Abstract
Chorioamnionitis is a major cause of prematurity as well as perinatal morbidity and mortality. The present study observed a marked increase in immunohistochemical staining for Colony Stimulating Factor 2 (CSF2; also known as granulocyte macrophage-colony stimulating factor), a potent neutrophil and macrophage chemoattractant and activator, in the decidua of patients with CAM compared with controls (n = 8; P = .001). To examine the regulation of this CSF2, cultured decidual cells primed with estradiol (E2) or E2 plus medroxyprogesterone acetate, were exposed to tumor necrosis factor-α or interleukin-1β and secreted CSF2 measured by ELISA. Levels of CSF2 in E2 plus MPA-treated cultures increased 18- and 245-fold following treatment with TNF or IL1B (n = 7, P < .05). Quantitative RT-PCR demonstrated parallel changes in mRNA levels. This study reveals that CSF2 is strongly expressed in decidua from patients with CAM and indicates TNF or IL1B as important regulators of CAM-related decidual leukocyte infiltration and activation.
Keywords: CSF2, GM-CSF, chorioamnionitis, TNF-α, IL-1β
Introduction
Preterm delivery (PTD) complicates 12.5% of live births and is a major antecedent of perinatal morbidity and mortality.1 Chorioamnionitis (CAM) is a leading cause of early PTD, contributing to more than 50% of cases.2,3 Such infections occur in 10% to 18% of women with term delivery and 20% to 33% of women delivering preterm.2,4 Ascending microorganisms, usually bacteria, reach the uterus from the vagina and the cervix to provoke an inflammatory reaction in the decidua.5 These organisms subsequently penetrate the chorion, amnion, and ultimately reach the amniotic fluid and fetus.5 Infections and the host inflammatory response to infections trigger, either directly or indirectly, the production of effector molecules including prostaglandin (PG)E2 and PGF2α as well as synthesis and secretion of matrix metalloproteinases.5-12 The resulting microenvironment, rich in uterotonic compounds and extracellular matrixdegrading enzymes, is thought to contribute to preterm labor, preterm premature rupture of membranes (PPROM), and to the onset of PTD.
Leukocyte infiltration and activation are classic hallmarks of the host response to bacterial infections. In CAM, the maternal inflammatory reaction results primarily in neutrophil and secondarily in macrophage accumulation and activation.13-15 Amniotic fluid from patients with intraamniotic infections contains elevated levels of leukocyte chemoattractants and activators including interleukin 6 (IL-6), IL-8, colony stimulating factors, CCL3 (macrophage inflammatory protein-1α), CXCL1 (growth-related oncogene-α), as well as elevated levels of the classic proinflammatory cytokines, tumor necrosis factor α (TNF) and interleukin 1β (IL-1β).16
Previous studies indicate a central role for the decidua in leukocyte recruitment and activation during intrauterine infections. Our laboratory demonstrated that cultured human decidual cells produce elevated levels of IL-8 (CXCL8), as well as monocyte chemoattractant protein-1 (CCL2) and colony stimulating factor 1 (CSF1) in response to TNF and IL-1β.17-19 Colony stimulating factor 2 (CSF2) was initially identified by its action in promoting proliferation and differentiation of progenitor cells of the myeloid lineage.20 It plays a crucial role in bolstering the host defense against invading microorganisms by enhancing trafficking of neutrophils and monocytes and by activating and/or augmenting many of the functional activities of mature neutrophils, monocyte/macrophages, and dendritic cells 20-29. Under the hypothesis that the expression of CFS2 by decidua cells contributes to the host defense during CAM, the current study assessed immunohistochemical staining for CSF2 in decidual cells of sections of placental-bed biopsies obtained from patients with CAM compared with gestational age-matched controls. The effects of TNF and IL-1β on CSF2 mRNA and protein levels were then evaluated in leukocyte-free monolayers of term decidual cells. In view of the high circulating progesterone levels during pregnancy, the potential interactions of a progestin with TNF and IL-1B were also assessed on CSF2 expression in the cultured decidual cells. Consistent with our hypothesis, the results indicate a previously undisclosed role of decidual cell-expressed CSF2 in leukocyte infiltration and activation in CAM.
Materials and Methods
Specimens
Eight placentas from patients with CAM, between 33 and 38 weeks of gestation, were selected from the case files of the Department of Human Pathology and Oncology of the University of Siena. Histological diagnosis of CAM was based on the presence of a rich inflammatory infiltrate of neutrophils in the membranes, decidua and cord in the setting of clinical infections. All infants displayed biochemical signs of acute phase response (ie, leukocytosis or leukopenia, C-reactive protein levels >1.0 mg/dL). In 4 cases, the presence of neonatal infections was confirmed by positive blood cultures for pathogens. All neonates were delivered vaginally. Eight control placentas from uncomplicated pregnancies were obtained following spontaneous delivery at term. Approval for this study was granted by the Institutional Human Investigation Committee (HIC) of the University of Siena. Informed consent was obtained from all women. Placenta sampling for histology included multiple full-thickness placental blocks and sections of the umbilical cord at 3 different levels. For each specimen, a block most representative of the decidua was selected for immunohistochemistry.
Immunohistochemistry
Sections (4 mm) of paraffin embedded placental and attached fetal membrane tissues were cut, deparaffinized, rehydrated, and washed in Tris-buffered saline (TBS: 20 mmol/L Tris–HCl, 150 mmol/L NaCl [pH 7.6]). Antigen retrieval was carried out by incubating sections in Target Retrieval Solution, pH 9.0 (DAKO, Copenhagen, Denmark), in a microwave oven at 750 Watts for 15 minutes. Sections were subsequently rinsed in 3% hydrogen peroxide to block endogenous peroxidase and incubated overnight at 4°C with monoclonal antibodies against CSF2 (R&D Systems, Minneapolis, Minn), vimentin, and cytokeratin (DAKO) diluted in TBS. Slides were then washed 3 times with TBS for 5 minutes and incubated with the Universal LSAB+ Kit (DAKO). After washing 3 times for 5 minutes in TBS, sections were incubated for 20 minutes with peroxidase conjugate streptavidin (DAKO) and then stained with 3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich, St. Louis, Mo) as chromogen substrate and counterstained with Meyer's hematoxylin. Staining specificity was tested by substituting nonimmune serum for the primary antibody. Immunostaining of CSF2 in the decidua was evaluated by one experienced pathologist (P.T.) employing a semi-quantitative method in accordance with the following scoring system: +++, intense staining; ++, marked staining; +, moderate staining; 0/+, weak staining.
Isolation and Culture of Decidual Cells
Placentas and attached fetal membranes were obtained from patients with uncomplicated pregnancies undergoing repeat cesarean deliveries near term and not in labor at the Yale-New Haven Hospital under approval by Yale's Institutional Review Board (the Human Investigation Committee). A small portion of each specimen was formalin fixed, paraffin embedded, and then examined histologically to rule out underlying acute or chronic inflammation. The decidua was scraped from the maternal surface of the chorion, minced and digested in Ham's F-10 + 10% charcoal-stripped calf serum (SCS; Flow Laboratories, Rockville, Md) containing 25 mg/mL of collagenase (200 U/mg; Worthington Biochemical Corp., Freehold, NJ) in a shaking water bath at 37°C for 30 minutes. After adding 6.25 units of DNase (Sigma-Aldrich) per mL of digestate, the incubation was continued for another 45 minutes. Cell clusters in the final digestate were dissociated via aspiration with a 23-gauge needle. The isolated cells were centrifuged at 1500 rpm for 5 minutes at 4°C then washed in Ham's F-10 × 3 and the resulting cell pellet was resuspended (1 g of tissue/mL) in 20% Percoll (Sigma-Aldrich), layered onto a (60%:50%:40%) discontinuous Percoll gradient, then centrifuged at 22 000 rpm for 20 minutes at 4°C. The top cell layer was collected, washed, resuspended in Ham's F-10, and centrifuged at 1500 rpm for 5 minutes at 4°C. After repeating this procedure, the cell pellet was resuspended in 40% Percoll (1 g of tissue/mL), layered on a discontinuous (55%:50%:40%) Percoll gradient, then centrifuged at 22,000 rpm for 20 min at 4°C. The top cell layer was washed twice in serum-free Ham's F-10 and then centrifuged at 500 rpm for 5 min at 4°C. The cell pellet was resuspended in Ham's F-10 + 10% SCS and decidual cells were counted in a hemocytometer. After the isolation procedure, trypan blue exclusion indicated that more than 95% of isolated cells were viable.
Isolated decidual cells (5 × 105 cells/mL) were suspended in Basal Medium (BM), a phenol red-free 1:1 vol/vol mix of DMEM (Invitrogen, Carlsbad, Calif) and Ham's F-12 (Flow Laboratories) with 100 U/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL fungizone supplemented with 10% SCS. The cells were seeded onto polystyrene tissue culture dishes coated with 2% type B gelatin (Sigma-Aldrich). The cultures were grown to confluence in a standard 95% air:5% CO2 incubator at 37°C and passaged 3 times. Fluorescent antibody cell sorting for the presence of CD45+ demonstrated that unpassaged cultures contained 12% to 15% CD45+ cells while passaged cultures were >99% negative for this common leukocyte marker. The latter were used for experimental cell incubations. The cultured cells were vimentin positive and cytokeratin negative. Cultured cells also showed decidualization-related morphological changes, and expressed biochemical endpoints of decidualization including enhanced tissue factor and plasminogen-activator inhibitor-1 and reduced interstitial collagenase and stromelysin-1 under the influence of progestin.
Experimental Cell Incubations
Confluent decidual cells were primed for 7 days in BM supplemented with 10% SCS containing either vehicle control (0.1% ethanol) or 10−8 M of estradiol (E2) or 10 −7 M of medroxyprogesterone acetate (MPA; Sigma-Aldrich) or E2 plus MPA with one change of medium. Because circulating levels of both E2 and progesterone are high during the third trimester, E2 was employed with MPA to mimic the gestational steroidal milieu. Medroxyprogesterone acetate was used in place of progesterone since the latter is rapidly metabolized in vitro.30 The cultures were washed twice with PBS and switched to a serum-free defined medium (DM) consisting of BM plus ITS+ premix (BD Biosciences, Bedford, Mass), 5 μM FeSO4, 0.5 μM ZnSO4, 1 nM CuSO4, 20 nM Na2SeO3, trace elements (Life Technologies Inc), 50 μg/mL ascorbic acid (Sigma-Aldrich), and 50 ng/mL epidermal growth factor (BD Biosciences). The corresponding vehicle or steroid(s) with or without TNF or IL-1B (R&D Systems) was added to DM.
ELISA
After 24-hour incubation, concentrations of immunoreactive CSF2 in conditioned DM were measured using an ELISA according to instructions provided by the manufacturer (R&D Systems). The sensitivity of this ELISA was <10 pg/mL. The intra- and inter-assay coefficients of variation were 2.6% and 5%, respectively. Levels of CSF2 were normalized to total cell culture protein as measured by a Bradford protein assay (Bio-Rad Laboratories, Hercules, Calif).31
Real Time-Quantitative RT-PCR
Total RNA was extracted from cultured cells with Tri-Reagent (Sigma-Aldrich). Reverse transcription was carried out with an AMV reverse transcriptase kit (Invitrogen, Carlsbad, Calif) on an Eppendorf Mastercycler (Eppendorf, Westbury, NY). To perform quantitative real time reverse transcriptase–polymerase chain reaction (RT-PCR) a standard curve was created between 500 pg and 250 ng of cDNA with a Roche Light Cycler (Roche, Indianapolis, Ind) by monitoring increasing fluorescence of PCR products during amplification. Upon establishing the standard curve, quantitation of the unknowns was determined with the Roche Light Cycler and adjusted to the quantitative expression of actin-β (ACTB) from the corresponding unknowns. For CSF2 mRNA detection in DC cultures the sense primer was 5′-CACAAGAGCCAGGAAGAAAC—3′ and the antisense primer was 5′-CTACAACAGACCCACACAATAC-3.′ The ACTB sense and antisense primers were 5′-CGTACCACTGGCATCGTGAT-3′ and 5′-GTGTTGGCGTACAGGTCTTTG-3,′ respectively. The expected sizes of the amplified fragments for CSF2 and ACTB mRNA were 452 and 459 base pairs, respectively.
Statistical Analysis
Comparisons of control and the various treatment groups were performed using the Kruskal-Wallis ANOVA on Ranks test followed by the Student-Newman-Keuls post hoc test with P value < .05 representing statistical significance. For immunohistochemistry, the intensity of staining in control vs. CAM cases was compared using the χ2 test, with significance set at a probability value of <.05.
Results
Decidual CSF2 Expression in CAM
Images of serial sections of a normal term placental specimen following immunostaining for CSF2, vimentin and cytokeratin are shown in Figure 1. Immunoreactivity for CSF2 was consistently found in the cytoplasm of cells with the morphological characteristics of decidual cells (Figure 1A). Most of these CSF2 positive cells also stained for vimentin (Figure 1B) but not for cytokeratin (Figure 1C) confirming their decidual phenotype.
Figure 1.
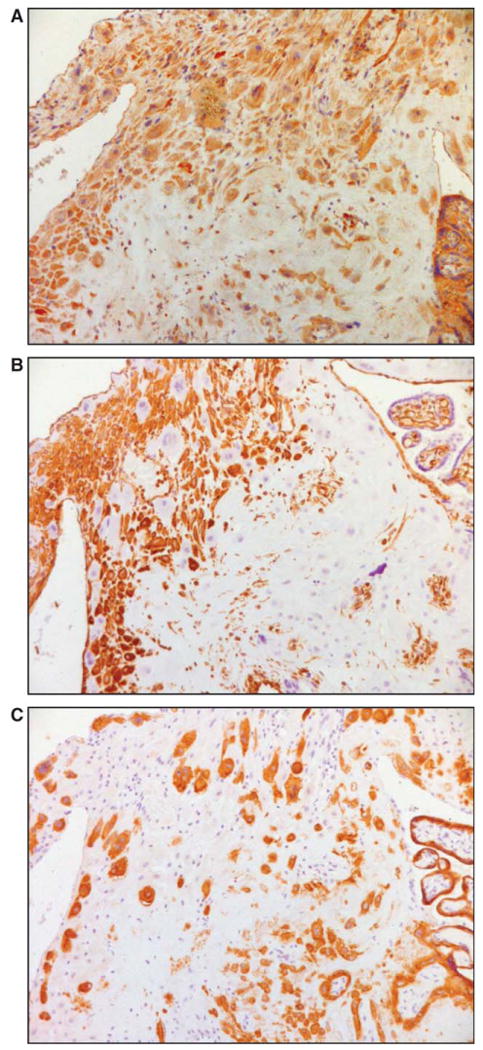
Immunohistochemical analysis of decidual CSF2-expressing cells. Serial sections of a placental bed specimens were stained for CSF2 (A), vimentin (B), and cytokeratin (C). Immunohistochemistry was performed by the labelled streptavidin–biotin (LSAB) method; ×200 original magnification. CSF2 = colony stimulating factor 2.
Staining for CSF2 in placental specimens from patients with CAM was then compared with controls. As depicted in Figure 2, decidual cells in CAM cases (Figure 2A) displayed more intense CSF2 immunostaining compared with controls (Figure 2C). In particular, intense staining was observed in 2 CAM cases, with marked staining observed in 6 CAM cases. Weak CSF2 immunostaining was evident in 3 control decidua, whereas 5 specimens displayed a moderate CSF2 positivity. As shown in Figure 2B and D, CSF2 immunopositive cells were also stained for vimentin. Table 1 summarizes the results, which are categorized by the intensity of immunostaining. Intense immunostaining for CSF2 was more frequently present in CAM versus control decidual specimens (χ2 = 16.0; df = 3; P = .001).
Figure 2.
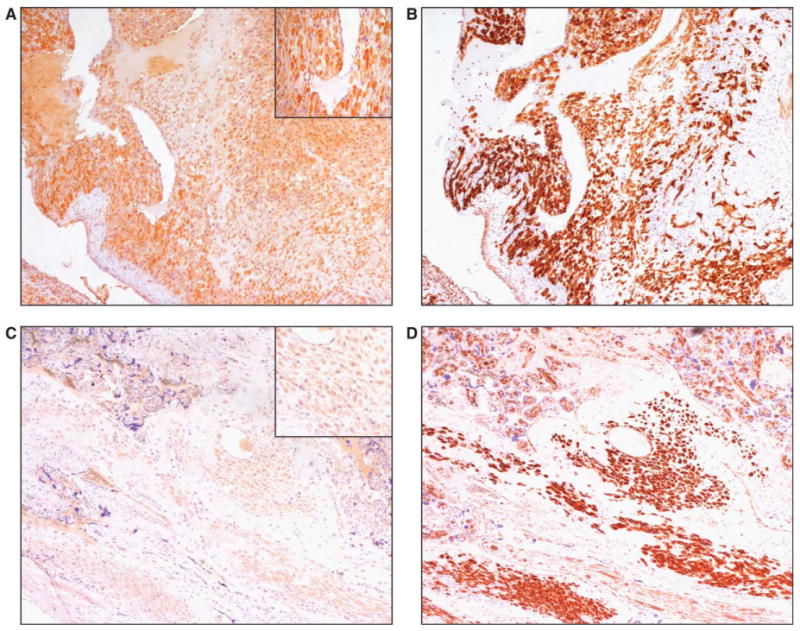
Immunohistochemical analysis of CSF2 expression in chorioamnionitis (CAM). Immunohistochemistry was performed by the labelled streptavidin–biotin (LSAB) method. Serial sections of decidua from a patient with CAM were stained for CSF2 (A) and vimentin (B); ×200 original magnification. Control decidua stained for CSF2 (C) and vimentin (D); ×200 original magnification). The inset in A and C shows a detail at higher magnification. CSF2 = colony stimulating factor 2.
Table 1. Immunohistochemical Analysis of Decidual Colony Stimulating Factor 2 (CSF2) Expression in Chorioamnionitis and Control Specimensa.
| CSF2 Immunostaining | ||||
|---|---|---|---|---|
| +++ | ++ | + | 0/+ | |
| Chorioamnionitis | 2 | 6 | 0 | 0 |
| Controls | 0 | 0 | 5 | 3 |
The following scoring system was used: +++, intense staining; ++, marked staining; +, moderate staining; 0/+, weak staining.
Regulation of CSF2 Expression in Decidual Cells
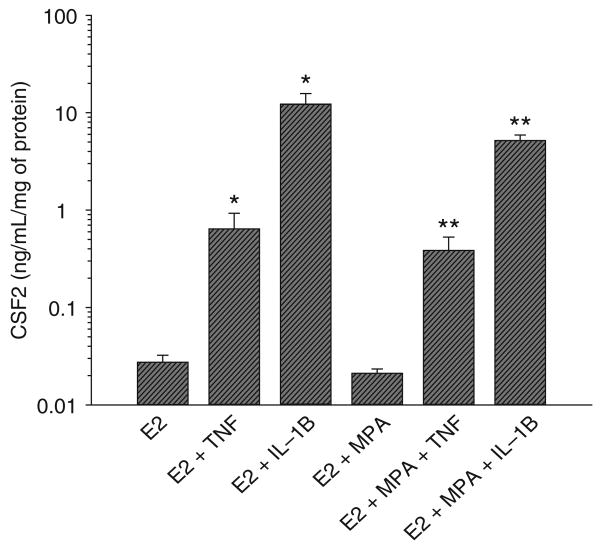
Figure 3 indicates that in cultures maintained with E2 alone, 1 ng/mL of TNF or IL-1B significantly enhanced CSF2 levels from 0.027 ± 0.0049 ng/mL/mg of protein (mean + SEM) in control cultures, to 0.63 ± 0.28 and 12.16 ± 3.4 ng/mL/mg of protein (P < .05; n = 8), respectively. This represents an increase CSF2 output by 23- and 450-fold, respectively. Similarly, in E2 + MPA treated cultures, CSF2 values increased from basal levels of 0.021 ± 0.0022 ng/mL/mg protein to 0.38 ± 0.14 and 5.15 ± 0.71 ng/mL/mg protein by TNF and IL-1B, respectively (P < .05; n = 8), representing increases in CSF2 output of 18- and 245-fold. In contrast to the marked elevation of CSF2 output elicited by the cytokines, the addition of MPA with E2 did not significantly alter basal levels compared to cultures treated with E2 alone and did not significantly affect the response to cytokines.
Figure 3.
Effects of estradiol (E2), medroxyprogesterone acetate (MPA), TNF, and IL-1B on CSF2 output by decidual cell monolayers. Confluent decidual cells were incubated for 7 days in 10−8 M E2 or E2 + 10−7 M MPA, then switched to DM with corresponding steroids ± 1 ng/mL of TNF or IL-1B for 24 hours. CSF2 levels were measured by ELISA in conditioned DM and normalized to cell protein (n = 8, mean + SEM). * versus E2 (P < .05); ** versus E2 + MPA (P < .05). Note that ordinate is log scale. CSF2 = colony stimulating factor 2; DM = defined medium; IL-1B = interleukin 1β; TNF = tumor necrosis factor α.
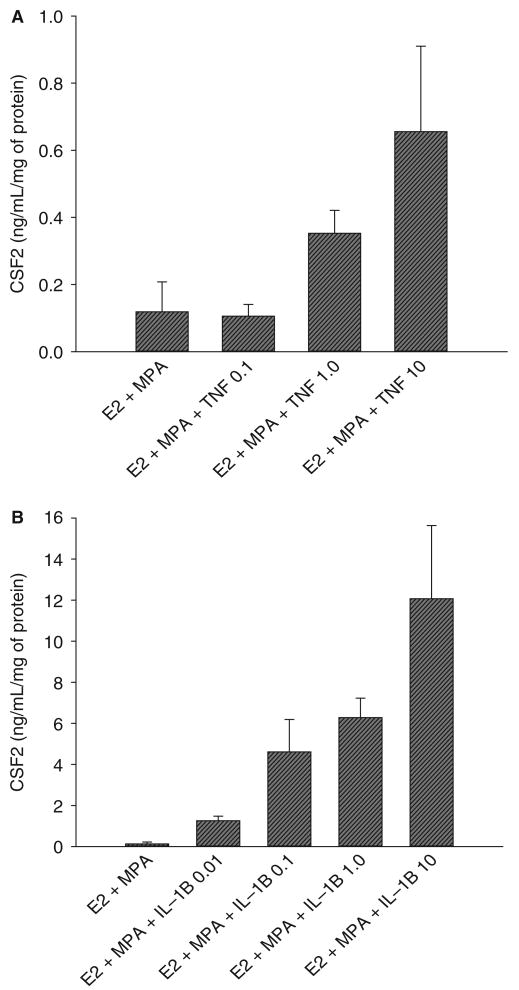
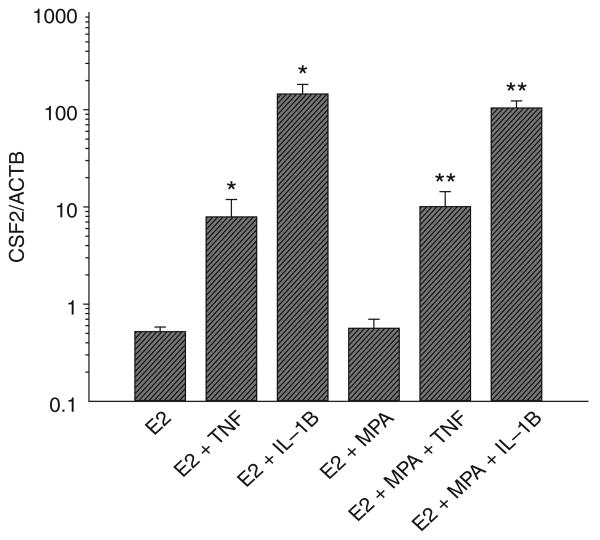
In view of the absence of a statistically significant progestin effect on either basal or cytokine-enhanced CSF2 output, further evaluation of the actions of TNF and IL-1B on CSF2 expression was carried out in decidual cell cultures primed with E2 + MPA. The induction of CSF2 protein levels by TNF (Figure 4A) and IL-1B (Figure 4B) was concentration-dependent with maximum effects for both cytokines observed at 10 ng/mL. As indicated in Figure 5, changes in the mRNA pattern for CSF2 corresponded to those of the protein. Thus, TNF and IL-1B each induced a statistically significant increase in CSF2 mRNA levels normalized to those of the ACTB whether added with E2 or with E2 plus MPA. In contrast, in the absence of the cytokines similar CSF2 mRNA levels were observed in parallel incubations with E2 or with E2 + MPA.
Figure 4.
Concentration-dependent effects of TNF and IL-1B on CSF2 output by decidual cell monolayers maintained in estradiol (E2) plus medroxyprogesterone acetate (MPA). Confluent decidual cells were incubated for 7 days in 10−8 M E2 + 10−7 M MPA, and then switched to DM with the steroids ± the indicated amount (in ng/mL) of TNF (A) or IL-1B (B). CSF2 levels were measured by ELISA in conditioned DM and normalized to cell protein. Shown are the mean ± SEM; n = 3. CSF2 = colony stimulating factor 2; DM = defined medium; IL-1B = interleukin 1β; TNF = tumor necrosis factor α.
Figure 5.
Quantitative RT-PCR of effects of estradiol (E2), medroxyprogesterone acetate (MPA), TNF and IL-1B on CSF2 mRNA levels in leukocyte-free term decidual cell monolayers. Confluent decidual cells were incubated for 7 days in 10−8 M E2 or E2 + 10−7 M MPA, and then switched to defined medium with corresponding steroids ± 1 ng/mL of TNF or IL-1B for 5 hours. CSF2 mRNA levels were measured by quantitative RT-PCR and normalized to ACTB mRNA levels. Ordinate: CSF2 mRNA/ACTB mRNA. (n = 7, mean ± SEM); * versus E2 (P < .05); ** versus E2 + MPA (P < .05). Note that ordinate is log scale. CSF2 = colony stimulating factor 2; IL-1B = interleukin 1β; RT-PCR = reverse transcriptase–polymerase chain reaction; TNF = tumor necrosis factor α.
Discussion
Inflammation involves migration and activation of leukocytes that are recruited down a gradient of chemotactic mediators. Circulating leukocytes, initially neutrophils and subsequently monocytes, marginate, adhere to endothelial cells, undergo diapedesis, and ultimately traffic to the inflammatory site. Upon their arrival at inflammatory sites, leukocytes are activated enabling them to respond to noxious stimuli via enhanced antimicrobial activity.32 Several studies have shown that leukocyte chemoattraction and activation depends on the coordinated actions of several factors, generated by bacteria, inflammatory cells, or by the resident cells of inflamed tissue. These include various chemokines. Among these, CSF2 plays a crucial and multifaceted role. The CSF2 molecule is a 22 000 kd glycoprotein that was discovered by virtue of its unique capacity to generate both granulocyte and macrophage colonies from precursor cells.20 In addition to promoting stem cell proliferation, CSF2 is a strong neutrophil and monocyte chemoattractant.21,22 It mediates the functional activity of mature neutrophils by enhancing release of arachidonic acid, leukotriene B4 synthesis, and superoxide anions.23,24 Furthermore, CSF2 increases phagocytosis by neutrophils and macrophages, as well as cytotoxicity, protease synthesis, and the generation of superoxide anions by monocytes and macrophages.25-29
A complex network of autocrine and paracrine interactions regulates the placental response to intrauterine infections. Inflammatory cytokines are secreted by reproductive tract tissues following exposure to bacteria or bacterial products. Several studies have shown that bacterial-derived lipopolysaccharide (LPS) enhances the synthesis and secretion of TNF and IL-1B by decidual cells,33 of IL-1B by chorionic cells34 and TNF by the amnion.35 Local macrophages also produce TNF following exposure to LPS.36 Consequently, levels of both cytokines are increased in the amniotic fluid during microbial invasion of the amniotic cavity and in gestational membranes in PTD.16
Tumor necrosis factor α and IL-1B enhance CSF2 production in several cell types such as fibroblasts, epithelial, and endothelial cells.37-40 Increased levels of CSF2 have been reported in the amniotic fluid of CAM patients compared to controls.41 In mice, CSF2 is responsible for the infiltration and activation of macrophages, dendritic cells, and neutrophils of the endometrial stroma that accompanies mating 42,43. Using immunostaining of gestationally age-matched placental sections, the present study provides the first demonstration that CSF2 is expressed by term decidua, and that its expression increases in CAM patients. In addition, our in vitro results show that TNF and IL-1B markedly augment CSF2 synthesis and secretion in decidual cells. Taken together, the current results indicate a novel role for decidual CSF2 in the TNF and IL-1B-driven inflammatory response that leads to leukocyte recruitment and activation in CAM.
Preterm labor with intact membranes has been associated with systemic activation of maternal granulocytes and monocytes.44 Epidemiological data also demonstrate an association between infiltration of the genital tract by maternal granulocytes and monocytes and an increased risk of preterm premature rupture of membranes (PPROM), suggesting that leukocyte accumulation and activation play a key role in its pathogenesis.45 Previous studies demonstrated a strong association between PPROM and increased protease activity in the fetal membranes.46,47 Activated neutrophils and macrophages are a well-documented source of elastase, collagenase, and MMP-9.48,49 These proteases degrade the extracellular matrix of the decidua and fetal membranes to reduce their tensile strength.45 Furthermore, the increased release of arachidonic acid by CSF2-primed neutrophils23 would result in enhanced availability of prostaglandins. These bioactive lipids target the myometrium to increase contractility. Thus, enhanced proteolytic degradation of the extracellular matrix of the decidua and fetal membranes is likely to synergize with bioactive lipid-mediated increased contractility to provide a potent signal that promotes PPROM and/or preterm labor and delivery.
In summary, the integration of our in vivo and in vitro provides strong supporting evidence that during CAM, leukocyte infiltration and activation of the decidua is associated with TNF and IL-1B-enhanced CSF2 expression in decidual cells. This increase in the leukocyte population and activity may play a role in PPROM and PTD.
Acknowledgments
This work was supported by grants from the National Institutes of Health 2 R01HD 33937–05 (CJL) and 1 R01 HL070004–03 (CJL), from the University of Siena (MC, FA), and the Italian Ministry of Education and Scientific Research (PT). This study was presented at the 53rd annual meeting of the Society for Gynecologic Investigation, Reno, Nevada, Poster #26; March 15, 2007.
References
- 1.Hamilton BE, Martin JA, Sutton PD. Births: preliminary data for 2003. Natl Vital Stat Rep. 2004;53:1–17. [PubMed] [Google Scholar]
- 2.Mueller-Heubach E, Rubinstein DN, Schwarz SS. Histologic chorioamnionitis and preterm delivery in different patient populations. Obstet Gynecol. 1990;75:622–626. [PubMed] [Google Scholar]
- 3.Cassell G, Hauth J, Andrews W, Cutter G, Goldenberg R. Chorioamnion colonization: correlation with gestational age in women delivered following spontaneous labor versus indicated delivery [abstract] Am J Obstet Gynecol. 1993;168:425. doi: 10.1016/0002-9378(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 4.Guzick DS, Winn K. The association of chorioamnionitis with preterm delivery. Obstet Gynecol. 1985;65:11–16. [PubMed] [Google Scholar]
- 5.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 6.So T, Ito A, Sato T, Mori Y, Hirakawa S. Tumor necrosis factor-alpha stimulates the biosynthesis of matrix metalloproteinases and plasminogen activator in cultured human chorionic cells. Biol Reprod. 1992;46:772–778. doi: 10.1095/biolreprod46.5.772. [DOI] [PubMed] [Google Scholar]
- 7.Van Meir CA, Sangha RK, Walton JC, Matthews SG, Keirse MJ, Challis JR. Immunoreactive 15-hydroxyprostaglandin dehydrogenase (PGDH) is reduced in fetal membranes from patients at preterm delivery in the presence of infection. Placenta. 1996;17:291–297. doi: 10.1016/s0143-4004(96)90052-1. [DOI] [PubMed] [Google Scholar]
- 8.Lei H, Furth EE, Kalluri R, et al. A program of cell death and extracellular matrix degradation is activated in the amnion before the onset of labor. J Clin Invest. 1996;98:1971–1978. doi: 10.1172/JCI119001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLaren J, Taylor DJ, Bell SC. Prostaglandin E(2)-dependent production of latent matrix metalloproteinase-9 in cultures of human fetal membranes. Mol Hum Reprod. 2000;6:1033–1040. doi: 10.1093/molehr/6.11.1033. [DOI] [PubMed] [Google Scholar]
- 10.Challis JR, Lye SJ, Gibb W, Whittle W, Patel F, Alfaidy N. Understanding preterm labor. Ann N Y Acad Sci. 2001;943:225–234. doi: 10.1111/j.1749-6632.2001.tb03804.x. [DOI] [PubMed] [Google Scholar]
- 11.Arechavaleta-Velasco F, Ogando D, Parry S, Vadillo-Ortega F. Production of matrix metalloproteinase-9 in lipopolysaccharide-stimulated human amnion occurs through an autocrine and paracrine proinflammatory cytokinedependent system. Biol Reprod. 2002;67:1952–1958. doi: 10.1095/biolreprod.102.004721. [DOI] [PubMed] [Google Scholar]
- 12.Fortunato SJ, Menon R, Lombardi SJ. Role of tumor necrosis factor-alpha in the premature rupture of membranes and preterm labor pathways. Am J Obstet Gynecol. 2002;187:1159–1162. doi: 10.1067/mob.2002.127457. [DOI] [PubMed] [Google Scholar]
- 13.Benirschke K, Kaufmann P. Pathology of the Human Placenta. New York, NY: Springer; 2000. pp. 591–659. [Google Scholar]
- 14.Matsubara S, Yamada T, Minakami H, Watanabe T, Takizawa T, Sato I. Polymorphonuclear leukocytes in the fetal membranes are activated in patients with preterm delivery: ultrastructural and enzyme-histochemical evidence. Placenta. 1999;20:185–188. doi: 10.1053/plac.1998.0366. [DOI] [PubMed] [Google Scholar]
- 15.Eis AL, Brockman DE, Myatt L. Immunolocalization of the inducible nitric oxide synthase isoform in human fetal membranes. Am J Reprod Immunol. 1997;38:289–294. doi: 10.1111/j.1600-0897.1997.tb00517.x. [DOI] [PubMed] [Google Scholar]
- 16.Saji F, Samejima Y, Kamiura S, Sawai K, Shimoya K, Kimura T. Cytokine production in chorioamnionitis. J Reprod Immunol. 2000;47:185–196. doi: 10.1016/s0165-0378(00)00064-4. [DOI] [PubMed] [Google Scholar]
- 17.Lockwood CJ, Arcuri F, Toti P, et al. Tumor necrosis factor-alpha and interleukin-1beta regulate interleukin-8 expression in third trimester decidual cells: implications for the genesis of chorioamnionitis. Am J Pathol. 2006;169:1294–1302. doi: 10.2353/ajpath.2006.060185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lockwood CJ, Matta P, Krikun G, et al. Regulation of monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha and interleukin-1beta in first trimester human decidual cells: implications for preeclampsia. Am J Pathol. 2006;168:445–452. doi: 10.2353/ajpath.2006.050082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arcuri F, Buchwalder L, Toti P, et al. Differential regulation of colony stimulating factor 1 and macrophage migration inhibitory factor expression by inflammatory cytokines in term decidua: implications for macrophage trafficking at the fetalmaternal interface. Biol Reprod. 2006;76:433–439. doi: 10.1095/biolreprod.106.054189. [DOI] [PubMed] [Google Scholar]
- 20.Burgess AW, Metcalf D. The nature and action of granulocyte-macrophage colony stimulating factors. Blood. 1980;56:947–958. [PubMed] [Google Scholar]
- 21.Gomez-Cambronero J, Horn J, Paul CC, Baumann MA. Granulocyte-macrophage colony-stimulating factor is a chemoattractant cytokine for human neutrophils: involvement of the ribosomal p70 S6 kinase signaling pathway. J Immunol. 2003;171:6846–6855. doi: 10.4049/jimmunol.171.12.6846. [DOI] [PubMed] [Google Scholar]
- 22.Wang JM, Colella S, Allavena P, Mantovani A. Chemotactic activity of human recombinant granulocyte-macrophage colony-stimulating factor. Immunology. 1987;60:439–444. [PMC free article] [PubMed] [Google Scholar]
- 23.DiPersio JF, Billing P, Williams R, Gasson JC. Human granulocyte-macrophage colony-stimulating factor and other cytokines prime human neutrophils for enhanced arachidonic acid release and leukotriene B4 synthesis. J Immunol. 1988;140:4315–4322. [PubMed] [Google Scholar]
- 24.Gadish M, Kletter Y, Flidel O, Nagler A, Slavin S, Fabian I. Effects of recombinant human granulocyte and granulocyte-macrophage colony-stimulating factors on neutrophil function following autologous bone marrow transplantation. Leuk Res. 1991;15:1175–1182. doi: 10.1016/0145-2126(91)90187-x. [DOI] [PubMed] [Google Scholar]
- 25.Fleischmann J, Golde DW, Weisbart RH, Gasson JC. Granulocyte-macrophage colony-stimulating factor enhances phagocytosis of bacteria by human neutrophils. Blood. 1986;68:708–711. [PubMed] [Google Scholar]
- 26.Coleman DL, Chodakewitz JA, Bartiss AH, Mellors JW. Granulocyte-macrophage colony-stimulating factor enhances selective effector functions of tissue-derived macrophages. Blood. 1988;72:573–578. [PubMed] [Google Scholar]
- 27.Wing EJ, Magee DM, Whiteside TL, Kaplan SS, Shadduck RK. Recombinant human granulocyte/macrophage colony-stimulating factor enhances monocyte cytotoxicity and secretion of tumor necrosis factor alpha and interferon in cancer patients. Blood. 1989;73:643–646. [PubMed] [Google Scholar]
- 28.Hamilton JA, Stanley ER, Burgess AW, Shadduck RK. Stimulation of macrophage plasminogen activator activity by colony-stimulating factors. J Cell Physiol. 1980;103:435–445. doi: 10.1002/jcp.1041030309. [DOI] [PubMed] [Google Scholar]
- 29.Perkins RC, Vadhan-Raj S, Scheule RK, Hamilton R, Holian A. Effects of continuous high dose rhGM-CSF infusion on human monocyte activity. Am J Hematol. 1993;43:279–285. doi: 10.1002/ajh.2830430410. [DOI] [PubMed] [Google Scholar]
- 30.Arici A, Marshburn PB, MacDonald PC, Dombrowski RA. Progesterone metabolism in human endometrial stromal and gland cells in culture. Steroids. 1999;64:530–534. doi: 10.1016/s0039-128x(99)00029-x. [DOI] [PubMed] [Google Scholar]
- 31.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 32.Janeway CA, Travers P, Walport M, Shlomchik M. Immunobiology: The Immune System in Health and Disease. New York: Garland Science Publishing; 2006. Innate Immunity; pp. 37–100. [Google Scholar]
- 33.Arntzen KJ, Egeberg K, Rahimipoor S, Vatten L, Austgulen R. LPS mediated production of IL-1, PGE2 and PGF2alpha from term decidua involves tumour necrosis factor and tumour necrosis factor receptor p55. J Reprod Immunol. 1999;45:113–125. doi: 10.1016/s0165-0378(99)00045-5. [DOI] [PubMed] [Google Scholar]
- 34.Menon R, Swan KF, Lyden TW, Rote NS, Fortunato SJ. Expression of inflammatory cytokines (interleukin-1 beta and interleukin-6) in amniochorionic membranes. Am J Obstet Gynecol. 1995;172:493–500. doi: 10.1016/0002-9378(95)90562-6. [DOI] [PubMed] [Google Scholar]
- 35.Zaga V, Estrada-Gutierrez G, Beltran-Montoya J, Maida-Claros R, Lopez-Vancell R, Vadillo-Ortega F. Secretions of interleukin-1beta and tumor necrosis factor alpha by whole fetal membranes depend on initial interactions of amnion or choriodecidua with lipopolysaccharides or group B streptococci. Biol Reprod. 2004;71:1296–1302. doi: 10.1095/biolreprod.104.028621. [DOI] [PubMed] [Google Scholar]
- 36.Vince G, Shorter S, Starkey P, et al. Localization of tumour necrosis factor production in cells at the materno/fetal interface in human pregnancy. Clin Exp Immunol. 1992;88:174–180. doi: 10.1111/j.1365-2249.1992.tb03059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leizer T, Cebon J, Layton JE, Hamilton JA. Cytokine regulation of colony-stimulating factor production in cultured human synovial fibroblasts: I. Induction of GM-CSF and G-CSF production by interleukin-1 and tumor necrosis factor. Blood. 1990;76:1989–1996. [PubMed] [Google Scholar]
- 38.Cromwell O, Hamid Q, Corrigan CJ, et al. Expression and generation of interleukin-8, IL-6 and granulocyte-macrophage colony-stimulating factor by bronchial epithelial cells and enhancement by IL-1 beta and tumour necrosis factor-alpha. Immunology. 1992;77:330–337. [PMC free article] [PubMed] [Google Scholar]
- 39.Sieff CA, Niemeyer CM, Mentzer SJ, Faller DV. Interleukin-1, tumor necrosis factor, and the production of colony-stimulating factors by cultured mesenchymal cells. Blood. 1988;72:1316–1323. [PubMed] [Google Scholar]
- 40.Bennett WA, Lagoo-Deenadayalan S, Brackin MN, Hale E, Cowan BD. Cytokine expression by models of human trophoblast as assessed by a semiquantitative reverse transcription-polymerase chain reaction technique. Am J Reprod Immunol. 1996;36:285–294. doi: 10.1111/j.1600-0897.1996.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 41.Stallmach T, Hebisch G, Joller H, Kolditz P, Engelmann M. Expression pattern of cytokines in the different compartments of the feto-maternal unit under various conditions. Reprod Fertil Dev. 1995;7:1573–1580. doi: 10.1071/rd9951573. [DOI] [PubMed] [Google Scholar]
- 42.Robertson SA, Mayrhofer G, Seamark RF. Uterine epithelial cells synthesize granulocyte-macrophage colony-stimulating factor and interleukin-6 in pregnant and nonpregnant mice. Biol Reprod. 1992;46:1069–1079. doi: 10.1095/biolreprod46.6.1069. [DOI] [PubMed] [Google Scholar]
- 43.Tremellen KP, Seamark RF, Robertson SA. Seminal transforming growth factor beta1 stimulates granulocyte-macrophage colony-stimulating factor production and inflammatory cell recruitment in the murine uterus. Biol Reprod. 1998;58:1217–1225. doi: 10.1095/biolreprod58.5.1217. [DOI] [PubMed] [Google Scholar]
- 44.Gervasi MT, Chaiworapongsa T, Naccasha N, Blackwell S, Yoon BH, Maymon E, et al. Phenotypic and metabolic characteristics of maternal monocytes and granulocytes in preterm labor with intact membranes. Am J Obstet Gynecol. 2001;185:1124–1129. doi: 10.1067/mob.2001.117681. [DOI] [PubMed] [Google Scholar]
- 45.Parry S, Strauss JF., 3rd Premature rupture of the fetal membranes. N Engl J Med. 1998;338:663–670. doi: 10.1056/NEJM199803053381006. [DOI] [PubMed] [Google Scholar]
- 46.Vadillo-Ortega F, Gonzalez-Avila G, Karchmer S, Cruz NM, Ayala-Ruiz A, Lama MS. Collagen metabolism in premature rupture of amniotic membranes. Obstet Gynecol. 1990;75:84–88. [PubMed] [Google Scholar]
- 47.Draper D, McGregor J, Hall J, et al. Elevated protease activities in human amnion and chorion correlate with preterm premature rupture of membranes. Am J Obstet Gynecol. 1995;173:1506–1512. doi: 10.1016/0002-9378(95)90640-1. [DOI] [PubMed] [Google Scholar]
- 48.Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000;80:617–653. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 49.Welgus HG, Campbell EJ, Cury JD, Eisen AZ, Senior RM, Wilhelm SM, et al. Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest. 1990;86:1496–1502. doi: 10.1172/JCI114867. [DOI] [PMC free article] [PubMed] [Google Scholar]