Abstract
Background
Persons with early kidney disease have an increased risk of cardiovascular events and mortality, but the importance of accelerated atherosclerosis in promoting these outcomes is unclear. We therefore explored whether serum cystatin C level is associated with carotid intima-media thickness (IMT) in ambulatory adults without clinical heart disease.
Study Design
Cross-sectional study.
Setting & Participants
We evaluated 6,557 ethnically diverse persons free of clinical cardiovascular disease aged 45 to 84 years at the baseline visit of the Multi-Ethnic Study of Atherosclerosis.
Predictors
Kidney function was estimated by using 2 methods: serum cystatin C level and estimated glomerular filtration rate, based on creatinine and cystatin C levels.
Outcomes & Measurements
Study outcomes were internal and common carotid IMT, measured by using high-resolution B-mode ultrasound. Multivariate linear and logistic regressions were used to evaluate the independent association of kidney function with carotid IMT.
Results
In unadjusted linear analysis, each SD (0.23 mg/L) greater cystatin C level was associated with 0.091-mm greater internal carotid IMT (P < 0.001), but this association was diminished by 70% after adjustment for age, sex, and race/ethnicity (0.027 mm; P < 0.001) and was no longer significant after adjustment for cardiovascular risk factors (0.005 mm; P = 0.5). Similarly, the strong unadjusted associations of cystatin C level with common carotid IMT disappeared after adjustment. Chronic kidney disease, defined by using either creatinine level or cystatin C–based estimated glomerular filtration rate less than 60 mL/min/1.73 m2, had no independent association with internal and common carotid IMT.
Limitations
There were few participants with severe kidney disease.
Conclusions
Cystatin C level had no independent association with carotid IMT in a population free of clinical heart disease. This observation suggests that accelerated atherosclerosis is unlikely to be the primary mechanism explaining the independent association of cystatin C level with cardiovascular risk.
Index Words: Cystatin C, intima-media thickness (IMT), atherosclerosis, cardiovascular diseases, kidney
Chronic kidney disease (CKD) is associated with an increased risk of cardiovascular events, including myocardial infarction, heart failure, stroke, and mortality, independent of traditional cardiovascular risk factors.1-6 For older adults without CKD, increased cystatin C levels in the “preclinical” range also are associated with increased risk of cardiovascular disease and death.7 Accelerated atherosclerosis has been postulated as an intermediate lesion that connects kidney disease with cardiovascular events because decreasing kidney function is accompanied by atherosclerotic risk factors, including hypertension,8 dyslipidemia,9 inflammation,9,10 and hyperhomocysteinemia.9
Carotid intima-media thickness (IMT) is an established measure of subclinical atherosclerosis that holds prognostic significance for cardiovascular events in the general population.11-14 In persons with end-stage renal disease, there is a progressive increase in carotid IMT with concomitant increased risk of adverse cardiovascular outcomes.15-22 The association of less severe kidney disease with carotid IMT has been less well characterized.23-30 We hypothesized that persons with mild kidney impairment and no clinical cardiovascular disease would have a greater quantity of atherosclerosis as measured by using carotid IMT compared with individuals with normal kidney function. Using cystatin C level as a measure of kidney function, we evaluated the association between kidney function and common and internal carotid IMT in 6,557 men and women in the Multi-Ethnic Study of Atherosclerosis (MESA).
Methods
Participants
MESA is a community-based prospective cohort study designed to characterize subclinical cardiovascular disease and its progression. Its participants include 6,814 men and women free of clinical cardiovascular disease, aged 45 to 84 years, from 4 different self-reported ethnic groups (white, African American, Hispanic, and Chinese) recruited to meet prespecified race/ethnicity proportions. Participants were enrolled between July 2000 and August 2002 from 6 US communities (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; northern Manhattan, NY; and St Paul, MN). Study design and sampling have been described previously.31 We included a sample of 6,557 participants (96%) with available baseline cystatin C, creatinine, and carotid IMT measurements. Institutional review board approval was obtained at each participating center. Informed consent was obtained from all participants.
Measurements
Predictors
All biochemistry assays were performed on plasma or serum drawn in the morning after an overnight fast during the initial visit and stored at −70°C. Cystatin C was measured using a BNII nephelometer on plasma specimens (N Latex Cystatin C; Dade Behring Inc, Deerfield, IL).32 The assay range is 0.195 to 7.330 mg/L, with the reference range for young healthy individuals reported as 0.53 to 0.95 mg/L.33 Intra-assay coefficients of variation range from 2.0% to 2.8%, and interassay coefficients of variation range from 2.3% to 3.1%. Cystatin C–based estimated glomerular filtration rate (eGFR) was calculated using the formula developed by Stevens et al.34
Serum creatinine was measured using the Vitros analyzer (Johnson & Johnson Clinical Diagnostics Inc, Rochester, NY). The reference range in adult women is 0.4 to 1.1 mg/dL, and in adult men, 0.5 to 1.2 mg/dL (serum creatinine in mg/dL may be converted to μmol/L by multiplying by 88.4). The laboratory analytical coefficient of variation was 2.2%. Creatinine levels were first calibrated with those from the Cleveland Clinic Laboratory, the core laboratory of the Modification of Diet in Renal Disease (MDRD) Study, as previously described,35 then used to calculate eGFR using the 4-variable MDRD Study equation.4 CKD was defined as eGFR less than 60 mL/min/1.73 m2 (GFR in mL/min/1.73 m2 may be converted to mL/s/1.73 m2 by multiplying by 0.01667).36
Covariates
All adjusted models included demographic factors (age, sex, and race/ethnicity). Further adjustment accounted for systolic blood pressure of 140 mm Hg or greater; diastolic blood pressure of 90 mm Hg or greater; diabetes, defined as fasting glucose level of 126 mg/dL or greater (glucose in mg/dL may be converted to mmol/L by multiplying by 0.05552) or use of hypoglycemic medications; current cigarette smoking, defined as having smoked a cigarette in the last 30 days; current use of antihypertensive medications; current use of statin medications; and body mass index, calculated as weight in kilograms divided by height in meters squared. Fasting serum samples were used to measure total cholesterol, high-density lipoprotein cholesterol, triglycerides, glucose, C-reactive protein, and interleukin 6. Low-density lipoprotein cholesterol was calculated using the Friedewald equation.37 A random urine sample was analyzed for albumin and creatinine by means of nephelometry and the rate Jaffé reaction, respectively. Spot urine albumin-creatinine ratio was calculated, with microalbuminuria defined as 30 mg/g or greater.
Outcomes
Internal and common carotid IMTs were measured using high-resolution B-mode ultrasound (Logiq 700; General Electric Co, UK) as the distance between the lumen-intima and media-adventitia interfaces. Internal carotid IMT was measured at the level of the carotid bifurcation (common carotid artery bulb and proximal internal carotid artery) on 3 projections at a frequency of 8 MHz, and common carotid IMT was measured over a distance of 10 mm proximal to the common carotid bulb on one projection at a frequency of 13 MHz. Carotid IMT was defined as the mean of the maximal IMT of the near and far walls on both the left and right sides. For internal carotid IMT measurements, intraobserver coefficient of variation was 6.93%, and interobserver coefficient of variation was 18.8%. For common carotid IMT measurements, intraobserver and interobserver coefficients of variation were 3.48% and 10.7%, respectively. Ultrasound imaging was performed at all centers during the same visit that blood samples were collected for biochemical assays. Central reading was conducted at the Department of Radiology, New England Medical Center, Boston, MA.
Statistical Analysis
Our analysis began by categorizing the cohort into quintiles based on cystatin C level. Descriptive statistics are expressed as mean ± SD for continuous variables and percentage for categorical variables. Skewed variables were represented as median with interquartile range. We compared differences in baseline characteristics across cystatin C quintiles by using analysis of variance or Kruskal-Wallis test for continuous variables and χ2 or Fisher exact test for categorical variables, as appropriate. We graphically show mean carotid IMT measurement before and after age adjustment by quintile of cystatin C level. Splines also were created from an ordinary least squares model by using restricted cubic splines to show the association of cystatin C level with carotid IMT in unadjusted and age-adjusted models. Univariate and multivariate linear regression models were then used to evaluate kidney function as a predictor of carotid IMT with cystatin C level initially modeled by quintile. We repeated these analyses using cystatin C–based eGFR, categorized as greater than 75, 60 to 75, and less than 60 mL/min/1.73 m2. Cystatin C–based eGFR was also dichotomized at 75 mL/min/1.73 m2. Additional analyses were conducted using creatinine-based eGFR dichotomized at the defined cutoff for CKD of 60 mL/min/1.73 m2.36 Initial adjustment accounted for age, sex, and race/ethnicity (demographic model) and then for all the mentioned characteristics as candidate predictors (full model). Covariates that changed the coefficient of cystatin C by 5% or more were retained in the final model. We also evaluated the predictive value of cystatin C as a linear variable per SD and dichotomized cystatin C level at the highest quintile to elucidate a possible threshold effect. Logistic regressions were similarly performed with high IMT defined by the highest quintile. For the internal carotid IMT outcome, we repeated the analyses in persons older than 65 years. Candidate interactions of cystatin C levels with both race/ethnicity and microalbuminuria were tested for each end point. All analyses were stratified on study site. Internal carotid IMT, interleukin 6 level, and C-reactive protein level were natural log-transformed to satisfy the assumption of normality and constant variance of residuals. Two-sided probability values of 0.05 or less are considered statistically significant. S-Plus (release 6.1; Insightful Inc, Seattle, WA) and SPSS statistical software (release 14.0.2; SPSS Inc, Chicago, IL) were used for analyses.
Results
For the 6,557 participants, mean age was 62 years, 47% were men, and race/ethnicity distribution was 39% white, 27% African American, 22% Hispanic, and 12% Chinese. Mean cystatin C level was 0.89 ± 0.23 mg/L, and mean eGFR was 79 ± 18 mL/min/1.73 m2. Baseline characteristics of the study sample by quintiles of cystatin C level are listed in Table 1. Compared with participants in the lowest cystatin C quintile (≤0.74 mg/L), those in the highest cystatin C quintile (≥1.03 mg/L) were older, more likely men and white, and had greater prevalences of diabetes, current smoking, and use of antihypertensives and statins. Greater cystatin C level was also associated with greater values for body mass index, systolic blood pressure, low-density lipoprotein cholesterol, C-reactive protein, interleukin 6, and urine albumin-creatinine ratio and a lower level of high-density lipoprotein cholesterol.
Table 1. Baseline Characteristics of Multi-Ethnic Study of Atherosclerosis Participants by Cystatin C Quintiles.
| Characteristic | Cystatin C Quintile | P for Trend | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Cutoff (mg/L) | ≤0.74 | 0.75-0.82 | 0.83-0.90 | 0.91-1.02 | ≥1.03 | |
| No. of participants | 1,412 | 1,336 | 1,246 | 1,328 | 1,235 | |
| Age (y) | 57 ± 9 | 59 ± 9 | 62 ± 9 | 65 ± 10 | 69 ± 9 | <0.001 |
| Men | 503 (36) | 632 (47) | 634 (51) | 701 (53) | 634 (51) | <0.001 |
| Race/ethnicity | 0.001 | |||||
| White | 470 (33) | 465 (35) | 512 (41) | 546 (41) | 550 (45) | |
| African American | 423 (30) | 393 (29) | 320 (26) | 321 (24) | 336 (27) | |
| Hispanic | 274 (19) | 309 (23) | 288 (23) | 315 (24) | 246 (20) | |
| Chinese | 245 (17) | 169 (13) | 126 (10) | 146 (11) | 103 (8) | |
| Diabetes | 192 (14) | 167 (13) | 139 (11) | 160 (12) | 250 (20) | <0.001 |
| Current smoker | 153 (11) | 178 (13) | 169 (14) | 171 (13) | 185 (13) | <0.001 |
| Medication use | ||||||
| Antihypertensives | 362 (26) | 381 (29) | 442 (36) | 532 (40) | 701 (57) | <0.001 |
| Statins | 152 (11) | 184 (14) | 184 (15) | 220 (17) | 230 (19) | <0.001 |
| Body mass index (kg/m2) | 26.8 ± 4.9 | 27.7 ± 5.0 | 28.3 ± 5.3 | 28.8 ± 5.5 | 29.7 ± 5.9 | <0.001 |
| Systolic blood pressure (mm Hg) | 122 ± 20 | 124 ± 20 | 126 ± 21 | 128 ± 21 | 133 ± 23 | <0.001 |
| Diastolic blood pressure (mm Hg) | 71 ± 10 | 72 ± 10 | 72 ± 10 | 72 ± 10 | 72 ± 11 | 0.8 |
| High-density lipoprotein cholesterol (mg/dL) | 56 ± 16 | 51 ± 15 | 51 ± 14 | 49 ± 14 | 48 ± 14 | <0.001 |
| Low-density lipoprotein cholesterol (mg/dL) | 117 ± 31 | 119 ± 30 | 118 ± 31 | 117 ± 32 | 114 ± 32 | 0.007 |
| C-Reactive protein* (mg/dL) | 1.49 (0.63-3.44) | 1.67 (0.71-3.92) | 1.74 (0.82-3.85) | 2.09 (0.95-4.63) | 2.61 (1.19-5.26) | <0.001 |
| Interleukin 6* (pg/mL) | 0.93 (0.61-1.49) | 1.04 (0.69-1.63) | 1.13 (0.77-1.76) | 1.35 (0.87-2.10) | 1.63 (1.14-2.57) | <0.001 |
| Urine albumin-creatinine ratio* | 5.1 (3.4-10.1) | 5.0 (3.2-9.2) | 4.8 (3.0-9.0) | 5.3 (3.3-10.5) | 7.3 (3.8-19.7) | <0.001 |
| Measures of kidney function | ||||||
| Creatinine (mg/dL) | 0.84 ± 0.15 | 0.91 ± 0.16 | 0.95 ± 0.17 | 0.99 ± 0.17 | 1.18 ± 0.43 | <0.001 |
| eGFRMDRD (mL/min/1.73 m2) | 90 ± 16 | 85 ± 14 | 80 ± 18 | 76 ± 13 | 64 ± 16 | <0.001 |
| eGFRcysC (mL/min/1.73 m2) | 123 ± 14 | 102 ± 4 | 91 ± 3 | 81 ± 3 | 63 ± 10 | <0.001 |
Note: Values expressed as mean ± SD for continuous variables and number (percent) for categorical variables. High-density and low-density lipoprotein cholesterol in mg/dL may be converted to mmol/L by multiplying by 0.02586; serum creatinine in mg/dL to μmol/L by multiplying by 88.4; GFR in mL/min/1.73 m2 to mL/s/1.73 m2 by multiplying by 0.01667.
Abbreviations: eGFRcysC, estimated glomerular filtration rate based on cystatin C level; estimated GFRMDRD, estimated glomerular filtration rate calculated by using the creatinine-based 4-variable Modification of Diet in Renal Disease Study equation.
Median (25th to 75th percentile).
Internal Carotid IMT
Median internal carotid IMT was 0.85 mm (25th percentile, 0.68; 75th percentile, 1.29). Higher cystatin C quintiles were associated with greater internal carotid IMTs; however, this effect was substantially attenuated by age adjustment (Fig 1). The association of cystatin C level with internal carotid IMT remained significant after adjustment for demographic factors, but only in the highest quintile. This association was no longer statistically significant after full adjustment (Table 2).
Figure 1.
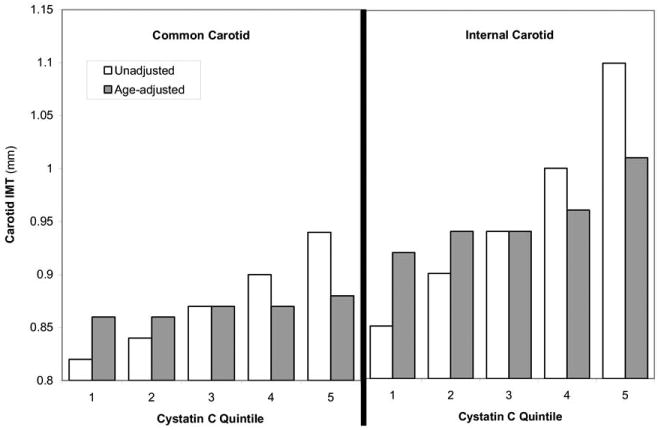
Association of cystatin C quintiles with unadjusted and age-adjusted mean common and internal carotid intima-media thickness (IMT) at the Multi-Ethnic Study of Atherosclerosis baseline visit.
Table 2. Association of Cystatin C Quintiles With Internal Carotid IMT at the Multi-Ethnic Study of Atherosclerosis Baseline Visit.
| Cutoff (mg/L) | Cystatin C Quintile | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||||
| ≤0.74 | 0.75-0.82 | 0.83-0.90 | 0.91-1.02 | ≥1.03 | ||||||
| Linear analyses | ||||||||||
| Model | β | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P |
| Unadjusted | 0.00 | Reference | 0.055 (0.020 to 0.090) | 0.002 | 0.099 (0.063 to 0.134) | <0.001 | 0.159 (0.124 to 0.194) | <0.001 | 0.261 (0.225 to 0.297) | <0.001 |
| Demographic* | 0.00 | Reference | 0.006 (−0.027 to 0.039) | 0.7 | 0.009 (−0.025 to 0.043) | 0.6 | 0.024 (−0.011 to 0.059) | 0.2 | 0.071 (0.034 to 0.108) | <0.001 |
| Full† | 0.00 | Reference | −0.003 (−0.036 to 0.029) | 0.8 | −0.010 (−0.043 to 0.024) | 0.6 | −0.010 (−0.044 to 0.025) | 0.6 | 0.005 (−0.032 to 0.043) | 0.8 |
| High internal carotid IMT (≥ 1.46 mm)‡ | OR | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | P for trend | ||||
| Unadjusted | 1.00 | 1.41 (1.13 to 1.76) | 1.77 (1.42 to 2.20) | 2.30 (1.86 to 2.85) | 3.46 (2.82 to 4.25) | <0.001 | ||||
| Demographic* | 1.00 | 1.14 (0.91 to 1.44) | 1.18 (0.94 to 1.49) | 1.25 (1.00 to 1.56) | 1.45 (1.16 to 1.82) | 0.02 | ||||
| Full† | 1.00 | 1.13 (0.89 to 1.43) | 1.14 (0.90 to 1.44) | 1.12 (0.89 to 1.41) | 1.09 (0.86 to 1.38) | 0.8 | ||||
Note: P values are by category unless otherwise noted.
Abbeviations: CI, confidence interval; IMT, intima-media thickness; OR, odds ratio.
Adjusted for age, sex, and race/ethnicity.
Adjusted for age, sex, race/ethnicity, diabetes, C-reactive protein level, interleukin 6 level, statin use, systolic blood pressure, diastolic blood pressure, antihypertensives, and microalbuminuria.
Defined by the highest quintile.
We used spline analysis to evaluate the association of cystatin C level and internal carotid IMT. The strong unadjusted association was almost entirely attenuated by age adjustment (Fig 2). We found similar results evaluating cystatin C as a linear variable per SD (0.23 mg/L): the strong association in unadjusted analysis (βunadjusted = 0.091; 95% confidence interval [CI], 0.079 to 0.103; P < 0.001) was largely attenuated by adjustment for demographics (βdemographic = 0.027; 95% CI, 0.015 to 0.040; P < 0.001) and was no longer significant with further addition of other risk factors (βfull = 0.005; 95% CI, −0.008 to 0.017; P = 0.5). Participants in the highest quintile of cystatin C had a more than 3-fold likelihood of high carotid IMT (≥1.46 mm) in unadjusted analysis, but this association was greatly attenuated by demographic adjustment and disappeared in the full model (Table 2). In individuals older than 65 years, we similarly found an association in unadjusted analysis, but only at the highest quintile of cystatin C (βquintile 5, unadjusted = 0.11; 95% CI, 0.04 to 0.18; P = 0.002); this association did not remain significant after demographic adjustment (βquintile 5, demographic = 0.04; 95% CI, −0.03 to 0.11; P = 0.3). There were no significant interactions between cystatin C and race/ethnicity or microalbuminuria for internal carotid IMT (race/ethnicity, P = 0.2; microalbuminuria, P = 0.5).
Figure 2.
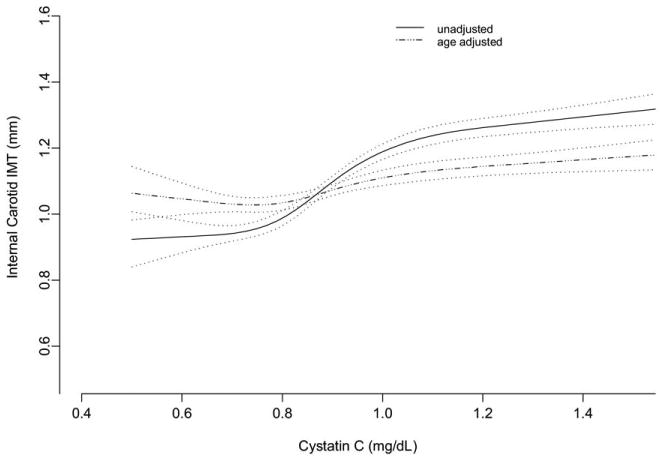
Spline plots (with 95% confidence interval bands) show the unadjusted and age-adjusted association of cystatin C level with internal carotid intima-media thickness (IMT) at the Multi-Ethnic Study of Atherosclerosis baseline visit.
When evaluated by using cystatin C–based eGFR increments of greater than 75, 60 to 75, and less than 60 mL/min/1.73 m2, lower levels of kidney function were associated with higher levels of internal carotid IMT in univariate analysis. Only the association with eGFR less than 60 mL/min/1.73 m2 remained statistically significant after demographic adjustment, but this association was lost after full adjustment (Table 3). Using an eGFR cutoff of 75 mL/min/1.73 m2, there again was an association after adjustment for demographic factors, but not after full adjustment. For comparison, CKD based on a creatinine-based eGFR less than 60 mL/min/1.73 m2 was associated with higher levels of internal carotid IMT in univariate analyses (βunadjusted = 0.142; 95% CI, 0.103 to 0.180; P < 0.001), but not after adjustment for demographic factors (βdemographic = 0.030; 95% CI, −0.007 to 0.067; P = 0.1).
Table 3. Association of Cystatin C–Based eGFR With Internal Carotid Intima-Media Thickness at the Multi-Ethnic Study of Atherosclerosis Baseline Visit.
| Cutoff value | Cystatin C-Based eGFR (mL/min/1.73 m2) | |||||
|---|---|---|---|---|---|---|
| >75 | 60-75 | <60 | ||||
| Linear analyses | ||||||
| Model | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P |
| Unadjusted | 0.00 | Reference | 0.144 (0.113 to 0.176) | <0.001 | 0.259 (0.209 to 0.309) | <0.001 |
| Demographic* | 0.00 | Reference | 0.034 (0.003 to 0.065) | 0.03 | 0.105 (0.056 to 0.154) | <0.001 |
| Full† | 0.00 | Reference | −0.005 (−0.036 to 0.025) | 0.7 | 0.04 (−0.009 to 0.089) | 0.1 |
Note: P values are by category.
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate.
Adjusted for age, sex, and race.
Adjusted for age, sex, race, diabetes, C-reactive protein level, interleukin 6, statin use, systolic blood pressure, diastolic blood pressure, antihypertensives, and microalbuminuria.
Common Carotid IMT
Mean common carotid IMT was 0.87 ± 0.19 mm. Similar to analyses with internal carotid IMT, we found an association between cystatin C level and common carotid IMT that was heavily attenuated by age adjustment (Fig 1). None of the quintiles were significantly associated with common carotid IMT after adjustment for demographic factors (P > 0.05). In linear analysis, each SD of cystatin C (0.23 mg/L) was associated with 0.040-mm higher carotid IMT in the unadjusted model (ie, βunadjusted = 0.040; 95% CI, 0.035 to 0.045; P < 0.001). The magnitude of this association was diminished by 90% after adjustment for demographic factors (βdemographic = 0.004; 95% CI, −0.001 to 0.009; P = 0.08). We did not observe significant interactions between cystatin C level and race/ethnicity (P = 0.2) or microalbuminuria (P = 0.4).
Similarly, using cystatin C–based eGFR, the associations between worse levels of kidney function and higher levels of common carotid IMT found in unadjusted linear analyses were lost after demographic adjustment at all increments, including eGFR less than 60 mL/min/1.73 m2 (βdemographic = 0.014; P = 0.2; βfull = −0.012; P = 0.2). Furthermore, CKD defined by using a creatinine-based eGFR was associated with higher levels of common carotid IMT (βunadjusted = 0.068; 95% CI, 0.052 to 0.083; P < 0.001), but not after adjustment for demographic factors (βdemographic = 0.007; 95% CI, −0.008 to 0.021; P = 0.4) or in the full model (P = 0.3).
Discussion
In individuals without clinical cardiovascular disease, mild kidney impairment measured using cystatin C level was strongly associated with common and internal carotid IMT in unadjusted analyses. However, this relationship was accounted for predominately by age. We found no independent association between either cystatin C level or CKD with common or internal carotid IMT. This observation suggests that accelerated atherosclerosis is unlikely to be the primary mechanism explaining the independent association of cystatin C level with cardiovascular risk.
Few prior studies have evaluated the association of mild kidney dysfunction with carotid IMT. Earlier work found that participants on hemodialysis therapy have greater atherosclerotic burden, manifested by higher carotid IMT,15-19 and IMT is an independent predictor of death and cardiovascular events in this population.20-22 Predialysis patients have an atherosclerotic burden similar to persons on dialysis therapy, suggesting that increased carotid IMT is a function of kidney disease, rather than dialysis itself.23 Similarly, several,24-27 but not all,28 studies have shown higher carotid IMT in participants with moderate to severe kidney disease. However, such an association was not observed in persons with milder kidney damage. Zhang et al29 examined 1,046 persons of Chinese descent and found that carotid IMT values in participants with eGFR of 30 to 60 mL/min/1.73 m2 and 60 to 90 mL/min/1.73 m2 were not significantly different from those with eGFR of 90 mL/min/1.73 m2 or greater after adjustment for cardiovascular risk factors. In 95 participants with kidney disease (mean chromium-51 EDTA-measured GFR, 36 ± 16 mL/min/1.73 m2), Briet et al30 found no difference in mean carotid IMT compared with healthy controls.
Although cystatin C has elucidated associations between kidney function and cardiovascular events that were not detected by using eGFR,7 initial studies have been inconclusive for its association with carotid IMT. Watanabe et al38 noted a correlation between serum cystatin C level and carotid IMT (r = 0.54; P < 0.001) in a study of 60 hypertensive patients, although there was no adjustment for potential confounders because it was not the primary end point. Rodondi et al39 recently found that cystatin C level was not independently associated with carotid atherosclerosis in a study population including 523 younger adults aged 35 to 64 years, primarily of African descent. Our study expands upon the findings of these prior studies to a larger and more diverse cohort with a broader range of cystatin C levels. The absence of an association in this group with more preserved kidney function suggests that impaired kidney function may have an association with accelerated atherosclerosis only in persons with severe kidney disease. Albuminuria, a manifestation of atherosclerotic microvascular disease, also had no association with carotid IMT in a study by Kramer et al40 in the MESA cohort. Conversely, a smaller study by Rodoni et al39 found a direct association in their participants.
These findings should be interpreted in the context of other studies that evaluated the association of mild to moderately impaired kidney function with subclinical cardiovascular disease measures. In MESA, Ix et al41,42 similarly found that cystatin C levels had a linear association with measures of vascular calcification (valvular and coronary artery calcium) in unadjusted analyses, yet had no association after multivariable adjustment for demographic characteristics and traditional risk factors. The absence of an association between cystatin C level with vascular calcification supports our findings that early kidney disease does not appear to be associated with increased levels of atherosclerosis. Conversely, cystatin C levels greater than 1.0 mg/L, the high quintile within MESA, were independently associated with greater left ventricular mass index and prevalent left ventricular hypertrophy.43 In addition, cystatin C levels had linear and independent associations with inflammatory marker44-47 and systolic blood pressure levels.48
The importance of this work is to elucidate the mechanisms underlying the remarkably strong association of cystatin C level with cardiovascular disease, which is independent of traditional risk factors,6,49 inflammatory biomarkers,44 and prevalent cardiovascular disease.6,7 Our null finding implies that accelerated atherosclerosis is unlikely to be the primary mechanism. However, there are other candidate mediators for which roles are yet to be clarified, such as vascular stiffness, left ventricular remodeling, and volume overload. Other cardiovascular lesions also have been described in people with CKD, including arteriolosclerosis, medial vascular calcification, and cardiac fibrosis.8,50 However, progressive atherosclerosis may have greater importance in persons with more advanced CKD.
There are several limitations to this study. First, this study is limited by its cross-sectional design. Therefore, we cannot determine whether kidney function predicts the progression of atherosclerosis over time. In addition, serum cystatin C level and carotid IMT may be manifestations of parallel processes of atherosclerosis. Another limitation is the potential for misclassification error in both the predictor and outcome variables. We do not have a direct measure of GFR and therefore cannot determine how accurately cystatin C or creatinine levels reflect actual kidney function in this study. Also, the range of cystatin C levels is lower than in the ambulatory cohorts in which cystatin C level has predicted increased cardiovascular risk.6,7,49 The absence of a statistically significant association therefore may reflect the healthier spectrum of kidney function in this population. Additionally, there may not be an independent association between cystatin C level and atherosclerosis burden in this particular population because limited atherosclerosis is present. However, our carotid IMT range is similar to studies that found associations between carotid IMT and cardiovascular events,11,12,14 and analyses were similar in persons older than 65 years, a subgroup with a higher IMT. Furthermore, our multivariate analysis of internal carotid IMT may have been overfit because some covariates, such as blood pressure, may be a result of kidney dysfunction. Finally, although carotid IMT has been independently associated with increased risk of cardiovascular events, carotid artery IMT is only one measure of atherosclerosis and may not represent all vascular disease in the body.
In conclusion, the evaluation of subclinical cardiovascular measures is important for elucidating the mechanisms for the strong association between impaired kidney function and increased cardiovascular risk. Based on our findings, processes other than accelerated atherosclerosis should be evaluated as candidate mechanisms.
Acknowledgments
The authors thank the other investigators, staff, and participants of MESA for valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Support: This research was supported by contracts N01-HC-95159 through N01-HC-95167 from the National Heart, Lung, and Blood Institute (NHLBI). Dr Shlipak is supported by R01 DK066488-01 and the American Heart Association Established Investigator Award. Dade Behring Inc donated to MESA and the NHLBI the reagents used to measure cystatin C.
Footnotes
Financial Disclosure: None.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 3.Shlipak MG, Simon JA, Grady D, Lin F, Wenger NK, Furberg CD. Renal insufficiency and cardiovascular events in postmenopausal women with coronary heart disease. J Am Coll Cardiol. 2001;38:705–711. doi: 10.1016/s0735-1097(01)01450-4. [DOI] [PubMed] [Google Scholar]
- 4.National Kidney Foundation K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(suppl 1):S1–S266. [PubMed] [Google Scholar]
- 5.Sarnak MJ, Levey AS. Cardiovascular disease and chronic renal disease: A new paradigm. Am J Kidney Dis. 2000;35(suppl 1):S117–S131. doi: 10.1016/s0272-6386(00)70239-3. [DOI] [PubMed] [Google Scholar]
- 6.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 7.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 8.D'Amico G. Statins and renal diseases: From primary prevention to renal replacement therapy. J Am Soc Nephrol. 2006;17(suppl 2):S148–S152. doi: 10.1681/ASN.2005121341. [DOI] [PubMed] [Google Scholar]
- 9.Muntner P, Hamm LL, Kusek JW, Chen J, Whelton PK, He J. The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Intern Med. 2004;140:9–17. doi: 10.7326/0003-4819-140-1-200401060-00006. [DOI] [PubMed] [Google Scholar]
- 10.Shlipak MG, Fried LF, Crump C, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107:87–92. doi: 10.1161/01.cir.0000042700.48769.59. [DOI] [PubMed] [Google Scholar]
- 11.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: The Rotterdam Study. Circulation. 1997;96:1432–1437. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 12.Burke GL, Evans GW, Riley WA, et al. Arterial wall thickness is associated with prevalent cardiovascular disease in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 1995;26:386–391. doi: 10.1161/01.str.26.3.386. [DOI] [PubMed] [Google Scholar]
- 13.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 14.Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: Prospective data from the Carotid Atherosclerosis Progression Study (CAPS) Stroke. 2006;37:87–92. doi: 10.1161/01.STR.0000196964.24024.ea. [DOI] [PubMed] [Google Scholar]
- 15.Stompor T, Krasniak A, Sulowicz W, et al. Changes in common carotid artery intima-media thickness over 1 year in patients on peritoneal dialysis. Nephrol Dial Transplant. 2005;20:404–412. doi: 10.1093/ndt/gfh597. [DOI] [PubMed] [Google Scholar]
- 16.Zoccali C, Benedetto FA, Maas R, et al. Asymmetric dimethylarginine, C-reactive protein, and carotid intima-media thickness in end-stage renal disease. J Am Soc Nephrol. 2002;13:490–496. doi: 10.1681/ASN.V132490. [DOI] [PubMed] [Google Scholar]
- 17.Burdick L, Periti M, Salvaggio A, et al. Relation between carotid artery atherosclerosis and time on dialysis. A non-invasive study in vivo. Clin Nephrol. 1994;42:121–126. [PubMed] [Google Scholar]
- 18.Hojs R. Carotid intima-media thickness and plaques in hemodialysis patients. Artif Organs. 2000;24:691–695. doi: 10.1046/j.1525-1594.2000.06466.x. [DOI] [PubMed] [Google Scholar]
- 19.Kawagishi T, Nishizawa Y, Konishi T, et al. High-resolution B-mode ultrasonography in evaluation of atherosclerosis in uremia. Kidney Int. 1995;48:820–826. doi: 10.1038/ki.1995.356. [DOI] [PubMed] [Google Scholar]
- 20.Benedetto FA, Mallamaci F, Tripepi G, Zoccali C. Prognostic value of ultrasonographic measurement of carotid intima media thickness in dialysis patients. J Am Soc Nephrol. 2001;12:2458–2464. doi: 10.1681/ASN.V12112458. [DOI] [PubMed] [Google Scholar]
- 21.Kato A, Takita T, Maruyama Y, Kumagai H, Hishida A. Impact of carotid atherosclerosis on long-term mortality in chronic hemodialysis patients. Kidney Int. 2003;64:1472–1479. doi: 10.1046/j.1523-1755.2003.00205.x. [DOI] [PubMed] [Google Scholar]
- 22.Nishizawa Y, Shoji T, Maekawa K, et al. Intima-media thickness of carotid artery predicts cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2003;41(suppl 3):S76–S79. doi: 10.1053/ajkd.2003.50090. [DOI] [PubMed] [Google Scholar]
- 23.Shoji T, Emoto M, Tabata T, et al. Advanced atherosclerosis in predialysis patients with chronic renal failure. Kidney Int. 2002;61:2187–2192. doi: 10.1046/j.1523-1755.2002.00372.x. [DOI] [PubMed] [Google Scholar]
- 24.Preston E, Ellis MR, Kulinskaya E, Davies AH, Brown EA. Association between carotid artery intima-media thickness and cardiovascular risk factors in CKD. Am J Kidney Dis. 2005;46:856–862. doi: 10.1053/j.ajkd.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 25.Shlipak MG, Fried LF, Crump C, et al. Cardiovascular disease risk status in elderly persons with renal insufficiency. Kidney Int. 2002;62:997–1004. doi: 10.1046/j.1523-1755.2002.00522.x. [DOI] [PubMed] [Google Scholar]
- 26.Szeto CC, Chow KM, Woo KS, et al. Carotid intima media thickness predicts cardiovascular diseases in Chinese predialysis patients with chronic kidney disease. J Am Soc Nephrol. 2007;18:1966–1972. doi: 10.1681/ASN.2006101184. [DOI] [PubMed] [Google Scholar]
- 27.Zoungas S, Ristevski S, Lightfoot P, et al. Carotid artery intima-medial thickness is increased in chronic renal failure. Clin Exp Pharmacol Physiol. 2000;27:639–641. doi: 10.1046/j.1440-1681.2000.03301.x. [DOI] [PubMed] [Google Scholar]
- 28.Leskinen Y, Lehtimaki T, Loimaala A, et al. Carotid atherosclerosis in chronic renal failure—The central role of increased plaque burden. Atherosclerosis. 2003;171:295–302. doi: 10.1016/j.atherosclerosis.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Zhao F, Yang Y, et al. Association between carotid artery intima-media thickness and early-stage CKD in a Chinese population. Am J Kidney Dis. 2007;49:786–792. doi: 10.1053/j.ajkd.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Briet M, Bozec E, Laurent S, et al. Arterial stiffness and enlargement in mild-to-moderate chronic kidney disease. Kidney Int. 2006;69:350–357. doi: 10.1038/sj.ki.5000047. [DOI] [PubMed] [Google Scholar]
- 31.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: Objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 32.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59:1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 33.Finney H, Newman DJ, Price CP. Adult reference ranges for serum cystatin C, creatinine and predicted creatinine clearance. Ann Clin Biochem. 2000;37:49–59. doi: 10.1258/0004563001901524. [DOI] [PubMed] [Google Scholar]
- 34.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: Comparison of traditional and novel risk factors. JAMA. 2005;293:1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 36.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 37.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 38.Watanabe S, Okura T, Liu J, et al. Serum cystatin C level is a marker of end-organ damage in patients with essential hypertension. Hypertens Res. 2003;26:895–899. doi: 10.1291/hypres.26.895. [DOI] [PubMed] [Google Scholar]
- 39.Rodondi N, Yerly P, Gabriel A, et al. Microalbuminuria, but not cystatin C, is associated with carotid atherosclerosis in middle-aged adults. Nephrol Dial Transplant. 2007;22:1107–1114. doi: 10.1093/ndt/gfl733. [DOI] [PubMed] [Google Scholar]
- 40.Kramer H, Jacobs DR, Jr, Bild D, et al. Urine albumin excretion and subclinical cardiovascular disease. The Multi-Ethnic Study of Atherosclerosis. Hypertension. 2005;46:38–43. doi: 10.1161/01.HYP.0000171189.48911.18. [DOI] [PubMed] [Google Scholar]
- 41.Ix JH, Shlipak M, Katz R, et al. Kidney function and aortic valve and mitral annular calcification in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2007;50:412–420. doi: 10.1053/j.ajkd.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 42.Ix JH, Katz R, Kestenbaum B, et al. Association of mild to moderate kidney dysfunction and coronary calcification. J Am Soc Nephrol. 2008;19:579–585. doi: 10.1681/ASN.2007070765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moran A, Katz R, Jenny NS, et al. Left ventricular hypertrophy in mild and moderate reduction in kidney function determined using cardiac magnetic resonance imaging and cystatin C: The Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. doi: 10.1053/j.ajkd.2008.06.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh D, Whooley MA, Ix JH, Ali S, Shlipak MG. Association of cystatin C and estimated GFR with inflammatory biomarkers: The Heart and Soul Study. Nephrol Dial Transplant. 2007;22:1087–1092. doi: 10.1093/ndt/gfl744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 46.Keller CR, Odden MC, Fried LF, et al. Kidney function and markers of inflammation in elderly persons without chronic kidney disease: The Health, Aging, and Body Composition Study. Kidney Int. 2007;71:239–244. doi: 10.1038/sj.ki.5002042. [DOI] [PubMed] [Google Scholar]
- 47.Shlipak MG, Katz R, Cushman M, et al. Cystatin-C and inflammatory markers in the ambulatory elderly. Am J Med. 2005;118:1416.e25–1416.e31. doi: 10.1016/j.amjmed.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 48.Peralta CA, Whooley MA, Ix JH, Shlipak MG. Kidney function and systolic blood pressure new insights from cystatin C: Data from the Heart and Soul Study. Am J Hypertens. 2006;19:939–946. doi: 10.1016/j.amjhyper.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ix JH, Shlipak MG, Chertow GM, Whooley MA. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: Data from the Heart and Soul Study. Circulation. 2007;115:173–179. doi: 10.1161/CIRCULATIONAHA.106.644286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Demer LL, Tintut Y, Parhami F. Novel mechanisms in accelerated vascular calcification in renal disease patients. Curr Opin Nephrol Hypertens. 2002;11:437–443. doi: 10.1097/00041552-200207000-00011. [DOI] [PubMed] [Google Scholar]


