one of two outcomes. For some genes belonging to redundant families, knockout mice were without phenotype. The remainder of the loss-of-function alleles and the cancer-related gain-of-function ones were embryonic or early neonatal lethal. Thus, the opportunity for RAS/MAPK mutations in inherited human developmental disorders was not apparent.
Noonan syndrome
In 1968, Jacqueline Noonan, a pediatric cardiologist, defined the syndrome that now bears her name (9). She initially observed a phenotype among children with pulmonary valve stenosis and noted other seminal features, including short stature, facial dysmorphia, and developmental delays. Subsequently, it became clear that other congenital heart defects are associated with Noonan syndrome (NS) and that 20% of NS patients develop hypertrophic cardiomyopathy (HCM). Several disorders with overlapping features were described subsequently — Costello syndrome, cardiofaciocutaneous (CFC) syndrome, and LEOPARD syndrome (LS). Phenotypic overlaps often made diagnosis challenging, and there were animated discussions about whether these were really separate traits.
In 2001, we ourselves identified protein tyrosine phosphatase, non-receptor type 11 (PTPN11) missense mutations as underlying NS, accounting for nearly 50% of cases (10). PTPN11 encodes SHP2, which is a non-receptor protein tyrosine phosphatase that primarily has positive regulatory roles in signal transduction, particularly for RAS signaling. NS-associated PTPN11 mutations have gain-of-function effects, primarily impairing SHP2’s activation/inactivation molecular switch, resulting in increased ERK1/2 activation. We later showed that different PTPN11 missense mutations with generally greater gain-of-function effects occur as somatic defects underlying childhood leukemias, particularly juvenile myelomonocytic leukemia (11, 12). The molecular and phenotypic specificity of these inherited and somatic mutations revealed the importance of RAS signaling levels, with respect to disease pathogenesis and organismal survival.
Several groups pursued the idea that the NS-related disorders were allelic. While no PTPN11 mutation was observed in Costello or CFC syndrome, nearly all individuals with LS were found to harbor PTPN11 missense defects that are distinct from those observed in NS (13, 14). Of interest, the LS-associated lesions reduce SHP2’s catalytic activity (12, 15).
Yoko Aoki, Yoichi Matsubara, and their colleagues pursued the hypothesis that Costello syndrome also result from perturbed RAS/MAPK signaling (16). They showed that gain-of-function HRAS mutations, most altering Gly12, cause this disorder. With perturbations of RAS signaling established as central to NS and related disorders, extensive candidate gene studies were undertaken to identify additional culprit mutations. In the past five years, these efforts have been fruitful, with the discovery of mutations in KRAS, NRAS, SOS1, RAF1, BRAF, MEK1, MEK2, SHOC2, and CBL among individuals with NS and related phenotypes (Figure 1 and ref. 17). Aside from enabling genetic testing for these RASopathies, genotype/phenotype associations have been established for many. For instance, SOS1 mutations, which only cause NS, are generally associated with normal or near-normal neurocognitive development and stature but marked ectodermal abnormalities, while RAF1 mutations, which cause NS and LS, are strongly associated with HCM. Collectively, these observations raise important biological and clinical questions. Why are certain organs affected in particular RASopathies, while others are spared? What is the basis of the specificity between altered gene, or even specific mutation, and the phenotype? Can interventions similar to RAS pathway inhibition in the setting of cancer ameliorate features of these disorders?
New models to gain insight
Investigators have been studying disease pathogenesis for the RASopathies using cell and animal models. The two articles in this issue of the JCI (18, 19), modeling an NS-associated RAF1 mutation and an LS-associated PTPN11 mutation in mice, substantially advance our understanding of those disorders, providing insights into the therapeutic opportunities but also glimpses of the magnitude of the challenges for developing such therapies.
Wu and colleagues generated a conditional murine Raf1L613V allele through homologous recombination, which requires Cre-mediated excision for activation of the mutation, and then studied mice in which the activated mutation was inherited through the germline, modeling the human condition (18). The Raf1L613V mouse faithfully recapitulated NS caused by this mutation, with postnatal growth retardation, craniofacial abnormalities, hematopoietic perturbation in the myeloid lines, and HCM. The authors focused on the HCM, showing that it results from cardiomyocyte hypertrophy, not hyperplasia, and is accompanied by increased cardiac contractility, similar to human HCM. Molecular characterization of this murine HCM showed a change in myosin heavy chain isoform expression, with an increased β-Mhc/α-Mhc ratio, which is typical for HCM. Assessment of Ras/Mapk signaling in vitro and in vivo revealed increased activation of Mek1/2 and Erk1/2 in hearts from mice heterozygous for the Raf1L613V allele. Of note, activation of other Mapks, p38 and Jnk, was not altered. These findings suggested that blockade of Ras/Mapk signaling could be efficacious in this mouse model. Postnatal treatment with the aforementioned MEK inhibitor, PD0325901, blocked emergence of HCM as well as reversed it, depending upon when therapy was initiated. Moreover, treatment normalized linear growth and craniofacial development.
Marin and colleagues generated a conditional Ptpn11Y279C allele in mice through homologous recombination, modeling one of the two most common mutations causing LS (19). When the Cre-activated allele was inherited through the germline, the resulting mice had a RASopathy phenotype, with postnatal growth retardation, craniofacial and sternal abnormalities, and HCM. While melanocytes in humans are dispersed among the epidermal keratinocytes, murine melanocytes reside deep in hair follicles and dermis. As a consequence, mice cannot develop lentigines, eliminating the possibility of the Ptpn11Y279C/+ mice exhibiting the hallmark feature of LS. This group also focused on HCM, showing myofiber disarray, increased fibrosis, increased cardiomyocyte width with normal length, and reexpression of the fetal cardiac gene program. Assessment of Ras/Mapk signaling revealed attenuated responses to stimulatory ligands such as Egf-1 and Ang II. A survey of relevant signaling pathways revealed activation of the Akt/mTor pathway as well as of Fak, Jnk1/2, and Stat3. To develop insights into the primary cause of the cardiac hypertrophy, the authors treated isolated cardiomyocytes from Ptpn11Y279C/+ mice with rapamycin, an mTor complex 1 (mTor1C) inhibitor, as well as with inhibitors of Jnk and Stat. They showed that rapamycin treatment rapidly normalized cardiomyocyte size in vitro, while the other inhibitors did not. Postnatal treatment of the Ptpn11Y279C/+ mice with rapamycin daily for four weeks and then weekly for four weeks prevented the emergence of HCM or reversed it, depending on the age at which the drug was started.
RAS in myocardial biology
The results of these two studies raise intriguing issues about myocardial biology. The role of signaling through ERK1/2 in inducing hypertrophy has been a subject of considerable debate. Physiologic cardiac hypertrophy, which results from exercise, does not result in reactivation of the fetal gene program or increased fibrosis and is primarily attributed to signaling from IGF-1 through PI3 kinase to AKT (20, 21). Pathologic hypertrophy is usually characterized as concentric or eccentric, the former associated with pressure overload and the latter associated with volume overload. Increased signaling through ERK1/2 is associated with concentric hypertrophy, while ERK5 activation is more relevant for eccentric hypertrophy. The HCM findings in the Raf1+/L613V mouse are compatible with this paradigm. Of interest, mice overexpressing activated Mek1 specifically in cardiomyocytes display HCM without increased cardiac fibrosis (22). Moreover, there is an emerging understanding that interactions between activated cardiac fibroblasts and cardiomyocytes contribute to the development of HCM. As noted by Wu and colleagues (18), a logical next set of experiments is to conditionally induce the Raf1L613V allele only in cardiomyocytes or cardiac fibroblasts and then examine the effects on disease pathogenesis. If mice expressing that mutation only in cardiomyocytes develop HCM, it would be useful to eliminate Erk1/2 genetically (23). If this genetic manipulation prevented HCM, this would provide even stronger evidence that signaling through Erk1/2 is necessary; a possible objection to the results obtained with MEK inhibitor is that the drug could have as-yet-unknown off-target effects.
The results concerning HCM from the Ptpn11Y279C/+ mouse are more difficult to align with the existing literature on the role of Erk1/2 in cardiac hypertrophy. Based on the work of Jeffery Molkentin’s group, elimination of Erk1/2 in murine cardiomyocytes results in a dilated cardiomyopathy with eccentric hypertrophy (23). Cardiomyocytes from these mice are elongated with normal width. Stimulation of hearts lacking Erk1/2 with pressure overload results in a normal hypertrophic response grossly but with cardiomyocytes that display even greater increased length. In the Ptpn11Y279C/+ mice, there was diminished signaling through Erk1/2, but HCM developed, with cardiomyocytes that were widened but not lengthened (19). Eliminating Erk1/2 genetically in the Ptpn11Y279C/+ cardiomyocytes would address the question of whether residual activation of Erk1/2 is necessary to drive the hypertrophic response with cell widening. If Erk1/2 is not necessary, then studies could focus on understanding how aberrant Y279C Shp2 function shifts signaling to mTOR1C, which cells with only normal Shp2 are apparently unable to achieve.
A potential therapy?
The therapeutic implications of these two JCI studies are also interesting. On the one hand, it is enormously exciting to learn that HCM in two RASopathies can be prevented or even reversed with small-molecule therapy. On the other, there are substantive issues concerning risks and benefits to consider. While there is a rare virulent form of HCM that progresses rapidly in infants with RASopathies, resulting in early death or need for heart transplantation, most RASopathy-associated HCM is associated with low mortality. As such, the risks associated with a novel therapy would need to be low, particularly as the treatment would be needed for many years. The same risk-benefit analysis would apply for other aspects of the RASopathies, such as developmental delays. This strategy contrasts with anti-RAS signaling efforts for cancers, diseases that are rapidly lethal and for which successful treatment can have a short duration. One approach to this problem is suggested by Marin et al., who suggest intense short-term therapy, followed by minimal maintenance dosing (19). Alternatively, it may be necessary to search for small molecules that have more modest effects on signaling, ones that would have failed screens for cancer drugs. Current efforts to use HMG-CoA reductase inhibitors (statins) to ameliorate neurocognitive delays associated with neurofibromatosis type I, in which there is increased RAS/MAPK signaling, are an example; this approach was successful in preclinical work with Nf1 mice (24) and is now in phase II clinical trials (25).
These two JCI papers also raise a cautionary note. While it has been gratifying to find numerous genetic causes along the RAS/MAPK pathway underlying phenotypically related disorders, the realization that HCM caused by two of those mutant alleles will likely require distinct therapeutic approaches is sobering. This provides further impetus to proceed with efforts to identify the other disease genes underlying these disorders and generate animal models as well as human cell models, such as was recently achieved with LS induced pluripotent stem cell–derived cardiomyocytes (26). Ultimately, the combination of a thorough understanding of pathogenesis and the identification of appropriate small molecules will enable us to engage affected individuals and their families to initiate clinical trials.
Acknowledgments
The authors thank Jeffery Molkentin and Beccy Josowitz for thoughtful discussions and/or reading of this commentary and apologize to colleagues whose work was not cited due to limited space. The work was supported in part by grants from Telethon-Italy (GGP10020), ERA-Net for research programmes on rare diseases (2009), “Convenzione Italia-Istituzioni USA 2010” to M. Tartaglia, and the NIH (HL71207) to B.D. Gelb.
Footnotes
Conflict of interest: Bruce D. Gelb and Marco Tartaglia receive royalties from several commercial laboratories for PTPN11 and RAF1 mutation testing for Noonan syndrome.
Citation for this article: J Clin Invest. 2011;121(3):844–847. doi:10.1172/JCI46399.
References
- 1. Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3(6):459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 2. Pratilas CA, Solit DB. Targeting the mitogen-activated protein kinase pathway: physiological feedback and drug response. Clin Cancer Res. 2010;16(13):3329–3334. doi: 10.1158/1078-0432.CCR-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bollag G, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467(7315):596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. A study of RO5185426 in comparison with dacarbazine in previously untreated patients with metastatic melanoma. NIH Web site. http://clinicaltrials.gov/ct2/show/NCT01006980 . Updated December 27, 2010. Accessed January 4, 2011. [Google Scholar]
- 5. Rao S, et al. Phase III double-blind placebo-controlled study of farnesyl transferase inhibitor R115777 in patients with refractory advanced colorectal cancer. . J Clin Oncol. 2004;22(19):3950–3957. doi: 10.1200/JCO.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 6. Lorusso PM, et al. Phase I and pharmacodynamic study of the oral MEK inhibitor CI-1040 in patients with advanced malignancies. J Clin Oncol. 2005;23(23):5281–5293. doi: 10.1200/JCO.2005.14.415. [DOI] [PubMed] [Google Scholar]
- 7. Rubin GM, et al. Signal transduction downstream from Ras in Drosophila. Cold Spring Harb Symp Quant Biol. 1997;62:347–352. [PubMed] [Google Scholar]
- 8. Sternberg PW, Han M. Genetics of RAS signaling in C. elegans. . Trends Genet. 1998;14(11):466–472. doi: 10.1016/S0168-9525(98)01592-3. [DOI] [PubMed] [Google Scholar]
- 9. Noonan JA. Hypertelorism with Turner phenotype. A new syndrome with associated congenital heart disease. Am J Dis Child. 1968;116(4):373–380. doi: 10.1001/archpedi.1968.02100020377005. [DOI] [PubMed] [Google Scholar]
- 10. Tartaglia M, et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29(4):465–468. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- 11. Tartaglia M, et al. Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia. Nat Genet. 2003;34(2):148–150. doi: 10.1038/ng1156. [DOI] [PubMed] [Google Scholar]
- 12. Tartaglia M, et al. Diversity and functional consequences of germline and somatic PTPN11 mutations in human disease. Am J Hum Genet. 2006;78(2):279–290. doi: 10.1086/499925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Digilio MC, et al. Grouping of multiple-lentigines/LEOPARD and Noonan syndromes on the PTPN11 gene. Am J Hum Genet. 2002;71(2):389–394. doi: 10.1086/341528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Legius E, Schrander-Stumpel C, Schollen E, Pulles-Heintzberger C, Gewillig M, Fryns JP. PTPN11 mutations in LEOPARD syndrome. J Med Genet. 2002;39(8):571–574. doi: 10.1136/jmg.39.8.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kontaridis MI, Swanson KD, David FS, Barford D, Neel BG. PTPN11 (Shp2) mutations in LEOPARD syndrome have dominant negative, not activating, effects. J Biol Chem. 2006;281(10):6785–6792. doi: 10.1074/jbc.M513068200. [DOI] [PubMed] [Google Scholar]
- 16. Aoki Y, et al. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet. 2005;37(10):1038–1040. doi: 10.1038/ng1641. [DOI] [PubMed] [Google Scholar]
- 17. Tartaglia M, Gelb BD. Disorders of dysregulated signal traffic through the RAS-MAPK pathway: phenotypic spectrum and molecular mechanisms. Ann N Y Acad Sci. 2010;1214:99–121. doi: 10.1111/j.1749-6632.2010.05790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu X, et al. MEK-ERK pathway modulation ameliorates disease phenotypes in a mouse model of Noonan syndrome associated with the Raf1L613V mutation. . J Clin Invest. 2011;121(3):1009–1025. doi: 10.1172/JCI44929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marin TM, et al. Rapamycin reverses hypertrophic cardiomyopathy in a mouse model of LEOPARD syndrome–associated PTPN11 mutation. . J Clin Invest. 2011;121(3):1026–1043. doi: 10.1172/JCI44972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation. 2010;122(25):2727–2735. doi: 10.1161/CIRCULATIONAHA.110.942268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kehat I, Molkentin JD. Extracellular signal–regulated kinase 1/2 (ERK1/2) signaling in cardiac hypertrophy. Ann N Y Acad Sci. 2010;1188:96–102. doi: 10.1111/j.1749-6632.2009.05088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bueno OF, et al. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19(23):6341–6350. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kehat I, et al. Extracellular signal–regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth [published online ahead of print December 7, 2010]. Circ Res. doi: 10.1161/CIRCRESAHA.110.231514. doi: 10.1161/CIRCRESAHA.110.231514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li W, et al. The HMG-CoA reductase inhibitor lovastatin reverses the learning and attention deficits in a mouse model of neurofibromatosis type 1. Curr Biol. 2005;15(21):1961–1967. doi: 10.1016/j.cub.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 25. A randomized placebo-controlled study of lovastatin in children with neurofibromatosis type 1 (STARS). NIH Web site. http://clinicaltrials.gov/ct2/show/NCT00853580 . Updated September 2, 2010. Accessed January 4, 2011. [Google Scholar]
- 26. Carvajal-Vergara X, et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature. 2010;465(7299):808–812. doi: 10.1038/nature09005. [DOI] [PMC free article] [PubMed] [Google Scholar]