Abstract
Purpose
The goals of this study were (a) to investigate whether preconditioning of immuno-competent mice with PC-61-mediated regulatory T-cell (Treg) depletion and interleukin-2 (IL-2) would enhance systemic delivery of reovirus into subcutaneous tumors and (b) to test whether cyclophosphamide (CPA), which is clinically approved, could mimic PC-61 for modification of Treg activity for translation into the next generation of clinical trials for intravenous delivery of reovirus.
Experimental Design
C57Bl/6mice bearing subcutaneous B16 tumors were treated with CPA or PC-61followed by 10 injections of low-dose IL-2. Mice were then treated with intravenous reovirus. Virus localization to tumor and other organs was measured along with tumor growth and systemic toxicity.
Results
Preconditioning with PC-61and IL-2 enhanced localization of intravenous oncolytic reovirus to tumors with significantly increased antitumor therapy compared with controls (P < 0.01). However, with the maximal achievable dose of reovirus, Treg modification + IL-2 was also associated with systemic toxicity. CPA (100 mg/kg) did not deplete, but did functionally inhibit, Treg. CPA also mimicked PC-61, in combination with IL-2, by inducing “hyperactivated” NK cells. Consistent with this, preconditioning with CPA + IL-2 enhanced therapy of intravenously delivered, intermediate-dose reovirus to a level indistinguishable from that induced by PC-61 + IL-2, without any detectable toxicity.
Conclusion
With careful reference to ongoing clinical trials with dose escalation of reovirus alone and in combinationwith CPA, we propose that future clinical trials of CPA + IL-2 + reovirus will allow for both improved levels of virus delivery and increased antitumor efficacy.
Replication-competent oncolytic viral vectors require, at least in theory, only low levels of seeding to initiate spreading infections to cover the tumor comprehensively (1, 2). In this respect, reoviruses (respiratory enteric orphan) are double-stranded RNA viruses isolated from the respiratory and gastrointestinal tracts of humans but not linked to disease (3–5). They do, however, cause fatal infections in neonatal and SCID NOD mice (5, 6), emphasizing the importance of an intact immune system as a component determining oncolytic specificity. Use of reovirus as a tumor selective oncolytic agent was proposed based on findings that an activated Ras pathway in tumor cells prevents PKR from aborting infection leading to lytic viral replication in tumors (but not in normal cells; refs. 3, 7, 8). Consistent with this, efficacy of reovirus as an antitumor agent has been shown in both immunocompetent and immunodeficient models (9–12).
We recently completed a phase I clinical trial of intravenous oncolytic reovirus (Dearing type 3) in heavily pretreated patients with advanced cancers. Reovirus was safely administered by intravenous injection at doses up to 3 × 1010 TCID50 for 5 days every 4 weeks without evidence of severe toxicities (13, 14). Encouragingly, both viral localization to and replication in metastatic tumor deposits was confirmed in several patients and antitumor activity was seen by radiologic and tumor marker evaluation. Neutralizing antibodies (NAb) were detected in all patients, which peaked at 4 weeks (13). We concluded that reovirus is a safe agent that warrants further evaluation in phase II studies. However, it is also clear that any additional interventions, which could enhance the delivery of reovirus into metastatic tumors, could add significantly to the therapeutic value of this approach.
Translational Relevance
We have completed a phase I trial of systemic delivery of oncolytic reovirus to treat patients with advanced cancer and have initiated a second trial using CPA in combination with reovirus. From these and other trials, it is clear that additional interventions, which could enhance the delivery of reovirus into metastatic tumors, could add significantly to the therapeutic value of this approach. The studies described in this article will allow us to proceed with carefully controlled dose escalation studies using a combination of CPA + IL-2 + reovirus to achieve both improved levels of virus delivery and increased antitumor efficacy.
In this respect, we recently hypothesized that the vascular leak syndrome (15, 16) associated with systemic treatment with interleukin-2 (IL-2; refs. 17–19) would enhance access of systemically delivered viruses into tumors. Indeed, treatment with nontoxic doses of IL-2 allowed for improved localization of intravenously delivered vesicular stomatitis virus (VSV) to subcutaneous tumors (20). Moreover, depletion of regulatory T cells (Treg) before IL-2 significantly enhanced VSV-mediated tumor regressions (20). Therapy was mediated by a population of NK/LAK cells, which become “hyperactivated” through IL-2-mediated expansion combined with loss of Treg-mediated suppression in vivo. These hyperactivated NK/LAK cells enhanced virus localization to the tumor site through induction of vascular leak syndrome, had direct antitumor activity, and conditioned the tumor to facilitate increased viral replication, spread, and oncolysis (20).
In those studies (20), we used PC-61, an antibody specific for CD25, a component of the high-affinity IL-2 receptor, to deplete Treg (21–23) before treatment with IL-2. Although PC-61 effectively depletes CD25hi Treg (21, 22), it also targets other CD25+ cells, such as activated T cells, which might themselves be positive effectors of tumor regression (22). Moreover, it has been proven difficult to develop effective reagents that can be used in patients to selectively deplete Treg. In this respect, ONTAK, an IL-2-diphteria toxin conjugate approved for human use, which would supposedly target CD25, was unable to deplete Treg in melanoma patients (24). However, several groups have shown that, at relatively low doses, cyclophosphamide (CPA) can enhance immune responses against tumors through selective, transient depletion of Treg (22, 25–32). There are several additional advantages of developing CPA in the context of systemic delivery of oncolytic viruses. There exists extensive experience with the drug in cancer patients (26) and CPA can both suppress local immune mechanisms that inhibit viral replication in tumors and control primary and anamnestic antiviral NAb responses (33–40). In this latter respect, we have shown that iterative injections of CPA and reovirus once every 6 days depressed, but did not ablate, levels of NAb (33). Thus, virus was still able to access tumors when delivered systemically, but the residual NAb levels protected normal tissues from toxicity resulting from subsequent systemic distribution of virus originating from ongoing replication in the tumor (33). This is of particular relevance to our clinical trials using intravenously delivered reovirus given the high levels of both preexisting and treatment-induced anti-reovirus antibodies observed in patients (13). As a result of these preclinical studies (33), clinical trials incorporating increasing doses of CPA along with intravenous reovirus are now under way. Finally, with particular importance to our proposed use of a combination of Treg depletion and IL-2 treatment to enhance viral delivery, IL-2 and CPA have been combined safely in patients (41, 42).
Therefore, the goals of this current study were (a) to investigate whether preconditioning of an immunocompetent host with Treg depletion and IL-2 would enhance the delivery of systemically delivered reovirus into metastatic tumors in a similar way to that observed with VSV (20) and (b), if so, to test whether CPA could be developed as a clinically approved reagent to mimic PC-61 for the depletion or phenotypic modification of Treg to allow for translation of these preclinical regimens into the next generation of phase I clinical trials for improved intravenous delivery of reovirus.
Materials and Methods
Cells and virus
Murine B16 melanoma cells (H2-Kb; ref. 43) were grown in DMEM (Life Technologies) supplemented with 10% (v/v) FCS (Life Technologies) and l-glutamine (Life Technologies). All cell lines were monitored routinely and found to be free of Mycoplasma infection.
Reovirus used in these studies is a wild-type reovirus type 3 (Dearing strain). Virus stock titers were measured by standard plaque assays of serially diluted samples on L929 cells.
Treg depletion and IL-2 treatment
The regimen of Treg depletion and IL-2 treatment was described by us previously (20). Briefly, for Treg depletion, 0.5 mg PC-61 antibody (Monoclonal Antibody Core Facility, Mayo Clinic) per mouse was given intraperitoneally as described (20, 21). Fluorescence-activated cell sorting analysis of spleens and lymph nodes confirmed depletion of CD4+, FoxP3+, and CD25+ cells. The control for PC-61 treatment was intraperitoneal injection of IgG control (ChromPure Rat IgG; Jackson ImmunoResearch). For mice treated with Treg depletion and IL-2, 24 h following the PC-61 antibody treatment, mice were injected intraperitoneally with recombinant human IL-2 at a dose of 75,000 units/injection (Proleukin; Novartis) three times a day for 3 days. On the fourth day, a single further injection of IL-2 was given. The control for IL-2 treatment was intraperitoneal injections of 100 µL PBS.
For studies using CPA in place of PC-61, mice were administered a single intraperitoneal injection of CPA at 100 mg/kg 24 h before the IL-2 treatments.
Virus titration from tumor and organs
Tumor and organs recovered from mice were harvested and weighed and, within 2 h of removal from the mouse, were lysed by three cycles of freezing/thawing. Virus was recovered from the lysates and titers were determined on L929 cells and expressed as TCID50 of reovirus/mg tissue.
Splenocyte preparation and antigen presentation
Splenocytes enriched in lymphocytes were prepared from spleens from treated/vaccinated animals by standard techniques (44). Freshly purified splenocyte populations were washed in PBS and 250,000 cells per well were incubated with target tumor cells (YAC or B16) typically at ratios of 100:1, 10:1, or 1:1. Then, 48 to 72 h later, cell free supernatants were tested for IFN-γ by ELISA (BD Pharmingen).
Reverse transcription-PCR
Splenocytes were recovered as described above and RNA was prepared with the Qiagen RNA extraction kit. Total cellular RNA (1 µg) was reverse transcribed in a 20 µL volume using oligo(dT) as a primer. A cDNA equivalent of 1 ng RNA was amplified by PCR for the matrix metalloproteinase-2 (MMP-2) gene using the MMP-2 primer pair purchased from R&D Systems.
Virus replication in explanted tumors
C57Bl/6 mice were seeded with subcutaneous B16 tumors. Ten to 15 days later, established tumors were excised and, without being dissociated, placed intact into wells of a 24-well tissue culture plate in DMEM.
Separately, splenocytes were recovered from C57Bl/6 mice, which had been treated as described in the text (with combinations of PC-61/ CPA/IL-2). Splenocytes (107) were then added to the wells containing explanted B16 tumors along with reovirus at 108 TCID50/well. Forty-eight hours later, tumors were recovered from the wells, dissociated, and subjected to three rounds of freeze/thaw treatment. Virus titers from these freeze/thaw lysates were determined as described above.
Antibody titration from mouse serum
Preheated mouse antiserum was mixed with an equal volume of reovirus (predetermined as killing 80% of target L929 cells) and incubated at 37°C for 2 h to allow antibody to bind to virus. The virus/antibody mix was transferred to L929 monolayers and cell survival was assayed at 48 h by MTT assay. The neutralizing titer is the highest dilution of serum that blocks the killing of L929 cells.
Flow cytometry
For analysis of phenotype, organs/tumors were recovered from mice and dissociated in vitro to achieve single-cell suspensions. Cells (1 × 106) were washed in PBS containing 0.1% bovine serum albumin (wash buffer), resuspended in 50 µL wash buffer, and exposed to directly conjugated primary antibodies for 30 min at 4°C. Cells were then washed and resuspended in 500 µL PBS containing 4% formaldehyde. Cells were analyzed by flow cytometry and data were analyzed using CellQuest software (BD Biosciences). Anti-CD8β FITC, anti-CD4 FITC, anti-NK1.1 PE, and their respective isotype controls where purchased from BD Pharmingen. Treg cells were analyzed using anti-CD4, anti-CD25, and anti-FOXP3 antibodies from E-Bioscience.
Assay for Treg activity in splenocyte populations
The OT-I mouse strain is on a C57Bl background (H-2Kb) and expresses a transgenic T-cell receptor Vα2 specific for the SIINFEKL peptide of ovalbumin in the context of MHC class I, H2-Kb (45). Preparation of activated OT-I cells was as described previously (46). Briefly, naive OT-I cells were isolated from spleen and lymph nodes. RBCs were lysed by ACK buffer [0.15 mol/L NH4Cl, 1.0 mmol/L KHCO3, 0.1 mmol/L EDTA (pH 7.2–7.4)], and the dissociated single-cell suspension was grown in Iscove’s modified Dulbecco’s medium plus 5% fetal bovine serum in the presence of 1 µg/mL SIINFEKL peptide, 50 µmol/L β-mercaptoethanol, 1% penicillin/streptomycin, and 50/mL IU recombinant IL-2. Three days after activation, cells were harvested and purified through centrifugation in Lympholyte-M density gradient (Cedarlane). OT-I cells (106), activated with SIINFEKL + IL-2, were then cocultured with 107 splenocytes harvested as described above from mice treated as described in the text. Forty-eight hours later, cell-free supernatants were tested for IFN-γ by ELISA (BD Pharmingen).
In vivo studies
All procedures were approved by the Mayo Foundation Institutional Animal Care and Use Committee. C57Bl/6 mice were age and sex matched for individual experiments and were purchased from The Jackson Laboratory at ages 6 to 8 weeks. To establish subcutaneous tumors, 2 × 105 B16 cells were injected subcutaneously (100 µL) into the flank region. Animals were examined daily until the tumor became palpable, and the diameter, in two dimensions, was measured thrice weekly using calipers. Animals were killed when tumor size was ~1.0 × 1.0 cm in two perpendicular directions. Mice were euthanized based on the double criteria of both active progression in measurable size over several days and reaching a diameter of 1.0 cm. This is based on our histologic experience that tumors that have progressively increased in size to a final size of 1.0 cm diameter represent actively growing tumor rather than predominantly necrotic tumor destruction. To establish systemic metastatic disease, C57Bl/6 mice were injected intravenously with 2 × 105 B16ova cells to form lung metastases. At the first sign of any distress mice were killed.
NK cell depletion
Anti-asialo-GM1 from Cedarlane was purchased and resuspended in 1 mL. Twenty-five microliters (~0.75 mg/mouse) were injected intraperitoneally once at the times indicated in the text. Mice treated with isotype received the same protein concentration of rabbit IgG (Jackson ImmunoResearch). NK depletion was verified by spleen NK1.1 flow cytometry analysis.
Statistics
Survival data from the animal studies were analyzed using the log-rank test (47), and the two-sample unequal variance Student’s t test analysis was applied for in vitro assays. Statistical significance was determined at the level of P < 0.05.
Results
Treg depletion + IL-2 enhances systemic delivery of reovirus
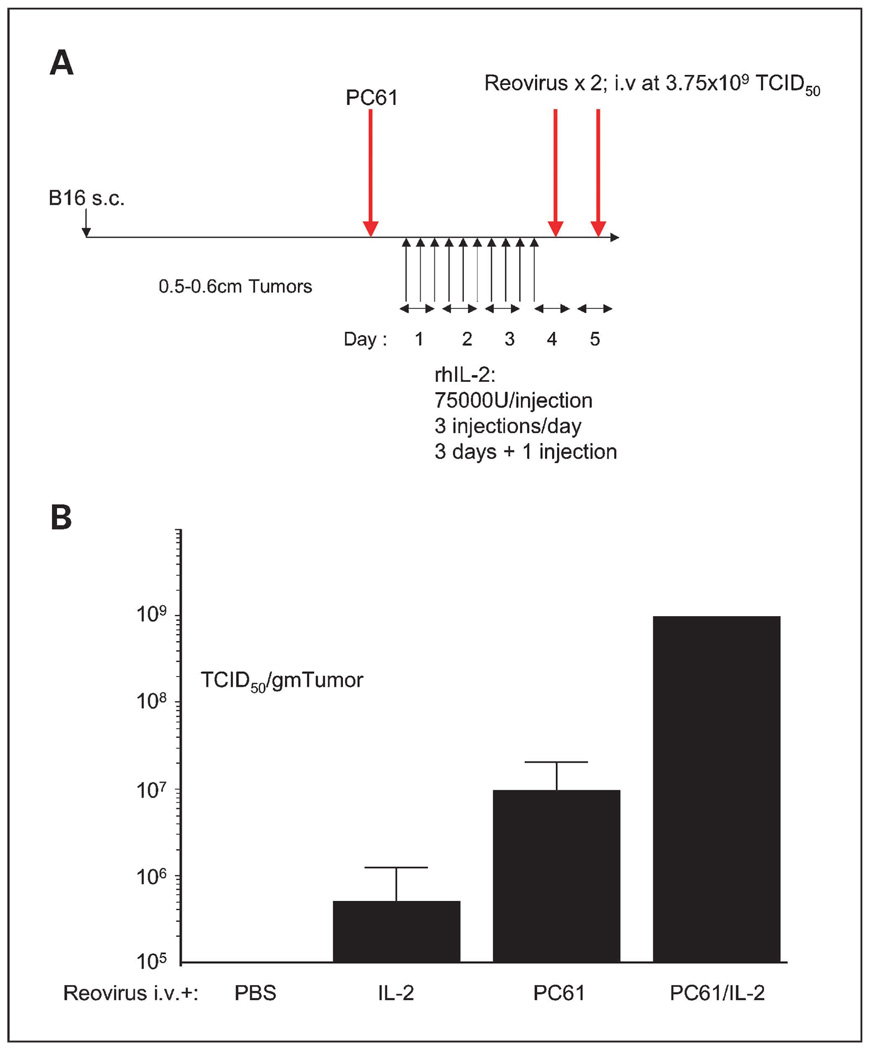
Preconditioning of C57Bl/6 mice with Treg depletion and IL-2 (Fig. 1A) significantly enhanced the localization of intravenously delivered reovirus to subcutaneous, established B16 tumors (Fig. 1B). Tumor sections from mice treated with CPA/IL-2/reovirus or PC-61/IL-2/reovirus reproducibly contained areas of extensive destruction of tumor architecture associated with virus infection. These necrotic areas were sometimes near blood vessels but were by no means exclusively perivascular. Overall, our histologic analyses were never convincingly able to associate CPA/IL-2 treatment solely with foci of increased vascular leak from these studies. Prior depletion of NK cells from mice treated with PC-61 + IL-2 + reovirus reduced levels of intratumoral reovirus to those in the absence of Treg depletion and IL-2 treatment (Table 1). These findings are consistent with our previous observations in which preconditioning with Treg depletion + IL-2 led to increased localization of intravenously delivered VSV to tumors, effects that were also dependent on an intact NK cell compartment (20).
Fig. 1.
Treg depletion + IL-2 enhances systemic delivery of reovirus to subcutaneous tumors. A, C57Bl/6 mice were seeded with subcutaneous B16 tumors. Nine days later, mice received an intraperitoneal injection of anti-CD25 antibody PC-61or a control IgG. Twenty-four hours later, mice were injected intraperitoneally with PBS or with recombinant human IL-2 at a dose of 75,000 units/injection three times a day for 3 d. On the fourth day, a single further injection of IL-2 was given. Two hours after this last injection of IL-2/PBS, mice received an intravenous injection of reovirus (3.75 × 109 TCID50) followed 24 hlater by a second similar injection of virus. B, 72 h later, tumors were explanted and dissociated and viral titers recovered from freeze/thaw lysates of tumors from mice treated as shown were determined (3 mice per group).
Table 1.
Reovirus localization to tumors is NK dependent
| Treatment | Reovirus titer (TCID50/gm tumor) |
|---|---|
| Control IgG/PC-61/IL-2/Reo | 9 × 106; 3 × 107; 3 × 107 |
| αGM-1/PC-61/IL-2/Reo | 2 × 104; 7 × 103 |
| Reovirus alone | 9 × 103; 4 × 103 |
NOTE: Experiment of A and B was repeated in three groups of mice, which were treated with PC-61 + IL-2 + reovirus (n = 3; in this case, with two injections of 1 × 108 TCID50), PC-61 + IL-2 + reovirus with prior depletion of NK cells using the anti-asialo-GM-1 antibody (αGM-1; n = 2), or reovirus alone (n = 2). Viral titers from freeze/thaw lysates of tumors from these mice are shown.
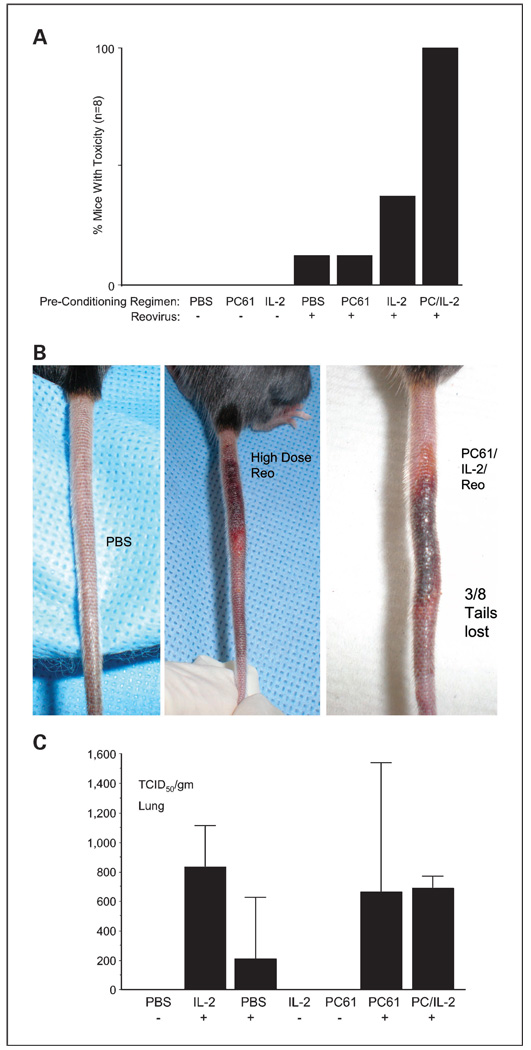
However, in addition to high levels of reovirus reaching and replicating in subcutaneous B16 tumors, we were unable to monitor antitumor therapy in these experiments using the maximum achievable dose of reovirus (3.75 × 109 TCID50) because of high levels of toxicity (Fig. 2A). Some toxicity was associated with high-dose reovirus alone, which was accentuated in the presence of IL-2, but was universally observed in all mice treated with virus in combination with PC-61 + IL-2 (Fig. 2A). Toxicities manifested as shortness of breath, shivering, and inactivity (Fig. 2A) as well as severe necrosis of the tails, which resulted in spontaneous tail loss in 3 of 8 animals treated with PC-61/IL-2/reovirus (Fig. 2B). Despite having previously observed cardiac toxicity associated with intravenous delivery of reovirus (32), no virus was detected in the hearts of any of the treated mice (data not shown). However, reovirus was detected in the lungs following intravenous delivery (Fig. 2C) but at levels considerably lower than in tumors (Fig. 1B and Fig. 2C). There were no significant differences in titers in lungs recovered from mice treated with reovirus + IL-2 alone, PBS alone, PC-61 alone, or PC-61 + IL-2 (Fig. 2C). Therefore, the increased toxicity in mice preconditioned with Treg depletion + IL-2 cannot be explained by increased virus localization to heart or lung.
Fig. 2.
PC-61 + IL-2 + high-dose reovirus leads to severe toxicity. Mice treated as described in Fig.1A and B (8 per group) were routinely monitored for signs of distress. In all groups that received the high-dose reovirus, a proportion of mice developed toxicities, manifested as shortness of breath, shivering, and inactivity, within 7 d of virus treatment. Generally, these toxicities resolved within 24h, although several mice were euthanized to prevent further distress. B, some mice also developed swelling of the tails along with sensitivity to touch (1mouse treated with reovirus alone and all 8 mice treated with PC-61 + IL-2 + reovirus). In 3 of 8 of those animals treated with PC-61 + IL-2 + reovirus, this toxicity resulted in spontaneous detachment of the tail. C, because of these systemic toxicities, mice from all groups were euthanized and viral titers recovered from the freeze/thaw lysates of lungs were determined.
CPA induces a “hyperactivated” NK cell phenotype
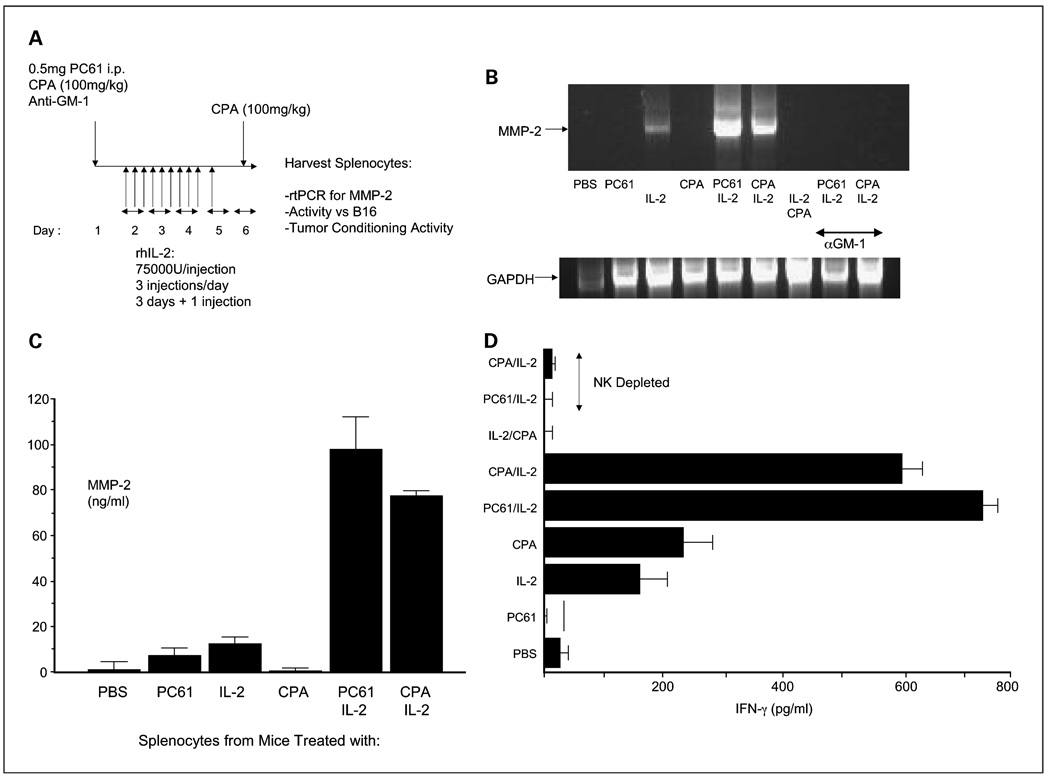
We investigated whether it would be possible to develop a preconditioning regimen with CPA, which would mimic that of the PC-61 antibody, for testing in clinical trials. Therefore, we screened for surrogate markers of a phenotype corresponding to that of “hyperactivated” NK cells, which are important for enhancing viral replication in and spread through established B16 tumors (20). Splenocytes from mice treated with CPA at 150 mg/kg, a dose that we have used previously to modulate anti-reovirus NAb levels in vivo (33), neither expressed MMP-2 by reverse transcription-PCR nor secreted IFN-γ on coculture with B16 targets in vitro (data not shown). However, splenocytes from mice treated with CPA at 100 mg/kg and IL-2 expressed MMP-2 at levels very similar to those induced by PC-61 + IL-2 (Fig. 3B and C). MMP-2 induction was dependent on NK cells in the splenocyte populations, as prior depletion of NK cells abolished its expression (Fig. 3B). In addition, treatment with CPA before IL-2 was critical, as CPA administration after the 10 injections of IL-2 failed to generate NK cells expressing MMP-2 (Fig. 3B). Similarly, NK cells from mice treated with CPA (100 mg/kg) + IL-2 secreted significant amounts of IFN-γ on coculture with B16 targets in vitro (Fig. 3D), similar to NK cells induced by PC-61 + IL-2 treatment, a phenotype that was central to promoting enhanced systemic delivery of VSV to tumors in vivo (20).
Fig. 3.
Low-dose CPA can replace PC-61in enhancing IL-2-activated NK function. A, additional groupswere added in the regimen of Fig.1A, inwhich a single intraperitoneal injection of CPA at100 mg/kg was administered either in place of the PC-61antibody or 24h after the final injection of IL-2. In addition, groups that received an injection of the NK cell depleting asialo-GM-1antibody 24h before treatment with PC-61or CPA were also included. B, 48 h following the final injection of IL-2, splenocytes from treated mice were recovered, cDNA was prepared, and expression of the MMP-2 gene was analyzed by PCR. Equal loading of RNA was shown using amplification of GAPDH. Representative of three mice per group and of multiple repeat experiments. C, experiment of A and B was repeated with fewer treatment groups. Five hundred thousand splenocytes from treated mice were recovered and plated for 48 h in vitro. Supernatants were then assayed forMMP-2 by ELISA (R&D Systems). D, splenocytes from the 3 mice per group in A and B above were also plated with B16 target cells at an effector splenocytes:target ratio of10:1, and 48 h later, supernatants were assayed for IFN-γ by ELISA.
Finally, and as observed previously for VSV (19), splenocytes from PC-61/IL-2-treated mice significantly enhanced the replication of reovirus through intact B16 tumor explants compared with reovirus replication supported by splenocytes from mice treated with PC-61 alone (data not shown), PBS, or IL-2 alone (Table 2). Although viral titers were not as high, splenocytes from CPA/IL-2-treated mice also strongly enhanced viral replication in B16 explants significantly over controls (Fig. 3D).
Table 2.
Cyclophosphamide mimics PC61 in enhancing viral spread through tumors
| Splenocytes from mice treated with |
Reovirus titer recovered from freeze/thawed lysate (TCID50/g tumor) |
|---|---|
| PBS | <1 |
| IL-2 | 1.3 |
| PC61/IL-2 | 1.1 × 105 |
| CPA/IL-2 | 2.4 × 104 |
NOTE: 107 splenocytes recovered from mice treated as shown were cocultured with freshly explanted, intact B16 tumors in vitro as described in Materials and Methods along with reovirus at 108 TCID50/well. Forty-eight hours later, virus titers from freeze/thaw lysates of the tumors were determined as shown. Mean of two tumors per group. Forty-eight hours later, virus titers from freeze/thaw lysates of the tumors were determined as shown. Mean of two tumors per group.
Therefore, preconditioning with CPA + IL-2 induces “hyper-activation” of NK cells, which includes expression of MMP-2, antitumor activity, and promotion of viral replication/spread through intact tumors.
CPA and IL-2 increase NK cells and modify Treg function
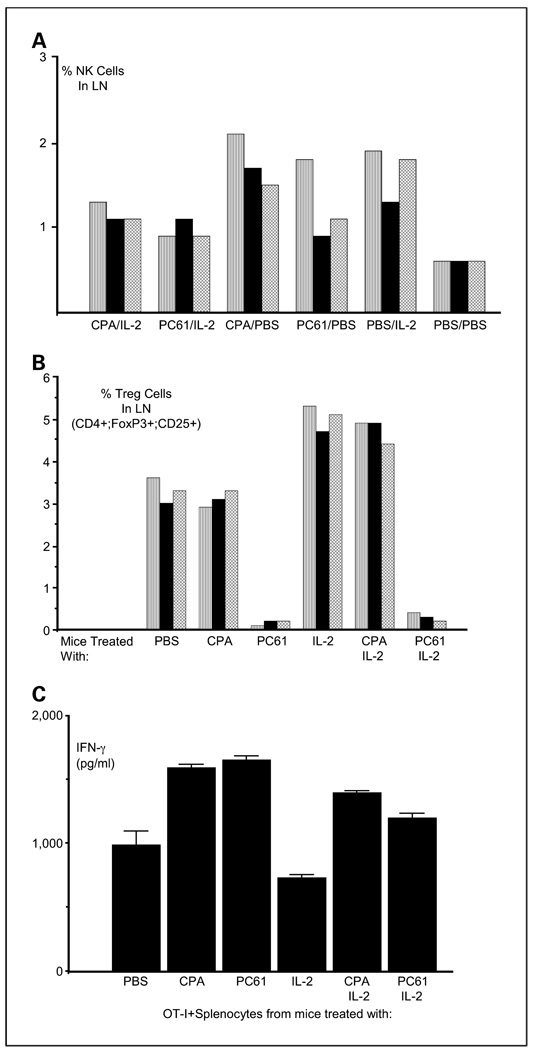
Interestingly, treatment with CPA alone significantly expanded NK cell numbers in lymph nodes compared with PBS-treated mice (P < 0.02) and to similar levels induced by IL-2 alone (Fig. 4A). PC-61 treatment increased NK cell numbers significantly over PBS treatment (P < 0.05) but less than with IL-2 or CPA alone (Fig. 4A). Both CPA + IL-2 and PC-61 + IL-2 increased NK cell numbers significantly over PBS treatment (P < 0.05) to similar levels, although these numbers were less than that induced by either CPA or IL-2 alone (Fig. 4A). However, there was a highly significant disparity between the activity of PC-61 and CPA on the numbers of Treg in the lymph nodes of treated mice (Fig. 4B). Whereas PC-61 effectively depleted the lymph nodes of CD4+;FOXP3+;CD25+ Treg, CPA did not (Fig. 4B). Although treatment with IL-2 following PC-61 did not rescue these Treg populations (Fig. 4B), IL-2 treatment following CPA led to significantly increased levels of CD4+;FOXP3+;CD25+ Treg numbers—to levels similar to those observed with IL-2 treatment alone (Fig. 4B). Despite not mediating depletion of Treg in vivo, treatment with CPA closely mimicked treatment with PC-61 in terms of removing Treg-mediated suppression of activated T cells in vitro. Thus, activated OT-I T cells cocultured with splenocytes from mice treated with either PC-61 or CPA secrete significantly more IFN-γ on stimulation with their cognate antigen than when cultured with splenocytes from PBS-treated mice (Fig. 4C). Splenocytes from mice treated with CPA + IL-2 also have significantly less suppressive effect on OT-I T cell function than splenocytes containing Treg (PBS-treated mice; P < 0.01) and, in fact, have significantly less suppressive effect than splenocytes from mice depleted of Treg by PC-61 and treated with IL-2 (P < 0.05; Fig. 4D).
Fig. 4.
Low-dose CPA does not deplete Treg but inhibits their suppressive activity. C57Bl/6 mice treated with intraperitoneal combinations of CPA, PC-61, and IL-2 as shown according to the regimen of Fig. 3A (3 per group) were euthanized 48 h following the last injection of IL-2. Lymph nodes were excised, dissociated, and analyzed for (A) NK or (B) Treg cells as a percentage of all lymph node cells using flow cytometry. Individual bars in the histograms represent the result from each independent mouse in the appropriate treatment group. C, 106 3-day activated OT-I cells were cocultured with 107 splenocytes harvested from mice treated as shown according to the regimen of Fig. 3A. Forty-eight hours later, cell-free supernatants were tested for IFN-γ by ELISA (BD Pharmingen). Activated OT-I secrete IFN-γ in response to coculture with their cognate antigen (SIINFEKL peptide); any increase in the levels of secretion of IFN-γ therefore represents removal of a Treg-like activity in the splenocyte populations used for coculture of the OT-I T cells (21).
CPA + IL-2 enhances the antitumor therapy of systemically delivered reovirus
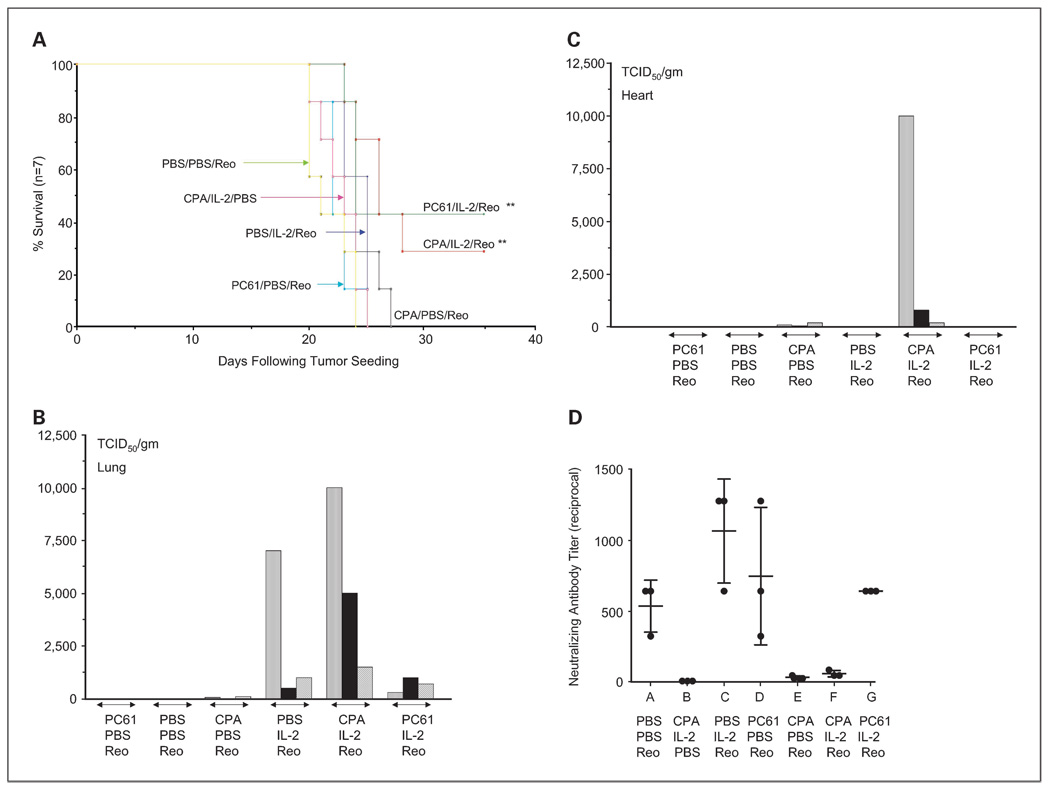
Because of the systemic toxicity observed with the maximum achievable dose of reovirus (3.75 × 109 TCID50; Fig. 1), to test therapeutic efficacy of CPA + IL-2 + reovirus, we reduced the viral dose to 1 × 108 TCID50 per injection. Under these conditions, we observed equivalent therapy of subcutaneous B16 tumors using either PC-61 + IL-2 or CPA + IL-2 at levels that were significantly better than any of the control treatments (P < 0.01; Fig. 5A). None of the mice treated with the preconditioning regimens and intravenous reovirus developed any of the toxicities observed using high-dose reovirus in Fig. 1. Despite the lack of observable toxicity, reovirus was, however, recovered from both the lungs and the hearts of mice treated with CPA + IL-2 + reovirus (Fig. 5B and C). This is in contrast to mice treated with PC-61 + IL-2 + reovirus where virus was recovered only from the lungs and not from the hearts (Fig. 5B and C). Therefore, preconditioning with CPA + IL-2 can enhance the therapy produced by systemic delivery of intravenously delivered reovirus to a level indistinguishable from that induced by PC-61 + IL-2.
Fig. 5.
CPA-mediatedTreg modification, with IL-2 and lower-dose reovirus, is therapeutic against established tumors. A, C57Bl/6 mice were seeded with subcutaneous B16 tumors. Nine days later, mice received an intraperitoneal injection of either CPA (100 mg/kg) or anti-CD25 antibody PC-61or PBS. Twenty-four hours later, mice were injected intraperitoneally with PBS or with recombinant human IL-2 at a dose of 75,000 units/injection three times a day for 3 d. On the fourth day, a single further injection of IL-2 was given. Two hours after this last injection of IL-2/PBS, mice received an intravenous injection of reovirus at a lower than maximal achievable dose of 1 × 108 TCID50 followed 24h later by a second similar injection of virus. Survival of mice (tumor <1.0 cm in any diameter) with time after tumor seeding is shown (n = 7 per group).The median survival times of groups treated with reovirus alone (median survival, 21d), CPA/IL-2 (23 d), PC-61/reovirus (22 d), or CPA/reovirus (21d) were not significantly different from each other and none of these treatments generated any long-term survivors. Median survival times of groups treated with IL-2/reovirus (25 d), PC-61/IL-2/reovirus (24d ), or CPA/IL-2/reovirus (25 d) were significantly longer (P = 0.04) than these other groups. However, only treatment with PC-61/IL-2/reovirus or CPA/IL-2/reovirus led to long-term survivors and both of these were significantly more therapeutic than any other group. **, P < 0.01. B and C, viral titers in the freeze/thaw lysates of lungs (B) orhearts (C) recovered 7 to10 d after the final viral injection frommice treated as described in A were determined (3mice per treatment group). Individual bars in the histograms represent the result from each independent mouse in the appropriate treatment group. D, serum recovered from the mice in B and C was assayed for neutralizing against reovirus as described in Materials and Methods.
Previously, we showed that a higher dose of CPA (150 mg/kg) can modulate levels of NAb against reovirus to allow for repeat administration of the virus (33). Therefore, although we do not believe that NAb to reovirus has any inhibitory role in the therapeutic effects seen in the virus-naive C57Bl/6 mice in Fig. 5A, we tested their serum for levels of NAb. As expected, serum from mice treated with reovirus alone contained high levels of neutralizing activity against reovirus but not VSV (data not shown; Fig. 5D). Pretreatment with either IL-2 or PC-61 showed a trend toward increasing the level of neutralizing activity in the serum, although these values were very variable (Fig. 5D). Pretreatment with CPA before reovirus administration reduced this neutralizing activity significantly (P < 0.01), which was maintained with the combination of CPA + IL-2 (Fig. 5D). Combination of Treg depletion by PC-61 + IL-2 maintained levels of neutralization at those observed in mice treated with reovirus alone (Fig. 5D). Therefore, use of CPA in combination with IL-2 + reovirus not only enhances antitumor therapy (Fig. 5A) but also modulates levels of anti-reovirus antibody.
Discussion
Here, we show that PC-61 + IL-2 enhanced intratumoral localization of systemically delivered reovirus by 2 to 3 logs compared with mice treated with PBS/reovirus alone (Fig. 1). One of our initial hypotheses was that IL-2-induced vascular leakage at the tumor site would increase the ability of systemically delivered virus to localize into established tumors. Conversely, however, others have shown that enhanced vascular leakage can increase the access of antiviral immune effectors and neutralizing agents into the tumor, which subsequently act to inhibit oncolytic virus replication and efficacy (48). In this respect, we have shown previously that PC-61 + IL-2 treatment induces a population of “hyperactivated” NK cells with potent antitumor activity and that express high levels of MMP-2, which are critical in enhancing VSV replication in and spread through established B16 tumors (19). In these current studies, increased reovirus localization to subcutaneous B16 tumors was equally dependent on NK cells (Table 1). We believe, therefore, that the increased vascular leakage induced by IL-2 is beneficial to the outcome of the virotherapy because of the added value provided by the infiltrating “hyperactivated” NK cells, which are themselves induced by the IL-2 treatment. Under other circumstances, where vascular leakage is not necessarily accompanied by activation of antitumor effectors, therapeutic outcome may, therefore, be very different.
However, using the maximum achievable dose of intravenously delivered reovirus, preconditioning with Treg depletion and IL-2 generated severe toxicities (Fig. 2). We did not recover any reovirus from the hearts of these mice despite cardiac-associated toxicities with systemically delivered reovirus in our previous studies (33). Because there were no significant differences in titers in lungs from mice treated with reovirus: +IL-2 alone, PBS alone, PC-61 alone, or PC-61 + IL-2 (Fig. 2C), the dramatic increased toxicity seen in mice preconditioned with Treg depletion + IL-2 cannot be explained by increased virus localization to heart or lung. The most dramatic toxicity we observed was severe swelling of the tails, which was pronounced in all 8 mice treated with PC-61 + IL-2 + reovirus. This culminated in spontaneous detachment of tails in 3 of 8 of these animals (Fig. 2B). A similar toxicity, “black foot syndrome,” has been reported previously in SCID or SCID/NOD mice treated with reovirus intratumorally (49–51). This syndrome involved necrosis of feet, tails, distal legs, and ears several weeks after injection of reovirus intratumorally (48). This pathogenesis was due to venous vasculitis secondary to reovirus infection, along with reovirus-induced myocarditis and heart failure (49, 51), and developed typically weeks or months after the reovirus therapy (49, 50). In our studies, a variant of black foot syndrome appeared much more rapidly, within 2 weeks of the reovirus therapy. The very high levels of virus we recovered from B16 tumors in Fig. 1A would be consistent with the tumor acting as a source for further dissemination of virus, which perhaps gains greater access to systemic tissues (such as the vasculature in the tail) as a result of Treg depletion + IL-2 preconditioning. More extensive toxicology studies are under way to understand the basis of these toxicities.
We reasoned that, if it were possible to mimic the biological effects of the PC-61 antibody with CPA, we would be able to develop novel clinical trials for enhanced systemic delivery of reovirus to metastatic tumors as a follow-up of our previous trials (13). Using screening for surrogate markers of the “hyperactivated” NK cell phenotype induced by PC-61 + IL-2 (20), we observed that a relatively low dose of CPA (100 mg/kg) could be combined with IL-2 to generate NK cells, which express elevated levels of MMP-2 (Fig. 3B and C), acquire antitumor activity (IFN-γ secretion on coculture with B16 targets; Fig. 3D), and enhance virus replication through an intact, excised B16 tumor in vitro (Table 2). We also showed that this dose of CPA + IL-2 expands NK cell numbers in vivo to similar levels to that produced by PC-61 + IL-2 (Fig. 4A). In contrast, whereas PC-61 clearly depletes Treg cells in vivo very efficiently, CPA does not (Fig. 4B). In fact, combination of CPA + IL-2 actually increased the numbers of Treg (Fig. 4B) compared with the extensive depletion seen with PC-61 + IL-2. However, CPA did induce a functional inhibition of Treg suppressive activity, which was similar, if not slightly more marked, than that induced by PC-61 in combination with IL-2 (Fig. 4C).
These data suggested that a low dose of CPA + IL-2 can mimic the functional effects of PC-61 + IL-2 in vivo. Consistent with this, preconditioning with CPA + IL-2 enhanced the therapy produced by systemic delivery of intermediate-dose reovirus to a level indistinguishable from that induced by PC-61 + IL-2 (Fig. 5A). Using this lower dose than that which is maximally achievable (Fig. 1), we observed highly significant antitumor therapy without any associated toxicity. Importantly, for future clinical translation, despite our findings that virus was recovered from both lung and heart tissues following CPA/IL-2 + reovirus, there was no overt toxicity in any of the treated mice. More extensive histologic and toxicologic studies will be required to investigate whether additional toxicities are induced at the cellular/tissue/organ level.
Previously, we have shown that a higher dose of CPA (150 mg/kg) can modulate levels of NAb against reovirus to allow for repeat administration of the virus (33). Those levels of CPA were not able to induce the “hyperactivated” NK cell phenotype induced by the lower levels of the drug as used in these current studies (100 mg/kg). However, most of the patients in our clinical trials will be immune to reovirus, and we observed increasing titers of anti-reovirus NAb in patients treated with intravenous reovirus (13). Therefore, we also examined the effects of this lower, NK-activating dose of CPA (100 mg/kg) on the development of NAb. Encouragingly in this regard, CPA + IL-2 not only enhanced the antitumor therapy induced by intravenous delivery of reovirus (Fig. 5A) but also significantly modulated the levels of anti-reovirus antibody (Fig. 5D). Our experiments using the higher dose of CPA (150 mg/kg) indicated that the most effective NAb-modulating activity of CPA was to lower levels of NAb, without ablating them completely (32). This allowed for further administrations of reovirus intravenously (with reduced NAb-mediated virus neutralization) but also maintained sufficiently high levels of NAb to protect against subsequent systemic dissemination of reovirus from tumors producing high levels of replicating virus (33). We show here that the reduced concentration of CPA (100 mg/kg), in combination with IL-2, can also lower, but not ablate, anti-reovirus NAb levels. This suggests that these levels may also help to overcome neutralization in the serum of patients with preexisting NAb, thereby enhancing systemic delivery while also continuing to provide systemic protection against tumor-derived, replicating virus. This hypothesis is currently being tested in mice that are preimmune to reovirus. As regards the final dose of CPA that will be used clinically, our current phase I clinical trials of systemic reovirus in combination with escalating doses of CPA will determine the levels of the drug that are safe in combination with the virus in patients. Correlative studies from these patients on toxicity, levels of NAb, and virus localization will help to determine the human equivalent dose of CPA that generates the most favorable in vivo conditions for systemic virus delivery as determined from these current preclinical studies.
In summary, preconditioning of immunocompetent hosts with Treg depletion and IL-2 enhances the localization of intravenously delivered oncolytic reovirus to established tumors, with associated increased antitumor therapy. We believe that these therapeutic effects derived from the effects of IL-2 expanded activity enhanced (by Treg depletion) NK activity, which promotes increased reovirus replication/spread/oncolysis within the tumor. Moreover, CPA can mimic the functional effects of the PC-61 Treg depleting antibody, when used at the appropriate dose, in combination with IL-2. However, the Treg-modulation + IL-2 can also be associated with increased virus-mediated toxicity. Therefore, with careful reference to our previous (13) and ongoing clinical trials with systemic delivery of reovirus alone and in combination with CPA, we propose that future clinical trials of CPA + IL-2 + reovirus may allow improved levels of virus delivery to metastatic tumors in patients.
Acknowledgments
We thank Toni L. Higgins for expert secretarial assistance.
Grant support: The Richard M. Schulze Family Foundation, Mayo Foundation, and NIH grants CA RO1107082-02 and CA130878-01.
Footnotes
Disclosure of Potential Conflicts of Interest
M. Coffey, employee/ownership interest, Oncolytics Biotech; K. Harrington, A. Melcher, R. Vile, research grants, Oncolytics Biotech.
References
- 1.Bell JC. Oncolytic viruses: what’s next? Curr Cancer Drug Targets. 2007;7:127–131. doi: 10.2174/156800907780058844. [DOI] [PubMed] [Google Scholar]
- 2.Kirn D, Martuza RL, Zwiebel J. Replication-selective virotherapy for cancer: biological principles, risk management and future directions. Nat Med. 2001;7:781–787. doi: 10.1038/89901. [DOI] [PubMed] [Google Scholar]
- 3.Norman KL, Lee PWK. Not all viruses are bad guys: the case for reovirus in cancer therapy. Drug Discov Today. 2005;10:847–856. doi: 10.1016/S1359-6446(05)03483-5. [DOI] [PubMed] [Google Scholar]
- 4.Rosen L, Evans HE, Spickard A. Reovirus infections in human volunteers. Am J Hyg. 1963;77:29–37. doi: 10.1093/oxfordjournals.aje.a120293. [DOI] [PubMed] [Google Scholar]
- 5.Tyler KL, Fields BN. Reoviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields virology. 3rd ed. Philadelphia: Lippincott-Raven; 1996. pp. 1597–1623. [Google Scholar]
- 6.Sherry B, Li XY, Tyler KL, Cullen JM, Virgin HWt. Lymphocytes protect against and are not required for reovirus-induced myocarditis. J Virol. 1993;67:6119–6124. doi: 10.1128/jvi.67.10.6119-6124.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strong JE, Lee PW. The v-erbB oncogene confers enhanced cellular susceptibility to reovirus infection. J Virol. 1996;70:612–616. doi: 10.1128/jvi.70.1.612-616.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 9.Wilcox ME, Yang W, Senger D, et al. Reovirus as an oncolytic agent against experimental human malignant gliomas. J Natl Cancer Inst. 2001;93:903–912. doi: 10.1093/jnci/93.12.903. [DOI] [PubMed] [Google Scholar]
- 10.Hirasawa K, Nishikawa SG, Norman KL, Alain T, Kossakowska A, Lee PW. Oncolytic reovirus against ovarian and colon cancer. Cancer Res. 2002;62:1696–1701. [PubMed] [Google Scholar]
- 11.Norman KL, Coffey MC, Hirasawa K, et al. Reovirus oncolysis of human breast cancer. Hum Gene Ther. 2002;13:641–652. doi: 10.1089/10430340252837233. [DOI] [PubMed] [Google Scholar]
- 12.Hirasawa K, Nishikawa S, Norman KL, et al. Systemic reovirus therapy of metastatic cancer in immune-competent mice. Cancer Res. 2003;63:348–353. [PubMed] [Google Scholar]
- 13.White CL, Twigger K, Vidal L, et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene Ther. 2008;15:911–920. doi: 10.1038/gt.2008.21. [DOI] [PubMed] [Google Scholar]
- 14.Vidal L, Pandha HS, Yap TA, et al. A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clin Cancer Res. 2008;14:7127–7137. doi: 10.1158/1078-0432.CCR-08-0524. [DOI] [PubMed] [Google Scholar]
- 15.Baluna R, Vitetta ES. Vascular leak syndrome: a side effect of immunotherapy. Immunopharmacology. 1997;37:117–132. doi: 10.1016/s0162-3109(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg SA, Mule JJ, Spiess PJ, Reichert CM, Schwarz SL. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin-2. J Exp Med. 1985;161:1169–1188. doi: 10.1084/jem.161.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoneda O, Imai T, Goda S, et al. Fractalkine-mediated endothelial cell injury by NK cells. J Immunol. 2000;164:4055–4062. doi: 10.4049/jimmunol.164.8.4055. [DOI] [PubMed] [Google Scholar]
- 18.Melencio L, McKallip RJ, Guan H, et al. Role of CD4(+)CD25(+) T regulatory cells in IL-2-induced vascular leak. Int Immunol. 2006;18:1461–1471. doi: 10.1093/intimm/dxl079. [DOI] [PubMed] [Google Scholar]
- 19.Renkonen R, Ristimaki A, Havry P. Interferon-γ protects human endothelial cells from lymphokine-activated killer cell-mediatedlysis. Eur J Immunol. 1998;18:1839–1842. doi: 10.1002/eji.1830181129. [DOI] [PubMed] [Google Scholar]
- 20.Kottke T, Galivo F, Wongthida P, et al. Treg depletion-enhanced IL-2 treatment facilitates therapy of established tumors by systemically delivered oncolytic virus. Mol Ther. 2008;16:1217–1226. doi: 10.1038/mt.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniels G, Sanchez-Perez L, Kottke T, et al. A simple method to cure established tumors by inflammatory killing of normal cells. Nat Biotechnol. 2004;22:1125–1132. doi: 10.1038/nbt1007. [DOI] [PubMed] [Google Scholar]
- 22.Matsushita N, Pilon-Thomas S, Martin L, Riker A. Comparative methodologies of regulatory T cell depletion in a murine melanoma model. J Immunol Methods. 2008;333:167–179. doi: 10.1016/j.jim.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 24.Attia P, Maker AV, Haworth LR, Rogers-Freezer L, Rosenberg SA. Inability of a fusion protein of IL-2 and diphtheria toxin (ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J Immunother. 2005;28:582–592. doi: 10.1097/01.cji.0000175468.19742.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 26.Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4(+)CD25(+) regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Paolo NC, Tuve S, Ni S, Hellstrom KE, Hellstrom IE, Lieber A. Effects of adenovirus-mediated heat shock protein expression and oncolysis in combination with low-dose cyclophosphamide treatment on anti tumor immune responses. Cancer Res. 2006;66:960–969. doi: 10.1158/0008-5472.CAN-05-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glaser M. Augmentation of specific immune response against a syngeneic SV40-induced sarcoma in mice by depletion of suppressor T cells with cyclophosphamide. Cell Immunol. 1979;48:339–345. doi: 10.1016/0008-8749(79)90128-x. [DOI] [PubMed] [Google Scholar]
- 29.Rollinghoff M, Starzinski-Powitz A, Pfizenmaier K, Wagner H. Cyclophosphamide-sensitive T lymphocytes suppress the in vivo generation of antigen-specific cytotoxic T lymphocytes. J Exp Med. 1977;145:455–459. doi: 10.1084/jem.145.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beyer M, Kochanek M, Darabi K, et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106:2018–2025. doi: 10.1182/blood-2005-02-0642. [DOI] [PubMed] [Google Scholar]
- 31.Ikezawa Y, Nakazawa M, Tamura C, Takahashi K, Minami M, Ikezawa Z. Cyclophosphamide decreases the number, percentage and the function of CD25+ CD4+ regulatory T cells, which suppress induction of contact hypersensitivity. J Dermatol Sci. 2005;39:105–112. doi: 10.1016/j.jdermsci.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 33.Qiao J, Wang X, Kottke T, et al. Cyclophosphamide facilitates anti tumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14:259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikeda K, Ichikawa T, Wakimoto H, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5:881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 35.Ikeda K, Wakimoto H, Ichikawa T, et al. Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant. J Virol. 2000;74:4765–4775. doi: 10.1128/jvi.74.10.4765-4775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith TA, White BD, Gardner JM, Kaleko M, McClelland A. Transient immunosuppression permits successful repetitive intravenous administration of an adenovirus vector. Gene Ther. 1996;3:496–502. [PubMed] [Google Scholar]
- 37.Fulci G, Breymann L, Gianni D, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedman A, Tian JP, Fulci G, Chiocca EA, Wang J. Glioma virotherapy: effects of innate immune suppression and increased viral replication capacity. Cancer Res. 2006;66:2314–2319. doi: 10.1158/0008-5472.CAN-05-2661. [DOI] [PubMed] [Google Scholar]
- 39.Wakimoto H, Fulci G, Tyminski E, Chiocca EA. Altered expression of antiviral cytokine mRNAs associated with cyclophosphamide’s enhancement of viral oncolysis. Gene Ther. 2004;11:214–223. doi: 10.1038/sj.gt.3302143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wakimoto H, Johnson PR, Knipe DM, Chiocca EA. Effects of innate immunity on herpes simplex virus and its ability to kill tumor cells. Gene Ther. 2003;10:983–990. doi: 10.1038/sj.gt.3302038. [DOI] [PubMed] [Google Scholar]
- 41.Kasamon Y, Flinn I, Grever M, et al. Phase I study of low-dose interleukin-2, fludarabine, and cyclophosphamide for previously untreated indolent lymphoma and chronic lymphocytic leukemia. Clin Cancer Res. 2005;11:8413–8417. doi: 10.1158/1078-0432.CCR-05-1612. [DOI] [PubMed] [Google Scholar]
- 42.Miller J, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 43.Linardakis E, Bateman A, Phan V, et al. Enhancing the efficacy of a weak allogeneic melanoma vaccine by viral fusogenic membrane glycoprotein-mediated tumor cell-tumor cell fusion. Cancer Res. 2002;62:5495–5504. [PubMed] [Google Scholar]
- 44.Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W. Current protocols in immunology. Wiley and Sons; 1998. [Google Scholar]
- 45.Hogquist KA, Jameson SC, Health WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonistic peptides induce positive selection. Cell. 1994;76:17. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 46.Cole C, Qiao J, Kottke T, et al. Tumor-targeted, systemic delivery of therapeutic viral vectors using hitchhiking on antigen-specific T cells. Nat Med. 2005;11:1073–1081. doi: 10.1038/nm1297. [DOI] [PubMed] [Google Scholar]
- 47.Altman DG. Practical statistics for medical research. London: Chapman Hall; 1991. Analysis of survival times; pp. 365–395. [Google Scholar]
- 48.Kurozumi K, Hardcastle J, Thakur R, et al. Effect of tumor microenvironment modulation on the efficacy of oncolytic virus therapy. J Natl Cancer Inst. 2007;99:1739–1741. doi: 10.1093/jnci/djm229. [DOI] [PubMed] [Google Scholar]
- 49.Loken SD, Norman K, Hirasawa K, Nodwell M, Lester WM, Demetrick DJ. Morbidity in immunosuppressed (SCID/NOD) mice treated with reovirus (Dearing 3) as an anti-cancer biotherapeutic. Cancer Biol Ther. 2004;3:734–738. doi: 10.4161/cbt.3.8.963. [DOI] [PubMed] [Google Scholar]
- 50.Kim M, Egan C, Alain T, et al. Acquired resistance to reoviral oncolysis in Ras-transformed fibrosarcoma cells. Oncogene. 2007;26:4124–4134. doi: 10.1038/sj.onc.1210189. [DOI] [PubMed] [Google Scholar]
- 51.DeBiasi RL, Robinson BA, Sherry B, et al. Caspase inhibition protects against reovirus-induced myocardial injury in vitro and in vivo. J Virol. 2004;78:11040–11050. doi: 10.1128/JVI.78.20.11040-11050.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]