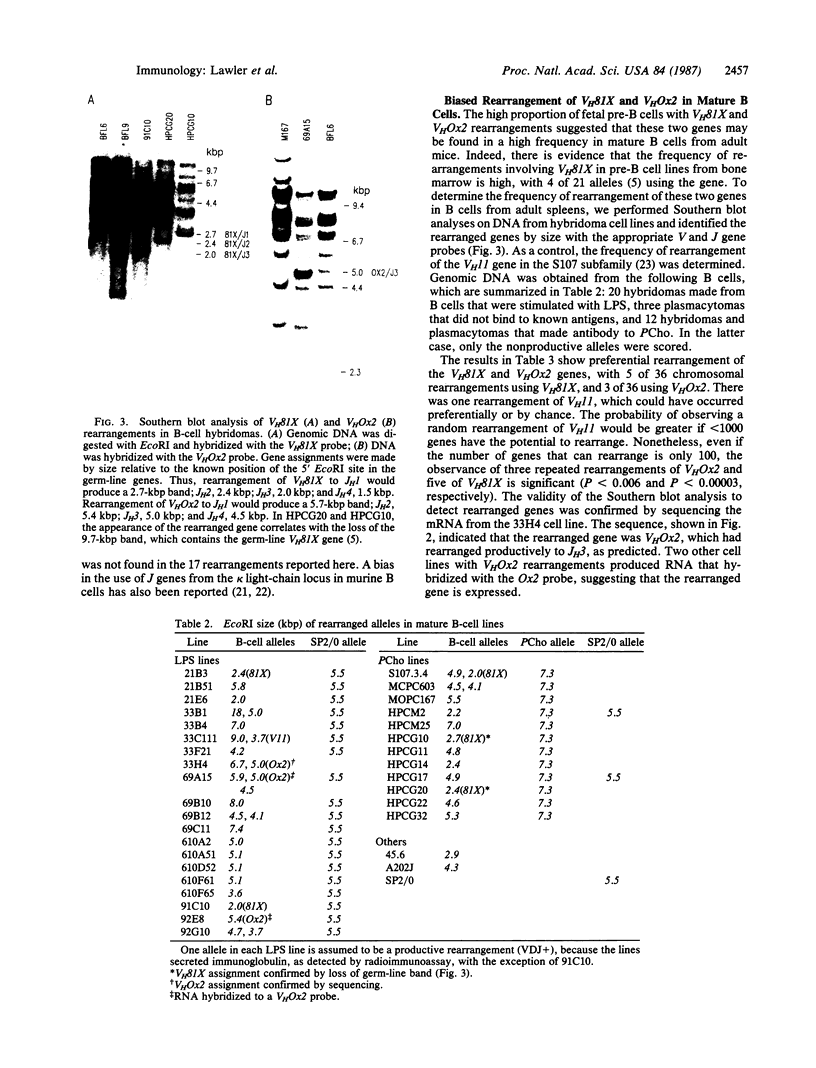
Abstract
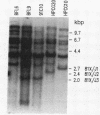
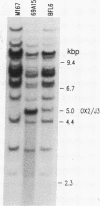
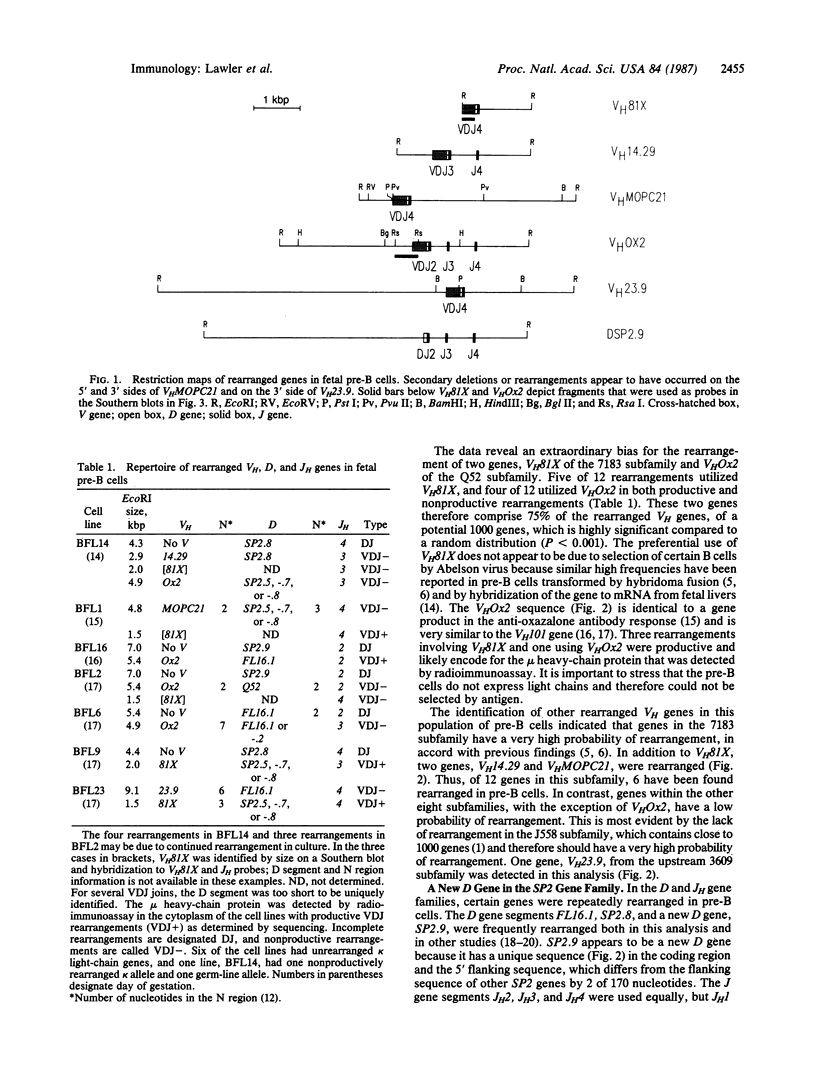
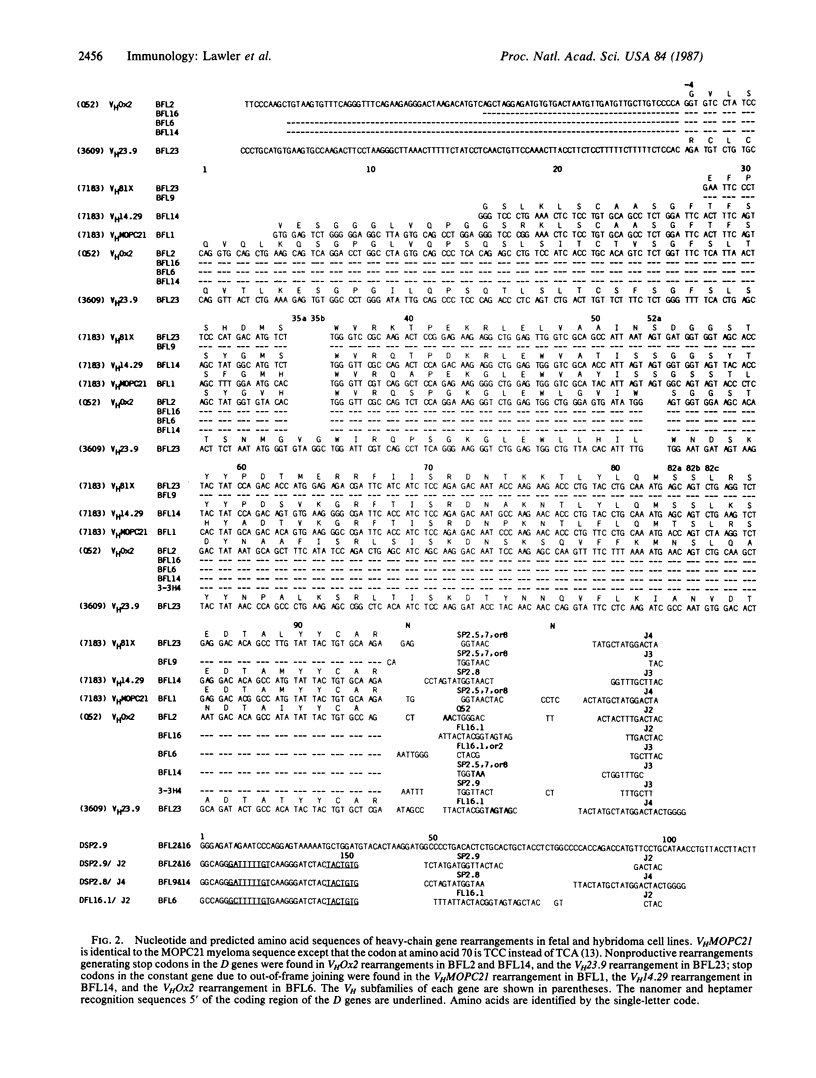
Fetal pre-B cells rearrange a very restricted set of immunoglobulin variable genes for the heavy chain (VH). To determine whether the adult B-cell repertoire is similarly skewed, we first identified the genes that rearrange in pre-B cells from BALB/c mice and then determined their frequency of rearrangement in adult B cells. In fetal pre-B cell lines, two genes, VH81X from the 7183 subfamily and VHOx2 from the Q52 subfamily, comprise 75% of the rearranged alleles of an estimated 1000 genes (P less than 0.001). Sequencing analyses revealed that rearrangements involving the two genes were both productive and nonproductive. The biased rearrangement of these two VH genes persists in B-cell hybridomas from adult mice at a frequency of 22%, as determined by Southern gel analysis and RNA sequencing. The sequence of one VHOx2 rearrangement from a hybridoma shows that the rearrangement is productive, suggesting that the gene encodes an antibody that could participate in the immune response. The data indicate that the adult B-cell repertoire is not random concerning usage of individual VH genes, and it may be shaped by the unknown mechanisms that cause preferential rearrangement of certain genes early in ontogeny.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F. W., Yancopoulos G. D., Blackwell T. K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984 Jun;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Brodeur P. H., Riblet R. The immunoglobulin heavy chain variable region (Igh-V) locus in the mouse. I. One hundred Igh-V genes comprise seven families of homologous genes. Eur J Immunol. 1984 Oct;14(10):922–930. doi: 10.1002/eji.1830141012. [DOI] [PubMed] [Google Scholar]
- Crews S., Griffin J., Huang H., Calame K., Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981 Jul;25(1):59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- Desiderio S. V., Yancopoulos G. D., Paskind M., Thomas E., Boss M. A., Landau N., Alt F. W., Baltimore D. Insertion of N regions into heavy-chain genes is correlated with expression of terminal deoxytransferase in B cells. Nature. 1984 Oct 25;311(5988):752–755. doi: 10.1038/311752a0. [DOI] [PubMed] [Google Scholar]
- Dildrop R., Krawinkel U., Winter E., Rajewsky K. VH-gene expression in murine lipopolysaccharide blasts distributes over the nine known VH-gene groups and may be random. Eur J Immunol. 1985 Nov;15(11):1154–1156. doi: 10.1002/eji.1830151117. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gearhart P. J., Johnson N. D., Douglas R., Hood L. IgG antibodies to phosphorylcholine exhibit more diversity than their IgM counterparts. Nature. 1981 May 7;291(5810):29–34. doi: 10.1038/291029a0. [DOI] [PubMed] [Google Scholar]
- Hagiya M., Davis D. D., Takahashi T., Okuda K., Raschke W. C., Sakano H. Two types of immunoglobulin-negative Abelson murine leukemia virus-transformed cells: implications for B-lymphocyte differentiation. Proc Natl Acad Sci U S A. 1986 Jan;83(1):145–149. doi: 10.1073/pnas.83.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlyn P. H., Browniee G. G., Cheng C. C., Gait M. J., Milstein C. Complete sequence of constant and 3' noncoding regions of an immunoglobulin mRNA using the dideoxynucleotide method of RNA sequencing. Cell. 1978 Nov;15(3):1067–1075. doi: 10.1016/0092-8674(78)90290-8. [DOI] [PubMed] [Google Scholar]
- Kaartinen M., Griffiths G. M., Markham A. F., Milstein C. mRNA sequences define an unusually restricted IgG response to 2-phenyloxazolone and its early diversification. 1983 Jul 28-Aug 3Nature. 304(5924):320–324. doi: 10.1038/304320a0. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Nikaido T., Miyata T., Moriwaki K., Honjo T. The nucleotide sequences of rearranged and germline immunoglobulin VH genes of a mouse myeloma MC101 and evolution of VH genes in mouse. J Biol Chem. 1982 Jan 10;257(1):277–285. [PubMed] [Google Scholar]
- Kleinfield R., Hardy R. R., Tarlinton D., Dangl J., Herzenberg L. A., Weigert M. Recombination between an expressed immunoglobulin heavy-chain gene and a germline variable gene segment in a Ly 1+ B-cell lymphoma. 1986 Aug 28-Sep 3Nature. 322(6082):843–846. doi: 10.1038/322843a0. [DOI] [PubMed] [Google Scholar]
- Livant D., Blatt C., Hood L. One heavy chain variable region gene segment subfamily in the BALB/c mouse contains 500-1000 or more members. Cell. 1986 Nov 7;47(3):461–470. doi: 10.1016/0092-8674(86)90603-3. [DOI] [PubMed] [Google Scholar]
- Near R. I., Manser T., Gefter M. L. The generation of major and minor idiotype-bearing families of anti-p-azophenylarsonate antibodies; stochastic utilization of VH gene segments. J Immunol. 1985 Mar;134(3):2004–2009. [PubMed] [Google Scholar]
- Nishi M., Kataoka T., Honjo T. Preferential rearrangement of the immunoglobulin kappa chain joining region J kappa 1 and J kappa 2 segments in mouse spleen DNA. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6399–6403. doi: 10.1073/pnas.82.19.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter R. M., Kearney J. F., Chang S. P., Hood L. E. Developmentally controlled expression of immunoglobulin VH genes. Science. 1985 Mar 29;227(4694):1597–1601. doi: 10.1126/science.3975629. [DOI] [PubMed] [Google Scholar]
- Reth M. G., Alt F. W. Novel immunoglobulin heavy chains are produced from DJH gene segment rearrangements in lymphoid cells. 1984 Nov 29-Dec 5Nature. 312(5993):418–423. doi: 10.1038/312418a0. [DOI] [PubMed] [Google Scholar]
- Reth M. G., Jackson S., Alt F. W. VHDJH formation and DJH replacement during pre-B differentiation: non-random usage of gene segments. EMBO J. 1986 Sep;5(9):2131–2138. doi: 10.1002/j.1460-2075.1986.tb04476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M., Gehrmann P., Petrac E., Wiese P. A novel VH to VHDJH joining mechanism in heavy-chain-negative (null) pre-B cells results in heavy-chain production. 1986 Aug 28-Sep 3Nature. 322(6082):840–842. doi: 10.1038/322840a0. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Dahan A., Weill J. C. A single rearrangement event generates most of the chicken immunoglobulin light chain diversity. Cell. 1985 Feb;40(2):283–291. doi: 10.1016/0092-8674(85)90142-4. [DOI] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. A quantitative assay for transformation of bone marrow cells by Abelson murine leukemia virus. J Exp Med. 1976 Jun 1;143(6):1453–1463. doi: 10.1084/jem.143.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal N. H., Gearhart P. J., Press J. L., Klinman N. R. Late acquisition of a germ line antibody specificity. Nature. 1976 Jan 1;259(5538):51–52. doi: 10.1038/259051a0. [DOI] [PubMed] [Google Scholar]
- Winter E., Radbruch A., Krawinkel U. Members of novel VH gene families are found in VDJ regions of polyclonally activated B-lymphocytes. EMBO J. 1985 Nov;4(11):2861–2867. doi: 10.1002/j.1460-2075.1985.tb04015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. L., Coleclough C. Different joining region J elements of the murine kappa immunoglobulin light chain locus are used at markedly different frequencies. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4756–4760. doi: 10.1073/pnas.81.15.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Desiderio S. V., Paskind M., Kearney J. F., Baltimore D., Alt F. W. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984 Oct 25;311(5988):727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]
- Yaoita Y., Matsunami N., Choi C. Y., Sugiyama H., Kishimoto T., Honjo T. The D-JH complex is an intermediate to the complete immunoglobulin heavy-chain V-region gene. Nucleic Acids Res. 1983 Nov 11;11(21):7303–7316. doi: 10.1093/nar/11.21.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]