Abstract
The primary surgical techniques used in myomectomy are open surgery, laparoscopic surgery, and, recently, robot-assisted (“robotic”) surgery. The optimal surgical treatment of myomas is still a subject of debate because of the limitations of minimally invasive techniques and the disadvantages of laparotomy. In this article, the authors discuss the technique and the application of robotic myomectomy in the treatment of uterine fibroids.
Key words: Robotic myomectomy, Uterine fibroids, Laparoscopic myomectomy, Minimally invasive surgery
Uterine fibroids (leiomyomas) are the most common pelvic tumor of the female genital tract whose occurrence tends to increase with age until menopause. The most frequent clinical symptoms are menorrhagia, pelvic pain, bloating, and infertility. Earlier diagnosis and a tendency to delay child-bearing have increased the need for uterine-sparing techniques in the surgical treatment of fibroids.1
The primary surgical techniques used in myomectomy are open surgery, laparoscopic surgery, and, recently, robot-assisted (“robotic”) surgery. The optimal surgical treatment of myomas is still a subject of debate because of the limitations of minimally invasive techniques and the disadvantages of laparotomy.
In this article, we discuss the technique and the application of robotic myomectomy in the treatment of uterine fibroids.
Robotic Surgery in Gynecology
Gynecologic surgery has traditionally been taught through laparotomy or a vaginal approach. During laparotomy, the surgeon has the benefit of depth perception and haptic feedback from the resistance of tissue. The human wrist affords 6 degrees of freedom for intra-abdominal suturing.2
The advent of laparoscopy created a minimally invasive alternative to laparotomy for cases that cannot be performed vaginally. Laparoscopy has evolved significantly over the last decades, with improved hand instrumentation, electrosurgical devices, and high-intensity light sources.3 Advantages of laparoscopy over laparotomy include decreased postoperative pain, a shorter hospital stay, faster return to normal activities, better cosmetic results, and less blood loss.4,5
In April 2005, the da Vinci® Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA) was the first robot approved by the US Food and Drug Administration (FDA) for gynecologic applications. This represents an enhancement along the continuum of laparoscopic technological advances. The instrumentation provides 7 degrees of freedom: 3 degrees provided by the robotic arms (insertion, pitch, yaw) and 4 degrees from the “wristed” instruments (pitch, yaw, roll, and grip).3 This improves dexterity and enables the surgeon to manipulate and dissect tissue in a delicate, controlled fashion. Robotic technology may improve efficiency, accuracy, ease, and comfort associated with the performance of laparoscopic operations such as hysterectomies for benign and malignant indications, myomectomies, tubal reanastomoses, complex endometriosis surgery, and sacrocolpopexies.2 Cited advantages of robotic technology over conventional laparoscopy include absence of tremor, a 3-dimensional image, superior instrument articulation, downscaling of movements, and comfort for the surgeon.6
Advocates of this technology report a faster operator learning curve with the robotic system, as documented by the fact that many surgeons with limited advanced laparoscopic skills have successfully converted their practice from primarily laparotomy to minimally invasive surgery using the da Vinci system.7
A recent retrospective study by Lenihan and colleagues8 addressed the learning curve when using the da Vinci Surgical System in benign gynecologic surgery. A team of 2 gynecologic laparoscopists offered the option of having their procedure performed laparoscopically with robotic assistance to 113 patients who would have otherwise been offered a transabdominal or conventional laparoscopic procedure. Robot setup times by the operative room staff, operative times for use of robot, total operative times, and perioperative outcome were analyzed. The learning curve was defined as the number of cases required to stabilize operative time to perform the various procedures. Total operative times for hysterectomies, the most commonly performed procedure in this study, sequentially stabilized at approximately 95 minutes after 50 cases. The authors concluded that, in the hands of surgeons with advanced laparoscopic skills, the learning curve for benign gynecologic interventions is 50 cases. It has been suggested that, because of the advantages of robotic technology, surgeons may be able to perform minimally invasive surgical procedures that they were unable to perform by laparoscopy.9
Comparison of Robotic (and Robot-Assisted), Open, and Laparoscopic Myomectomy
Laparotomy has long been the standard surgical approach to myomectomy because it allows easy access to the uterus for the removal of large fibroids. However, it usually requires a large incision and, compared with minimally invasive surgery, is associated with longer hospitalization, considerably higher levels of postoperative analgesia, and increased morbidity.7,8
Moreover, second-look laparoscopic studies examining adhesion formation after an open myomectomy have demonstrated the presence of adhesions in as many as 55.5% to 93.7% of cases, depending on hysterotomy location.10 Similar studies following laparoscopic myomectomy have reported a much lower incidence of adhesions, ranging from 29.4% to 35.6% per patient, and of 11.2% to 16.7% per myomectomy site.11
In spite of these considerations, and although the laparoscopic approach is increasingly used by some groups,12 most myomectomies today are still performed via laparotomy.13
Several studies on the role of the robot in the surgical removal of fibroids have been reported. Advincula and colleagues14 introduced robotassisted myomectomy in 2004 as a safe and reproducible surgical approach to uterine fibroids. In 2007, the same group compared short-term surgical outcomes and costs of robotic-assisted and open myomectomy.15 The 58 patients with symptomatic fibroids included in their retrospective analysis were case-matched based on age, body mass index (BMI), and myoma weight. Patients who underwent robot-assisted laparoscopic myomectomy had significantly decreased estimated blood loss, complication rates, and length of stay, but the operative times were significantly longer in the robotic group. Professional charges and hospital charges were significantly higher in the robotic group, with mean hospital charges of approximately $30,000 versus $13,000, respectively. Professional reimbursement was not significantly different between the groups, but mean hospital reimbursement rates for the robotic group were significantly higher. In their conclusions, the authors raised the question whether the observed benefits may prove to have a significant societal benefit that will outweigh the upfront financial impact.15
Two small, retrospective studies have recently compared robotic myomectomy with standard laparoscopic myomectomy. Bedient and colleagues16 reported a chart review of 81 patients undergoing robotic (n = 40) or laparoscopic (n = 41) myomectomy. When they adjusted for uterine size and fibroid size and number, they did not find significant differences between robotic and laparoscopic groups for short-term surgical outcomes such as mean operating time (141 vs 166 minutes), mean blood loss (100 vs 250 mL), intraoperative or postoperative complications (2% vs 20% and 11% vs 17%, respectively), hospital stay more than 2 days (12% vs 23%), readmissions, or symptom resolution.16 Long-term outcomes and costs were not assessed in this study.
A similar study by Nezhat and coworkers matched 15 cases of robotic myomectomies (RALM) with a control group of 35 standard laparoscopic myomectomies.17 The 2 groups were matched by age, BMI, parity, previous abdominopelvic surgery, size, number, and location of myomas. The RALM required a significant prolonged mean surgical time over laparoscopic myomectomy (234 vs 203 minutes). There were no significant differences in blood loss, hospitalization time, and postoperative complications. The authors concluded that RALM does not offer any significant advantages in the hands of a skilled laparoscopic surgeon.17
An important caveat in the interpretation of these studies is that neither offers insight into the level of expertise of the robotic myomectomy teams compared with the standard laparoscopic myomectomy teams. As we mentioned before, the teams’ status within the learning curve is a fundamental variable to be addressed when comparing proficiency levels and complication rates of robotic procedures. 8 However, at this time there are no data demonstrating superiority of the robotic approach over standard laparoscopy for myomectomy. Larger retrospective (and, ideally, prospective) studies are needed that compare robotic and laparoscopic myomectomy performed by teams that are beyond reasonable learning curves for both techniques.
It is very likely that the different techniques will continue to coexist in the future, and that open, laparoscopic, robotic, and robot-assisted myomectomy will be performed based on the clinical scenario and surgeon expertise. More research is needed to define preoperative factors that make one approach superior to another for a given clinical situation, both in terms of patient outcomes and costeffectiveness.
Technique of Robotic Myomectomy
Given that robotic myomectomy is a relatively new operation, variations in technique exist among centers. In this article, we describe the techniques used at Brigham and Women’s Hospital (Boston, MA).
Patient Selection
Candidates for robotic myomectomy are patients with any single myoma smaller than 15 cm and with fewer than 15 myomas in total. The procedure is currently not offered to patients with a uterine fundus that is palpable above the umbilicus, or with diffuse adenomyosis by magnetic resonance imaging (MRI) or whose uterine cavity cannot be clearly visualized by MRI. Preoperative MRI is very useful to determine myoma size, number, and locations, and to rule out adenomyosis. It is also used to assist in deciding whether a standard robotic myomectomy or a hybrid robotic myomectomy will be performed. In the hybrid procedure, a conventional laparoscopic myomectomy is followed by reconstruction with the da Vinci robot.
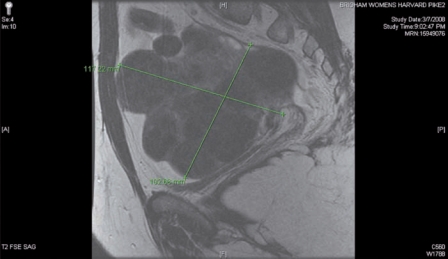
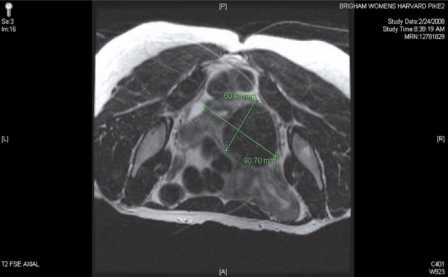
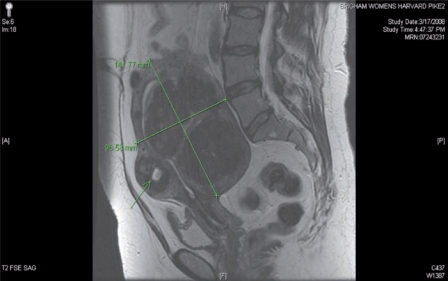
The presence of diffuse myomatosis makes the patient a poor candidate for robotic myomectomy (Figure 1). A broad ligament myoma makes the patient a good candidate for a standard robotic myomectomy (Figure 2). A large intramural myoma makes the patient a good candidate for a hybrid robotic myomectomy (Figure 3).
Figure 1.
The presence of diffuse myomatosis makes the patient a poor candidate for robotic myomectomy.
Figure 2.
The presence of a broad ligament myoma makes the patient a good candidate for a classic robotic myomectomy.
Figure 3.
The presence of a large intramural myoma makes the patient a good candidate for a hybrid robotic-assisted myomectomy. Note that the uterine fundus is just below the patient’s umbilicus.
Basic Setup
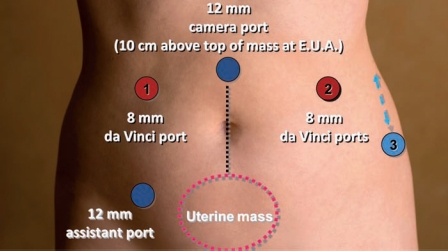
The basic robotic setup consists of the patient-side robot, a vision cart, and the robotic master console.3 Patient positioning and setup are identical to conventional laparoscopy, in dorsal lithotomy position in Allen stirrups with the arms padded and tucked. Using a combination of hand controls and foot pedals, the robotic surgeon operates from the remote master console. Our preferred trocar sites for both standard and hybrid robotic myomectomy are shown in Figure 4.
Figure 4.
Robotic trocar placement.
After trocar placement, the patient is placed in Trendelenburg position and the docking process is undertaken. For this, a patient-side cart with robotic arms is brought either between the patient’s legs or to the outside of the left Allen stirrup and each robotic arm is connected to one trocar. The right lower quadrant trocar is left undocked and used by the bedside assistant as a conventional laparoscopic port for suction/irrigation, passage of needles, tissue retraction, and morcellation. The bedside assistant also performs instrument exchanges on the robotic arms. Our preferred robotic instruments for this operation include the tenaculum forceps, the Maryland bipolar forceps, the harmonic shears, and the large and mega needle drivers.
Hysterotomy and Myoma Retrieval
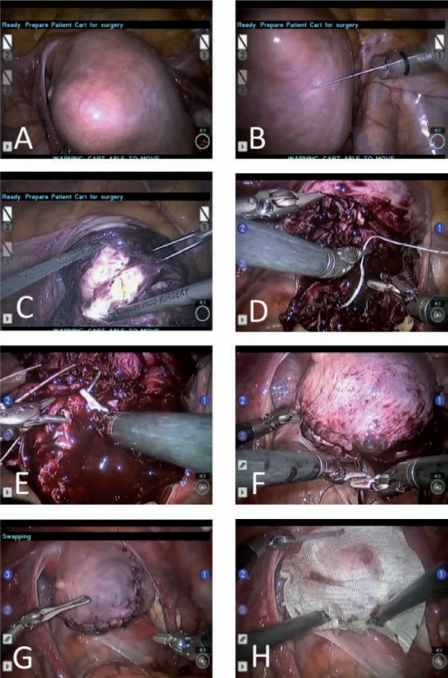
After the fibroid location has been exactly determined by visual inspection and MRI review in the operating room, a dilute concentration of vasopressin is injected into the myometrium surrounding the myoma (Figure 5A and B). Using the robotic harmonic shears, a hysterotomy is made over the myoma (Figure 5C). The incision can be made in a longitudinal or horizontal axis. Using the robot, the operator can enucleate the myoma in an identical fashion to open myomectomy, employing a robotic tenaculum and/or a bipolar coagulator in addition to the harmonic shears. The bedside assistant can provide additional traction on the myoma using a laparoscopic tenaculum. In the hybrid technique (usually preferred for myomata larger than 10 cm in maximum diameter), this portion of the operation is performed by conventional laparoscopy. In our view, the advantages of conventional laparoscopic enucleation of large myomata before robotic repair of the hysterotomy are several: (1) preservation of tactile sensation while separating heavy tumoral masses from delicate reproductive structures, (2) use of a rigid (not articulated) tenaculum that is capable of exerting significant pull at every angle with the benefit of haptic feedback and without risk of equipment damage, and (3) effective operation outside of the pelvis and into the upper abdominal quadrants.
Figure 5.
Technique of robotic myomectomy. (A, B) After the fibroid location has been exactly determined, a dilute concentration of vasopressin is injected into the myometrium surrounding the myoma. (C) Using the robotic harmonic shears, a hysterotomy is made over the myoma. (D–G) A multilayer closure is performed employing sutures and suturing techniques that are identical to those of an open myomectomy. (H>) An adhesion barrier may be placed onto the closed hysterotomy to prevent future scar tissue formation.
The removed myomata are placed in the posterior cul-de-sac or in the paracolic gutters for retrieval and morcellation at the end of the case. A precise written count of all enucleated myomata is kept by the surgical team to avoid the risk of leaving myoma tissue behind.
Hysterotomy Closure and Myoma Morcellation
After removal of each myoma, a multilayer closure is performed employing sutures and suturing techniques that are identical to those of an open myomectomy (Figure 5D-G). For the deep layers, interrupted or running sutures of 0 polyglactin 910 are used, prior to closure of the uterine serosa with a baseball-stitch technique using 2-0 poliglecaprone 25 or a bidirectional barbed suture.18 An intracorporeal technique is used, utilizing the accessory port for suture passage.
In hybrid robotic myomectomy, the robot is swiftly docked to accomplish the above-described uterine repair after 1 or sometimes more than 1 of the largest myomata are enucleated by conventional laparoscopy. This technique entails a variable time lag between the completion of the conventional laparoscopic myoma enucleation and the timepoint at which the operator is sitting at the console with the ability to control the resulting uterine bleeding. This “docking time” is a function of the team’s proficiency and decreases significantly throughout the learning curve. Because of this consideration in particular, a hybrid robotic myomectomy has to be regarded as an advanced robotic technique and should not be planned during the robotic team’s initial learning curve.
Following hysterotomy closure, the specimens are morcellated and retrieved through the accessory port, usually after the robot itself has been undocked.
An adhesion barrier may be placed onto the closed hysterotomy to prevent future scar tissue formation (Figure 5H).
Postoperative Care
Postoperative aftercare is identical to that used for laparoscopic gynecologic procedures, with the goal of early mobilization and same-day discharge. At the follow-up appointment, the patient may be counseled on the need for cesarean delivery in future pregnancies, given the uterine rupture risk because of weakening of the endometrium. Although a successful term pregnancy after robotic myomectomy has been reported,19 larger studies on pregnancy outcomes after robotic myomectomy are not currently available. Until specific data do become available, surgeons may still counsel patients of reproductive age with reassuring data from large, multicenter studies on standard laparoscopic myomectomy.20
Conclusions and Practice Points
The ultimate role of laparoscopic myomectomy, be it conventional or robotic, is to supplant open myomectomy as the standard of care for conservative surgical treatment of uterine fibroids.
Robotic surgery has the potential to become an enabler for gynecologic minimally invasive surgery, especially for microsurgical and suture-intensive operations such as myomectomy. The role of robotic technology in this specific operation is to guarantee to the patient that she will have a procedure that is as effective as a classic open myomectomy, but is as safe and acceptable as a laparoscopic operation.
Large, retrospective studies and prospective, cohort studies comparing conventional and robotic myomectomy are underway, as well as multicenter studies on reproductive safety of robotic myomectomy. It will be vital to define preoperative factors that make one approach superior to another for a given clinical situation, both in terms of patient outcomes and cost effectiveness.
Improvements in robotic technology are also expected in terms of affordability, miniaturization, haptic feedback, assisted docking, singleincision applications, and 3-dimensional imaging fusion. This will likely make robotic myomectomy a moving target in terms of comparing it with standard laparoscopic techniques, with plenty of opportunity for clinical investigation.
Main Points.
The advent of laparoscopy created a minimally invasive alternative to laparotomy for cases that cannot be performed vaginally. Advantages of laparoscopy over laparotomy include decreased postoperative pain, a shorter hospital stay, faster return to normal activities, better cosmetic results, and less blood loss.
Cited advantages of robotic technology over conventional laparoscopy include absence of tremor, a 3-dimensional image, superior instrument articulation, downscaling of movements, and comfort for the surgeon.
It is very likely that the different techniques will continue to coexist in the future, and that open, laparoscopic, robotic, and robotassisted myomectomy will be performed based on the clinical scenario and surgeon expertise. More research is needed to define preoperative factors that make one approach superior to another for a given clinical situation, both in terms of patient outcomes and cost effectiveness.
References
- 1.Manyonda I, Sinthamoney E, Belli AM. Controversies and challenges in the modern management of uterine fibroids. BJOG. 2004;111:95–102. doi: 10.1046/j.1471-0528.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- 2.Chen CC, Falcone T. Robotic gynecologic surgery: past, present, and future. Clin Obstet Gynecol. 2009;52:335–343. doi: 10.1097/GRF.0b013e3181b08adf. [DOI] [PubMed] [Google Scholar]
- 3.Visco AG, Advincula AP. Robotic gynecologic surgery. Obstet Gynecol. 2008;112:1369–1384. doi: 10.1097/AOG.0b013e31818f3c17. [DOI] [PubMed] [Google Scholar]
- 4.Mais V, Ajossa S, Guerriero S, et al. Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996;174:654–658. doi: 10.1016/s0002-9378(96)70445-3. [DOI] [PubMed] [Google Scholar]
- 5.Paraiso MF, Walters MD, Rackley RR, et al. Laparoscopic and abdominal sacral colpopexies: a comparative cohort study. Am J Obstet Gynecol. 2005;192:1752–1758. doi: 10.1016/j.ajog.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 6.Magrina JF. Robotic surgery in gynecology. Eur J Gynaecol Oncol. 2007;28:77–82. [PubMed] [Google Scholar]
- 7.Holloway RW, Patel SD, Ahmad S. Robotic surgery in gynecology. Scand J Surg. 2009;98:96–109. doi: 10.1177/145749690909800205. [DOI] [PubMed] [Google Scholar]
- 8.Lenihan JP , Jr, Kovanda C, Seshadri-Kreaden U. What is the learning curve for robotic assisted gynecologic surgery? J Minim Invasive Gynecol. 2008;15:589–594. doi: 10.1016/j.jmig.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Monsarrat N, Collinet P, Narducci F, et al. [Robotic assistance in gynaecological surgery: State-of-the-art] Gynecol Obstet Fertil. 2009;37:415–424. doi: 10.1016/j.gyobfe.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Tulandi T, Murray C, Guralnick M. Adhesion formation and reproductive outcome after myomectomy and second-look laparoscopy. Obstet Gynecol. 1993;82:213–215. [PubMed] [Google Scholar]
- 11.Takeuchi H, Kinoshita K. Evaluation of adhesion formation after laparoscopic myomectomy by systematic second-look microlaparoscopy. J Am Assoc Gynecol Laparosc. 2002;9:442–446. doi: 10.1016/s1074-3804(05)60516-6. [DOI] [PubMed] [Google Scholar]
- 12.Hackethal A, Brüggmann D, Leis A, et al. Surgical management of uterine fibroids in Hesse, Germany, between 1998 and 2004. Fertil Steril. 2009;91:862–868. doi: 10.1016/j.fertnstert.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Hurst BS, Matthews ML, Marshburn PB. Laparoscopic myomectomy for symptomatic uterine myomas. Fertil Steril. 2005;83:1–23. doi: 10.1016/j.fertnstert.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Advincula AP, Song A, Burke W, Reynolds RK. Preliminary experience with robot-assisted laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 2004;11:511–518. doi: 10.1016/s1074-3804(05)60085-0. [DOI] [PubMed] [Google Scholar]
- 15.Advincula AP, Xu X, Goudeau S , 4th, Ransom SB. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of shortterm surgical outcomes and immediate costs. J Minim Invasive Gynecol. 2007;14:698–705. doi: 10.1016/j.jmig.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Bedient CE, Magrina JF, Noble BN, Kho RM. Comparison of robotic and laparoscopic myomectomy. Am J Obstet Gynecol. 2009;201:566.e1–e5. doi: 10.1016/j.ajog.2009.05.049. [DOI] [PubMed] [Google Scholar]
- 17.Nezhat C, Lavie O, Hsu S, et al. Robotic-assisted laparoscopic myomectomy compared with standard laparoscopic myomectomy-a retrospective matched control study. Fertil Steril. 2009;91:556–559. doi: 10.1016/j.fertnstert.2007.11.092. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg JA, Einarsson JI. The use of bidirectional barbed suture in laparoscopic myomectomy and total laparoscopic hysterectomy. J Minim Invasive Gynecol. 2008;15:621–623. doi: 10.1016/j.jmig.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Bocca S, Stadtmauer L, Oehninger S. Uncomplicated full term pregnancy after da Vinci-assisted laparoscopic myomectomy. Reprod Biomed Online. 2007;14:246–249. doi: 10.1016/s1472-6483(10)60794-8. [DOI] [PubMed] [Google Scholar]
- 20.Sizzi O, Rossetti A, Malzoni M, et al. Italian multicenter study on complications of laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14:453–462. doi: 10.1016/j.jmig.2007.01.013. [DOI] [PubMed] [Google Scholar]