Abstract
While patients with Alzheimer’s disease (AD) show deficits in attention, manifested by inefficient performance on visual search, new visual talents can emerge in patients with frontotemporal lobar degeneration (FTLD), suggesting that, at least in some of the patients, visual attention is spared, if not enhanced. To investigate the underlying mechanisms for visual talent in FTLD (behavioral variant FTD [bvFTD] and semantic dementia [SD]) patients, we measured performance on a visual search paradigm that includes both feature and conjunction search, while simultaneously monitoring saccadic eye movements. AD patients were impaired relative to healthy controls (NC) and FTLD patients on both feature and conjunction search. BvFTD patients showed less accurate performance only on the conjunction search task, but slower response times than NC on all three tasks. In contrast, SD patients were as accurate as controls and had faster response times when faced with the largest number of distracters in the conjunction search task. Measurement of saccades during visual search showed that AD patients explored more of the image, whereas SD patients explored less of the image before making a decision as to whether the target was present. Performance on the conjunction search task positively correlated with gray matter volume in the superior parietal lobe, precuneus, middle frontal gyrus and superior temporal gyrus. These data suggest that despite the presence of extensive temporal lobe degeneration, visual talent in SD may be facilitated by more efficient visual search under distracting conditions due to enhanced function in the dorsal frontoparietal attention network.
Keywords: Alzheimer’s disease, frontotemporal dementia, conjunction search, voxel-based morphometry, eye movements
Introduction
Humans have evolved to become largely visual animals and vision occupies more volume in the brain than does any other sense. Despite the large proportion of brain anatomy devoted to visual processing, humans are limited in terms of the number of objects that they are able to perceive in detail at any given time. Borrowing from William James, “each of us literally chooses, by his ways of attending to things, what sort of universe he shall appear himself to inhabit” (James, 1890). James’ observation underscores the central role that attention plays in visual processing, and the influence that attention wields on cognition, identity and memory. Understanding visual attention processes, then, may provide a key to understanding many other brain functions.
When viewing a visual stimulus, features such as color, orientation, spatial frequency, brightness, and direction of movement are separable, registered early, automatically, and in parallel across the visual field (Treisman, Sykes, & Gelade, 1977). When only one feature is necessary to recognize an object, automatic recognition or pop-out occurs. When conjunctions of more than one separable feature are needed to distinguish the target from surrounding distracters, however, focused attention must be directed serially to each stimulus in the display in order to find the target (Treisman, et al., 1977). Object recognition requires recombining and synthesizing features for each object, and therefore demands focal visual attention (Treisman, et al., 1977). Deficits in visual attention commonly occur in patients with Alzheimer’s disease (AD) and contribute strongly to their clinical syndrome (Hao, et al., 2005; Hof, Bouras, Constantinidis, & Morrison, 1990; Kiyosawa, et al., 1989; Mendez, Mendez, Martin, Smyth, & Whitehouse, 1990; O'Brien, et al., 2001; Pignatti, et al., 2005).
These deficits affect visual search (Baddeley, Baddeley, Bucks, & Wilcock, 2001 ; Daffner, et al., 2001 ; Foster, Behrmann, & Stuss, 1999 ; Mosimann, Felblinger, Ballinari, Hess, & Muri, 2004; Nebes & Brady, 1989; Rosler, Mapstone, Hays-Wicklund, Gitelman, & Weintraub, 2005; Rosler, et al., 2000 ; Tales, et al., 2002) shifts of covert attention (Filoteo, et al., 1992), visually-guided and voluntary saccades (Boxer, et al., 2006; Shafiq-Antonacci, Maruff, Masters, & Currie, 2003 ) and smooth pursuit (Garbutt, et al., 2008; Hutton, Nagel, & Loewenson, 1984 ). Visual attention relies on the function of a dorsal frontoparietal network including the frontal and parietal eye fields (Corbetta & Shulman, 2002), regions that are selectively vulnerable to AD pathology (Giannakopoulos, et al., 1998; Giannakopoulos, et al., 1999; Kiyosawa, et al., 1989; Lewis, Campbell, Terry, & Morrison, 1987; Thompson, et al., 2003). Few studies, however, have linked visual search deficits to degeneration within specific brain networks.
In contrast to AD, some patients with frontotemporal lobar degeneration (FTLD) appear to exhibit a paradoxical facilitation of visual function. Although visual distractibility has been noted in behavioral variant frontotemporal dementia (bvFTD) patients (Krawczyk, et al., 2008), individuals with the semantic dementia (SD) subtype of FTLD have normal visual and oculomotor function (Garbutt, et al., 2008). In addition to anomia, patients with SD also experience obsessive behaviors, (Rosen, et al., 2006) and typically choose visually-based games and activities, such as playing solitaire on the computer, completing jigsaw puzzles and collecting coins (Green, & Patterson, 2009; Kertesz, Blair, McMonagle, & Munoz, 2007; Seeley, et al., 2005). Some of these patients have even shown enhanced visual artistry (Miller, Boone, Cummings, Read, & Mishkin, 2000). Each of these tasks requires intact visual attention skills, and all involve visual search.
Recently, a FTLD patient with enhanced visual artistic talent was found to have increased gray matter in dorsal parietal regions, suggesting a possible anatomical correlate of paradoxical facilitation of visuospatial function (Seeley, et al., 2008). This finding, along with decreased inhibition of the right frontoparietal attention network due to selective degeneration of the left anterior temporal lobe, has been proposed as a possible mechanism of facilitated visual function in FTLD (Miller, et al., 2000) and in savants (Snyder, 2009). Savant-like numerosity skills (ie., counting matches) have been observed with transcranial magnetic pulses to the left anterior temporal lobe (Snyder, et al., 2006) suggesting that loss of function in the anterior temporal lobe region may actually enhance certain forms of visual search. This paradoxical enhancement might be due to increased network connectivity in dorsal parietal regions as has been demonstrated to occur in bvFTD (Zhou et al., 2010).
Given these observations, we hypothesized that SD patients might show enhancements in visual search as compared to other dementia patients and possibly even normal controls. The visual search task used in this study was adapted from protocols used by Treisman and colleagues (Treisman, et al., 1977). Subjects had to identify a target as differing from an increasing number of distracters by a single separable feature (such as color) thereby obviating the need for serial search and leading to ‘pop-out’ (automatic parallel search), or by a conjunction of features, thus demanding serial, focused search of each item in the display. Under pop-out conditions, when feature search is sufficient, performance should not be affected by increasing numbers of distracters. During conjunction search, in contrast, increasing numbers of distracters should increase response times and decrease accuracy, indicating greater difficulty. Since some SD patients excel in real world visually-demanding tasks, we hypothesized that any paradoxical enhancements in visual search would be most apparent in conjunction search.
Materials and Methods
Participants
Patients and healthy normal controls (NC) were recruited and evaluated through the Memory and Aging Center at the University of California, San Francisco (UCSF). Each patient and NC underwent extensive neurological and neuropsychological examination, with a diagnosis made by a team of neurologists, neuropsychologists and nurses. Participants who were red/green colorblind or who had undergone eye surgery were excluded. All subjects gave informed consent to participate in the experimental procedures, and the study was approved by the UCSF Institutional Review Board.
Neuropsychological Battery
Subjects were administered a comprehensive neuropsychological battery as previously described (Garbutt, et al., 2008). Tests of general cognition and functional abilities included the Mini-Mental State Examination (MMSE) (Folstein, Folstein, & McHugh, 1975) and the Clinical Dementia Rating Scalre (CDR) (Morris, 1993). Neuropsychological tests included the longest correct backward digit span (Wechsler, 1997); a 15-item Boston Naming Test (BNT)(Kaplan, Goodglass, & Wintraub, 1983); measures of verbal fluency including phonemic fluency (number of D words in 1 minute), category fluency (number of animals in 1 minute) and design fluency (DF; number of novel designs using unique patterns of lines on the same template in 1 minute); the Number Location condition from the Visual Object Spatial Perception (VOSP) battery (Warrington & James, 1991); a copy and 10-minute recall of a simplified version of the Rey-Osterrieth; trial 1 of the Design Fluency subtest and Stroop (Interference condition, # correct) from the Delis-Kaplan Executive Function System (Delis, Kaplan, & Kramer, 2001); and a modified Trailmaking (MT) test (time to make all correct lines) (Kramer, et al., 2003). Subjects were also evaluated with the Geriatric Depression Scale (GDS) (Yesavage, et al., 1983) and the Neuropsychiatric Inventory (NPI) (Cummings, 1997).
Visual Search Task
Stimuli
Stimuli consisted of colored letters displayed on a computer screen. In each task, there were four different display sizes, with 1, 5, 15 or 30 items, one of which was a target on ‘target present’ trials. For ‘target absent trials,’ no target was present. The target could appear at any spatial location on the screen, and the distance between targets and distracters was variable. Letters included Os, Ns and Xs, depending on the task. Colors included orange, green and blue. Sample displays from each task with a display size of 15 items are shown in Figure 1. Stimuli were programmed in Eprime (version 2.0, Psychology Software Tools, Inc.) and displayed on a 19 inch LCD monitor at a distance of 65 cm. In each task, there were four different display sizes, with 1, 5, 15 or 30 items, one of which was a target on ‘target present’ trials. Participants viewed 3 blocks of trials, corresponding to letter pop-out, color pop-out and conjunction search tasks. In the letter pop-out task, for example, an orange “O” was the target, embedded amongst orange “N”s and green “X”s. In the color pop-out task, a blue letter was embedded in a display of orange and green letters. In the conjunction search task, a green “N” was embedded amongst orange “N”s and green “X”s. (see Figure 1a). There were 48 trials in each block, 12 at each display size, with 6 target absent and 6 target present. The order of trials was randomized within each block. Each trial lasted 3500 msec, with 500 msec of fixation followed by 3000 msec of search (see Figure 1b).
Figure 1. Search Task.
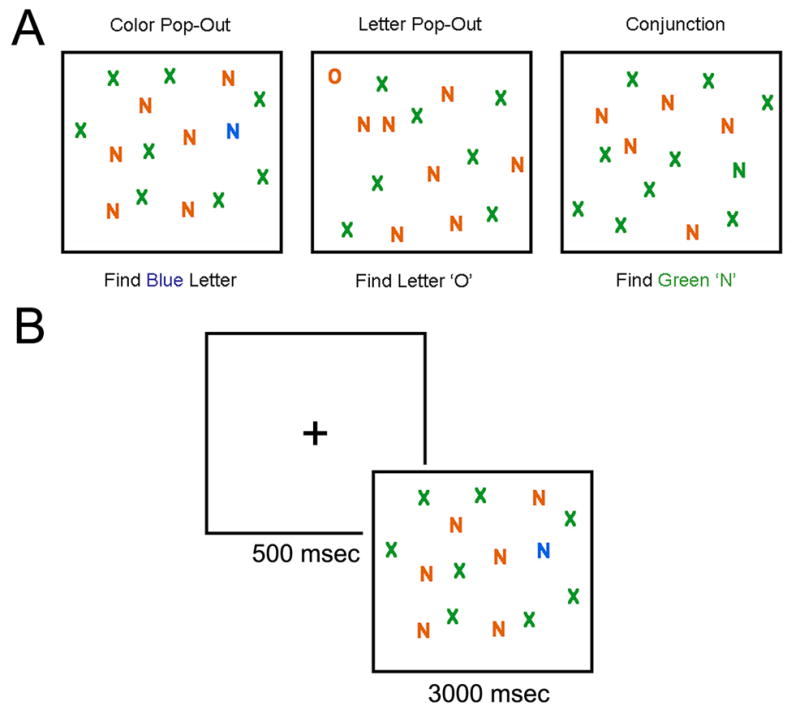
Examples of displays from each task with a display size of 15 items are seen in panel A. Panel B shows the timeline of a single trial.
Procedure
Participants were instructed at the beginning of each task to look for a particular target. Instructions were presented on the screen, and reiterated verbally before each task. During the color pop-out task, the target was any blue letter amongst orange and green letters. During the letter pop-out task, the target was the letter O amongst Xs and Ns. During the conjunction search task, participants were told to find the ‘green N’ amongst brown Ns and green Xs. Participants were told to press the space bar as soon as they see the target, or any other key when they are confident that the display does not include the target. Their fingers were then placed such that the thumb or index finger was resting on the space bar and other fingers were located on other keys. This procedure ensured that patients for whom traditional button press tasks are difficult would still be able to respond fairly quickly and accurately during this task.
Analysis
Accuracy
Because the task involved a yes/no decision (ie., Is the target present?), and signal and noise distributions of responses had different standard deviations, we used the non-parametric measure A’ (Pollack & Norman, 1964) to denote accuracy, as suggested by signal detection theory. To calculate A’, we used the following formula, where H = hit rate and F = false positive rate, as suggested by Snodgrass and Corwin (Snodgrass & Corwin):
Response Times
Response times were noted by the Eprime software program, and reflect the difference between the time at which the stimulus was presented and the recording of a button press.
Oculomotor data
Eye tracking
Eye position versus time data were collected for the left eye using an Applied Science Laboratories infrared eye tracker (model 504HS). Data were sampled at a rate of 120Hz. Analysis was performed using customized software written in Matlab (version 7.7 for Windows). Any data points with coordinates outside the bounds of the maximum gaze dimensions were excluded from analysis along with data points for which combined recognition of pupil and corneal reflection were not achieved. Missing data were interpolated as necessary up to a maximum duration of 4 missing samples (0.033s). Gaze coordinates were then transformed to pixel coordinates by linear transformation according to target image resolution. These data constitute the "raw" coordinates as referenced in the methods below.
Raw coordinate data were further consolidated into “event” data by recording the length of time that contiguous data points remained within a distance of 10 gaze coordinate units (approx. 1 degree of arc) from an initial reference point. When this boundary was passed, the reference point position and the duration value were recorded as an event, a new reference point was created from the next valid raw coordinate, and the process continued until the last data point was reached, at which point a final event was registered.
Visual inspection analysis
A 20 by 20 matrix of rectangular bins was used to sum event data durations for proximal events and visualized as a 3D plot with the proportion of time spent in each bin represented by the vertical dimension.
A two dimensional matrix with dimensions equal to the target image resolution was used to sum all event data durations for each [X,Y] pixel coordinate. The resultant data was then passed through a Gaussian smoothing algorithm (kernel size = 60x60; sigma=10; background image size=256x290) and scaled to fit a standard 256 color indexed heat map image.
Calculating the time to target ROI
Polygonal regions of interest (ROI) were first defined for each target image using pixel coordinates. These ROIs were square boxes surrounding the target letter with a margin of approximately 50% of the letter size. The raw coordinate data was then sequentially evaluated to determine whether the given [X,Y] pixel coordinate fell within the specified ROI. The first data point to satisfy this condition was used to calculate the time elapsed from the beginning of data sampling, yielding a measure of how quickly the patient’s gaze traveled to the target.
Fixation analysis
The event data was first filtered to remove events with durations of less than 0.05s. These events largely represent the capture of saccadic eye movements which span several sampling cycles, and which would otherwise overwhelm the statistical analysis of the less numerous fixation events, here defined as events with durations >0.05s. The data was further filtered to represent only events that preceded the registration of the subject’s response for a given trial. Therefore, fixation data were not diluted by eye movements that followed a patient’s response, as these movements were not directly related to task performance. Trials that yielded no fixations were removed from consideration and the average fixation duration for each given trial was then aggregated for further analysis.
Saccade spread analysis
Raw data were first filtered to represent only events that preceded the registration of the subject’s response for a given trial. The remaining [X,Y] pixel coordinates were used to determine the min(X), max(X), min(Y), and max(Y) values for each individual trial. This information was then used to define a bounding box for the trial coordinate data with width=max(X)-min(X) and height=max(Y)-min(Y). The saccade spread for each trial was subsequently defined as the ratio of the area of this bounding box to the total area of the target image.
Voxel-based Morphometry
Structural images were acquired on a 1.5T Siemens Magnetom Vision system (Siemens, Iselin, NJ) equipped with a standard quadrature head coil (n=9), or a 3T Siemens TIM Trio scanner Siemens, Iselin, NJ) also equipped with the manufacturer’s standard 12 channel head coil (n=13), or a 4T MRI scanner (Bruker/Siemens) equipped with a head birdcage RF transmission coil and an 8-channel receiver coil (n=34). At the 1.5T scanner, a volumetric magnetization prepared rapid gradient echo (MPRAGE) sequence was used to acquire T1 images of the whole brain (164 coronal slices; slice thickness = 1.5 mm; FOV = 256 mm; matrix 256 x 256; voxel size 1.0 x 1.5 x 1.0 mm3; TR = 10 ms; TE = 4 ms; flip angle = 15°). At the 3T scanner, the MPRAGE sequence consisted of 160 sagittal slices, slice thickness = 1mm; FOV = 256 mm; matrix 256 x 256; voxel size 1.0 x 1.0 x 1.0 mm3; TR = 2300 ms; TE = 2.98ms; flip angle = 9°. Finally, on the 4 T Bruker MedSpec system, images were acquired using the following MPRAGE sequence: 176 sagittal slices; slice thickness = 1 mm; FOV = 256 × 256 mm; matrix = 256 × 256; voxel size = 1.0 × 1.0 × 1.0 mm; TR = 2300 ms; TE = 3 ms; flip angle = 7°. All T1 structural images were segmented, bias corrected and spatially normalized to MNI space using a unified segmentation procedure (Ashburner & Friston, 2005). These images were analyzed by an optimized method of VBM using SPM5 software (http://www.fil.ion.ucl.ac.uk/spm/software/spm5; Friston et al., 2007), wherein the gray matter value in each voxel was multiplied by the Jacobian determinant derived from the spatial normalization in order to preserve the total amount of gray matter from the original images. The modulated gray matter images were smoothed with a Gaussian kernel (8mm FWHM). A general linear model was then fitted at each voxel, including one variable of interest (accuracy) and three nuisance covariates (age, gender and total intracranial volume). To ensure that the use of images from three different scanners did not account for our effects, we performed a series of additional analyses, including scanner type as a covariate, and found no difference in the overall pattern of results. A threshold of p < 0.05, family-wise error corrected at the voxel level was accepted as statistically significant.
Statistics
To investigate how the different task types and the display size affected performance in the different diagnostic groups, we conducted separate 3 (task) by 4 (display size) by 4 (diagnostic group) repeated measures ANOVAs for each measure of interest using SPSS Statistics 17.0 software (www.spss.com). When a significant interaction between diagnostic group and task was found, post-hoc ANOVAs were then conducted on each task type separately. To examine the data further, post-hoc Tukey tests were used to investigate significant main effects (alpha level set to p <0.05).
Results
Participants
All participants underwent neurological examination, neuropsychological testing, and brain MRI scans within 3 months of visual search evaluation and were categorized as control, FTLD or AD. FTLD subjects met diagnostic criteria of Neary et al. (Neary, et al., 1998) for frontotemporal dementia (also called behavioral variant [bvFTD]) or semantic dementia (SD). AD subjects met National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association criteria for probable AD (McKhann, et al., 1984). Control (NC) subjects had no neurological complaints, normal neurological and neuropsychological examinations, and clinical dementia rating (CDR) scores of 0 (Morris, 1993). Our population contained 21 control, 12 bvFTD, 6 SD, and 17 AD subjects.
There were no differences between groups in terms of age or education. All patient groups differed from controls on the CDR sum of boxes score, but patient groups did not differ from each other. AD patients had lower MMSE scores than bvFTD patients and healthy controls (p < 0.05). It should be noted, however, that when we excluded the AD patients with the lowest MMSE scores, there was no change to the overall pattern of results. No other demographic differences were found between the groups.
Accuracy
We performed a 3 (task) by 4 (display size) by 4 (diagnostic group) repeated measures ANOVA with accuracy as the dependent measure on trials in which there was a target present. This analysis revealed a significant group by task by display size interaction (F[18, 210] = 2.9, p < 0.01). Therefore, we proceeded to analyze each task separately (see Figure 2).
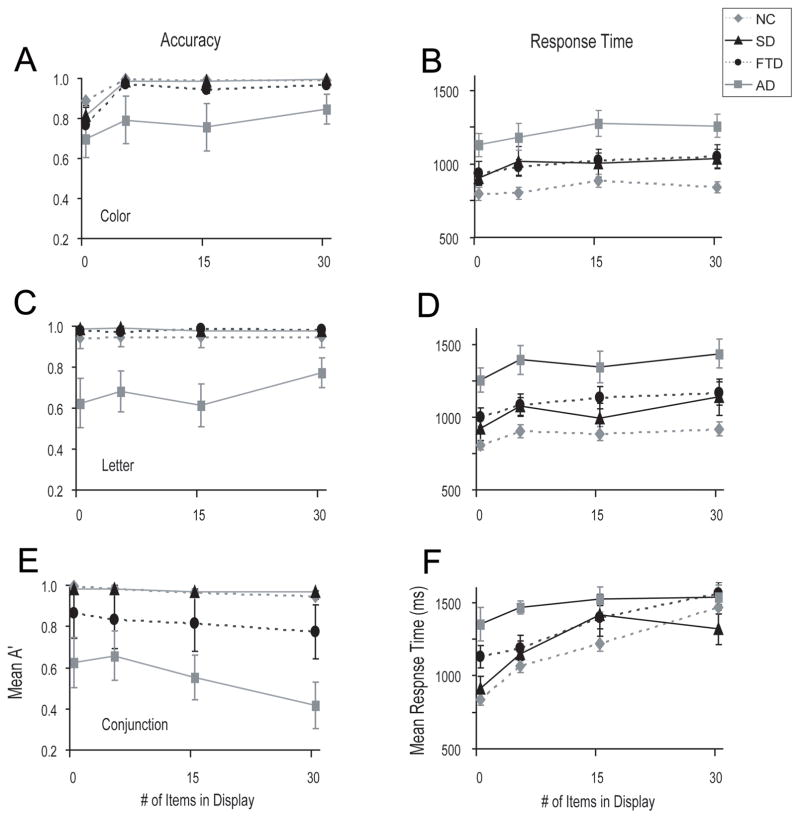
Figure 2. Behavioral Performance on the search tasks.
Panels show accuracy (A’) and response time performance on the color pop-out task (A, B), the letter pop-out task (B, C), and the conjunction search task (D, E). Error bars indicate standard error of the mean.
Color Pop-Out
A 4 (display size) by 4 (group) ANOVA revealed a main effect of display size (F[3, 132] = 20.5, p < 0.01) and a main effect of group (F[3, 44] = 3.9, p < 0.05)]. Post-hoc tests revealed that AD patients were less accurate than NC (p < 0.01). There were no performance differences between other groups (Figure 2A).
Letter Pop-Out
There was a main effect of group (F[3, 46] = 6.0, p < 0.01) and no other effects or interactions on letter pop-out. Post-hoc tests revealed that AD patients were less accurate than healthy controls (p <0.01), bvFTD patients (p <0.01) and SD patients (p <0.05) (Figure 2C). There were no other group differences.
Conjunction Search
There was a linear relationship between display size and accuracy (F[1, 39] = 9.7, p.<.01). Performance on the conjunction search task was worse in all groups with increasing display size (F[3, 117] = 5.1, p < 0.01). There was also a main effect of diagnostic group (F[3, 39] = 8.8, p < 0.01) on conjunction search. Post-hoc tests revealed that AD patients were less accurate than healthy controls (p < 0.01), bvFTD (p < 0.05) and SD patients (p < 0.01), with no other differences between subject groups (Figure 2E).
Response Time
We performed a 3 (task) by 4 (display size) by 4 (diagnostic group) repeated measures ANOVA with response time as the dependent measure on trials in which there was a target present. This analysis revealed main effects of task (F [2, 94] = 57.2, p < 0.01), display size (F [3, 141] = 48.6, p < 0.01), and group (F [3, 47] = 12.8, p.<.01), a task by group interaction (F[6, 94] = 2.6, p.<. 05), a task by display size interaction (F[6, 282] = 9.1, p < 0.01) and a task by display size by group interaction (F[18, 282] = 2.4, p <0.01). Given this triple interaction, we further analyzed each block separately (see Figure 2).
Color Pop-Out
Response times were slower with increasing display size (F[3, 153] = 6.0, p < 0.01) and there was an effect of diagnostic group (F[3, 51] = 8.6, p <0.01). Post-hoc tests revealed that AD patients had longer response times than healthy controls (p < 0.01). There were no other group differences. These results are shown in Figure 2B.
Letter Pop-Out
As with color pop-out, there was a linear relationship between response time and display size (F[1, 50] = 21.0, p < 0.01) such that response times were slower with increasing display size (F[3, 150] = 9.6, p <0.01). There was also a main effect of diagnostic group (F[3, 50] = 11.1, p.<.01). Post-hoc tests revealed that AD patients showed significantly longer response times than NC (p.<.01), bvFTD patients and SD patients (p < 0.05 for both). These results are shown in Figure 2D.
Conjunction Search (Figure 2F)
As with color pop-out, there was a linear relationship between response time and display size (F[3, 141] = 35.0, p < 0.01) such that response times were slower with increasing display size (F[1, 47] = 121.8, p < 0.01]. There was also a main effect of diagnostic group (F[1, 47] = 8.1, p < 0.01) and notably, a diagnostic group by display size interaction (F[9, 141] = 302, p <0.01), which had a significant linear component. AD patients had longer response times overall than SD (p <0.05) and healthy controls (p < 0.01). Given the interaction between display size and group, we performed a repeated measures ANOVA for each group separately and found an effect of display size in NC (F[3, 60] = 60.0, p <0.01), bvFTD (F[3, 30] = 10.3, p < 0.01) and SD groups: (F[3, 15] = 6.0, p.<.01), but not in the AD group (p > 0.2). Of note, however, the AD group performed at chance in terms of accuracy at 15 and 30 distracters, indicating that they were likely guessing.
At the largest display size, SD patients were both more accurate (t[1, 16] =2.7, p.<.01) and faster (t[1, 15]= 1.9, p < 0.05) than bvFTD patients (independent samples t-tests; equal variances not assumed). In addition, there was a significant interaction between SD and NC groups at the two largest display sizes, such that the effect of increasing the display size from 15 to 30 items was not the same for SD patients as for NC (F[1, 22] = 5.94, p < 0.05). Specifically, whereas NC showed slower response times with more distracters, SD patients showed faster response times (Figure 3b). SD patients and healthy controls showed near perfect accuracy at all display set sizes in the conjunction task, making it difficult to measure enhanced accuracy in the SD patients. Numerically, however, it is interesting to note that the SD patients did show better performance than controls in terms of accuracy. Furthermore, 5 out of 6 patients showed faster performance at 30 items than 15 items, whereas only 2 out of 21 healthy controls showed the same effect. This result is statistically significant (two-tailed Fisher’s exact test: p<.0014).
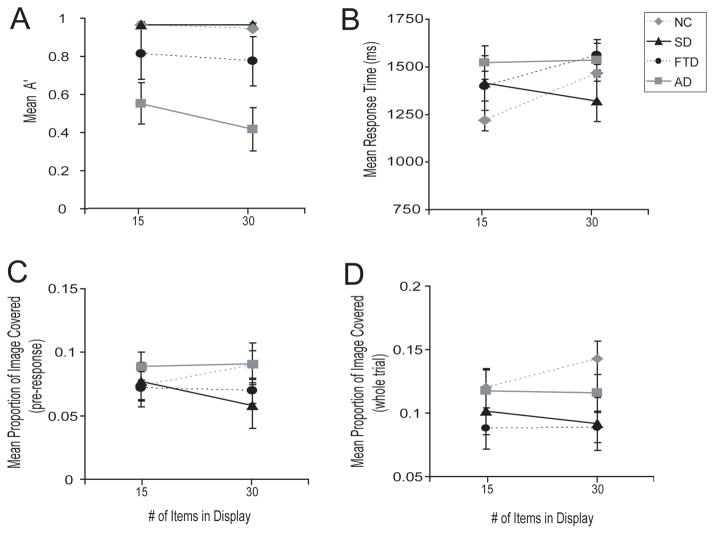
Figure 3. Interaction between groups and the display size increase from 15–30 items in the conjunction search task on four measures.
Panel A indicates accuracy, panel B indicates response time, panel C shows saccade spread until the response was detected, and panel C shows saccade spread across the whole trial. Note that in panels B-D, SD patients and healthy controls show opposite effects of the increase in display size from 15–30. Error bars indicate standard error of the mean.
Saccade analysis
Inspection of visual search patterns (Figure 4)
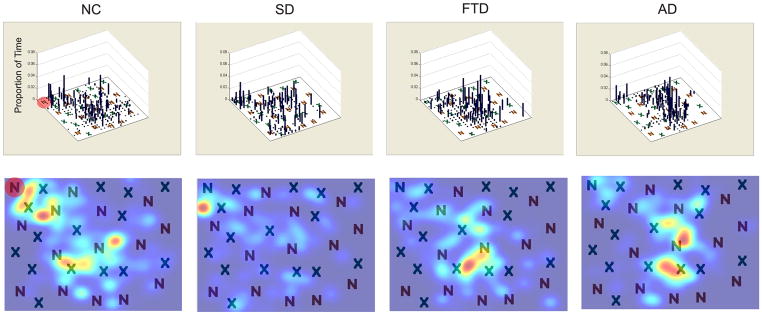
Figure 4. Examples of visual search patterns during conjunction search.
Representative plots eye movements during a conjunction search trial with a display size of 30 items. Target is shaded in red in the NC figures. The top panel shows a histogram the proportion of time spent in each part of the figure, with a density map (smoothed Gaussian distribution) of the same data shown below. The data shown here are representative of 10–14 trials in each patient group.
We first manually-inspected the visual search patterns of our patients and controls and observed that patient groups looked very similar in terms of their eye movements. In order to assess whether a particular patient group found the targets faster, or searched less of the image, additional quantitative analyses were designed as described below.
Time to target region-of-interest (ROI)
We performed a 3 (trial type) by 4 (display size) by 4 (diagnostic group) repeated measures ANOVA with the time until each individual shifted gaze into the ROI surrounding the target on trials in which there was a target present as the dependent measure. This analysis revealed main effects of trial type (F[2, 72] = 6.9, p.<.01) and display size (F[3, 108] = 14.6, p < 0.01) but not of diagnostic group. Because some patients had missing data for one of the blocks, we further analyzed each block separately. For the color pop-out stimuli the display size did not influence the amount of time it took to shift gaze to look at the target, however with letter pop-out stimuli, the amount of time that was required to reach the target increased linearly (F[1, 39] = 21.4, p < 0.01) with increasing display size (F[3, 117] = 4.03, p.<.01). Similarly, in the conjunction search condition, the time to reach the target increased linearly (F[1, 38] = 13.9, p < 0.01) with increasing display size (F[3, 114] = 8.4, p < 0.01) .
Fixation Duration
Mean fixation durations were calculated for each trial. A 2 (target present or absent) by 3 (trial type) by 4 (display size) ANOVA, demonstrated main effects of target presence (F[1, 34] = 9.02, p < 0.01) and display size (F[3, 102] = 22.85, p < 0.01) as well as a significant interaction between target presence and trial type (F [2, 68] = 3.28, p.<.05). There was no effect of diagnostic group or interaction with group. Post-hoc tests indicated that under conjunction search conditions, mean fixation durations were longer when the target was present than when it was absent (p < 0.001). Fixation durations were longer with greater numbers of distracters (14 or 29) in the conjunction but not pop-out conditions (p < 0.05). In the absence of a significant group effect or interaction with group, no further post-hoc tests were conducted.
Size of visual search area
In order to quantify how much of the image was viewed, a bounding box was created from the extent of visual fixations prior to a subject’s indicating that a target was present or absent. The mean proportion of the image covered by this box was calculated for each trial. These data were included in a 2 (target present or absent) by 3 (trial type) by 4 (display size) ANOVA, which showed a main effect of target presence (F[1, 38] = 65.55, p < 0.01), trial type (F[2, 76] = 10.00, p < 0.01), and of display size (F[3, 114] = 33.42, p < 0.01). There was also an interaction between the presence of target, display size and diagnosis (F[9, 114] = 2.28, p < 0.05) as well as an interaction between trial type and display size (F[6, 228] = 6.64, p < 0.01). To further explore these interactions, each target condition was analyzed separately.
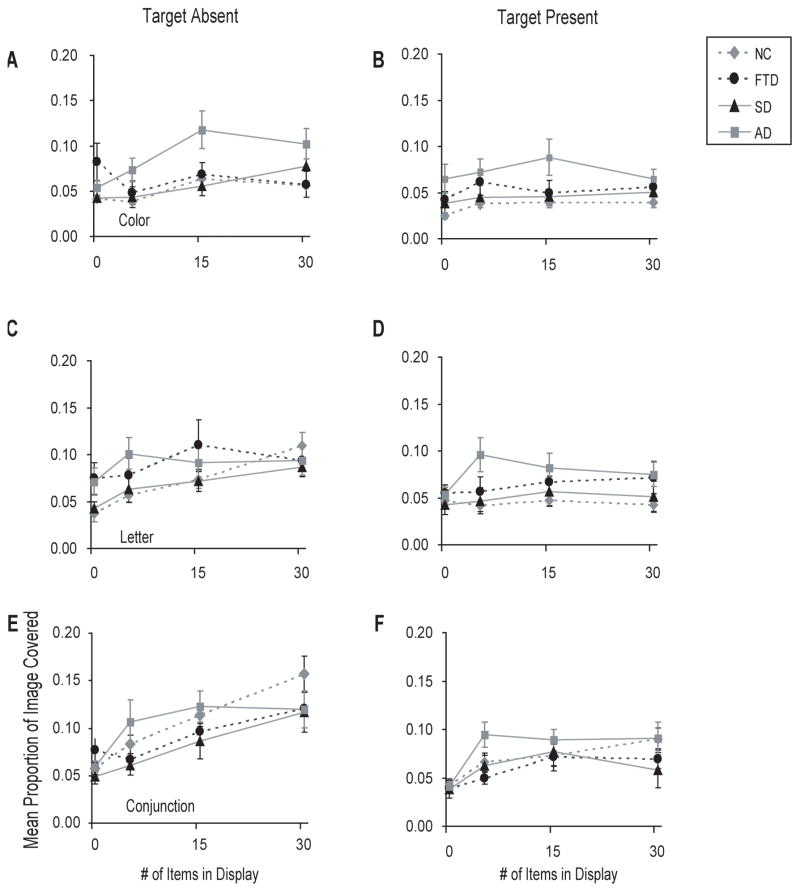
Color pop-out (Figure 5)
Figure 5. Saccade spread by diagnostic group.
Error bars represent the standard error of the mean.
A 4 (diagnostic group) by 4 (display size) ANOVA revealed a main effect of display size (F[3, 117] = 7.66, p < 0.01), and an interaction with diagnostic group (F[9, 117] = 3.39, p < 0.01). Post-hoc tests indicated that AD patients made saccades that covered a larger area of the image than NC whether or not the target was present (p < 0.05).
Letter pop-out
A 4 (diagnostic group) by 4 (display size) ANOVA revealed a main effect of display size (F[3, 123] = 11.08, p <0.01), and a display size by diagnosis interaction (F[9, 123] = 2.31, p <0.05). Post-hoc tests indicated that AD patients looked at more of the image than NC (p <0.05) when there were there were 1 or 5 items in the display when the target was absent, and when there were 5 or 15 items in the display and the target was present (p <0.05).
Conjunction search (Figure 5C)
Under conditions when the target was absent from the image, the area explored by all subjects increased in proportion to the display size (F[3, 120] = 29.45, p < 0.01). Post-hoc tests indicated that no patient groups differed in any condition. When there was a target present, the area of the image viewed was also linearly related (F[1, 40] = 31.69, p < 0.01) to the display size (F[3, 120] = 16.22, p <.01). Post-hoc tests indicated that AD patients covered a larger proportion of the image than NC (p <0.05) when there were 5 or 15 items in the display and SD patients when there were 30 items.
Since there was an interaction between response times to the two most difficult conjunction search conditions (15 and 30 items) such that SD and controls differed in terms of the effect of 30 items on response time, we investigated whether the difference in response time was reflected in a more efficient visual search, as measured by the visual search area. As with the response times, search areas were smaller in SD than NC subjects (F[1, 20] = 6.11, p < 0.05) under these conditions (Figure 3c). Similar results were obtained with data from the entire trial, including the period after the target was identified (Figure 3d).
Brain structural correlates of visual search performance
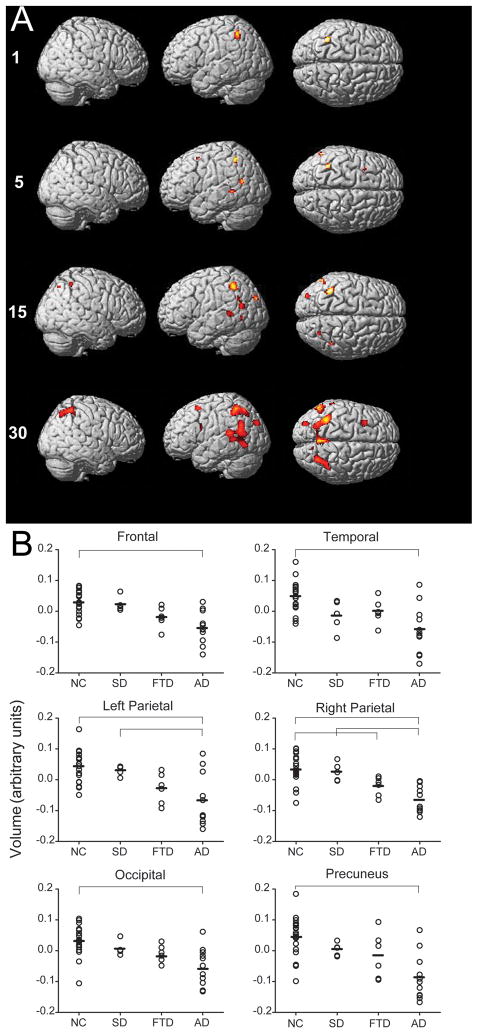
VBM was used to investigate the gray matter correlates of visual search performance. Accuracy on the conjunction, but not letter or color pop-out tasks, was correlated with gray matter volume in dorsal frontoparietal regions (p < 0.05, FWE corrected; Figure 6; Table 2). Other search variables were not correlated with gray matter volume. The number and extent of gray matter voxels that were correlated with conjuction search accuracy increased with increasing number of distracters. When only the target was on the screen (no distracters), conjunction search accuracy correlated with gray matter volume only in the left dorsal parietal lobe. With 5 items in the display, conjunction search accuracy correlated with gray matter in the right frontal and left superior temporal regions, along with left dorsal parietal lobe. With 15 items present, gray matter regions correlated with conjunction search accuracy included the bilateral dorsal parietal lobes, left superior temporal lobe and left occipital lobe. With 30 items present, conjunction search accuracy correlated with a larger extent of voxels in these regions as well as with regions in the precuneus, and left prefrontal regions.
Figure 6.
Conjunction visual search accuracy correlates with dorsal frontoparietal gray matter volume. (A) Highlighted voxels show gray matter regions that were significantly correlated with better performance on the conjunction search task (family-wise error rate of p <.05). (B) Gray matter concentrations for each patient at the peak voxel in six significant regions correlated with search performance are indicated with open circles. Means for each diagnostic group are represented by horizontal dashes. Horizontal lines indicate which group differences were significant (p.<.05). MNI coordinates for each region are: Frontal (−32,14,56), Temporal (−58,–60,16), Left Parietal (−38,−50,−54), Right Parietal (−28, −64, −52), Occipital (−30, −84, −32) and Precuneus (0, −58, 44).
Table 2.
Brain regions where gray matter volume is correlated with search performance by VBM.
| Region | MNI Coordinates | Max T (voxel-level) | ||
|---|---|---|---|---|
| X | Y | Z | ||
| Display Size: 1 | ||||
| Left Parietal Lobe | −38 | −50 | 52 | 5.88 |
| Display Size: 5 | ||||
| Left Parietal Lobe | −38 | −50 | 52 | 5.62 |
| Left Temporal Lobe | −56 | −44 | 2 | 5.35 |
| −58 | −62 | 18 | 5.24 | |
| −60 | −40 | 16 | 5.07 | |
| Right Frontal Lobe | −34 | 10 | 54 | 5.22 |
| Display Size: 15 | ||||
| Left Parietal Lobe | −40 | −50 | 50 | 6.33 |
| −52 | −58 | 32 | 5.16 | |
| Right Parietal Lobe | 44 | −48 | 56 | 5.41 |
| 28 | −68 | 52 | 5.35 | |
| Left Temporal Lobe | −58 | −64 | 0 | 5.21 |
| −56 | −44 | 4 | 5.28 | |
| −60 | −64 | 18 | 5.38 | |
| Left Occipital Lobe | −32 | −88 | 32 | 5.65 |
| Display Size: 30 | ||||
| Left Parietal Lobe | −36 | −50 | 54 | 6.74 |
| −30 | −62 | 52 | 5.72 | |
| −52 | −58 | 30 | 5.95 | |
| Right Parietal Lobe | 28 | −64 | 52 | 6.60 |
| 36 | −52 | 58 | 5.80 | |
| Left Superior Temporal Lobe | −58 | −60 | 16 | 5.53 |
| −58 | −62 | −4 | 5.50 | |
| Left Frontal | −32 | 14 | 56 | 6.14 |
| −48 | 8 | 24 | 5.23 | |
| Precuneus | 0 | −58 | 44 | 6.05 |
| 12 | −72 | 44 | 5.22 | |
| Left Occipital Lobe | −30 | −84 | 32 | 5.93 |
In order to assess whether there were any group differences in gray matter volume in the regions that significantly correlated with accuracy in the highest display size condition during the conjunction search task, we conducted an ANOVA using the gray matter volume at the most significant voxel in each of the six significant regions. This ANOVA demonstrated that gray matter volume differed between diagnostic groups in each of the brain regions that were correlated with conjunction search performance with 30 items (all F [3, 38] > 7.00, p < 0.001). The NC group had higher gray matter concentrations than AD patients in the regions correlated with task performance in the bilateral parietal lobes, precuneus, occipital, temporal and frontal lobes (p < 0.05, Tukey post hoc tests). The SD patients also had more gray matter than AD patients in the left and right parietal lobes and the frontal lobes (p < 0.05). The NC group also had more gray matter in the right parietal lobe than the bvFTD subjects (p < 0.05). There were no significant differences in gray matter volume in these regions between NC and SD patients or between bvFTD and SD patients.
Discussion
FTLD is a progressive neurodegenerative disorder that sometimes leads to a paradoxical facilitation of visual function, manifested in activities that rely heavily on the processing complex visual images such as completing jigsaw puzzles, finding coins, playing solitaire or ball sports and even creating compelling works of visual art. We measured visual search performance in FTLD as a way to identify mechanisms of enhanced visual function and found evidence of enhanced visual search in the SD subtype of FTLD. Unlike AD subjects who displayed uniformly inaccurate and slow visual search, we found that visual search was impaired in some FTLD subjects only under the most difficult search conditions and that performance differed according to FTLD clinical syndrome. bvFTD subjects were less accurate than NC only in the most difficult conjunction search task, whereas SD patients were as accurate as NC on all search tasks, performing at ceiling with numerically superior performance to NC. Furthermore, whereas bvFTD patients and NC showed an increase in response time with greater numbers of distracters, SD patients did not. Hence, under the most difficult conjunction search condition (a target embedded amongst 30 items), SD patients responded more quickly than NC while looking at a smaller proportion of the image. These results suggest that SD patients do not find the conjunction search more difficult with increasing numbers of distracters, and have more efficient search strategies than NC perhaps due to being less distracted by a larger number of irrelevant items. As conjunction search with the highest numbers of distracters correlated with gray matter volume in a dorsal frontoparietal network commonly associated with visual attention, and SD subjects showed no evidence of atrophy in this network, we hypothesize that enhanced visual search in SD arises not from more gray matter, but rather from alterations in network connectivity in the setting of decreased ventral stream visual information flow.
Comparison with other studies of visual search in neurodegenerative disease
We have replicated findings by several groups (Baddeley, et al., 2001; Foldi, Jutagir, Davidoff, & Gould, 1992; Foster, et al., 1999) demonstrating an impairment in visual search in AD. Previously we showed that AD patients have increased latencies and decreased accuracy and gain when making visually-guided saccades, antisaccades and smooth pursuit eye movements (Garbutt, et al., 2008). AD subjects were less accurate and slower in their visual search performance than the other groups. Although none of the patient groups differed from controls in the duration of visual fixations, or how quickly their gaze was recorded within the target area of each image, AD patients looked at a greater proportion of the image before making a decision about the presence of the target than the other groups. These findings parallel visual search impairments noted during clock reading in AD (Mosimann, et al., 2004) and suggest that AD patients’ ability to encode visual information or to convert visual information to action is less efficient than in FTLD or control subjects.
These visual search results in SD are consistent with our previous findings of normal saccade and smooth pursuit function in SD as compared to other neurodegenerative dementias (Garbutt, et al., 2008) and are in line with reports of enhanced visual abilities in SD subjects (Green, et al., 2009; Kertesz, 2006; Seeley, et al., 2005). Together with our previous results, the subtle enhancements in SD subjects’ performance as compared to NC observed in this study suggest that enhanced visual abilities in SD arise from alterations in the control of visual search and not in basic oculomotor function. Alternatively, it is possible that SD subjects accomplish visual search by different basic mechanisms than the other groups, potentially taking advantage of more salient bottom-up processing to achieve more efficient results.
Structural basis of visual search impairments
Our VBM results show that larger gray matter volumes in the superior parietal lobe bilaterally, the precuneus, a portion of the middle frontal gyrus and higher visual regions in the occipital lobe correlate with better performance in the conjunction search with increasing numbers of distracters. The differences we observed in visual search between AD and FTLD are likely to be explained by the greater degree of volume loss of dorsal parietal lobe structures in AD than in FTLD (Du, et al., 2007), particularly SD (Boxer, Kramer, et al., 2003; Boxer, Rankin, et al., 2003). Given that conjunction search conditions rely heavily on the binding of separable features into a coherent trace for accurate performance, our results are also in line with a wealth of research suggesting that the parietal lobe, in particular, is involved in the process of binding together stimulus features into a coherent percept.
Patients with lesions to the parietal lobe sometimes show binding deficits evident in conditions such as Balint’s syndrome and hemispatial neglect (for a review, see (Chan, Robertson, & Crawford, 2003). In addition, hyperactive binding, as seen in people with grapheme-color synaesthesia, has been correlated with neural activity and more gray matter in the parietal lobe (Weiss & Fink, 2009). Similarly, more dorsal parietal gray matter has been associated with enhanced visual artistic talent in a painter with FTLD (Seeley, et al., 2008).
Our results suggest that enhanced function, but not altered structure, of gray matter in the parietal lobe is likely to account for the paradoxical facilitation of conjunction search in these patients. SD patients and NC had more gray matter in these regions than AD and bvFTD patients, who showed impairments in conjunction search with large display sizes, however there was no statistically-significant difference in gray matter volume in these regions between NC and SD groups. It is possible that this study did not have adequate power to detect increased volume in the SD group. We further suggest that the subtle enhancements in conjunction visual search performance observed in SD as compared to NC reflect altered neural activity in this structurally intact dorsal frontoparietal network. Enhancements in dorsal parietal network connectivity have recently been demonstrated in bvFTD (Zhou et al., 2010). Such enhanced activity might also be a function of enhanced compulsivity in the SD subjects as was observed on the NPI (Table 1) as compared to NC. A limitation of this work was that both the NC and SD showed ceiling effects in accuracy on most of the tasks and so to test this hypothesis, further studies assessing neural activity in this network will need to employ more difficult visual search tasks.
Table 1.
Neuropsychological and Demographic Characteristics of Patients and Controls.
| AD | bvFTD | SD | NC | Overall ANOVA | |
|---|---|---|---|---|---|
| n | 17 | 12 | 6 | 21 | |
| Gender (m/f) | 7/10 | 10/2 | 2/4 | 8/13 | |
| Age (years) | 62.4 ± 7.7 | 61.2 ± 7.7 | 66.8 ± 6.0 | 60.7 ± 10.3 | NS |
| Mini Mental Status Examination (max = 30) | 21.5 ± 5.3^* | 26.9 ± 2.7 | 24.0 ± 3.1* | 29.2 ± 0.9 | F (3,5) = 17.1 |
| Education (years) | 15.8 ± 2.6 | 15.3 ± 3.2 | 14.5 ± 1.8 | 16.7 ± 2.1 | NS |
| Clinical Dementia Rating Scale (CDR) (max = 3) | 0.85 ± 0.39* | 0.91 ± 0.44* | 0.83 ± 0.26* | 0.03 ± | F (3,50) = 30.8 |
| CDR sum of cognitive-functional impairment (max = 18) | 4.79 ± 2.10* | 5.45 ± 2.01* | 5.17 ± 2.07* | 0.10 ± | F (3,50) = 38.6 |
| Geriatric Depression Scale (max =30) | 9.14 ± 7.26 | 6.45 ± 5.85 | 11.0 ± 6.99 | 3.24 ± | F (3,48) = 4.9 |
| Memory | |||||
| Modified Rey Recall (max = 18) | 5.18 ± 3.84^* | 10.8 ± 2.7 | 8.50 ± 5.4 | 11.8 ± 2.6 | F (3,52) = 12.9 |
| Language | |||||
| Boston Naming Test (max = 15) | 11.3 ± 4.4 | 12.4 ± 1.7 | 3.00 ± 3.03#^* | 14.3 ± 1.0 | F (3,50) = 26.0 |
| Verbal Repetition (max = 3) | 2.31 ± 0.95 | 2.83 ± 0.39 | 2.67 ± 0.52 | 2.90 ± | NS |
| Visuospatial | |||||
| Modified Rey Copy (max = 18) | 10.2 ± 5.6^@* | 14.6 ± 1.6 | 16.0 ± 0.6 | 15.3 ± 0.9 | F (3,52) = 9.9 |
| Calculations (max = 5) | 3.06 ± 1.30* | 3.83 ± 0.94 | 3.83 ± 0.75 | 4.85 ± 0.49 | F (3,51) = 11.6 |
| Executive, Attention, Speed & Working Memory | |||||
| Digits Backward (number) | 3.12 ± 1.36* | 4.08 ± 1.44* | 4.17 ± 1.17 | 6.11 ± 1.45 | F (3,50) = 14.4 |
| Modified Trails Time (seconds) | 85.1 ± 39.3* | 54.3 ± 30.3 | 52.8 ± 32.9 | 31.5 ± 13.6 | F (3,47) = 9.7 |
| Modified Trails Correct (number of lines) | 9.29 ± 5.85* | 12.9 ± 3.62 | 14.0 ± 0.00 | 14.0 ± | F (3,47) = 5.6 |
| Design Fluency Correct (number) | 5.81 ± 3.83* | 5.75 ± 2.93* | 7.00 ± 3.22 | 10.7 ± 3.6 | F (3,51) = 8.1 |
| Design Fluency Repetitions (number) | 1.06 ± 1.39 | 5.25 ± 5.94#* | 2.50 ± 2.59 | 1.00 ± 1.26 | F (3,51) = 5.7 |
| Stroop Color Naming (number) | 41.9 ± 24.8* | 60.0 ± 13.7 | 58.2 ± 16.4 | 82.8 ± 16.9 | F (3,47) = 13.9 |
| Stroop Interference (number) | 17.1 ± 15.6 | 35.0 ± 14.0 | 32.0 ± 8.1 | 49.4 ± 10.9 | F (3,46) = 18.0 |
| D Words (number) | 7.24 ± 4.18* | 7.50 ± 3.32* | 4.00 ± 2.68* | 16.8 ± 5.5 | F (3,52) = 22.7 |
| Animals (number) | 9.82 ± 5.56* | 12.8 ± 4.5 | 4.67 ± 4.68* | 21.6 ± 5.1 | F (3,52) = 26.3 |
| Abstraction (max = 7) | 2.07 ± 1.39* | 2.33 ± 1.67* | 1.50 ± 1.00* | 4.55 ± 0.95 | F (3,47) = 15.2 |
| Compulsivity | |||||
| Distractibility + Aberrant motor behaviors score (Neuropsychiatric Inventory) | 4.00± 5.40^ | 13.55±6.52#* | 12.17 ± 9.30* | 0 ± 0 | F (3, 42) = 11.3 |
p < 0.01 vs. AD
p < 0.01 vs. bvFTD
p < 0.01 vs. SD
p < 0.01 vs. NC
The findings in this study support an evolving literature on the variability of strengths and weaknesses associated with different neurodegenerative conditions. While AD is associated with profound deficits in visual search, in bvFTD this cognitive function is relatively spared, while in SD it may be enhanced. Dysfunction, sparing or even paradoxical facilitation of a frontoparietal attentional network may represent the physiological mechanism for these distinctions.
Supplementary Material
Mean search slopes for each diagnostic group for target present and absent trials of each task type. Error bars indicated standard error of the mean.
Acknowledgments
We would like to thank our patients and their families for generously donating their time and effort to our study. This work was funded by the following sources: The National Institute on Aging and the National Institute on Health (P50 AG023501 [BLM], R01AG038791 [ALB], R01AG031278 [ALB], P01AG019724 [BLM]). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health. This work was also supported by grants from the John Douglas French Foundation [ALB]; the Hellman Family Foundation [ALB]; the Larry L. Hillblom Foundation [BLM] and the McBean Family Foundation [IVV].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Baddeley HA, Bucks RS, Wilcock GK. Attentional control in Alzheimer's disease. Brain. 2001;124(Pt 8):1492–1508. doi: 10.1093/brain/124.8.1492. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Garbutt S, Rankin KP, Hellmuth J, Neuhaus J, Miller BL, et al. Medial versus lateral frontal lobe contributions to voluntary saccade control as revealed by the study of patients with frontal lobe degeneration. J Neurosci. 2006;26(23):6354–6363. doi: 10.1523/JNEUROSCI.0549-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer AL, Kramer JH, Du AT, Miller BL, Schuff N, Weiner MW, et al. Focal right inferotemporal atrophy in AD patients with disproportionate visual constructive impairment. Neurology. 2003;61:1485–1491. doi: 10.1212/01.wnl.0000090568.34810.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer AL, Rankin KP, Miller BL, Schuff N, Weiner M, Gorno-Tempini ML, et al. Cinguloparietal atrophy distinguishes Alzheimer disease from semantic dementia. Arch Neurol. 2003;60(7):949–956. doi: 10.1001/archneur.60.7.949. [DOI] [PubMed] [Google Scholar]
- Chan RC, Robertson IH, Crawford JR. An application of individual subtest scores calculation in the Cantonese version of the Test of Everyday Attention. Psychol Rep. 2003;93(3 Pt 2):1275–1282. doi: 10.2466/pr0.2003.93.3f.1275. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48(5 Suppl 6):S10–16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Rentz DM, Scinto LF, Faust R, Budson AE, Holcomb PJ. Pathophysiology underlying diminished attention to novel events in patients with early AD. Neurology. 2001;56(10):1377–1383. doi: 10.1212/wnl.56.10.1377. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan EB, Kramer JH. The Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Du AT, Schuff N, Kramer JH, Rosen HJ, Gorno-Tempini ML, Rankin K, et al. Different regional patterns of cortical thinning in Alzheimer's disease and frontotemporal dementia. Brain. 2007;130(Pt 4):1159–1166. doi: 10.1093/brain/awm016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filoteo JV, Delis DC, Massman PJ, Demadura T, Butters N, Salmon DP. Directed and divided attention in Alzheimer's disease: impairment in shifting of attention to global and local stimuli. J Clin Exp Neuropsychol. 1992;14(6):871–883. doi: 10.1080/01688639208402541. [DOI] [PubMed] [Google Scholar]
- Foldi NS, Jutagir R, Davidoff D, Gould T. Selective attention skills in Alzheimer's disease: performance on graded cancellation tests varying in density and complexity. J Gerontol. 1992;47(3):P146–153. doi: 10.1093/geronj/47.3.p146. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Foster JK, Behrmann M, Stuss DT. Visual attention deficits in Alzheimer's disease: simple versus conjoined feature search. Neuropsychology. 1999;13(2):223–245. doi: 10.1037//0894-4105.13.2.223. [DOI] [PubMed] [Google Scholar]
- Garbutt S, Matlin A, Hellmuth J, Schenk AK, Johnson JK, Rosen H, et al. Oculomotor function in frontotemporal lobar degeneration, related disorders and Alzheimer's disease. Brain. 2008;131(Pt 5):1268–1281. doi: 10.1093/brain/awn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakopoulos P, Duc M, Gold G, Hof PR, Michel JP, Bouras C. Pathologic correlates of apraxia in Alzheimer disease. Arch Neurol. 1998;55(5):689–695. doi: 10.1001/archneur.55.5.689. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, Gold G, Duc M, Michel JP, Hof PR, Bouras C. Neuroanatomic correlates of visual agnosia in Alzheimer's disease: a clinicopathologic study. Neurology. 1999;52(1):71–77. doi: 10.1212/wnl.52.1.71. [DOI] [PubMed] [Google Scholar]
- Green HA, Patterson K. Jigsaws-A preserved ability in semantic dementia. Neuropsychologia. 2009 Jan;47(2):569–76. doi: 10.1016/j.neuropsychologia.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Hao J, Li K, Zhang D, Wang W, Yang Y, Yan B, et al. Visual attention deficits in Alzheimer's disease: an fMRI study. Neurosci Lett. 2005;385(1):18–23. doi: 10.1016/j.neulet.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Hof PR, Bouras C, Constantinidis J, Morrison JH. Selective disconnection of specific visual association pathways in cases of Alzheimer's disease presenting with Balint's syndrome. J Neuropathol Exp Neurol. 1990;49(2):168–184. doi: 10.1097/00005072-199003000-00008. [DOI] [PubMed] [Google Scholar]
- Hutton JT, Nagel JA, Loewenson RB. Eye tracking dysfunction in Alzheimer-type dementia. Neurology. 1984;34(1):99–102. doi: 10.1212/wnl.34.1.99. [DOI] [PubMed] [Google Scholar]
- James W. Principles of Psychology. Boston: Harvard University Press; 1890. [Google Scholar]
- Kaplan E, Goodglass H, Wintraub S. The Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Kertesz A. Progress in clinical neurosciences: Frontotemporal dementia-pick's disease. Can J Neurol Sci. 2006;33(2):141–148. doi: 10.1017/s0317167100004893. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Blair M, McMonagle P, Munoz DG. The Diagnosis and Course of Frontotemporal Dementia. Alzheimer Dis Assoc Disord. 2007;21(2):155–163. doi: 10.1097/WAD.0b013e31806547eb. [DOI] [PubMed] [Google Scholar]
- Kiyosawa M, Bosley TM, Chawluk J, Jamieson D, Schatz NJ, Savino PJ, et al. Alzheimer's disease with prominent visual symptoms. Clinical and metabolic evaluation. Ophthalmology. 1989;96(7):1077–1085. doi: 10.1016/s0161-6420(89)32769-2. discussion 1085–1076. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16(4):211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC, Morrison RG, Viskontas I, Holyoak KJ, Chow TW, Mendez MF, et al. Distraction during relational reasoning: the role of prefrontal cortex in interference control. Neuropsychologia. 2008;46(7):2020–2032. doi: 10.1016/j.neuropsychologia.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Campbell MJ, Terry RD, Morrison JH. Laminar and regional distributions of neurofibrillary tangles and neuritic plaques in Alzheimer's disease: a quantitative study of visual and auditory cortices. J Neurosci. 1987;7(6):1799–1808. doi: 10.1523/JNEUROSCI.07-06-01799.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer disease: Report of the NINCDS-ARDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Mendez MA, Martin R, Smyth KA, Whitehouse PJ. Complex visual disturbances in Alzheimer's disease. Neurology. 1990;40(3 Pt 1):439–443. doi: 10.1212/wnl.40.3_part_1.439. [DOI] [PubMed] [Google Scholar]
- Miller BL, Boone K, Cummings JL, Read SL, Mishkin F. Functional correlates of musical and visual ability in frontotemporal dementia. Br J Psychiatry. 2000;176:458–463. doi: 10.1192/bjp.176.5.458. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Mosimann UP, Felblinger J, Ballinari P, Hess CW, Muri RM. Visual exploration behaviour during clock reading in Alzheimer's disease. Brain. 2004;127(Pt 2):431–438. doi: 10.1093/brain/awh051. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Brady CB. Focused and divided attention in Alzheimer's disease. Cortex. 1989;25(2):305–315. doi: 10.1016/s0010-9452(89)80045-0. [DOI] [PubMed] [Google Scholar]
- O'Brien HL, Tetewsky SJ, Avery LM, Cushman LA, Makous W, Duffy CJ. Visual mechanisms of spatial disorientation in Alzheimer's disease. Cereb Cortex. 2001;11(11):1083–1092. doi: 10.1093/cercor/11.11.1083. [DOI] [PubMed] [Google Scholar]
- Pignatti R, Rabuffetti M, Imbornone E, Mantovani F, Alberoni M, Farina E, et al. Specific impairments of selective attention in mild Alzheimer's disease. J Clin Exp Neuropsychol. 2005;27(4):436–448. doi: 10.1080/13803390490520427. [DOI] [PubMed] [Google Scholar]
- Pollack L, Norman DA. Non-parametric analysis of recognition experiments. Psychonomic Science. 1964;1:161–173. [Google Scholar]
- Rosen HJ, Allison SC, Ogar JM, Amici S, Rose K, Dronkers N, et al. Behavioral features in semantic dementia vs other forms of progressive aphasias. Neurology. 2006;67(10):1752–1756. doi: 10.1212/01.wnl.0000247630.29222.34. [DOI] [PubMed] [Google Scholar]
- Rosler A, Mapstone M, Hays-Wicklund A, Gitelman DR, Weintraub S. The "zoom lens" of focal attention in visual search: changes in aging and Alzheimer's disease. Cortex. 2005;41(4):512–519. doi: 10.1016/s0010-9452(08)70191-6. [DOI] [PubMed] [Google Scholar]
- Rosler A, Mapstone ME, Hays AK, Mesulam MM, Rademaker A, Gitelman DR, et al. Alterations of visual search strategy in Alzheimer's disease and aging. Neuropsychology. 2000;14(3):398–408. doi: 10.1037//0894-4105.14.3.398. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Bauer AM, Miller BL, Gorno-Tempini ML, Kramer JH, Weiner M, et al. The natural history of temporal variant frontotemporal dementia. Neurology. 2005;64(8):1384–1390. doi: 10.1212/01.WNL.0000158425.46019.5C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Matthews BR, Crawford RK, Gorno-Tempini ML, Foti D, Mackenzie IR, et al. Unravelling Bolero: progressive aphasia, transmodal creativity and the right posterior neocortex. Brain. 2008;131(Pt 1):39–49. doi: 10.1093/brain/awm270. [DOI] [PubMed] [Google Scholar]
- Shafiq-Antonacci R, Maruff P, Masters C, Currie J. Spectrum of saccade system function in Alzheimer disease. Arch Neurol. 2003;60(9):1272–1278. doi: 10.1001/archneur.60.9.1272. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117(1):34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Snyder A. Explaining and inducing savant skills: privileged access to lower level, less-processed information. Philos Trans R Soc Lond B Biol Sci. 2009;364(1522):1399–1405. doi: 10.1098/rstb.2008.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A, Bahramali H, Hawker T, Mitchell DJ. Savant-like numerosity skills revealed in normal people by magnetic pulses. Perception. 2006;35(6):837–845. doi: 10.1068/p5539. [DOI] [PubMed] [Google Scholar]
- Tales A, Butler SR, Fossey J, Gilchrist ID, Jones RW, Troscianko T. Visual search in Alzheimer's disease: a deficiency in processing conjunctions of features. Neuropsychologia. 2002;40(12):1849–1857. doi: 10.1016/s0028-3932(02)00073-8. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, et al. Dynamics of gray matter loss in Alzheimer's disease. J Neurosci. 2003;23(3):994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman A, Sykes M, Gelade G. Selective attention and stimulus integration. In: Dornic S, editor. Attention and Performance VI. Hillsdale, NJ: Lawrence Erlbaum; 1977. pp. 333–361. [Google Scholar]
- Warrington EK, James M. The Visual Object and Space Perception Battery. Bury St Edmunds: Thames Valley Test Company; 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Weiss PH, Fink GR. Grapheme-colour synaesthetes show increased grey matter volumes of parietal and fusiform cortex. Brain. 2009;132(Pt 1):65–70. doi: 10.1093/brain/awn304. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rolse TL, Lum O, Huang V, Adey M, et al. Development and validity of a Geriatric Depression Scale: A preliminary report. J Psychiatric Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain. 2010;133:1352–67. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mean search slopes for each diagnostic group for target present and absent trials of each task type. Error bars indicated standard error of the mean.