Abstract
Several attempts have been made in the last two decades to investigate ulcerative colitis (UC) patients during the natural course of the disease so as to identify appropriate surrogate markers of disease activity. Most patients with quiescent inflammatory bowel disease have low grade inflammation and it is possible that relapse occurs only once the inflammatory process crosses a critical intensity. Since inflammation is a continuous process, its direct assessment may provide us a quantitative pre-symptomatic measure of imminent relapse. If substantial, it may allow targeted treatment early, to avert relapse or formulate newer therapeutic strategies to maintain symptomatic remission. It is clinically very important to identify these patients at a subclinical stage, noninvasively, by various biomarkers. Biomarkers help to gain an objective measurement of disease activity as symptoms are often subjective. Biomarkers also help to avoid invasive procedures which are often a burden to the patient and the health care system. If an ideal biomarker existed for UC, it would greatly facilitate the work of the gastroenterologist treating these patients. Both “classical” and “emerging” biomarkers of relevance for UC have been studied, but the quest for an ideal biomarker still continues. In this brief review we describe various biomarkers of clinical importance.
Keywords: biomarkers, ulcerative colitis, inflammation, serum C-reactive protein, fecal markers, myeloperoxidase (MPO), calprotectin, lactoferrin, review
Abstract
In den letzten beiden Dekaden sind mehrere Versuche unternommen worden, geeignete Marker für die Diagnose und den Verlauf der Colitis ulcerosa zu finden. In den Ruhephasen der Erkrankung haben die meisten Patienten schwach ausgeprägte Zeichen der Entzündung. Es ist möglich, dass der Rückfall nur auftritt, wenn der Entzündungsprozess eine kritische Intensität überschreitet. Da die Entzündung ein kontinuierlicher Prozess ist, könnte ihre direkte Erfassung eine quantitative Messgröße für den drohenden Rückfall bilden. Wenn wesentliche Veränderungen auftreten, könnte eine frühe gezielte Therapie eingeleitet werden, um den Rückfall zu vermeiden oder neuere therapeutische Strategien entwickelt werden, um eine symptomatische Remission zu erzielen. Es ist klinisch von großer Bedeutung, auf nicht-invasivem Wege mit Hilfe von Biomarkern diese Patienten bereits im subklinischen Stadium der Erkrankung zu erfassen. Biomarker ermöglichen, die Erkrankungsaktivität objektiv zu erfassen, weil die Symptome häufig subjektiv sind. Biomarker helfen auch, invasive Prozeduren, die für den Patienten sehr belastend und kostenträchtig sind, zu vermeiden. Wenn ein idealer Biomarker für die ulcerative Colitis existieren würde, könnte dieser bei der gastroenterologischen Behandlung vieler Patienten hilfreich sein. Sowohl klassische als auch neu gefundene Biomarker von Bedeutung wurden untersucht, aber die Suche nach einem idealen Biomarker wird weiter gehen. In diesem kurzen Übersichtsartikel werden verschiedene Biomarker von klinischer Bedeutung beschrieben.
Introduction
Ulcerative colitis has remissions and relapses, so both the patient and the treating physician need to have a coordinated approach. Treatment modalities may range from drugs with lot of side effects to surgical interventions. The disease is usually chronic and the aim of treatment in active disease is to induce remission. The gastrointestinal advisory panel of the American Food and Drug Administration has proposed that remission be defined as an absence of inflammatory symptoms (rectal bleeding or diarrhea) in conjunction with evidence of mucosal healing (absence of ulceration, significant granularity or friability). While repeated endoscopic examination is not always thought desirable by patient or clinician, a simple marker or a combination of biomarkers of intestinal inflammation would be of great help in monitoring the response to treatment. The shortcomings of clinical indices in assessing disease activity are widely known. Also in subjects with previously diagnosed UC it is sometimes not clear whether symptoms are due to active inflammatory bowel disease or an overlying functional bowel disorder, which commonly co-exists in patients with inflammatory bowel disease [1]. There is no single “Gold Standard” test or examination for diagnosis of UC, instead physicians apply a combination of symptoms, clinical examination, laboratory indices, endoscopy and histology to make the diagnosis and to assess severity as well as predict the outcome of disease [2]. Biomarkers have been studied in the past decade firstly to gain an objective measurement of disease activity as symptoms are often subjective and secondly to avoid invasive (endoscopic) procedures which are often a burden to the patient and to health care system as well.
Markers in serum
Serum C-reactive protein
A large number of changes, distant from the site of inflammation and involving many organ systems, may accompany inflammation. In 1930, interest was focused on these changes by the discovery of C-reactive protein [3] (CRP; so called because it reacted with the plasma of the patients during the acute phase of pneumococcal pneumonia). Denovo hepatic synthesis starts very rapidly after a single stimulus, serum concentration rising above 5 mg/L by about 6 hrs and peaking around 48 hours. The plasma half life of CRP is about 19 hours and is constant under all conditions of health and disease, so that the sole determinants of circulating CRP concentration is the synthesis rate which thus directly reflects the intensity of the pathological process stimulating CRP production. When the stimulus for increased production completely ceases, the circulating CRP concentration falls rapidly, at almost the rate of plasma CRP clearance [4]. Twin studies show a highly significant hereditable component in base line CRP values that are independent of age and body mass index (BMI). In most, though not all diseases, the circulating value of CRP reflect on going inflammation and tissue damage much more accurately than do other laboratory parameters of the acute phase response, such as plasma viscosity and the erythrocyte sedimentation rate (ESR). Importantly, acute phase CRP values show no diurnal variation and are unaffected by eating. Furthermore, no anti-inflammatory or immunosuppressive drug affects CRP production. Therefore, modification of CRP response during treatment occurs only as a result of the effect of drug on underlying disorder. Serum CRP as a biomarker in the course of UC has been studied by number of workers. Prantera et al. [5] studied 60 UC patients and concluded that the disease severity and the presence of signs and symptoms of toxicity seem likely to be determined by the amount of colonic tissue involved by inflammation, both in depth and in extent. CRP appeared the most reliable factor reflecting activity and extension of lesion. In a study from Mayo Clinic [6], a cohort of 43 UC patients were studied. The authors concluded that serum CRP levels were associated with increase in biomarkers of inflammation (except platelets) and an active disease at ileocolonoscopy. However, histological activity was not associated with CRP concentrations in UC patients. In another study Chouhan et al. [7] concluded that measurement of CRP levels is a simple method of assessing disease activity and extent in UC. CRP level >12 mg/L is indicative of severe and extensive disease. A change in CRP following therapy is a good parameter to assess the effect of the drug on the underlying inflammation. A decrease in CRP in response to therapy is objective evidence that the drug has a beneficial effect on gut inflammation even in patients with little change in symptoms. On the other hand, persistently raised CRP indicates failure of the therapy to control mucosal inflammation [8]. A very important clinical scenario in the course of UC is severe colitis: when a clinician is faced with a challenge of sparing the colon and saving the life. Studies have shown that on day 3 of intensive management with steroids and serum CRP >45 mg/L , stool frequency greater than 8, a surgical intervention may become life saving. However, patterns of colitis, radiographic appearance, age of the patient and last but not the least experience of the treating physician can never be underestimated in this decision.
Fecal markers as non-invasive markers for assessment of severity of UC
The presence of active gut inflammation in patients with UC is associated with an acute phase reaction and migration of leucocytes to the gut, and this is transferred into the production of several proteins, which may be detected in serum or stools [9]. Evaluation of whether mucosal inflammation is present or not is very important in the management of patient with UC because relapse rates are very high if minimal mucosal inflammation remains in a patient who has clinical remission [10], [11]. Even colonoscopy does not detect inflammation if it is minimal or localized. It is conceivable that feces reflect the state of all sites in the digestive tract, since feces are formed during transit of the bowel [12]. Fecal markers fulfill all the criteria of being non-invasive, simple, in-expensive, sensitive and specific parameters to detect gastrointestinal inflammation. Fecal neutrophil derived proteins have been the subjects of a number of reports; Sugi et al. [13] concluded in their study that lactoferrin is the most suitable of these proteins to be used as a neutrophil-derived fecal marker of inflammation for clinical applications. Chronic inflammation of the large intestine predominantly comprises of lymphocytes and plasma cells while in acute exacerbation neutrophils migrate and degranulate substances like myeloperoxidase (MPO), lactoferrin, eosinophilic chemotactic factors etc. The levels of these substances indicate activation of neutrophils along gut mucosa during exacerbation. These substances are stable in fecal matter and measurement of these can give indirect evidence of colonic inflammation in a non-invasive manner [13]. Fecal markers comprise a heterogeneous group of substances that either leak from, or are generated by the inflamed intestinal mucosa. The main use of these markers is likely to be in diagnosing and assessing disease activity in difficult cases. They may also have a role in assessing treatment effect and prediction of relapse. Many potential markers have been assessed to date; however, an ideal one is yet to be identified [14].
Fecal myeloperoxidase (MPO)
Neutrophil accumulation in the inflamed intestinal mucosa is a prominent feature in ulcerative colitis. The granules of neutrophil granulocytes contain a number of enzymes, for example myeloperoxidase which are important in the combat against bacteria. These granule enzymes, some of which are proteolytic can be released upon stimulation, together with cytotoxic oxygen metabolites. Therefore activated neutrophils may contribute to tissue damage at sites of inflammation. The factors responsible for the mucosal recruitment of neutrophils in ulcerative colitis are not certain [15]. Myeloperoxidase activity is directly related to cell number, down to as few as 500 cells. Myeloperoxidase activity was assayed in two animal models of inflammation: acetic acid induced colitis in rats and Clostridium difficile enterotoxin induced enteritis in hamsters. In both models, the activity of myeloperoxidase solubilized from the inflamed tissue was directly proportional to the number of neutrophils seen in histologic sections [16], [17]. Raab et al. [18] studied the concentration of myeloperoxidase, a neutrophil granule constituent, in the perfusion fluid from sigmoid and rectal segments in patients with ulcerative colitis. The concentrations of myeloperoxidase were increased several fold in the patients with ulcerative colitis compared with healthy controls pointing to an enhanced neutrophil activity. The release of myeloperoxidase correlated to an enhanced local release of the neutrophil activating peptide interleukin-8 (IL8). The results obtained are compatible with the hypothesis that local mucosal recruitment/activation of neutrophil in ulcerative colitis is mediated by an enhanced IL8 synthesis. TNF-α may be one relevant factor as a stimulus to IL8 synthesis. Bustos et al. [19] solubilized fecal MPO with hexadecyl trimethylammonium bromide and the MPO activity was measured by a diamisidine H2O2 assay. In their study, fecal leukocytes were not found in healthy controls and stool MPO activity ranged from 1.6 x 103 to 2.83 x 103 MPO per gram of feces; the authors concluded that fecal MPO activity is a simple biochemical assay for the detection and quantification of fecal leukocytes. Later Saiki et al. [20] studied MPO levels in stool extracts which were measured using a radio-immunoassay in 33 patients with ulcerative colitis and 15 normal controls. Stool levels of MPO in active UC patients increased significantly and correlated with laboratory parameters and endoscopic grade of inflammation. A paired analysis showed a decrease in MPO levels after the resolution of disease exacerbation. These results suggest that stool MPO is a simple, non-invasive and relevant marker of disease activity. Fecal MPO levels can detect intestinal healing after treatment in a non invasive manner. This can be reassuring to the treating physician and high levels can predict relapse in a given patient. In a study by Sangfelt et al. [21] the rectal release of the neutrophil MPO and eosinophil constituents (eosinophilic cationic protein ECP, eosinophil peroxidase EPO granule) were measured in 11 patients using intraluminal segmental perfusion of the rectum. The released amounts of MPO, ECP and EPO in the perfusion fluids were determined by radio-immunoassays before and during prednisolone enema treatment and correlated to clinical, endoscopic and histopathological data in addition to treatment outcome. In their study rectally released MPO seemed to be an early marker of treatment response in patients with ulcerative colitis. Further more, when the different granule products monitored in this study were compared, MPO was best associated with inflammatory activity and disease response to treatment.
Fecal lactoferrin
Lactoferrin, an iron binding protein with a molecular weight of approximately 80,000, is present in the intestinal mucus besides other body fluids. It has an antibacterial effect. Uchide et al. developed ELISA (enzyme-linked immunosorbent assay) and have shown that fecal lactoferrin level is high in patients with colorectal disease [22]. Later Langhorst et al. [23] studied 76 fecal specimens from 31 patients with UC in times of active and inactive status of disease. Disease activity was determined with the colitis activity index, which includes a combination of laboratory parameters and clinical symptoms, with a score of at least 6 indicating active disease. Lactoferrin showed increased levels in samples from patients with active disease compared with those in remission. In another study Kane et al. [24] studied fresh stool samples of 80 UC patients and 31 irritable bowel syndrome (IBS) patients. Fecal lactoferrin concentrations were determined using a polyclonal antibody based on ELISA. Mean fecal lactoferrin concentrations for each group and sensitivity and specificity were determined by the authors. The authors concluded that fecal lactoferrin was 90% specific for identifying inflammation in patients with active UC and 100% specific in ruling out IBS.
Fecal calprotectin
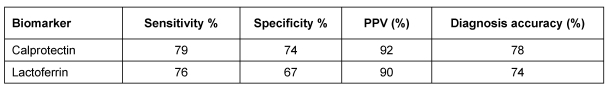
Calprotectin is a calcium-binding protein that inhibits metalloproteinases. It has antibacterial and antifungal activities [25]. It is an abundant protein found in all body fluids. Calprotectin has many clinical advantages to be used as a non-invasive marker. It is resistant to bacterial degradation in the gut and is stable in stool for up to one week at room temperature, overcoming delays in transporting the sample to the laboratory. Furthermore, calprotectin can be readily quantified using ELISA. Since calprotectin is primarily derived from neutrophils, its concentration is directly proportional to neutrophil migration toward the intestinal tract. From this comparison, levels of all of the fecal leukocyte markers in IBS were found to be in the range of healthy controls. Fecal calprotectin was found to be strongly associated with colorectal inflammation, indicating the presence of organic disease [26] and can differentiate inflammatory bowel disease (IBD) from irritable bowel syndrome. Studies have shown that fecal calprotectin differentiated an inactive disease from mild, moderate and highly active diseases, highlighting its usefulness for monitoring activity [27]. In a follow-up study of 163 patients (89 Crohn's disease (CD), 74 UC) over a period of 12 months Gisbert et al. [28] evaluated the role of fecal calprotectin and lactoferrin in the prediction of IBD relapse. In patients who relapsed during follow-up calprotectin concentrations were found to be higher than in patients who had not (239 ±150 versus 136 ±158 µg/g (P<0.001). The relapse risk was higher in patients that had high (>150 µg/g) calprotectin concentrations (30% versus 7.8%; P<0.001) or positive lactoferrin (25% versus 10%; P<0.05). The sensitivity and specificity of fecal calprotectin (>150 µg/g) to predict relapse were 69% and 69%, respectively. The corresponding values for lactoferrin were 62% and 65%, respectively. The authors concluded that these markers are effective in predicting the relapse. Fecal calprotectin is an easy, inexpensive, sensitive and specific way to evaluate IBD. Despite the fact that levels of fecal calprotectin have an important role in diagnosis, follow-up, prediction of relapses and assessment of response to treatment, it still has some disadvantages and can only be used as a complementary test. Fecal calprotectin levels get affected by nonsteroidal anti-inflammatory drug (NSAID) intake, bleeding more than 100 ml and by malignancy. Renata et al. [29] compared fecal lactoferrin with calprotectin in 46 UC patients (26 had extensive colitis and 20 had left sided colitis); 36 (78%) patients had active disease at colonoscopy and calprotectin was above normal in 28 patients, as was lactoferrin in 27. In their study, endoscopic and histologic scores correlated significantly with lactoferrin. The authors concluded that both calprotectin and lactoferrin tests appear to be useful in detecting bowel inflammation in symptomatic patients, achieving a similar diagnostic accuracy as shown in Table 1 (Tab. 1). The proportion of patients positive for calprotectin or lactoferrin was not influenced by the extent of disease when a similar disease activity was considered.
Table 1. Comparison of fecal lactoferrin and calprotectin as biomarkers.
Comparison of serum and fecal biomarkers in assesing disease severity in UC
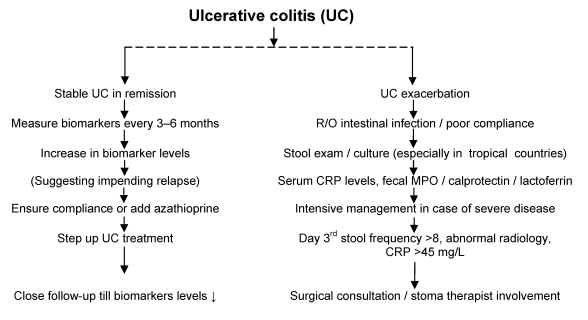
We studied both serum and fecal biomarkers in assessing disease severity of UC and response to treatment. In this prospective study [30] cases had significantly higher levels of serum CRP, fecal lactoferrin and MPO levels than controls, making biomarkers an excellent tool in differentiation of functional bowel syndromes from inflammatory bowel disorders. We observed that cases with severe UC had higher CRP, MPO and fecal lactoferrin levels than those with mild disease. All three markers showed a high degree of correlation with each other. There was significant correlation between severity of UC and serum CRP levels and it was 100% specific. Fecal lactoferrin was 94% sensitive and 100% specific and fecal MPO was 89% sensitive and 51% specific. The clinical improvement correlated with significant fall in all three biomarkers on follow up depicting intestinal healing. We found these biomarkers to be excellent in assessing disease severity and response to treatment in a non-invasive manner.There are no guidelines for clinical use of biomarkers yet. The algorithm in Figure 1 (Fig. 1) may be useful for clinical practice.
Figure 1. Algorithm for clinical use of biomarkers in ulcerative colitis.
Future biomarkers
In a recent study [31] of gene expression profiles from blood of patients with ulcerative colitis, Crohn's disease and non-inflammatory diarrheal disorders, Burakoff et al. demonstrated that different genes existed for each of these disorders. Gene profiles could differentiate these disorders in a given patient in a non-invasive manner. However more studies are required before these biomarkers will come into clinical practice. The cost factor may, however, become a limiting factor in their wider use. Colorectal carcinoma is the most severe complication in ulcerative colitis. In an experimental animal model Talero et al. [32] observed that dysplasia and adenocarcinomas correlated with number of cycles of dextran sulfate sodium (DSS) induced colonic inflammation. The authors observed that adrenomedullin is implicated in carcinogenesis. This molecule or similar other molecule may become one of the future biomarkers in the management of ul-cera-tive colitis so that patients with ulcerative colitis are treated before dysplasia progresses to malignancy.
Conclusion
In conclusion, biomarkers are useful in differentiating functional bowel disorders from inflammatory bowel disease. These are useful in assessing disease activity and severity of ulcerative colitis. Using biomarkers, intestinal healing after treatment can be documented on follow-up in a non-invasive manner. This would be of great help in monitoring response to treatment. Biomarkers can play a role in determining relapse and can help in tailoring treatment without repeating colonoscopy. Disease assessment by biomarkers may lead to new management approaches in UC if a perfect one or a combination of biomarkers are discovered, as the quest for a perfect biomarker in ulcerative colitis still continues.
Notes
Competing interests
The authors declare that they have no competing interests.
References
- 1.Nielsen OH, Vainer B, Madsen SM, Seidelin JB, Heegaard NH. Established and emerging biological activity markers of inflammatory bowel disease. Am J Gastroenterol. 2000;95(2):359–367. doi: 10.1111/j.1572-0241.2000.t01-1-01790.x. Available from: http://dx.doi.org/10.1111/j.1572-0241.2000.t01-1-01790.x. [DOI] [PubMed] [Google Scholar]
- 2.Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119(1):15–22. doi: 10.1053/gast.2000.8523. Available from: http://dx.doi.org/10.1053/gast.2000.8523. [DOI] [PubMed] [Google Scholar]
- 3.Vermeire S, Van Assche G, Rutgeerts P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis. 2004;10(5):661–665. doi: 10.1097/00054725-200409000-00026. Available from: http://dx.doi.org/10.1097/00054725-200409000-00026. [DOI] [PubMed] [Google Scholar]
- 4.Castell JV, Gómez-Lechón MJ, David M, Fabra R, Trullenque R, Heinrich PC. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology. 1990;12(5):1179–1186. doi: 10.1002/hep.1840120517. Available from: http://dx.doi.org/10.1002/hep.1840120517. [DOI] [PubMed] [Google Scholar]
- 5.Prantera C, Davoli M, Lorenzetti R, Pallone F, Marcheggiano A, Iannoni C, Mariotti S. Clinical and laboratory indicators of extent of ulcerative colitis. Serum C-reactive protein helps the most. J Clin Gastroenterol. 1988;10(1):41–45. doi: 10.1097/00004836-198802000-00010. Available from: http://dx.doi.org/10.1097/00004836-198802000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Solem CA, Loftus EV, Jr, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11(8):707–712. doi: 10.1097/01.MIB.0000173271.18319.53. Available from: http://dx.doi.org/10.1097/01.MIB.0000173271.18319.53. [DOI] [PubMed] [Google Scholar]
- 7.Chouhan S, Gahlot S, Pokharna RK, Mathur KC, Saini K, Pal M. Severity and extent of ulcerative colitis: role of C-reactive protein. Indian J Gastroenterol. 2006;25(1):46–47. [PubMed] [Google Scholar]
- 8.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805–1812. doi: 10.1172/JCI18921. Available from: http://dx.doi.org/10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bischoff SC, Grabowsky J, Manns MP. Quantification of inflammatory mediators in stool samples of patients with inflammatory bowel disorders and controls. Dig Dis Sci. 1997;42(2):394–403. doi: 10.1023/A:1018886423475. Available from: http://dx.doi.org/10.1023/A:1018886423475. [DOI] [PubMed] [Google Scholar]
- 10.Riley SA, Mani V, Goodman MJ, Dutt S, Herd ME. Microscopic activity in ulcerative colitis: what does it mean? Gut. 1991;32(2):174–178. doi: 10.1136/gut.32.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Modigliani R. Endoscopic management of inflammatory bowel disease. Am J Gastroenterol. 1994;89(8 Suppl):S53–S65. [PubMed] [Google Scholar]
- 12.Niederau C, Backmerhoff F, Schumacher B, Niederau C. Inflammatory mediators and acute phase proteins in patients with Crohn's disease and ulcerative colitis. Hepatogastroenterology. 1997;44(13):90–107. [PubMed] [Google Scholar]
- 13.Sugi K, Saitoh O, Hirata I, Katsu K. Fecal lactoferrin as a marker for disease activity in inflammatory bowel disease: comparison with other neutrophil-derived proteins. Am J Gastroenterol. 1996;91(5):927–934. [PubMed] [Google Scholar]
- 14.van der Sluys Veer A, Biemond I, Verspaget HW, Lamers CB. Faecal parameters in the assessment of activity in inflammatory bowel disease. Scand J Gastroenterol Suppl. 1999;230:106–110. doi: 10.1080/003655299750025624. Available from: http://dx.doi.org/10.1080/003655299750025624. [DOI] [PubMed] [Google Scholar]
- 15.Williams JG, Hughes LE, Hallett MB. Toxic oxygen metabolite production by circulating phagocytic cells in inflammatory bowel disease. Gut. 1990;31(2):187–193. doi: 10.1136/gut.31.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87(6):1344–1350. [PubMed] [Google Scholar]
- 17.Weissmann G, Smolen JE, Korchak HM. Release of inflammatory mediators from stimulated neutrophils. N Engl J Med. 1980;303(1):27–34. doi: 10.1056/NEJM198007033030109. Available from: http://dx.doi.org/10.1056/NEJM198007033030109. [DOI] [PubMed] [Google Scholar]
- 18.Raab Y, Hällgren R, Knutson L, Krog M, Gerdin B. A technique for segmental rectal and colonic perfusion in humans. Am J Gastroenterol. 1992;87(10):1453–1459. [PubMed] [Google Scholar]
- 19.Bustos D, Greco G, Yapur V, Facente A, Di Carlo M, Bustos F, Dapia L, Ascione A, Negri G. Quantification of fecal neutrophils by MPO determination (myeloperoxidase) in patients with invasive diarrhea. Acta Gastroenterol Latinoam. 2000;30(2):85–87. [PubMed] [Google Scholar]
- 20.Saiki T. Myeloperoxidase concentrations in the stool as a new parameter of inflammatory bowel disease. Kurume Med J. 1998;45(1):69–73. doi: 10.2739/kurumemedj.45.69. [DOI] [PubMed] [Google Scholar]
- 21.Sangfelt P, Carlson M, Thörn M, Lööf L, Raab Y. Neutrophil and eosinophil granule proteins as markers of response to local prednisolone treatment in distal ulcerative colitis and proctitis. Am J Gastroenterol. 2001;96(4):1085–1090. doi: 10.1111/j.1572-0241.2001.03743.x. Available from: http://dx.doi.org/10.1111/j.1572-0241.2001.03743.x. [DOI] [PubMed] [Google Scholar]
- 22.Uchida K, Matsuse R, Tomita S, Sugi K, Saitoh O, Ohshiba S. Immunochemical detection of human lactoferrin in feces as a new marker for inflammatory gastrointestinal disorders and colon cancer. Clin Biochem. 1994;27(4):259–264. doi: 10.1016/0009-9120(94)90027-2. Available from: http://dx.doi.org/10.1016/0009-9120(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 23.Langhorst J, Elsenbruch S, Mueller T, Rueffer A, Spahn G, Michalsen A, Dobos GJ. Comparison of 4 neutrophil-derived proteins in feces as indicators of disease activity in ulcerative colitis. Inflamm Bowel Dis. 2005;11(12):1085–1091. doi: 10.1097/01.MIB.0000187980.08686.18. Available from: http://dx.doi.org/10.1097/01.MIB.0000187980.08686.18. [DOI] [PubMed] [Google Scholar]
- 24.Kane SV, Sandborn WJ, Rufo PA, Zholudev A, Boone J, Lyerly D, Camilleri M, Hanauer SB. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am J Gastroenterol. 2003;98(6):1309–1314. doi: 10.1111/j.1572-0241.2003.07458.x. Available from: http://dx.doi.org/10.1111/j.1572-0241.2003.07458.x. [DOI] [PubMed] [Google Scholar]
- 25.Steinbakk M, Naess-Andresen CF, Lingaas E, Dale I, Brandtzaeg P, Fagerhol MK. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet. 1990;336(8718):763–765. doi: 10.1016/0140-6736(90)93237-J. Available from: http://dx.doi.org/10.1016/0140-6736(90)93237-J. [DOI] [PubMed] [Google Scholar]
- 26.Erbayrak M, Turkay C, Eraslan E, Cetinkaya H, Kasapoglu B, Bektas M. The role of fecal calprotectin in investigating inflammatory bowel diseases. Clinics (Sao Paulo) 2009;64(5):421–425. doi: 10.1590/S1807-59322009000500009. Available from: http://dx.doi.org/10.1590/S1807-59322009000500009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoepfer AM, Beglinger C, Straumann A, Trummler M, Renzulli P, Seibold F. Ulcerative colitis: Correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis. 2009;15(12):1851–1858. doi: 10.1002/ibd.20986. Available from: http://dx.doi.org/10.1002/ibd.20986. [DOI] [PubMed] [Google Scholar]
- 28.Gisbert JP, Bermejo F, Pérez-Calle JL, Taxonera C, Vera I, McNicholl AG, Algaba A, López P, López-Palacios N, Calvo M, González-Lama Y, Carneros JA, Velasco M, Maté J. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm Bowel Dis. 2009 Aug;15(8):1190–1198. doi: 10.1002/ibd.20933. Available from: http://dx.doi.org/10.1002/ibd.20933. [DOI] [PubMed] [Google Scholar]
- 29.D'Incà R, Dal Pont E, Di Leo V, Ferronato A, Fries W, Vettorato MG, Martines D, Sturniolo GC. Calprotectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int J Colorectal Dis. 2007;22(4):429–437. doi: 10.1007/s00384-006-0159-9. Available from: http://dx.doi.org/10.1007/s00384-006-0159-9. [DOI] [PubMed] [Google Scholar]
- 30.Masoodi I, Kochhar R, Dutta U, Vaishnavi C, Prasad KK, Vaiphei K, Kaur S, Singh K. Fecal lactoferrin, myeloperoxidase and serum C-reactive are effective biomarkers in the assessment of disease activity and severity in patients with idiopathic ulcerative colitis. J Gastroenterol Hepatol. 2009;24(11):1768–1774. doi: 10.1111/j.1440-1746.2009.06048.x. Available from: http://dx.doi.org/10.1111/j.1440-1746.2009.06048.x. [DOI] [PubMed] [Google Scholar]
- 31.Burakoff R, Chao S, Perencevich M, Ying J, Friedman S, Makrauer F, Odze R, Khurana H, Liew CC. Blood-based biomarkers can differentiate ulcerative colitis from crohn's disease and noninflammatory diarrhea. Inflamm Bowel Dis. 2011 Jan 6; doi: 10.1002/ibd.21574. Available from: http://dx.doi.org/10.1002/ibd.21574. [DOI] [PubMed] [Google Scholar]
- 32.Talero E, Sánchez-Fidalgo S, Villegas I, de la Lastra CA, Illanes M, Motilva V. Role of different inflammatory and tumor biomarkers in the development of ulcerative colitis-associated carcinogenesis. Inflamm Bowel Dis. 2010 Aug 18; doi: 10.1002/ibd.21420. Available from: http://dx.doi.org/10.1002/ibd.21420. [DOI] [PubMed] [Google Scholar]