Summary
Background
The mechanism of assembly of the platelet glycoprotein (GP) Ib-IX complex from GPIbα, GPIbβ and GPIX subunits is not entirely clear. In this complex, ectodomains of both GPIbβ and GPIX subunits contain two leucine-rich repeats (LRR) and share high sequence similarity. However, they differ noticeably in stability, hampering further analysis of their interaction.
Objectives and Methods
Guided by analysis of the LRR structure, we report a well-folded Ibβ/IX chimera and its usage in dissecting GPIX function.
Results
In this chimera, three non-contiguous sequences that may constitute the putative convex surface of the GPIbβ ectodomain are replaced by their GPIX counterparts. Like GPIbβ but unlike GPIX ectodomain, it can secrete from transfected Chinese hamster ovary cells and fold into a stable conformation. Furthermore, replacing the ectodomain in GPIX with the Ibβ/IX chimera, but not the GPIbβ ectodomain, preserved its interaction with GPIbβ as demonstrated by its native-like GPIbβ-induced increase in surface expression and coimmunoprecipitation.
Conclusions
The putative convex surface of the LRR domain in GPIX is sufficient, in the context of full-length subunit, to mediate its association with GPIbβ.
Keywords: GPIb-IX complex, complex assembly, leucine-rich repeats, homologous sequence swapping
Introduction
Both glycoprotein (GP) Ibβ and IX are integral parts of the GPIb-IX complex that is identified as the platelet receptor for von Willebrand factor, although the ligand-binding site is exclusively located in GPIbα, another subunit in the complex[1, 2]. GPIbβ and GPIX are indispensable for efficient expression of GPIbα in the plasma membrane, a conclusion drawn from extensive studies in transfected cell lines as well as naturally occurring mutations identified in patients with Bernard-Soulier syndrome (BSS), a hereditary bleeding disorder characterized by deficiency of GPIbα in platelets[3, 4]. Moreover, several studies have reported evidence supporting trans-subunit regulation of GPIbα activity by GPIbβ[5-7]. However, how GPIbβ and GPIX support surface expression of GPIbα and modulate its activity is not clear, partly due to our lack of understanding of inter-subunit interactions in the GPIb-IX complex.
The interaction among transmembrane helices in the GPIb-IX complex has recently been identified as one of the forces driving complex assembly. Replacing the transmembrane domain of any subunit with a generic poly-leucine-alanine sequence disrupted native interaction, altered formation of juxtamembrane disulfide bonds between GPIbα and GPIbβ, and led to a significant decrease in surface expression of GPIbα[8, 9]. Isolated transmembrane peptides derived from each subunit can interact spontaneously with one another and form a native-like complex in appropriate detergent micelles[10, 11]. Another interaction, the one between GPIbβ and GPIX ectodomains, has been thought to contribute also to complex assembly[12, 13]. However, direct detection of this interaction has not been possible, partly due to the difficulty in obtaining isolated ectodomains. Although the GPIbβ ectodomain can be expressed in insect cells and secreted into the culture medium, the GPIX ectodomain, despite its impressive sequence homology with GPIbβ, can not be produced readily in mammalian or insect cells[14, 15]. Instead, the interaction between GPIbβ and GPIX ectodomains is inferred from the observation that only in the presence of GPIbβ can GPIX be expressed in the plasma membrane of transfected mammalian cells[14] and further attested by the large number of missense mutations in GPIbβ and GPIX ectodomains identified in BSS patients[4]. The reliance of GPIX expression on GPIbβ also afforded further dissection of the GPIbβ ectodomain that reported GPIbβ residues 15-32 as critical for the interaction with GPIX[13].
Both GPIbβ and GPIX ectodomains contain two leucine-rich repeats (LRR)[16], but no other structural information is available. In this paper, guided by structural models generated for the two domains, we swapped homologous sequences and identified a stable and well-folded Ibβ/IX chimera. The chimera was subsequently used to map the regions in GPIX that likely mediate its association with GPIbβ.
Materials and Methods
Materials
The CHO K1 cell line was obtained from ATCC. Monoclonal anti-HA antibody, anti-myc antibody, anti-actin antibody and HRP-conjugated anti-HA antibody were purchased from Sigma (St. Louis, MO). Monoclonal anti-GPIX antibody FMC25 was from Chemicon (Billerica, MA), and polyclonal anti-GPIX antibody Santa Cruz Biotechnology (Santa Cruz, CA).
Molecular modeling of GPIbβ and GPIX ectodomains
Analysis of the protein sequences indicated an assembly of two LRRs using a consensus start sequence of LxLxxN. Inspection of the N-terminal capping region of GPIbβ and GPIX revealed the greatest similarity with the decorin crystal structure[17] and the C-terminal capping region with Nogo-66 receptor[18]. The GPIbβ and GPIX ectodomains were modeled on a single template produced by manual assembly of residues 22-56 of decorin (PDB ID: 1XKU) and residues 231-311 of Nogo-66 receptor (1P8T). Sequences of human GPIbβ (residues C1-A117) and GPIX (residues T1-G120) were modeled using the program xlook and energy minimized using segmod[19] based on the pre-assembled template.
GPIbβ and GPIX constructs
All cDNAs described in this study were included in the pDX vector for transient transfection to CHO K1 cells[3, 14]. The pDX vectors containing cDNAs encoding IXE, HA-Ibβ (GPIbβ with N-terminal HA epitope tag, YPYDVPDYA), and Ibβ-myc (GPIbβ with C-terminal c-myc epitope tag, EQKLISEEDL) have been described[9, 10, 20]. To obtain the HA-IbβE construct (Fig. 2A), the codon for residue Cys122 in pDX-HA-Ibβ was changed to a stop codon.
Figure 2. Both ectodomain and transmembrane domain of GPIbβ are required for expression of GPIX in CHO cells.
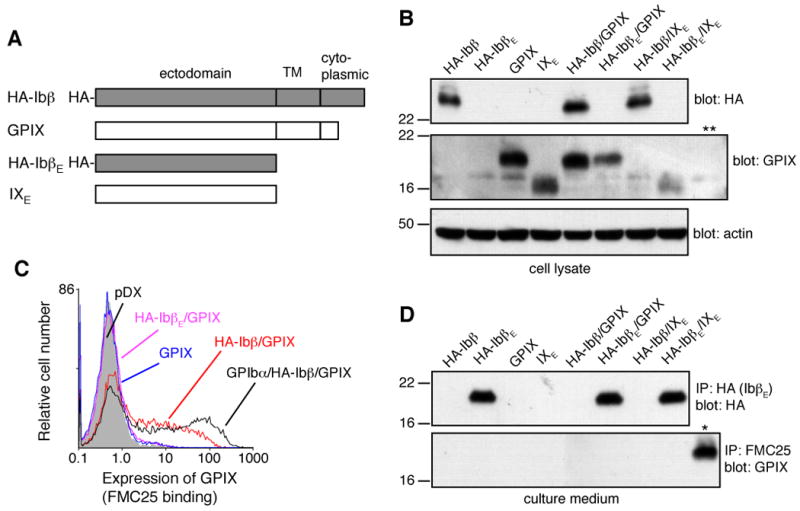
(A) Illustration of GPIbβ and GPIX constructs used in the study. An HA epitope tag was attached to the N-terminus of either full-length GPIbβ or the isolated GPIbβ ectodomain (IbβE) for detection. HA-IbβE and IXE contain only the ectodomain of GPIbβ and GPIX, respectively. (B) Production of GPIbβ and GPIX proteins in transfected CHO cells. After transient transfection, cells were lysed by 1% Triton X-100 lysis buffer, and proteins were separated in a 12% Tris-glycine SDS gel, transferred to PVDF membrane and immunoblotted by HRP-conjugated anti-HA antibody (for HA-Ibβ), goat anti-GPIX polyclonal antibody (for GPIX and IXE), or anti-actin antibody. Each lane is identified on top by the subunits transfected into the CHO cell. Cells transfected with empty vector pDX were included as a negative control for GPIX detection (** lane). Each gel in this figure is a representative of 3-5 independent experiments. (C) Overlaid histograms showing surface expression levels of GPIX, in the presence of noted GPIbβ variants, as measured by flow cytometry after immunostaining with anti-GPIX antibody FMC25. The expression level of GPIX in CHO cells transfected with GPIb-IX complex (GPIbα/HA-Ibβ/GPIX) and empty vectors (pDX) were shown as positive and negative controls, respectively. The plot is a representative of no less than 4 independent experiments. (D) Secretion of HA-IbβE and lack of secretion of IXE in the culture medium. Proteins in the culture medium of various transiently transfected cells were precipitated by anti-HA antibody (for HA-IbβE) or FMC25 (IXE), separated in a 12% Tris-glycine SDS gel, transferred to PVDF membrane and blotted by HRP-conjugated anti-HA antibody or goat anti-GPIX polyclonal antibody. As a positive control for detection of GPIX (* lane), CHO cells transiently transfected with GPIbα/HA-Ibβ/GPIX were lysed, and GPIX in the lysate was immunoprecipitated and blotted as the other samples.
To generate the HA-IbβEa gene, one of the IbβE/IXE chimeric constructs illustrated in Figure 3A, the DNA fragment encoding GPIX residues T1-R36, amplified from pDX-IXE, was fused to that encoding GPIbβ residues T32-L121, amplified from pDX-HA-IbβE, by a series of PCR reactions. The resulting DNA fragment was then used as a template in subsequent PCR reactions to further replace the coding sequence for GPIX residues T1-T28 with that for GPIbβ residues C1-T20. HA-IbβEb and HA-IbβEc genes (Fig. 3A) were constructed via similar multi-step PCR. DNA fragments containing these single-swapped sequences were cleaved by BglII and XmaI and ligated back into the pDX-HA-IbβE vector that had been digested by the same restriction enzymes. They were also used as PCR templates to generate HA-IbβEab and HA-IbβEabc constructs (Fig. 3A).
Figure 3. Identification of a stable IbβE/IXE chimera.
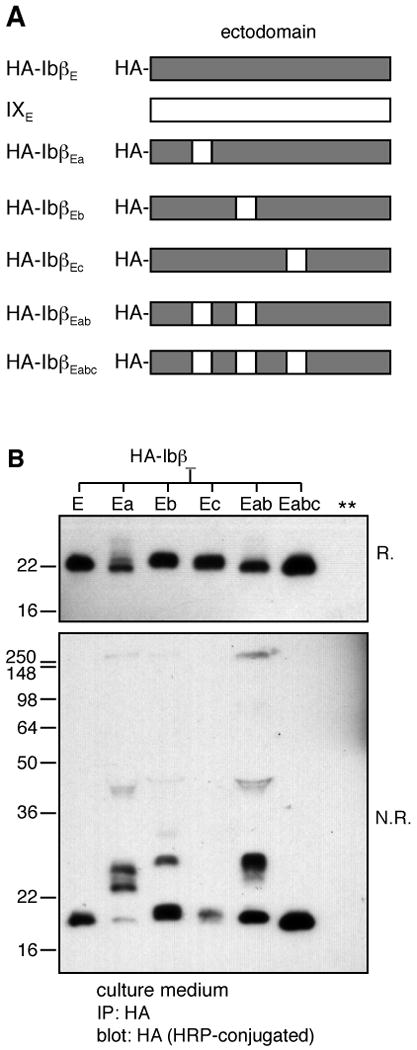
(A) Illustration of various chimeric IbβE/IXE constructs. The HA tag was attached to the N-terminus of all chimeric constructs for detection. Three convex loops in GPIX (residues A29-R36, S49-Q60, and S76-D86) were identified as loop a, b, and c, respectively. The subscript in the name for each chimera indicates the grafted IX loop(s). (B) Only with all three convex loops grafted could the IbβE/IXE chimera form no intermolecular but only intramolecular disulfide bonds, as the well-folded HA-IbβE. The chimera protein in the culture medium, identified on the top of each lane, was immunoprecipitated by anti-HA antibody after transient transfection, separated in a 12% Tris-glycine SDS gel under either reducing (R.) or non-reducing (N.R.) conditions, transferred to PVDF membrane and blotted by HRP-conjugated anti-HA antibody. This figure is a representative of 3 independent experiments.
To construct the HA-IX gene (Fig. 4A), the sequence encoding the HA tag was inserted, in multiple steps using a Quikchange mutagenesis kit (Stratagene, La Jolla, CA), into the pDX-IX vector between those encoding the signal peptide and mature protein. To avoid spontaneous mutations that may occur during the insertion, the gene fragment containing the inserted HA tag was amplified from the vector, cleaved by BbvCI (located upstream of the GPIX signal sequence) and SapI (within the GPIX transmembrane domain), and ligated back into a clean pDX-IX vector. To construct HA-IbβE-IXTC and HA-IbβEabc-IXTC genes (Fig. 4A), DNA fragments encoding HA-IbβE and HA-IbβEabc were fused by PCR, respectively, with that encoding the GPIX signal sequence at the 5′-end and that encoding GPIX transmembrane and cytoplasmic domains at the 3′-end. The entire fragments were then ligated back into pDX-IX as the BbvCI-SapI fragment. All constructs were confirmed by DNA sequencing (SeqWright, Houston, TX).
Figure 4. The convex loops of GPIX preserve the interaction of GPIX with GPIbβ, in the context of full-length subunits.
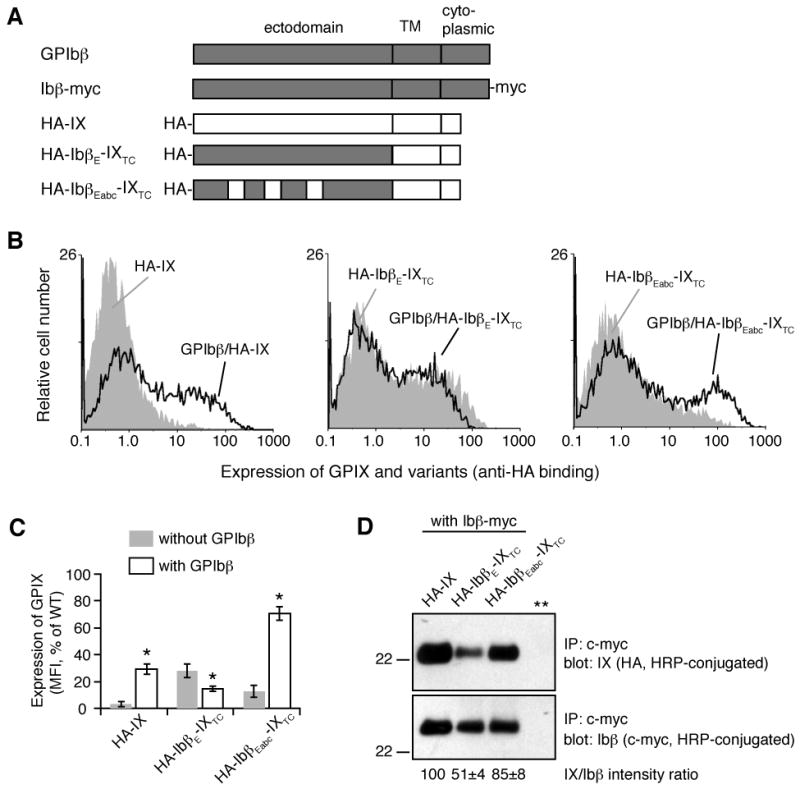
(A) Illustration of GPIbβ and GPIX constructs used. The HA tag was attached to the N-terminus of mature GPIX and its variants for consistent measurements. The c-myc tag was appended to the C-terminus of GPIbβ for the co-IP experiment. IXTC denotes the GPIX transmembrane and cytoplasmic domains. HA-IX is essentially HA-IXE-IXTC. (B) Overlaid histograms showing effects of co-expressing GPIbβ on the expression level of HA-IX (left), HA-IbβE-IXTC (middle), or HA-IbβEabc-IXTC (right) on the surface of transiently transfected CHO cells as detected by flow cytometry after immunostaining with anti-HA antibody. Grey peak: without GPIbβ; solid line: with GPIbβ. Expression levels of GPIX derivatives in CHO cells transfected with GPIbα/GPIbβ/HA-IX or with empty vectors were not shown here but essentially the same as those shown in Figure 2C. (C) Quantitative representation of surface expression levels of GPIX. The measured mean fluorescence intensity (MFI), obtained for the entire cell population (10,000 cells per sample), was normalized with the GPIX expression level in CHO cells transfected with GPIbα/GPIbβ/HA-IX being 100% and cells transfected with empty vectors 0%[8]. All data are presented as the mean ± SD from 4 independent experiments. Groups were compared using the nonpaired t test; *, p < 0.01. (D) IbβEabc helps to preserve GPIX association with GPIbβ. After transient transfection with Ibβ-myc and individual GPIX variants as noted, proteins in cells lysate were immunoprecipitated by anti-myc antibody, separated in a 12% Tris-glycine SDS gel, transferred to PVDF membrane and blotted by HRP-conjugated anti-HA antibody (for GPIX variants) or HRP-conjugated anti-myc antibody (for Ibβ-myc). The negative control (** lane) is as described in Figure 2B. The ratio of IX and Ibβ band intensities was quantitated and expressed as a percentage of that for HA-IX. The data are presented as the mean ± SD from 4 independent experiments.
Transient transfection of Chinese hamster ovary (CHO) cells
Vectors containing wild-type or mutant GPIbα, GPIbβ and GPIX cDNAs were transiently transfected into CHO K1 cells using Lipofectamine 2000 (Invitrogen) as described[8, 10]. When only one or two subunit genes were transfected, empty pDX vector was included to keep the amount of total DNA a constant. Other parameters for each transfection were kept the same as described earlier to allow proper comparison of expression levels[8]. After transfection, cells were grown for additional 48 hours before being analyzed for protein expression.
Flow cytometry
Surface expression of GPIX and its variants was detected with FMC25 or anti-HA antibody and measured on a Beckman-Coulter Epics XL flow cytometer as described before[10]. Groups were compared using the nonpaired t test.
Immunoprecipitation and SDS-polyacrylamide gel electrophoresis
Two days after transfection, either 1 ml cell culture media or 500 μl cell lysate in the 1% Triton X-100 lysis buffer[8] was mixed with 1:50 (v/v) diluted protease inhibitor cocktail for mammalian tissues (Sigma, St. Louis, MO), and centrifuged at 4 °C for 30min. After pre-incubation with 20 μl protein G agarose beads (Invitrogen) for 30 min at 4°C, the samples were mixed with 1 μg indicated antibody and 20 μl protein G agarose beads and incubated 4°C overnight. After 4 washes with 1 ml phosphate-buffered saline containing 0.5% bovine serum albumin (for cell culture media) or 0.1% Triton X-100 (cell lysate), immunoprecipitated proteins were eluted by 20 μl SDS sample buffer in the presence or absence of reducing agent, separated on a 12% Tris-glycine SDS gel, transferred to PVDF membrane, and blotted by desired antibody.
Results
Modeling of GPIbβ and GPIX ectodomains
This study began with a close examination of both GPIbβ and GPIX ectodomains, which display high degree of sequence similarity and are therefore expected to take on similar structures[16, 21]. Based on their similarity to LRR-containing decorin and Nogo receptor for which crystal structures have been determined[17, 18], we have generated structural models for both domains. Both models, resembling a cylinder with approximately 5 layers (Fig. 1B), share the same overall fold as that previously predicted for GPIbβ[16]. Containing the LRR motifs, layers 2-4 constitute the main body of the cylinder, which can be further divided to a short and two long sides (Fig. 1C). The long sides are often referred to as the concave and convex surface in LRR proteins, with the former in a parallel β-sheet and the latter comprised of loops with disparate conformations. The short side links the concave to the convex side. The LRR motifs are located in the concave and short sides of the cylinder, helping to maintain the structure. In particular, the Asn ladder (i.e. GPIbβ-N40, N64, and GPIX-N45, N69), characteristic of many LRR proteins[22], is located on the short side (Fig. 1B,C). Its importance to the stability and structural integrity of the LRR domain is exemplified by documented BSS-causing mutations[23-25]. In contrast, sequences on the convex side are more varied and less predictable than those in LRR motifs.
Figure 1. Sequence analysis and structural models of GPIbβ and GPIX ectodomains.
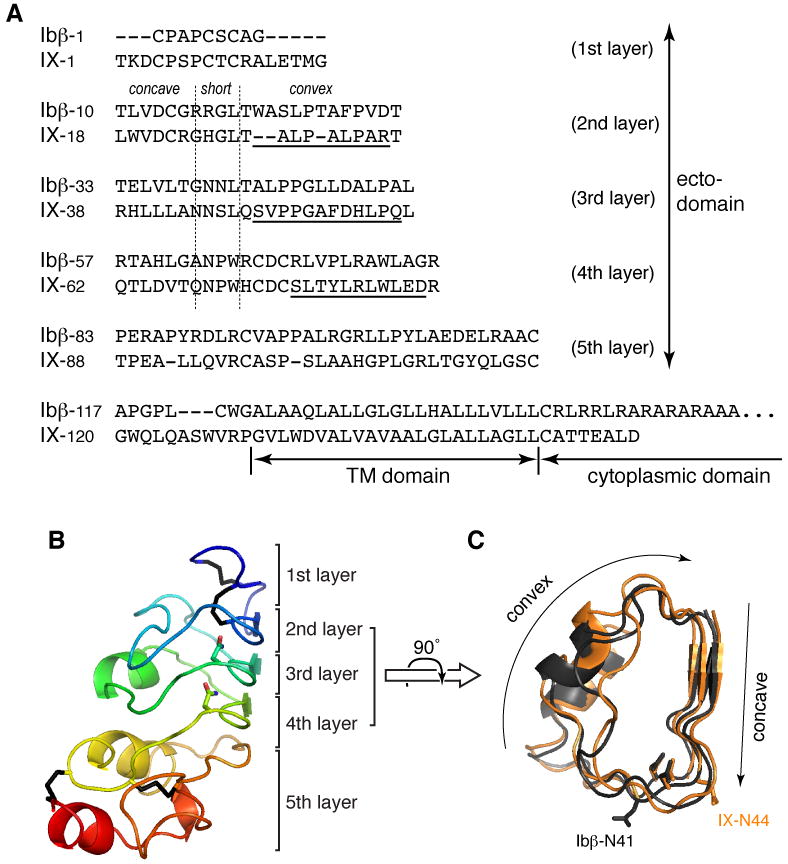
(A) Alignment of human GPIbβ and GPIX sequences. Ectodomain residues are organized into rows corresponding to the 5 layers of the LRR domain. Vertical dashed lines mark the junctions between concave, short and convex sides of the domain. (B) Structural model of the GPIbβ ectodomain, shown in the ribbon diagram with rainbow coloring. The 5-layer organization of the domain is marked on the right. Four disulfide bonds are colored black; side chains of stacked Asn residues in LRR motifs are also shown. (C) A top view of superimposed 2-4 layers of GPIbβ (dark gray) and GPIX (light orange) ectodomains. Concave and convex surfaces are marked. Side chains of Asn ladder point to the inside, while those of glycosylated Asn residues (Ibβ-N41 and IX-N44) point outside.
For both GPIbβ and GPIX, their LRR domains are linked via a short sequence to respective transmembrane domains. Since GPIbβ and GPIX transmembrane domains associate closely in a parallel fashion[9, 11], it is most likely for the ectodomains to interact sideways. Moreover, the cylinder shape of GPIbβ and GPIX ectodomains implies that the inter-domain binding interface should be restricted to one side of the cylinder.
Both transmembrane and ectodomains of GPIbβ are required for surface expression of GPIX in transfected CHO cells
Partly due to the unavailability of a recombinant GPIX ectodomain, the interaction between GPIbβ and GPIX ectodomains has not been detected directly. Instead, it is inferred from the reliance of GPIX surface expression on GPIbβ[14]. In the absence of GPIbβ, little GPIX is detected on the cell surface[14], although it is clearly produced in transfected cells (Fig. 2). It is thought that, upon coexpression, GPIbβ forms a complex with GPIX, stabilizing GPIX conformation and facilitating its trafficking to the plasma membrane.
To test whether the GPIbβ ectodomain alone can rescue surface expression of GPIX in CHO cells, we constructed cDNAs for IbβE and IXE (Fig. 2A). IbβE and IXE contain only the ectodomain of GPIbβ (residues C1-L121) and GPIX (residues T1-Q124), respectively. To facilitate detection, the HA epitope tag was added to the N-terminus of full-length GPIbβ or IbβE. The resulting constructs are designated in this paper as HA-Ibβ and HA-IbβE, respectively. The GPIX or IXE cDNA was cotransfected transiently with HA-Ibβ, HA-IbβE, or empty vector pDX into CHO cells. Production of full-length proteins or their respective ectodomains was confirmed by immunoblotting of the cell lysate (Fig. 2B). Since a significant portion of target proteins is not transported to the cell surface and instead takes on non-native forms in the cell[20, 26], expression of native full-length receptors and ectodomains were assessed by expression level on the cell surface and protein secretion into the culture media, respectively. HA-IbβE alone was secreted efficiently from transfected CHO cells into the medium. Like HA-Ibβ[20], its expression was not affected by coexpression of GPIX or IXE (Fig. 2D). On the other hand, unlike HA-Ibβ, HA-IbβE could not enhance surface expression of GPIX (Fig. 2C). Neither HA-Ibβ nor HA-IbβE could induce secretion of native IXE (Fig. 2D). Thus, the transmembrane domains are indispensable for coexpression of GPIX with GPIbβ. Considering numerous BSS-causing mutations in the GPIbβ ectodomain, the simplest explanation is that the strong interaction of GPIbβ with GPIX comprises two components — one between transmembrane domains and another between ectodomains. Without the former, the latter may not suffice to stabilize GPIX, which could explain the ability of cells to coexpress GPIbβ and GPIX but not their ectodomains. For the purpose of this study, these results suggest that the interaction between GPIbβ and GPIX ectodomains be probed in the context of full-length subunits.
A stable IbβE/IXE chimera
For disulfide-containing globular proteins, their folding often depends on and is manifested by formation of native disulfide bonds[27]. Both GPIbβ and GPIX ectodomains contain 8 Cys residues that are expected to form 4 disulfide bonds (Fig. 1B). If any native disulfide bond is not formed, which typically results in formation of non-native inter-molecular disulfide bonds that can be detected by SDS-PAGE under nonreducing conditions, the ectodomain will not adopt the native conformation.
HA-IbβE secreted from transfected CHO cells was stable and well-folded as it formed no inter-molecular disulfide bonds (Fig. 3B). This is consistent with our recent characterization of the recombinant GPIbβ ectodomain purified from insect cell media (McEwan et al. manuscript in preparation). In contrast, despite its high sequence homology with IbβE, IXE was not secreted from transfected cells (Fig. 2D). We reasoned that it may be due to the low stability of IXE. It is thus possible that a IbβE/IXE chimeric protein is sufficiently stable to sustain folding and secretion. Figure 3 shows that, when IXE residues that were predicted to constitute all three convex loops (residues A29-R36, S49-Q60 and S76-D86) were grafted onto HA-IbβE to replace corresponding GPIbβ residues (W21-D31, A44-A55 and R71-G81), the resulting chimeric protein, designated HA-IbβEabc, was readily secreted and, more importantly, did not form any inter-molecular disulfide bonds (Fig. 3B). In comparison, grafting any one of the three convex loops of IXE onto HA-IbβE did not produce a stable chimera devoid of inter-molecular disulfide bonds (HA-IbβEa, HA-IbβEb, HA-IbβEc and HA-IbβEab in Fig. 3B). Replacing the HA epitope tag with the c-myc tag did not alter the secretion or the expression level of IbβEabc (data not shown). These results indicate that HA-IbβEabc is stable and well-folded, like HA-IbβE rather than IXE.
The IbβEabc chimera preserves the interaction of GPIX with GPIbβ
We next tested whether IbβEabc can replace IXE in the GPIbβ/GPIX complex. HA-IbβEabc was fused to transmembrane and cytoplasmic domains of GPIX to produce HA-IbβEabc-IXTC, which was then transfected transiently into CHO cells with or without GPIbβ (Fig. 4). For direct comparison, HA-IX, in which an HA tag was added to the N-terminus of GPIX, and HA-IbβE-IXTC, in which HA-IbβE is fused to GPIX transmembrane and cytoplasmic domains, were included in the same transfection experiment. Surface expression of GPIX or its chimera was measured by flow cytometry with anti-HA antibody immunostaining and quantified as described in earlier studies[8, 10]. Consistent with prior observations[14], when transfected alone, little HA-IX was detected on the cell surface (Fig. 2C, 4B). In contrast, both HA-IbβE-IXTC and HA-IbβEabc-IXTC were detectable, with the former at a higher level (Fig. 4B,C). This correlated well with the expression level and stability of isolated ectodomains. When co-transfected with GPIbβ, HA-IX appeared on the cell surface, at approximately 30% of the mean fluorescence intensity observed for GPIX in the wild type GPIb-IX complex (Fig. 2C, 4C). While surface expression of HA-IbβE-IXTC was decreased by the presence of GPIbβ, surface expression of HA-IbβEabc-IXTC was enhanced by the extent similar to that of HA-IX.
To further characterize the effect of loop-swapping on the interaction between GPIbβ and GPIX, a c-myc epitope tag was appended to the C-terminus of GPIbβ and the resulting Ibβ-myc construct used in co-immunoprecipitation (co-IP) experiments (Fig. 4D). Addition of the c-myc tag to GPIbβ did not decrease significantly the expression levels of the other subunits[10], suggesting that the epitope tag did not interfere with inter-subunit interactions. In transfected CHO cells, not all GPIbβ and GPIX proteins were transported to the plasma membrane[20, 26]. Given the uneven expression levels of target proteins, we compared the relative amount of GPIX variants pulled down with Ibβ-myc by co-IP. It is clear from Figure 4D that while HA-IbβE-IXTC was in association with Ibβ-myc, presumably through transmembrane domain interactions, the extent of their association was significantly lower than those between Ibβ-myc and HA-IbβEabc-IXTC or HA-IX.
Overall, these results indicate that, despite its high sequence similarity to HA-IbβE-IXTC, HA-IbβEabc-IXTC retained elements in the GPIX ectodomain that adequately support GPIbβ-induced increase in its surface expression and mediate native-like association with GPIbβ. In other words, GPIX residues A29-R36, S49-Q60, and S76-D86, which were predicted to form the three convex loops of the LRR domain in GPIX, may be considered the interfacial region with GPIbβ.
Discussion
Swapping sequences between homologous proteins to probe their structure and function is an approach often adopted because of its implicit and the usually correct assumption that swapping does preserve, not disrupt, the overall conformation and stability of the host protein. However, its application to GPIbβ and GPIX has been limited despite their high sequence similarity that was recognized upon their cloning two decades ago[21]. The main obstacle is the stark contrast of their abilities to express in the plasma membrane of transfected cells that, to a large extent, reflects the difference in stability or foldability between GPIbβ and GPIX ectodomains. Since swapping homologous sequences between a protein domain that is stable and well-folded (i.e. IbβE) and another that is not (i.e. IXE) could produce a chimera that behaves like the latter, it is necessary to assess the folding and stability of all swapped constructs. Utilizing disulfide formation as an effective and accurate indicator for native conformation, we have in this study identified IbβEabc as a stable and well-folded IbβE/IXE chimera. Structural models of GPIbβ and GPIX suggest that, in HA-IbβEabc, the IbβE scaffold supports GPIX residues A29-R36, S49-Q60, and S76-D86 to make up the convex surface of its cylinder-shaped domain. GPIbβ forms a complex with HA-IbβEabc-IXTC, in which HA-IbβEabc is fused to GPIX transmembrane and cytoplasmic domains, and boosts its surface expression in the native-like manner. The functional similarity of HA-IbβEabc-IXTC to HA-IX rather than HA-IbβE-IXTC, led us to conclude that three putative convex loops contain the GPIbβ-binding site in the GPIX ectodomain.
Given the high degree of sequence similarity between GPIbβ and GPIX ectodomains, our conclusion that three convex loops mediate GPIX interaction with GPIbβ suggests the convex loops in GPIbβ may also participate in interaction with GPIX. This is consistent with an earlier finding that GPIbβ residues 15-32 are critical for GPIbβ interaction with GPIX[13]. It was found that swapping GPIbβ residues 1-14 with GPIX counterparts did not affect surface expression of GPIX but swapping GPIbβ residues 1-32 did. According to the predicted structure, GPIbβ residues 15-32 cover both the short and convex sides in the second layer (Fig. 1). It is thus likely that swapping GPIbβ residues 1-32 could modify the GPIX-binding site. However, swapping residues 1-32 could also destabilize or disrupt folding of the GPIbβ ectodomain, which in turn impacts its interaction with GPIX and is unable to facilitate surface expression of GPIX.
A number of BSS-associated mutations have been mapped to GPIbβ and GPIX ectodomains. Although these mutations clearly highlight the importance of both ectodomains in assembly of the GPIb-IX complex, how they disrupt complex assembly and surface expression is not entirely clear. While mutations involving Cys are straightforward to comprehend, as they most likely interfere with formation of native disulfide bonds, others are more puzzling. Our study found the putative convex loops of GPIX as the GPIbβ-binding site, suggesting that mutations in these regions may disrupt association between GPIbβ and GPIX. Indeed, two mutations in GPIX convex loops have been reported[25, 28]. Two additional BSS mutations are located in the putative convex loops of GPIbβ[29, 30]. Whether these mutations cause BSS by disrupting GPIbβ/GPIX binding or by other mechanisms (e.g. disrupting folding of the host subunit) will require further investigation.
In contrast to grafting all three GPIX convex loops at once onto IbβE to generate IbβEabc, grafting these loops in any other combination failed to create a stable well-folded chimera (Fig. 3B). This result suggests that the interaction between three loops contribute critically to the domain stability. Such inter-layer interaction is consistent with those observed in concave and short sides and exemplified by the Asn ladder. Without concrete knowledge about their structure, it is difficult at the present time to further reduce the identified GPIbβ-binding site to specific residues in GPIX. Nonetheless, our demonstration of IbβEabc as a stable protein domain and as a capable substitute for IXE in its interaction with GPIbβ has made it possible to circumvent the obstacle of IXE instability and to obtain a suitable protein complex with the native GPIbβ/GPIX binding interface for detailed structural analysis.
Despite numerous trials, we have failed to detect a stable IbβE/IbβEabc complex by co-IP (data not shown). This indicates that the binding affinity between GPIbβ and GPIX ectodomains is weak and below the detection limit of a co-IP experiment. It could explain the inability of HA-IbβE to rescue secretion of IXE or surface expression of GPIX (Fig. 2). Furthermore, the interaction between GPIbβ and GPIX ectodomains is only detectable in the presence of their adjacent transmembrane domains, indicating that both ectodomain and transmembrane domain participate in the interaction. Synergistic association through both domains results in strong binding of GPIbβ and GPIX. Together with covalent and noncovalent forces linking GPIbα and GPIbβ, the molecular basis for the tightly integrated GPIb-IX complex has begun to emerge.
Acknowledgments
This work was supported by a grant from NIH (HL082808). We thank Dr. Sankaranarayanan Srinivasan for assistance with Figure 1.
References
- 1.Clemetson KJ. A short history of platelet glycoprotein Ib complex. Thromb Haemost. 2007;98:63–8. [PubMed] [Google Scholar]
- 2.Huizinga EG, Tsuji S, Romijn RA, Schiphorst ME, de Groot PG, Sixma JJ, Gros P. Structures of glycoprotein Ibα and its complex with von Willebrand factor A1 domain. Science. 2002;297:1176–9. doi: 10.1126/science.107355. [DOI] [PubMed] [Google Scholar]
- 3.Lopez JA, Leung B, Reynolds CC, Li CQ, Fox JEB. Efficient plasma membrane expression of a functional platelet glycoprotein Ib-IX complex requires the presence of its three subunits. J Biol Chem. 1992;267:12851–9. [PubMed] [Google Scholar]
- 4.Lopez JA, Andrews RK, Afshar-Kharghan V, Berndt MC. Bernard-Soulier syndrome. Blood. 1998;91:4397–418. [PubMed] [Google Scholar]
- 5.Bodnar RJ, Xi X, Li Z, Berndt MC, Du X. Regulation of glycoprotein Ib-IX-von Willebrand factor interaction by cAMP-dependent protein kinase-mediated phosphorylation at Ser 166 of glycoprotein Ibβ. J Biol Chem. 2002;277:47080–7. doi: 10.1074/jbc.M208329200. [DOI] [PubMed] [Google Scholar]
- 6.Othman M, Notley C, Lavender FL, White H, Byrne CD, Lillicrap D, O'Shaughnessy DF. Identification and functional characterization of a novel 27-bp deletion in the macroglycopeptide-coding region of the GPIbα gene resulting in platelet-type von Willebrand disease. Blood. 2005;105:4330–6. doi: 10.1182/blood-2002-09-2942. [DOI] [PubMed] [Google Scholar]
- 7.Mo X, Luo SZ, Munday AD, Sun W, Berndt MC, Lopez JA, Dong Jf, Li R. The membrane-proximal intermolecular disulfide bonds in glycoprotein Ib influence receptor binding to von Willebrand factor. J Thromb Haemost. 2008;6:1789–95. doi: 10.1111/j.1538-7836.2008.03088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mo X, Lu N, Padilla A, López JA, Li R. The transmembrane domain of glycoprotein Ibβ is critical to efficient expression of glycoprotein Ib-IX complex in the plasma membrane. J Biol Chem. 2006;281:23050–9. doi: 10.1074/jbc.M600924200. [DOI] [PubMed] [Google Scholar]
- 9.Luo SZ, Mo X, López JA, Li R. Role of the transmembrane domain of glycoprotein IX in assembly of the glycoprotein Ib-IX complex. J Thromb Haemost. 2007;5:2494–502. doi: 10.1111/j.1538-7836.2007.02785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo SZ, Mo X, Afshar-Kharghan V, Srinivasan S, Lopez JA, Li R. Glycoprotein Ibα forms disulfide bonds with 2 glycoprotein Ibβ subunits in the resting platelet. Blood. 2007;109:603–9. doi: 10.1182/blood-2006-05-024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo SZ, Li R. Specific heteromeric association of four transmembrane peptides derived from platelet glycoprotein Ib-IX complex. J Mol Biol. 2008;382:448–57. doi: 10.1016/j.jmb.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenny D, Morateck PA, Gill JC, Montgomery RR. The critical interaction of glycoprotein (GP) Ibβ with GPIX — a genetic cause of Bernard-Soulier syndrome. Blood. 1999;93:2968–75. [PubMed] [Google Scholar]
- 13.Kenny D, Morateck PA, Montgomery RR. The cysteine knot of platelet glycoprotein Ibβ (GPIbβ) is critical for the interaction of GPIbβ with GPIX. Blood. 2002;99:4428–33. doi: 10.1182/blood.v99.12.4428. [DOI] [PubMed] [Google Scholar]
- 14.Lopez JA, Weisman S, Sanan DA, Sih T, Chambers M, Li CQ. Glycoprotein (GP) Ibβ is the critical subunit linking GP Ibα and GP IX in the GP Ib-IX complex. Analysis of partial complexes. J Biol Chem. 1994;269:23716–21. [PubMed] [Google Scholar]
- 15.Finch CN, Lyle VA, Cunningham D, Miller JL. Expression of human platelet glycoprotein Ibβ in insect cells. Thromb Res. 1996;81:679–86. doi: 10.1016/0049-3848(96)00045-x. [DOI] [PubMed] [Google Scholar]
- 16.Tang J, Stern-Nezer S, Liu PC, Matyakhina L, Riordan M, Luban NL, Steinbach PJ, Kaler SG. Mutation in the leucine-rich repeat C-flanking region of platelet glycoprotein Ib beta impairs assembly of von Willebrand factor receptor. Thromb Haemost. 2004;92:75–88. doi: 10.1160/TH04-02-0071. [DOI] [PubMed] [Google Scholar]
- 17.Scott PG, McEwan PA, Dodd CM, Bergmann EM, Bishop PN, Bella J. Crystal structure of the dimeric protein core of decorin, the archetypal small leucine-rich repeat proteoglycan. Proc Natl Acad Sci USA. 2004;101:15633–8. doi: 10.1073/pnas.0402976101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barton WA, Liu BP, Tzvetkova D, Jeffrey PD, Fournier AE, Sah D, Cate R, Strittmatter SM, Nikolov DB. Structure and axon outgrowth inhibitor binding of the Nogo-66 receptor and related proteins. EMBO J. 2003;22:3291–302. doi: 10.1093/emboj/cdg325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levitt M. Accurate modeling of protein conformation by automatic segment matching. J Mol Biol. 1992;226:507–33. doi: 10.1016/0022-2836(92)90964-l. [DOI] [PubMed] [Google Scholar]
- 20.Mo X, Luo SZ, López JA, Li R. Juxtamembrane basic residues in glycoprotein Ibβ cytoplasmic domain are required for assembly and surface expression of glycoprotein Ib-IX complex. FEBS Lett. 2008;582:3270–4. doi: 10.1016/j.febslet.2008.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez JA. The platelet glycoprotein Ib-IX complex. Blood Coagul Fibrinolysis. 1994;5:97–119. [PubMed] [Google Scholar]
- 22.Bella J, Hindle KL, McEwan PA, Lovell SC. The leucine-rich repeat structure. Cell Mol Life Sci. 2008;65:2307–33. doi: 10.1007/s00018-008-8019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clemetson JM, Kyrle PA, Brenner B, Clemetson KJ. Variant Bernard-Soulier syndrome associated with a homozygous mutation in the leucine-rich domain of glycoprotein IX. Blood. 1994;84:1124–31. [PubMed] [Google Scholar]
- 24.Strassel C, Pasquet JM, Alessi MC, Juhan-Vague I, Chambost H, Combrie R, Nurden P, Bas MJ, De La Salle C, Cazenave JP, Lanza F, Nurden AT. A novel missense mutation shows that GPIbβ has a dual role in controlling the processing and stability of the platelet GPIb-IX adhesion receptor. Biochemistry. 2003;42:4452–62. doi: 10.1021/bi026213d. [DOI] [PubMed] [Google Scholar]
- 25.Drouin J, Carson NL, Laneuville O. Compound heterozygosity for a novel nine-nucleotide deletion and the Asn45Ser missense mutation in the glycoprotein IX gene in a patient with Bernard-Soulier syndrome. Am J Hematol. 2005;78:41–8. doi: 10.1002/ajh.20236. [DOI] [PubMed] [Google Scholar]
- 26.Lopez JA, Li CQ, Weisman S, Chambers M. The glycoprotein Ib-IX complex-specific monoclonal antibody SZ1 binds to a conformation-sensitive epitope on glycoprotein IX: implications for the target antigen of quinine/quinidine-dependent autoantibodies. Blood. 1995;85:1254–8. [PubMed] [Google Scholar]
- 27.Sela M, White FH, Jr, Anfinsen CB. Reductive cleavage of disulfide bridges in ribonuclease. Science. 1957;125:691–2. doi: 10.1126/science.125.3250.691. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki K, Hayashi T, Yahagi A, Akiba J, Tajima K, Satoh S, Sasaki H. Novel point mutation in the leucine-rich motif of the platelet glycoprotein IX associated with Bernard-Soulier syndrome. Br J Haematol. 1997;99:794–800. doi: 10.1046/j.1365-2141.1997.4753275.x. [DOI] [PubMed] [Google Scholar]
- 29.Kunishima S, Tomiyama Y, Honda S, Fukunishi M, Hara J, Inoue C, Kamiya T, Saito H. Homozygous Pro74-->Arg mutation in the platelet glycoprotein Ibβ gene associated with Bernard-Soulier syndrome. Thromb Haemost. 2000;84:112–7. [PubMed] [Google Scholar]
- 30.Hillmann A, Nurden A, Nurden P, Combrie R, Claeyssens S, Moran N, Kenny D. A novel hemizygous Bernard-Soulier Syndrome (BSS) mutation in the amino terminal domain of glycoprotein (GP)Ibβ--platelet characterization and transfection studies. Thromb Haemost. 2002;88:1026–32. [PubMed] [Google Scholar]


