Abstract
Cervical, endometrial, and breast cancers are paradigms for global health disparity. The paucity of well-designed and -used cancer registries obstructs accurate assessment and planning for international agency resource allocation. Increasing rates of both smoking and obesity in resource-poor nations will lead to increases in the incidence of cancers in developing nations. Women residing in the developing world continue to present in later stages of disease and have fewer options for treatment than those in developed countries.
Key words: Cervical cancer, Endometrial cancer, Breast cancer, Treatment inequity
Poor health within countries and inequities between countries “... are largely caused by the unequal distribution of power, income, goods, and services,” resulting from a combination of poor social policies, unfair economic arrangements, and bad politics.1 It is the downstream effect of social factors (such as nutritious food, clean water, sanitation, shelter, health care, literacy, and meaningful work) that act in synergistic ways with genetics to determine the life expectancy of a population. With respect to access and financing, the health care system itself is a social determinant of individual health, and currently only 5% of global spending on cancer is directed toward developing countries.2
Burden of Disease
By 2020, the International Agency for Research on Cancer, a branch of the World Health Organization (WHO), predicts 16 million new cases of cancer per year, with cancer overtaking heart disease to become the world’s number 1 killer. Currently, 12.5% of all deaths are caused by cancer, which is more than HIV/acquired immunodeficiency syndrome (AIDS), tuberculosis, and malaria combined. Emerging nations, which are experiencing increased use of tobacco and the adoption of Western lifestyles and diets, will bear the brunt of the crisis—up to 70% of all new cases.3 We define developed countries to include North America, Europe (including Russia), Australia, New Zealand, and Japan. Those countries in remaining regions are considered developing or emerging nations.4
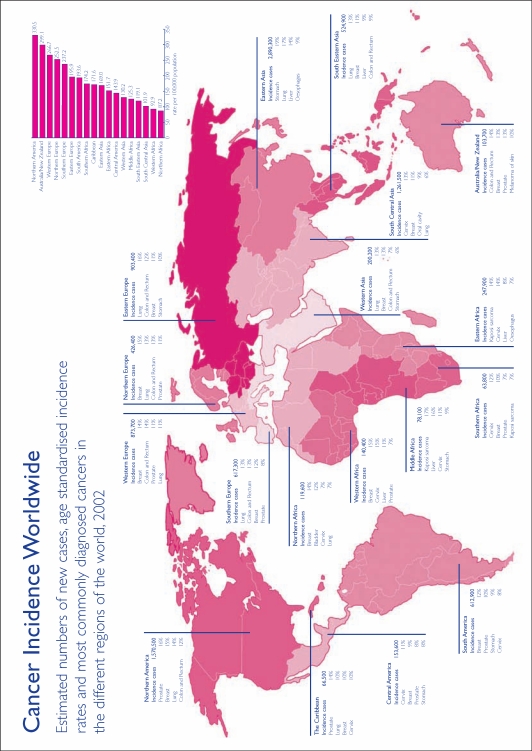
Documenting the scope of the problem is beset by the paucity of cancer registries in many low-resource countries. This is the major impediment to evidence-based selection of priorities for early diagnosis and intervention. In 1995, only 16% of the world’s population (64% of developed and 5% of developing country populations) were included in registries.4 It is estimated that over half of incident cancer cases and 70% of global cancer deaths occur in these settings (Figure 1).
Figure 1.
Many areas of the world have national or regional associations of cancer registries. These associations collaborate in setting methodological standards and publishing data on cancer incidence. They are a useful source of information on cancer and cancer registration. Reproduced with permission from Cancer Research UK.
Cancers that affect primarily women are a special subset of health inequity, as many women in developing countries lack access to screening and treatment as a consequence of discriminatory beliefs and practices.5 Breast and cervical cancer alone accounted for almost 1 million of the close to 6 million cancer cases estimated to have occurred in developing countries in 2002.6 Although the position of women has improved substantially over the past century in many countries, progress has been uneven and multiple challenges remain.1 Despite great improvements in health in the past 30 years, many countries have yet to see and/or benefit from these advances. Women’s low literacy level, cultural and religious factors, competing health needs, discriminatory feeding patterns, limited resources, poorly developed health care services, and limited information on cancer prevention are contributory. In developing countries, specific pathologies, such as cervical cancer, demonstrate much greater prevalence and more advanced stage at diagnosis than in the developed world. Economically disadvantaged women may also give less attention to their symptoms and/or be unable to use preventive measures due to a historical focus on curative medicine rather than preventive care.7 This was also found to be the case in investigations during the early phase of the global HIV/AIDS epidemic.8
Exemplifying the growing inequality between rich and poor nations regarding cancer cure and care is Austria, which houses the International Atomic Energy Agency headquarter and possesses 1 radiotherapy machine for every 200,000 people or fewer. In contrast, many low-resource countries have only 1 treatment machine for up to 10 million people. Some of the world’s poorest nations have no radiotherapy facilities whatsoever.9
Established treatment modalities that are widely available in developed countries, such as chemotherapy, can be difficult to administer in low-resource settings. The availability and cost of anticancer drugs vary considerably among nations.10 Even where these barriers do not exist, delivery of drugs is hindered by widespread lack of health care professionals skilled in administering chemotherapeutic agents, access to laboratories for blood count analyses, and effective antiemetic treatments. Most chemotherapeutic regimens have been tested in clinical trials in the developed world, and may not be generalizable to the developing world, where patient and tumor characteristics may differ markedly. Additional common challenges obstructing cancer therapy are listed in Table 1.
Table 1.
Common Challenges to Cancer Therapy in the Developing World
|
Another aspect of care that has been poorly studied in the developing world is palliative care. Physicians in these countries are restricted in their ability to provide comfort care and pain relief, especially as part of end-of-life care, secondary to financial and time constraints. In addition, many common and effective pain medications, such as morphine, are not readily available.11
In this review, we highlight some of the issues regarding diagnosis, prevention, and treatment of cancer in the developing world, utilizing cervical, endometrial, and breast cancer as examples. Space would not permit an adequate discussion of the entire spectrum of cancers that affect primarily women and the disparities that exist within countries, including in the United States.
Cervical Cancer
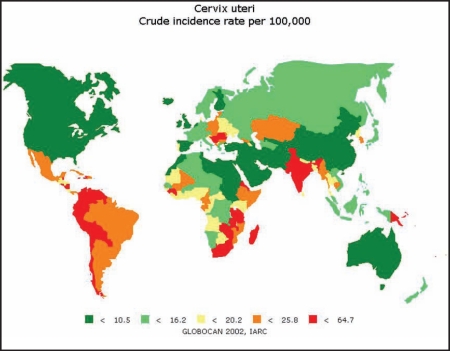
The global disparity in incidence rates is shown in Figure 2 and Table 2. It has been estimated that 529,000 incident cases and 275,000 deaths due to malignant neoplasm of the cervix uteri will have occurred among women globally in the year 2008.12 Over 80% of this estimated burden will have occurred in less developed countries, where cervical cancer is the leading cause of malignancy among women. Among gynecologic cancers, cervical cancer offers great potential for prevention, early detection, and cure due to its long preinvasive phase.
Figure 2.
Global distribution of cancer of the uterine cervix (crude incidence rates). Reproduced with permission from the International Agency for Research on Cancer.
Table 2.
Malignant Neoplasm of the Cervix Uteri Age-Standardized Incidence and Mortality Rates per 100,000 Population6
| Region | Incidence Rate | Mortality Rate |
| Eastern Africa | 44.32 | 24.24 |
| Middle Africa | 25.08 | 14.16 |
| Northern Africa | 16.77 | 9.08 |
| Southern Africa | 30.32 | 16.45 |
| Western Africa | 20.28 | 10.87 |
| Caribbean | 35.78 | 16.84 |
| Central America | 40.28 | 17.03 |
| South America | 30.92 | 11.97 |
| North America | 7.88 | 3.23 |
| Eastern Asia | 6.44 | 3.19 |
| S. Eastern Asia | 18.26 | 9.65 |
| S. Central Asia | 26.47 | 14.95 |
| Western Asia | 4.77 | 2.50 |
| Eastern Europe | 16.81 | 6.20 |
| Northern Europe | 9.84 | 4.00 |
| Southern Europe | 10.18 | 3.25 |
| Western Europe | 10.43 | 3.74 |
| Australia | 7.72 | 2.66 |
| Melanesia | 43.81 | 23.78 |
| Micronesia | 12.31 | 6.16 |
| Polynesia | 28.98 | 15.20 |
International advocacy and resources have been mobilized toward prevention of cervical cancer since 1999 through the efforts of the Alliance for Cervical Cancer Prevention. A cervical cancer screening program based on the Papanicolaou test depends on high-quality sampling, well-trained cytologists, adequate follow-up and diagnosis of women with a positive cytology result, and broad coverage of at-risk populations. Cytology quality-control studies reveal significant variation on positive rates, as well as histopathological and gold standard test reliability.13
Recognition of the failure of cytology-based approaches in low-resource settings has focused attention on the development of alternative approaches to screening. Visual inspection of the cervix, performed with either Lugol’s iodine (VILI) or acetic acid (VIA), are sensitive but nonspecific screening modalities. VIA had a sensitivity of 79% and specificity of 85% for identification of cervical intraepithelial neoplasia 2/3, whereas VILI added 10% to both sensitivity and specificity in a meta-analysis of studies in rural Africa and India.14 Cryotherapy is a cost-effective treatment of small lesions that do not extend into the endocervical canal.
Human papilloma virus (HPV) DNA tests have proven to have higher sensitivity and negative predictive value compared with cytology. This allows for longer screening intervals and reduced costs. Automation and high throughput reduce quality control problems related to human error.15 Low cost or rapid screening for HPV or other biomarkers is under investigation. Development of a self-administered screening test would enhance acceptability.16
The effectiveness of the HPV vaccine for reduction in global mortality remains unknown at present. Programmatic challenges include variable disease burdens, ability to sustain an immunization program for adolescents, affordability, cost effectiveness, cultural acceptability, lack of political will, and public support.17 Screening will continue to be required for several decades to come.
Sub-Saharan Africa
The rising prevalence of HIV/AIDS, from 32.5% in 1999 to 38.5% in 2003, has resulted in a paradoxical decline in the incidence of cervical cancer, and is likely related to the rise in HIV-related mortality.18 Previous studies that suggested a causal association between the 2 entities suffered from the bias inherent in cross-sectional analysis.
Asia
Cervical cancer incidence rates have been decreasing in China and Japan as a result of screening programs, creation of registries, and health education. The age-adjusted incidence rate in China was 29.0 in 1973 versus 5.6 in 2002; corresponding rates for Japan were 17.3 versus 6.7. Unfortunately, rates continue to be high in Thailand (23.8), India (18.2), and the Philippines (19.8). In the former country, commercial sex workers serve as reservoirs of oncogenic HPV, and among monogamous women cervical cancer is associated with their husbands’ frequenting of massage parlors and brothels.19 A cluster randomized trial in India compared the effectiveness of 3 alternatives for cervical cancer screening. Among 131,178 women enrolled, VIA was the most cost effective at $3917 per 1000 women eligible, cytology was intermediate at $6609 per 1000 women, and HPV testing was most expensive at $11,799 per 1000 women.20 Implementation and administrative costs were 22% of the total expenditure. The success of the program was attributed to its cultural appropriateness and time invested in recruitment. The potential benefit that can be attributed to this model of care can be appreciated upon recognition that 14% of the women in the world reside in rural India.
Latin America and the Caribbean
Incidence (age-standardized rate [ASR], 29.2/100,000) and mortality rates (ASR, 13.6/100,000) of cervical cancer in Latin America and the Caribbean (LAC) are high compared with other regions in the world, with the exception of Africa.13
Cervical cancer mortality rates have remained almost unchanged between 1975 and 1990 in the Americas, with the exceptions of Canada and the United States. In 2000, it was estimated that 77,291 cases and 30,570 deaths occurred among women, accounting for roughly 17% and 13.6% of total cancer cases and deaths, excluding skin cancers.6
LAC countries have not achieved the requirements of an organized screening program, and offer opportunistic screening in urban areas, usually through public family planning and reproductive health care facilities or private practices.
Among the countries reporting cytologic coverage within the last 3 years, El Salvador exhibits the lowest rate (19% in 1999) whereas Puerto Rico the highest (72% in 2002). Only Chile and Cuba have national data for follow-up of positive screening results with a performance of over 90%.
Utilization of immediate in-office loop electrosurgical excision procedure treatment of high-grade or persistently positive low-grade lesions was found to reduce the loss to follow-up rate in Honduran demonstration projects by 20.5% compared with the standard multistep model of care.21
United States
Despite state-of-the-art technology and informatics in urban locations, incidence and mortality rates are higher among minority women, especially in rural areas and among recent immigrants. Between the years 1998 and 2002, 60,000 diagnoses of cervical cancer were made, with age-adjusted incidence rates of 8.5 among white women, 13.5 among African American women, and 14.8 among Hispanic women.22 African American women may lack access to screening and treatment, whereas Latinas face cultural and language barriers. Thirteen percent of incident cancers were caused by failure of follow-up. National Breast and Cervical Cancer Early Detection programs have enrolled only 12% to 15% of eligible women. Elucidating the root causes of these disparities continues to be confounded by the interaction of behavioral, social, economic, and environmental demographics, such as poor health literacy, limited transportation, and mistrust of the care provider.23
Endometrial Cancer
Cancer of the uterine corpus is the most common pelvic gynecologic malignancy in developed countries, with wide variation in incidence globally.24 Approximately 199,000 cases of uterine cancer were estimated worldwide in 2002. The number of deaths yearly is approximately 45,000, with 50% of these in developed countries.25 It is the seventh most common tumor in women globally.25 The incidence of endometrial cancer has been rising in developing countries and is consistent with countries in socioeconomic transition.24 This increase may be attributed to lifestyle changes, such as increasing rates of obesity, as well as greater life expectancy that results in an aging population.26
Postmenopausal bleeding is often a presenting sign of early endometrial cancer, allowing prompt diagnosis in developed countries. In the United States, 70% to 75% of women are diagnosed with stage 1 disease. Delayed diagnosis, leading to a more advanced stage at presentation, continues to plague developing countries. This is most often attributable to lack of resources, but also to women’s misperceptions regarding the hopelessness of a cancer diagnosis.8
Progesterone therapy has been used as potential treatment of early-stage endometrial cancer, particularly in younger patients desiring fertility-sparing therapy.27 This treatment option may be of great use in developing countries where surgical options are often unavailable to women due to prohibitive cost and lack of proximity to medical centers. However, this option would be of greatest utility in early-stage disease, which is, unfortunately, the rare situation in developing countries.
South Asia
Although multiple cancer registries are available on the Indian subcontinent, data on the incidence of endometrial cancer are difficult to ascertain, as breast and cervical cancers are much more prevalent and deadly in this population.
A Pakistani study demonstrated internal differences among endometrial cancers within its population as compared with developed countries. Earlier onset of endometrial carcinoma was observed in Karachi, Pakistan, with 15% of cancers occurring in women younger than 40 years with a mean age of 53.7 years, nearly 10 years younger than that observed in developed countries.24 Fortunately, the majority of incident cases were diagnosed in early stages. Pakistan is considered a moderate-risk area, and the etiology of these earlier-onset endometrial cancers needs to be elucidated. It is interesting to note that a similar early onset of colon cancer has been reported in Karachi, perhaps suggesting a role for hereditary nonpolyposis coli in these younger cases.
Mexico
A case-control study noted increased rates of endometrial cancer in Mexico City, Mexico, perhaps secondary to a corresponding increased rate of obesity and resultant diabetes mellitus. It has been established that the incidence of endometrial cancer is 3 times greater in diabetic women compared with nondiabetic women. In addition, reduced birth rates may also be a contributing factor via prolonged estrogen exposure.26
The increased incidence of endometrial cancer in developing countries may also be a result of modernization. Such changes may lead to obesity and/or a greater life expectancy, and therefore an aging population. As both obesity and increasing age are known risk factors for type 1 endometrial carcinoma, it is feasible that higher rates of this cancer are secondary to lifestyle changes.
China
Endometrial cancer is estimated to be the tenth most common tumor among women in China. A case-cohort study evaluated the incidence of endometrial cancer in textile workers in China, with 176 cases reported in the cohort of 267,400 active and retired female workers. There was a trend toward greater risk for endometrial cancer associated with increasing years of work in the silk industry. Although these results did not achieve statistical significance, the study validates the need for evaluating these and other potential occupational and/or environmental exposures for endometrial cancer in developing countries.28 No associations have been found to date between industrial solvents, which are known endocrine disruptors, or insecticides, and incident endometrial cancer.28
Sub-Saharan Africa
The paucity of data on endometrial cancer prevalence is most evident in sub-Saharan Africa, where histopathology services are very limited in more than 20 African countries. In 32 African countries with populations of > 157 million, no radiotherapy services are available.6 One potential explanation for this dearth of information may be the enormous burden of infectious diseases, including AIDS, and malnutrition, which taken together claim the lives of millions of Africans annually. Local and international aid and research efforts are justifiably directed toward these areas.
Breast Cancer
Over a million new cases of breast cancer are diagnosed yearly. Worldwide, breast cancer is the most common cause of cancer-related death among women; 55% occur in developed nations, which have age standardized rates 3 times that of developing nations.29 However, the incidence and mortality rates have been increasing rapidly in developing countries, especially in recent birth cohorts, coinciding with rising urbanization, population aging, and adoption of Western diet and lifestyles. Risk for breast cancer increases by 2% per unit of body mass index, in association with weight gain after age 18.19 The combination of decreased parity, delayed childbearing, early menarche, and late menopause may be causative via prolonged estrogenic exposure. Five percent to 10% of breast cancers demonstrate familial clustering; half of these involve BRCA1 or BRCA2 gene mutations. The prevalence of these has not been extensively studied in the developing world to date, but will certainly become so in the future. Acknowledging the burden of breast cancer in developing countries, the Breast Health Global Initiative (BHGI), comprised of a panel of breast cancer experts representing 17 countries, was created in 2002. Its goal was the development of evidence-based, economically feasible, and culturally appropriate breast health guidelines for countries with limited resources to improve breast cancer outcomes.30 Their updated 2005 findings and recommendations are incorporated in the following sections.
Similar to the case of cervical cancer, early detection and intervention have been established as effective at decreasing mortality from breast cancer and reducing costs for treatment through multiple randomized, controlled trials and meta-analyses. For example, in Africa and Asia, treatment of stage 1 to 3 tumors costs US $390 per disability adjusted life years compared with US $3500 for stage 4 tumors.8 However, incident cases in developing nations are characterized by late presentation and high mortality: a dismal 80% of patients in Nigeria and 50% to 70% in India present in an advanced stage, requiring mastectomy; the majority are either dead or lost to follow-up within a year.31 Low-income areas, such as sub-Saharan Africa, have the highest mortality rate in ages 45 to 59 years, with devastating effects on family structure and income.32 Explanations for delayed clinical presentation include lack of education and awareness, superstition, denial, and fears of diagnosis with consequent disfiguring surgery. In addition, the majority of women in the world do not have access to screening mammography.33 From a practical standpoint, the BHGI found that mammography is unavailable in most developing countries because of its expense. In addition, because mammographic equipment is dedicated to breast imaging and cannot be used for other applications, resource-limited nations and hospitals are reluctant to invest in it. In such countries, breast ultrasound as an initial diagnostic test may be more practical, as ultrasound is relatively inexpensive and can be used for a variety of applications.30
Given the strong association among tumor size, advanced-stage disease, and prognosis, timely diagnosis and improved breast health awareness are key to reducing mortality from breast cancer worldwide. The National Cancer Institute (NCI) has supported an ongoing trial of trained primary health workers performing VIA cervical screening with clinical breast examination (CBE) in India; cluster randomization of 150,000 women was used to assess the effect of these economical screening methods, in combination with an educational intervention, on case fatality rates. There was a low level of attrition over the 4-year period to date, with a 73% compliance with confirmatory diagnostic procedures and a 92% treatment compliance for the breast cancer cases.34 Neither CBE nor breast self-examination has yet been established as a screening tool, but trials are underway to address this issue. The utility in promoting CBE and breast self-examination in limited resource areas may lie in promoting breast health awareness.33
Cost-effective, tailored approaches to breast cancer treatment in low resource settings have included: a 9-week trial of trastuzumab (Herceptin®; Genentech, Inc., South San Francisco, CA), which was similar in survival outcome to the standard of 52 weeks; prolonged infusion of low-dose gemcitabine; interrupted courses of aromatase inhibitors; and prolonged low doses of cyclophosphamide and methotrexate as palliative treatment. The latter conventional drugs are inexpensive compared with the newer generation of biopharmaceuticals.35 An alternative to the expensive personal burden of medications is surgical or radiation-induced oophorectomy. Many of the latter treatments, however, are effective in the setting of cancers that express estrogen and progesterone receptors (ER, PR). Therefore, stratifying patients by testing for these receptors would enable countries to reserve their limited resources for those patients who would benefit from targeted therapies, as racial groups vary in their incidence of ER-positive cancers.30
Discussion
The inequities in cancer diagnosis and treatment are multifactorial, as highlighted in the limited case studies available. It may be useful to construct a framework around which solutions may be proposed. The first logical step would be to accurately characterize the extent of the problem, which would require the existence of universal cancer registries. The information gathered could then be used to determine priorities and implement targeted initiatives. The creation and sustaining of registries in countries lacking basic infrastructure is a daunting task. National cancer control programs have been implemented in several countries. However, as seen in Table 3, only 48% of countries worldwide have cancer control policies or plans. In addition, those countries that do have these programs in place still have difficulty achieving optimal effectiveness. For example, Vietnam, which has a National Cancer Control Program in place, lacks laws requiring physicians to use it.36 In addition, there are insufficient staff to maintain the programs. This would seem to be a good starting point for international health organizations desiring to take a leadership role in ensuring adequate and just distribution of women’s global health care.
Table 3.
Global Cancer Policy41
| Region | Countries With Cancer Policy or Plan |
| Africa | 15% |
| The Americas | 50% |
| Eastern Mediterranean | 56% |
| Europe | 62% |
| Southeast Asia | 78% |
| Western Pacific | 64% |
| Overall | 48% |
Although the full extent of the regional disparities in gynecologic cancer care is still unknown, the lack of skilled health care providers in preventive and therapeutic services is well documented. For example, in sub-Saharan Africa, a mere 1.3% of the world’s health care workers reside, alongside 13.8% of the world’s population. Factors contributing to the lack of trained physicians in women’s health in developing countries include low wages, political instability, and employment in fields other than those of their expertise. The net result is the “brain drain” of valuable skilled professionals from developing countries to resource-rich countries.37 Even clinicians from developing countries who visit cancer centers in developed countries to gain additional training often learn new innovative medical and surgical technologies rather than more helpful scientific methodology and preventive measures. The latter would be more relevant and applicable upon return to their home countries.11 Many of these factors are not easily amenable to change; therefore, greater utilization of midlevel and trained lay health care workers may provide partial solutions. Preventive care, routine gynecologic examinations, VIA, and education have been successfully provided in low resource settings.8 In addition, research personnel and skilled scientists are in limited supply. It is not unusual for low-income countries to spend less than 1% of national budgets on research, have no doctoral training programs, and have 1 or fewer scientists per million persons.38
Table 4 lists some of the features that should be components of model outreach programs to ensure their success. There is little utility in screening patients if treatment cannot be offered at diagnosis, secondary to finances, geography, or unavailability. Short intervals between screening and treatment are optimal to minimize loss to follow-up. Innovative and novel cost-effective treatments must be pursued; health care costs for women and girls are not always considered in the budgets of many families in developing countries. Women may conceal their symptoms to protect the integrity of a family’s marginal finances.8
Table 4.
Features of Model Outreach Programs
|
Lessons learned from the HIV/AIDS epidemic, as well as successful immunization and family planning programs, may be applicable here. Any new health care programs must arise within existing health care service infrastructures. Public awareness through education is the best method of prevention. If patients are not aware that they may be at risk for cancer, they cannot and will not seek out ways to prevent it. Culturally and socially relevant educational material must be provided to women in developing countries (keeping in mind that the majority of these women may be illiterate, necessitating person-to-person discussions regarding these issues). The WHO framework for chronic disease management, which incorporates the patient, family, and community, can serve as a model for programmatic development in this area.39
Conclusions
There is a dearth of information regarding health care disparity among gynecologic cancers worldwide. Nevertheless, one thing is clear-women in developing countries have access to fewer resources and are more likely to suffer serious morbidity and mortality from cancer than their counterparts in the developed world, partly due to the social stigma associated with cancers that affect primarily women. Only 5% of the world’s total resources for cancer control reach the developing world.11
Perhaps the most glaring discrepancy may be found in simply comparing the data available to analyze the problem of disparate diagnosis and treatment throughout the world. To effect change, steps must be taken to ensure that the extent of the problem can be accurately documented. It is also clear that the health problems of developing countries ultimately become the health problems of developed countries. This was evident in the HIV/AIDS epidemic, which began in sub-Saharan Africa and has become a global disease. The converse is also true: smoking and obesity, which began as health concerns of the developed world, are now playing great roles in disease causality in developing countries. It is the responsibility and moral obligation of international health organizations, as well as resource-rich and developed countries, to provide capital resources, mentorship, and training to aid developing countries in control efforts.40 In this technologically connected world, mentoring and training colleagues who are thousands of miles away is no longer a daunting task. Many organizations have initiated programs to help with cancer education and screening programs abroad. The goal of providing equal access to prevention and treatment of cancers that affect primarily women is a clear issue of social justice.
Main Points.
It has been estimated that 529,000 incident cases and 275,000 deaths due to malignant neoplasm of the cervix uteri will have occurred among women globally in the year 2008. Over 80% of this estimated burden will have occurred in less developed countries, where cervical cancer is the leading cause of malignancy among women.
The incidence of endometrial cancer and breast cancer has been rising in developing countries. This increase may be attributed to lifestyle changes, as well as greater life expectancy.
It is the responsibility and moral obligation of international health organizations, as well as resource-rich and developed countries, to provide capital resources, mentorship, and training to aid developing countries in control efforts. The goal of providing equal access to prevention and treatment of cancers that affect primarily women is a clear issue of social justice.
Related Websites
| Access to Treatment (Axios International) | www.accesstotreatment.org |
| Alliance for Cervical Cancer Prevention (ACCP) | www.alliance-cxca.org |
| Center to Reduce Cancer Health Disparities (CRCHD) | www.crchd.nci.nih.gov |
| International Agency for Research on Cancer (IARC) | www.iarc.fr |
| International Network for Cancer Treatment and | www.inctr.org |
| Research (INCTR) | |
| National Cancer Institute Surveillance Epidemiology | www.seer.cancer.gov |
| and End Results (SEER) | |
| Program of Action for Cancer Therapy (PACT) | www.iaea.org/pact |
| Union for International Cancer Control (UICC) | www.uicc.org |
| International Cancer Fellowships | |
| World Health Organization (WHO) | www.who.int/cancer |
References
- 1.Marmot M, Friel S, Bell R, et al. Commission on Social Determinants of Health. Closing the gap in a generation: health equity through action on the social determinants of health. Lancet. 2008;372:1661–1669. doi: 10.1016/S0140-6736(08)61690-6. [DOI] [PubMed] [Google Scholar]
- 2.Breast cancer in developing countries. Lancet. 2009;374:1567. doi: 10.1016/S0140-6736(09)61930-9. [DOI] [PubMed] [Google Scholar]
- 3.Lodge M. The evidence base for cancer control in developing countries: what is to be done? The Newsletter of the International Network for Cancer Treatment and Research. 2005;6(3) http://www.inctr.org/publications/2005_v06_n03_w02.shtml. [Google Scholar]
- 4.Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: the size of the problem. Best Pract Res Clin Obstet Gynaecol. 2006;20:207–225. doi: 10.1016/j.bpobgyn.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Wakabi W. Research collaboration boosts women’s health in Ethiopia. Lancet. 2008;372:1534. doi: 10.1016/s0140-6736(08)61637-2. [DOI] [PubMed] [Google Scholar]
- 6.Parkin DM, Whelan SL, Ferlay J, et al., editors. Cancer Incidence in Five Continents. Vol. VIII. Geneva: World Health Organization; 2007. (International Agency for Research on Cancer Publication No. 155). [Google Scholar]
- 7.Pinotti JA, Faúndes A. Obstetric and gynecological care for Third World women. Int J Gynaecol Obstet. 1984;22:449–455. doi: 10.1016/0020-7292(84)90037-7. [DOI] [PubMed] [Google Scholar]
- 8.Reeler A, Qiao Y, Dare L, et al. Women’s cancers in developing countries: from research to an integrated health systems approach. Asian Pac J Cancer Prev. 2009;10:519–526. [PubMed] [Google Scholar]
- 9.Raising the profile of the global cancer epidemic: Monaco dedicates gala evening to IAEA’s Cancer Initiative. IAEA Cancer Initiative Newsletter. 2008. http://www.iaea.org/NewsCenter/News/2008/cancerprofile.html.
- 10.Basile S, Angioli R, Manci N, et al. Gynecological cancers in developing countries: the challenge of chemotherapy in low-resources settings. Int J Gynecol Cancer. 2006;16:1491–1497. doi: 10.1111/j.1525-1438.2006.00619.x. [DOI] [PubMed] [Google Scholar]
- 11.Tobias JS, Mittra I. Improving cancer care world wide. Ann Oncol. 1993;4:283–287. doi: 10.1093/oxfordjournals.annonc.a058483. [DOI] [PubMed] [Google Scholar]
- 12.GLOBOCAn 2008 Cancer Fact Sheet. Lyon, France: International Agency for Research on Cancer Section of Cancer Information; [Accessed September 14, 2010]. http://globocan.iarc.fr/factsheets/cancers/cervix.asp. [Google Scholar]
- 13.Murillo R, Almonte M, Pereira A, et al. Cervical cancer screening programs in Latin America and the Caribbean. Vaccine. 2008;26(suppl 11):L37–L48. doi: 10.1016/j.vaccine.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Arbyn M, Sankaranarayanan R, Muwonge R, et al. Pooled analysis of the accuracy of five cervical cancer screening tests assessed in eleven studies in Africa and India. Int J Cancer. 2008;123:153–160. doi: 10.1002/ijc.23489. [DOI] [PubMed] [Google Scholar]
- 15.Cuzick J, Arbyn M, Sankaranarayanan R, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008;26(suppl 10):K29–K41. doi: 10.1016/j.vaccine.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Tsu VD, Pollack AE. Preventing cervical cancer in low-resource settings: how far have we come and what does the future hold? Int J Gynaecol Obstet. 2005;89(suppl 2):S55–S59. doi: 10.1016/j.ijgo.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Agosti JM, Goldie SJ. Introducing HPV vaccine in developing countries-key challenges and issues. N Engl J Med. 2007;356:1908–1910. doi: 10.1056/NEJMp078053. [DOI] [PubMed] [Google Scholar]
- 18.Moodley M. Reduction in prevalence of invasive cervical cancer in KwaZulu-Natal, South Africa: impact of the human immunodeficiency virus epidemic. Int J Gynecol Cancer. 2006;16:1036–1040. doi: 10.1111/j.1525-1438.2006.00588.x. [DOI] [PubMed] [Google Scholar]
- 19.Park S, Bae J, Nam B, Yoo K. Aetiology of cancer in Asia. Asian Pac J Cancer Prev. 2008;9:371–380. [PubMed] [Google Scholar]
- 20.Legood R, Gray AM, Mahé C, et al. Screening for cervical cancer in India: how much will it cost? A trial based analysis of the cost per case detected. Int J Cancer. 2005;117:981–987. doi: 10.1002/ijc.21220. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh K, Segura A, Crispen C, Montz FJ. Use of the “see and treat” technique for the management of high-risk abnormal Pap smears in a Third World country. Int J Gynecol Cancer. 1997;7:144–150. [Google Scholar]
- 22.Hoover K, Koumans EH, Montaño D, et al. Access of Black, Hispanic, and nonprivately insured women to liquid-based cytology, human papillo-mavirus DNA testing, and on-site colposcopy in the United States. J Low Genit Tract Dis. 2009;13:17–27. doi: 10.1097/LGT.0b013e318194b87e. [DOI] [PubMed] [Google Scholar]
- 23.Betancourt JR, Green AR, Carrillo JE, Park ER. Cultural competence and health care disparities: key perspectives and trends. Health Aff. 2005;24:499–505. doi: 10.1377/hlthaff.24.2.499. [DOI] [PubMed] [Google Scholar]
- 24.Bhurgri Y, Nazir K, Shaheen Y, et al. Pathoepidemiology of cancer corpus uteri in Karachi South “1995–1997”. Asian Pac J Cancer Prev. 2007;8:489–494. [PubMed] [Google Scholar]
- 25.Pecorelli S, Favalli G, Zigliani L, Odicino F. Cancer in women. Int J Gynaecol Obstet. 2003;82:369–379. doi: 10.1016/s0020-7292(03)00225-x. [DOI] [PubMed] [Google Scholar]
- 26.Salazar-Martínez E, Lazcano-Ponce EC, Lira-Lira GG, et al. Case-control study of diabetes, obesity, physical activity and risk of endometrial cancer among Mexican women. Cancer Causes Control. 2000;11:707–711. doi: 10.1023/a:1008913619107. [DOI] [PubMed] [Google Scholar]
- 27.Chiva L, Lapuente F, Gonázlez-Cortijo L. Sparing fertility in young patients with endometrial cancer. Gynecol Oncol. 2008;111(2 suppl):S101–S104. doi: 10.1016/j.ygyno.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 28.Wernli KJ, Ray RM, Gao DL, et al. Occupational risk factors for endometrial cancer among textile workers in Shanghai, China. Am J Ind Med. 2008;51:673–679. doi: 10.1002/ajim.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bray F, McCarron P, Parkin DM. The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res. 2004;6:229–239. doi: 10.1186/bcr932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson BO, Shyyan R, Eniu A, et al. Breast cancer in limited-resource countries: an overview of the Breast Health Global Initiative 2005 guidelines. Breast J. 2006;12(suppl 1):S3–S15. doi: 10.1111/j.1075-122X.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- 31.Anyanwu SN. Temporal trends in breast cancer presentation in the third world. J Exp Clin Cancer Res. 2008;27:17. doi: 10.1186/1756-9966-27-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Igene H. Global health inequalities and breast cancer: an impending public health problem for developing countries. Breast J. 2008;14:428–434. doi: 10.1111/j.1524-4741.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- 33.Smith RA, Caleffi M, Albert US, et al. Global Summit Early Detection and Access to Care Panel. Breast cancer in limited-resource countries: early detection and access to care. Breast J. 2006;12(suppl 1):S16–S26. doi: 10.1111/j.1075-122X.2006.00200.x. [DOI] [PubMed] [Google Scholar]
- 34.Mittra I, Mishra GA, Singh S, et al. A cluster randomized, controlled trial of breast and cervix cancer screening in Mumbai, India: methodology and interim results after three rounds of screening. Int J Cancer. 2010;126:976–984. doi: 10.1002/ijc.24840. [DOI] [PubMed] [Google Scholar]
- 35.Elzawawy A. The “win-win” initiative: a global, scientifically based approach to resource sparing treatment for systemic breast cancer therapy. World J Surg Oncol. 2009;7:44. doi: 10.1186/1477-7819-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webb S. Customizing anticancer strategies to local needs. J Natl Cancer Inst. 2009;101:842–844. doi: 10.1093/jnci/djp169. [DOI] [PubMed] [Google Scholar]
- 37.Serour GI. Healthcare workers and the brain drain. Int J Gynaecol Obstet. 2009;106:175–178. doi: 10.1016/j.ijgo.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 38.Mellstedt H. Cancer initiatives in developing countries. Ann Oncol. 2006;17(suppl 8):viii24–viii31. doi: 10.1093/annonc/mdl984. [DOI] [PubMed] [Google Scholar]
- 39.Feuerstein M, editor. Handbook of Cancer Survivorship. New York: Springer; 2007. [Google Scholar]
- 40.Kerr F, Kerr D. Do we bear any moral responsibility for improving cancer care in Africa? Ann Oncol. 2006;17:1730–1731. doi: 10.1093/annonc/mdl442. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization (WHO), authors National Cancer Control Programmes: Policies and Managerial Guidelines. Geneva: WHO; 2002. [Google Scholar]