Abstract
As lipofilling of the female breast is becoming more popular in plastic surgery, the use of MRI to assess breast volume has been employed to control postoperative results. Therefore, we sought to evaluate the accuracy of magnetic resonance imaging (MRI)-based breast volumetry software tools by comparing the measurements of silicone implant augmented breasts with the actual implant volume specified by the manufacturer. MRI-based volume analysis was performed in eight bilaterally augmented patients (46 ± 9 years) with three different software programs (Brainlab© I plan 2.6 neuronavigation software; mass analysis, version 5.3, Medis©; and OsiriX© v.3.0.2. 32-bit). The implant volumes analysed by the BrainLab© software had a mean deviation of 2.2 ± 1.7% (r = 0.99) relative to the implanted prosthesis. OsiriX© software analysis resulted in a mean deviation of 2.8 ± 3.0% (r = 0.99) and the Medis© software had a mean deviation of 3.1 ± 3.0% (r = 0.99). Overall, the volumes of all analysed breast implants correlated very well with the real implant volumes. Processing time was 10 min per breast with each system and 30 s (OsiriX©) to 5 min (BrainLab© and Medis©) per silicone implant. MRI-based volumetry is a powerful tool to calculate both native breast and silicone implant volume in situ. All software solutions performed well and the measurements were close to the actual implant sizes. The use of MRI breast volumetry may be helpful in: (1) planning reconstructive and aesthetic surgery of asymmetric breasts, (2) calculating implant size in patients with missing documentation of a previously implanted device and (3) assessing post-operative results objectively.
Key words: MRI, volumetry, mamma, breast, lipofilling, silicone implant, BrainLab, OsiriX, Medis
Introduction
Breast volume assessment is not routinely performed prior to breast surgery; however, it may be helpful in optimizing the postoperative outcomes1. As symmetry of breasts is one main objective in aesthetic and reconstructive breast surgery, having a simple tool to assess breast volume is desirable. Indeed, in pathological conditions with substantial asymmetry, for example Poland syndrome or following mastectomies, the calculation of breast size and volume differences preoperatively, offers a better chance for more symmetric breasts, postoperatively. Furthermore, lipofilling for breast modification highlights another clinical indication for volume analysis of the breast as a method is needed to evaluate the remaining fat tissue during routine follow-up.
Various methods used to measure breast volume have been previously described2,3. The anthropometric method is based on the end-to-end measurement of the thorax. The breast is visualised as half of an ellipse and the necessary parameters, according to the mathematical formula, are measured directly on the patient or from a photograph4,5. Alternatively, similar measurements can be obtained from mammographies6,7. Thermoplastic sheets may be used to generate a three-dimensional cast of the breast in order to determine breast volumes8,9. Breast volume can also be assessed using the displaced water technique, whereby the patient bends over a water-filled container and the displaced water is collected3,10. In addition, a non-invasive, three-dimensional body surface imaging capability is available12. Advantages of this technique include the quantitative evaluation of symmetry, volume, shape, contour, surface and length1,11–13. Finally, widely available radiological procedures, for example computer tomography (CT) and magnetic resonance imaging (MRI) are accepted diagnostic and volumetric tools for the female breast14–20. Apart from contraindications, i.e. pacemaker or claustrophobia, breast MRI is a safe, non-invasive and non-irradiative method to obtain substantial information on the breast.
In addition to MRI as a diagnostic tool, volume assessment through MRI imaging has been described before21. In breast volumetry, the border between mammary tissue and the thoracic wall can be easily determined with axial slices. Furthermore, calculating volumes of other organs, tissue-types and brain regions with MRI is possible due to the unique densities of these tissues22,23.
With respect to the volume calculation, demarcation only of the lesion or area to be assessed is necessary. Furthermore, DICOM data sets consisting of MRI data of the breast can be processed in a similar way as cranial MRI data with Brainlab© I plan 2.6 neuronavigation software. Modern radiological image processing systems including Mass analysis, version 5.3, Medis© also contain a volume tool. Similarly, computer software not limited to hospital use, for example OsiriX©, offers another alternative for volume calculation.
Breast MRI is a powerful diagnostic tool and is currently considered the gold standard for diagnosing breast implant rupture17. Recently, this method has become more integrated into radiographical diagnostics of the breast. While the use of MRI for the assessment of breast volume is appealing, it remains equivocal whether the analysis is both simple and convenient. To this point, the study presented here, evaluates breast volume measurements using three commonly used MRI software packages. To validate the calculations, MRI data of augmented breasts were compared to the computed volume analysis with respect to the real implant dimensions.
Material and Methods
Patients
Eight healthy female patients who had previously received silicone implant based breast augmentation were included in the study. MRI examinations of both breasts were already available. There were no radiological implications for an implant rupture in any of the 16 breast implants. Retrospectively, a volume analysis was performed. Mean age of the patients was 46 ± 9 years at time of examination. IRB approval was obtained by the ethics committee of Hannover Medical University before beginning any research.
MRI
MRI data was available digitally. Examinations were performed using a 1.5 T clinical whole body scanner (Sigma Advantage Echo Speed, GE Health Care, Milwaukee, WI, USA).
The patient was placed in a prone position with a standard breast case to prevent compression. A standard, four-channel receiver breast coil was used in all cases. Images were acquired with a standard IR-GRE sequence shimmed to the silicon peak to enable silicon selective imaging. Sequence parameters were as follows: TR/TE 5,000/100 ms; TI: 150 ms; field of view 37 × 37 cm; matrix 512 × 256; section thickness 4.0 mm without intersection gap. Examinations covered the entire breast in an axial scan direction. No intravascular contrast agent was used.
Volume Analysis
All volume analyses were performed with three different software programs. Examiners were blinded to the implant size prior to performing the volume analysis.
Brainlab© I plan 2.6 neuronavigation software was installed on a Windows computer. The regular preoperative neuronavigational planning process was abbreviated for our use and object marking and volume analysis was used. Overall system costs including hardware, i.e. infrared scanners, computers, in addition to neurosurgical specific instruments, totals approximately $500,000. Brainlab© I plan 2.6 neuronavigation software adds the chosen voxels and uses, due to information provided by the company`s customer support, the algorithm of Cavalieri between the sections and in border areas for segmentation and volume measurement.
Mass analysis, version 5.3, Medis© is a professional medical segmentation software package currently used by our hospital’s radiology department. Software cost is approximately $20,000. The Simpsons`s rule is the algorithm used to calculate the volume, due to information provided by the company`s customer support.
OsiriX© v.3.0.2. 32-bit is an image processing software program used specifically with DICOM images generated from the following medical instruments: MRI, CT, positron emission tomography (PET), PET-CT. It is typically installed on Apple computers and is free of charge under the GNU open-source licensing agreement. It is only approved for scientific use under American and European law and not for clinical use, as there are no FDA/CE-1 certifications. The same software is available for clinical use in Europe at a cost of 89 Euro a month inclusive support by Aycan Digitalsysteme GmbH, Würzburg, Germany. OsiriX© v.3.0.2 uses the Power Crust algorithm due to the customer support. General information about the software is to be found in a publication by Rosset et al.24.
Historical MRI data from patients were uploaded from the DICOM data saved on CD Rom or PACS system. Figures 1, 2 and 3 show the process of marking the borders of the breast implant. The implant itself was easy to outline and differentiate from breast tissue due to an implant specific intensity on the MRI. The breast itself was determined to include areola, nipple and skin on the surface as the superior border and to lie above the pectoral muscle, as the internal border. The medial border parasternal was identified, and the lateral border was defined at the lateral thorax wall, where the subcutaneous fat of the breast reached the same height as the subcutaneous fat of the thorax wall. From this point, a straight line was drawn radial to the pectoral muscle.
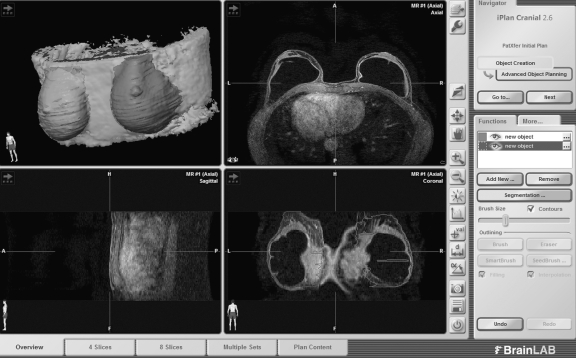
Fig 1.
MRI breast volumetry using Brainlab© I plan 2.6 neuronavigation software, installed on a Windows computer. The entire augmented breast with the elliptical silicone implant in situ is traced. The upper left window contains a 3D reconstruction.
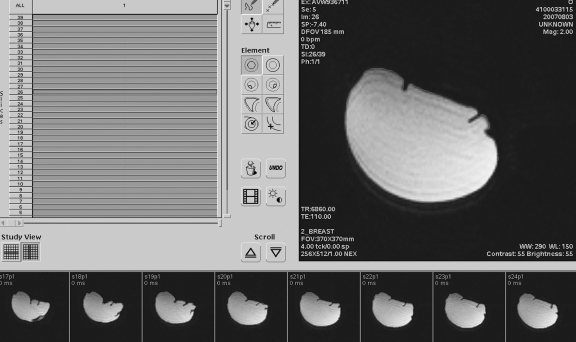
Fig 2.
MRI breast volumetry using Mass analysis, version 5.3, Medis©. The silicon breast implant (elliptical area) is traced onto axial slices.
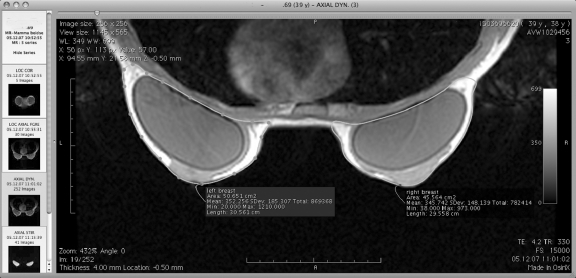
Fig 3.
MRI breast volumetry using OsiriX© v.3.0.2. 32-bit. The breasts with the elliptical silicone implant inside are traced on a bilateral axial slice. After marking all slices, the volume is calculated by the software.
Statistical Analyses
Statistical Analysis was performed using the SPSS standard software package (version 15; SPSS Inc., Chicago, IL, USA). Statistical differences were determined using the Pearson’s correlation test and the Greenhouse–Geisser correlation test as a general linear model for repeated measurements. This test measures the differences between measurements, in order to assess the within subject variability. A p value of <0.05 was considered significant.
Results
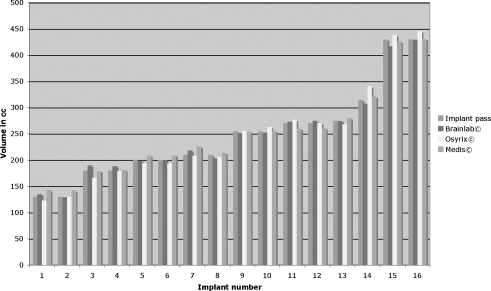
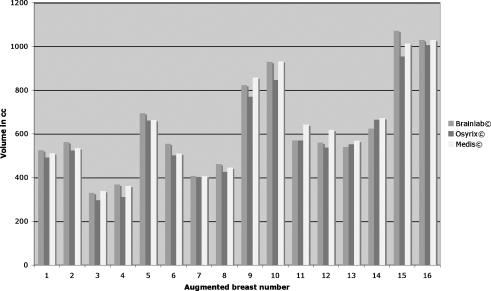
All eight patients had previously undergone a bilateral breast augmentation. Seven patients received implants of equal size (130–430 cc) and one patient received a 275 cc implant on the left and a 315 cc implant on the right side (mean implant volume 246 ± 88 cc). Figure 4 depicts the volume analysis of the implants placed in order of size. All 16 silicon implants were intact.
Fig 4.
Implant volumes determined by all three software packages were positively correlated with the volumes indicated in the official implant dimensions (Pearson correlation coefficient r = 0.99). No differences were observed between the different software tools (p = 0.516).
The mean implant volume analysed by the BrainLab© software was 246 ± 85 cc with a mean measurement deviation of 2.2 ± 1.7%. Analysis using OsiriX© software resulted in a mean implant volume of 248 ± 95 cc with a mean deviation of 2.8 ± 3.0%. Mean implant volume determined by Medis© software was 249 ± 85 cc. These measurements had a mean deviation of 3.1 ± 3.0%.
The volumes of all analysed breast implants correlated well with the volumes specified by the manufacturers. Pearson correlation coefficients calculated for the given implant size were r = 0.99 (p < 0.05) for volume analysis using all three software packages. No volume differences among the different software packages (p = 0.516) were observed, suggesting that all three can be useful tools for studying breast volumetry. Average time for volume analysis of each breast implant was 5 min with BrainLab© and Medis© software programs and 30 s with the OsiriX© software package.
The same three software packages were used to determine the volume of the entire breast, including implant (Fig. 5). The average volumes of all analysed breasts were 594 ± 211 cc (OsiriX©), 627 ± 225 cc (BrainLab©) and 631 ± 221 cc (Medis©). Similar to the data reported above, Pearson correlation coefficients for all analyses were r = 0.99 (p < 0.05). Average time for each breast volume analysis with all three systems was 10 min. In whole breasts there is no uniform intensity like in silicone implants, so OsiriX© is not faster than the other software in calculating the volume of whole breasts.
Fig 5.
Correlation of breast volume. Pearson correlation coefficient of all analyses was r = 0.99.
Discussion
The study described herein evaluated the feasibility of MRI-based evaluation of breast volume on augmented breasts. The three different software tools assessed, currently used for other medical applications, offer a relatively simple and fast method for the surgeon, while being convenient and safe for the patient.
Our study design offered us the possibility to correlate the calculated volume of the implant in situ, to the actual implant volume. Whereas other studies, measuring breast volume, only compared calculations obtained from different methods. In addition, the whole breast was also analysed to demonstrate the applicability of this method for its original intention—volume analysis of the human breast.
We evaluated both the accuracy of the software-generated volume to the actual implant volume, in addition to the precision of the three different software packages in calculating silicone implant and total breast volumes. Accuracy with respect to the known implant volumes was encouraging: mean deviations were 2.2% with the BrainLab© software, 2.8% with the OsiriX© software and 3.1% with the Medis© software. Furthermore, serial measurements of both the implants and the entire breast using the different software packages showed significant precision.
The most accurate software program was BrainLab©, although all packages performed almost equally well. Purchasing cost is the main disadvantage of the BrainLab© software package; however, it is a commonly used system in neurosurgical departments so access, and thus cost may not be limiting. Data obtained from Medis© software analysis were also accurate with respect to the known implant volumes. In addition, the software cost is affordable and can be found in most radiology departments.
Analysis of the MRI data using all three systems was quick (approximately 10 min/breast), in addition to a 5 min analysis per implant with Medis© and BrainLab©. OsiriX© offers an integrated tool for marking regions of the same intensity in MRI images, and consequently, was the fastest software for silicone implant volumetry of the three (<30 s/implant). An advantage of this program, in addition to the analytic speed, is the free, open access version. Despite this, it has not yet been legally approved for clinical use in the USA and Europe.
Practicality and reproducibility are limiting factors of the different methods currently used for breast volume measurement3. Anthropomorphic methods are based on individual measurements employing end-to-end values and geometrical shapes that do not always correlate to the human anatomy. Furthermore, although not cost intensive, the mean volume deviation for this method is reported as 6.26%2. Thermoplastic casts are inflexible and lead to the compression of the breast against the thorax wall. While patient discomfort is subjective, the objective difficulties, i.e. filling the forms after production with water or sand, misrepresent the convex nature of the thorax. Furthermore, resource requirements, including material costs ($300/patient), time and manpower, in addition to an average mean volume deviation of 7.97%, do not promote this technique2. Archimedean methods of water displacement are relatively simple and do not generate costs, but require the patients to conform to standard procedures, making measurement precision and reproducibility a challenge. Non-invasive 3D-body scanning is fast; however, the analysis of the scan is time intensive and only the surface of the breast can be visualised25. There is a wide range of 3D-body scanning systems available which differ enormously in quality and cost2, single camera systems are available from $20,000 to $100,00026. The running costs are depending on the software license.
The advantage of using MRI for breast volumetry stems from the fact that MRI is a powerful diagnostic tool. At our hospital costs of a mamma MRI is round about $280 each patient. The ability to utilise all images, even at different times, adds to the cost effectiveness of this method. In contrast to 3D scanning in combination with mammography of the breast where multiple hospital visits may be necessary, MRI allows for both the examination and evaluation of the breast in the same exam. This not only benefits the physician with respect to time, but also the patient. Furthermore and in addition to decreasing costs associated with MRI, MRI equipment is available in most hospitals, including the analytical software designed for clinical use.
In conclusion, the results of this study are encouraging and are in line with previously reported data using this technique2,20. Ultimately, MRI of the breast offers information regarding breast tissue without exposing the patient to radiation and/or uncomfortable examination techniques as with the aforementioned methods.
Limitations
As with other techniques, subjectivity of the program user, may confound the data obtained. To this point, tracing of the implant on the axial slices using volumetric software is still dependent on the accuracy of the examiner. Despite this, silicone can be differentiated from native breast tissue quite easily on MRI images and implants, making this a minor problem in MRI volume assessment of the implant. In contrast, definition of the entire breast can be rather subjective. Careful tracing of both the right and left breast in the same way, utilizing the same anatomic landmarks, may help to maximise accuracy.
Breast MRI is not routinely performed during regular appointments and so the number of patients with MRI examinations is small in comparison to patients undergoing mammographic examination. Therefore, the number of MRI examinations available and appropriate for this study was limited, leading to the non-randomised study design. However, these data are an encouraging foundation for future MRI-based volumetry studies, which in turn, will help determine the clinical benefit of MRI-based breast volumetry.
Conclusion
As presented in this paper, MRI-based breast volumetry is simple and convenient. The comparison of three different software tools resulted in a positive correlation, with low measurement deviations, between actual implant volume and calculated volume. The utility of MRI-based breast volumetry is especially highlighted in the planning of breast surgery, specifically the reconstructive and aesthetic surgery of asymmetric breasts. Furthermore, in the absence of original operative notes, MRI-based breast volumetry may provide pertinent information. In the emerging field of breast augmentation and reconstruction with lipofilling or hyaluronic acid, this method offers an objective tool for initial operative planning and post-operative follow-up, to assess volume and composition of breast tissue, in situ, in one step.
Acknowledgements
We would like to thank Suzanne Dorfman Ph.D. for editing the manuscript.
Footnotes
The current “Guide for Authors” has been read.
The authors have seen and agreed to the submitted version of the paper, and bear responsibility for it.
All who have been acknowledged as contributors or as providers of personal communications have agreed to their inclusion.
The material is original; and it has been neither published elsewhere nor submitted for publication simultaneously.
The paper will not be published elsewhere in the same or similar form, in English or in any other language, without written consent of the copyright holder.
There have been no financial supports.
References
- 1.Kovacs L, Zimmermann A, Papadopulos NA, et al. Re: Factors determining shape and symmetry in immediate breast reconstruction. Ann Plast Surg. 2004;53:192–194. doi: 10.1097/01.sap.0000132572.72432.74. [DOI] [PubMed] [Google Scholar]
- 2.Kovacs L, Eder M, Hollweck R, et al. Comparison between breast volume measurement using 3D surface imaging and classical techniques. Breast (Edinburgh, Scotland) 2007;16:137–145. doi: 10.1016/j.breast.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Bulstrode N, Bellamy E, Shrotria S. Breast volume assessment: Comparing five different techniques. Breast (Edinburgh, Scotland) 2001;10:117–123. doi: 10.1054/brst.2000.0196. [DOI] [PubMed] [Google Scholar]
- 4.Brown RW, Cheng YC, Kurtay M. A formula for surgical modifications of the breast. Plast Reconstr Surg. 2000;106:1342–1345. doi: 10.1097/00006534-200011000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Qiao Q, Zhou G, Ling Y. Breast volume measurement in young Chinese women and clinical applications. Aesthetic Plast Surg. 1997;21:362–368. doi: 10.1007/s002669900139. [DOI] [PubMed] [Google Scholar]
- 6.Kalbhen CL, McGill JJ, Fendley PM, et al. Mammographic determination of breast volume: Comparing different methods. AJR Am J Roentgenol. 1999;173:1643–1649. doi: 10.2214/ajr.173.6.10584814. [DOI] [PubMed] [Google Scholar]
- 7.Malini S, Smith EO, Goldzieher JW. Measurement of breast volume by ultrasound during normal menstrual cycles and with oral contraceptive use. Obstet Gynecol. 1985;66:538–541. [PubMed] [Google Scholar]
- 8.Campaigne BN, Katch VL, Freedson P, et al. Measurement of breast volume in females: Description of a reliable method. Ann Hum Biol. 1979;6:363–367. doi: 10.1080/03014467900003741. [DOI] [PubMed] [Google Scholar]
- 9.Edsander-Nord A, Wickman M, Jurell G. Measurement of breast volume with thermoplastic casts. Scand J Plast Reconstr Surg Hand Surg. 1996;30:129–132. doi: 10.3109/02844319609056394. [DOI] [PubMed] [Google Scholar]
- 10.Schultz RC, Dolezal RF, Nolan J. Further applications of Archimedes' principle in the correction of asymmetrical breasts. Ann Plast Surg. 1986;16:98–101. doi: 10.1097/00000637-198602000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Galdino GM, Nahabedian M, Chiaramonte M, et al. Clinical applications of three-dimensional photography in breast surgery. Plast Reconstr Surg. 2002;110:58–70. doi: 10.1097/00006534-200207000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Losken A, Fishman I, Denson DD, et al. An objective evaluation of breast symmetry and shape differences using 3-dimensional images. Ann Plast Surg. 2005;55:571–575. doi: 10.1097/01.sap.0000185459.49434.5f. [DOI] [PubMed] [Google Scholar]
- 13.Nahabedian MY, Galdino G. Symmetrical breast reconstruction: Is there a role for three-dimensional digital photography. Plast Reconstr Surg. 2003;112:1582–1590. doi: 10.1097/01.PRS.0000085818.54980.C4. [DOI] [PubMed] [Google Scholar]
- 14.Bartella L, Smith CS, Dershaw DD, et al. Imaging breast cancer. Radiol Clin North Am. 2007;45:45–67. doi: 10.1016/j.rcl.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Blair S, McElroy M, Middleton MS, et al. The efficacy of breast MRI in predicting breast conservation therapy. J Surg Oncol. 2006;94:220–225. doi: 10.1002/jso.20561. [DOI] [PubMed] [Google Scholar]
- 16.DeMartini W, Lehman C, Partridge S. Breast MRI for cancer detection and characterization: A review of evidence-based clinical applications. Acad Radiol. 2008;15:408–416. doi: 10.1016/j.acra.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Gorczyca DP, Gorczyca SM, Gorczyca KL. The diagnosis of silicone breast implant rupture. Plast Reconstr Surg. 2007;120:49S–61S. doi: 10.1097/01.prs.0000286569.45745.6a. [DOI] [PubMed] [Google Scholar]
- 18.Planche K, Vinnicombe S. Breast imaging in the new era. Cancer Imaging. 2004;4:39–50. doi: 10.1102/1470-7330.2003.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tozaki M. Diagnosis of breast cancer: MDCT versus MRI. Breast Cancer. 2008;15:205–211. doi: 10.1007/s12282-008-0049-9. [DOI] [PubMed] [Google Scholar]
- 20.Fowler PA, Casey CE, Cameron GG, et al. Cyclic changes in composition and volume of the breast during the menstrual cycle, measured by magnetic resonance imaging. Br J Obstet Gynaecol. 1990;97:595–602. doi: 10.1111/j.1471-0528.1990.tb02546.x. [DOI] [PubMed] [Google Scholar]
- 21.Mineyev M, Kramer D, Kaufman L, et al. Measurement of breast implant volume with magnetic resonance imaging. Ann Plast Surg. 1995;34:348–351. doi: 10.1097/00000637-199504000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Bergin PS, Raymond AA, Free SL, et al. Magnetic resonance volumetry. Neurology. 1994;44:1770–1771. doi: 10.1212/wnl.44.9.1770-b. [DOI] [PubMed] [Google Scholar]
- 23.Kruggel F. MRI-based volumetry of head compartments: Normative values of healthy adults. Neuroimage. 2006;30:1–11. doi: 10.1016/j.neuroimage.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 24.Rosset A, Spadola L, Ratib O. OsiriX: An open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17:205–216. doi: 10.1007/s10278-004-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovacs L, Yassouridis A, Zimmermann A, et al. Optimization of 3-dimensional imaging of the breast region with 3-dimensional laser scanners. Ann Plast Surg. 2006;56:229–236. doi: 10.1097/01.sap.0000197774.80832.24. [DOI] [PubMed] [Google Scholar]
- 26.Tepper OM, Choi M, Small K, et al. An innovative three-dimensional approach to defining the anatomical changes occurring after short scar-medial pedicle reduction mammaplasty. Plast Reconstr Surg. 2008;121:1875–1885. doi: 10.1097/PRS.0b013e31817151db. [DOI] [PubMed] [Google Scholar]