Abstract
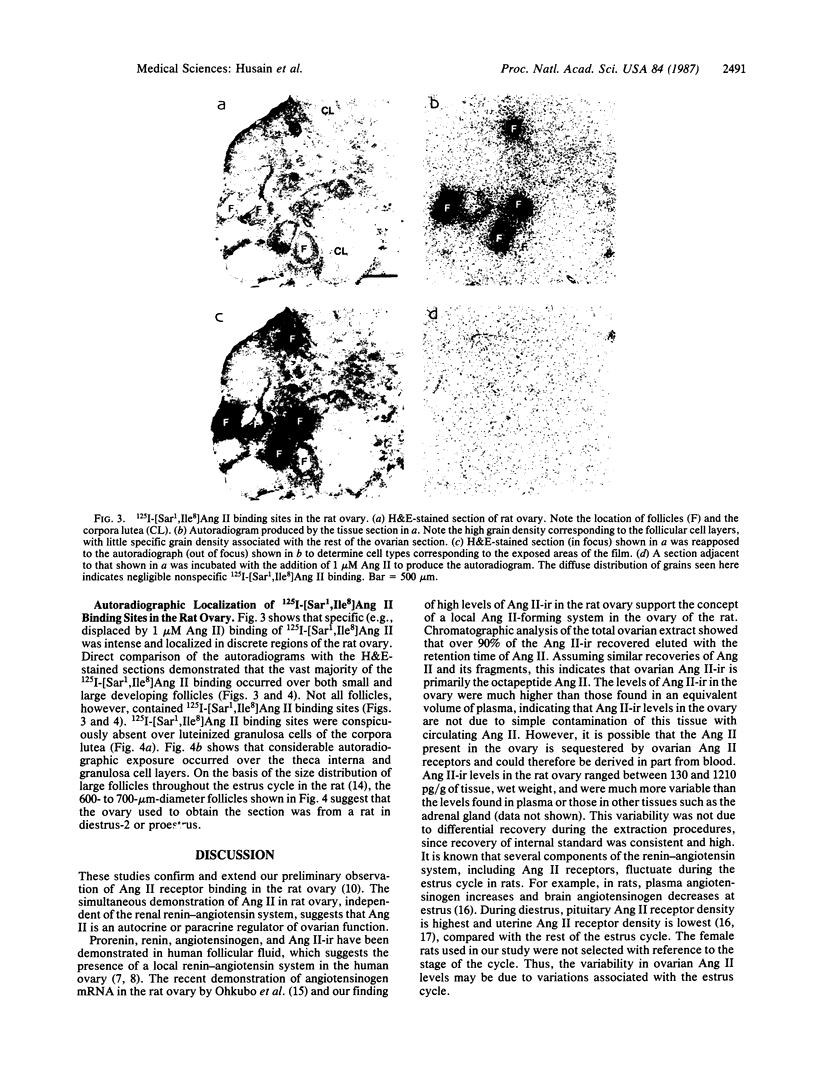
Specific, high-affinity (Kd approximately equal to 0.6 nM), and saturable (3.3 fmol/mg of tissue, wet weight) binding of 125I-labeled [Sar1,Ile8]angiotensin II to rat ovarian membranes was observed. Displacement of 125I-labeled [Sar1,Ile8]angiotensin II binding to rat ovarian membranes by angiotensin II analogs and fragments resembled the potency order of these compounds on angiotensin II receptors in other tissues: [Sar1,Ile8]angiotensin II greater than angiotensin II greater than des-Asp1-angiotensin II greater than angiotensin I greater than des-Asp1,Arg2-angiotensin II. Several unrelated peptides, including follicle-stimulating hormone at 10 microM, did not displace ovarian 125I-labeled [Sar1,Ile8]angiotensin II binding. Autoradiograms of 125I-labeled [Sar1,Ile8]angiotensin II binding to ovarian sections indicated that the angiotensin II receptor binding sites were localized exclusively to a subpopulation of follicles, occurring on the granulosa and theca interna cells. Other follicles were devoid of 125I-labeled [Sar1,Ile8]angiotensin II binding sites. Angiotensin II immunoreactive material was also identified in the ovary. The concentration of ovarian Ang II immunoreactivity was 8- to 75-fold greater than that of plasma, was not reduced in bilaterally nephrectomized rats, and was shown by high-pressure liquid chromatographic analysis to be the native angiotensin II octapeptide. The presence of angiotensin II and its receptor binding sites in the ovary suggests a role for angiotensin II as a regulator of ovarian function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B. Regulation of water intake. Physiol Rev. 1978 Jul;58(3):582–582. doi: 10.1152/physrev.1978.58.3.582. [DOI] [PubMed] [Google Scholar]
- Brentjens J. R., Matsuo S., Andres G. A., Caldwell P. R., Zamboni L. Gametes contain angiotensin converting enzyme (kininase II). Experientia. 1986 Apr 15;42(4):399–402. doi: 10.1007/BF02118626. [DOI] [PubMed] [Google Scholar]
- Culler M. D., Tarlatzis B. C., Lightman A., Fernandez L. A., Decherney A. H., Negro-Vilar A., Naftolin F. Angiotensin II-like immunoreactivity in human ovarian follicular fluid. J Clin Endocrinol Metab. 1986 Mar;62(3):613–615. doi: 10.1210/jcem-62-3-613. [DOI] [PubMed] [Google Scholar]
- De Léan A., Ong H., Gutkowska J., Schiller P. W., McNicoll N. Evidence for agonist-induced interaction of angiotensin receptor with a guanine nucleotide-binding protein in bovine adrenal zona glomerulosa. Mol Pharmacol. 1984 Nov;26(3):498–508. [PubMed] [Google Scholar]
- Eng J., Yalow R. S. Evidence against extrapancreatic insulin synthesis. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4576–4578. doi: 10.1073/pnas.78.7.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorioso N., Atlas S. A., Laragh J. H., Jewelewicz R., Sealey J. E. Prorenin in high concentrations in human ovarian follicular fluid. Science. 1986 Sep 26;233(4771):1422–1424. doi: 10.1126/science.3529392. [DOI] [PubMed] [Google Scholar]
- Hirshfield A. N., Midgley A. R., Jr Morphometric analysis of follicular development in the rat. Biol Reprod. 1978 Oct;19(3):597–605. doi: 10.1095/biolreprod19.3.597. [DOI] [PubMed] [Google Scholar]
- Hsueh A. J., Adashi E. Y., Jones P. B., Welsh T. H., Jr Hormonal regulation of the differentiation of cultured ovarian granulosa cells. Endocr Rev. 1984 Winter;5(1):76–127. doi: 10.1210/edrv-5-1-76. [DOI] [PubMed] [Google Scholar]
- Khosla M. C., Leese R. A., Maloy W. L., Ferreira A. T., Smeby R. R., Bumpus F. M. Synthesis of some analogs of angiotensin II as specific antagonists of the parent hormone. J Med Chem. 1972 Aug;15(8):792–795. doi: 10.1021/jm00278a003. [DOI] [PubMed] [Google Scholar]
- LARAGH J. H., ANGERS M., KELLY W. G., LIEBERMAN S. Hypotensive agents and pressor substances. The effect of epinephrine, norepinephrine, angiotensin II, and others on the secretory rate of aldosterone in man. JAMA. 1960 Sep 17;174:234–240. doi: 10.1001/jama.1960.03030030014003. [DOI] [PubMed] [Google Scholar]
- Mendelsohn F. A. Localization and properties of angiotensin receptors. J Hypertens. 1985 Aug;3(4):307–316. doi: 10.1097/00004872-198508000-00002. [DOI] [PubMed] [Google Scholar]
- Nakamaru M., Misono K. S., Naruse M., Workman R. J., Inagami T. A role for the adrenal renin-angiotensin system in the regulation of potassium-stimulated aldosterone production. Endocrinology. 1985 Nov;117(5):1772–1778. doi: 10.1210/endo-117-5-1772. [DOI] [PubMed] [Google Scholar]
- Ohkubo H., Nakayama K., Tanaka T., Nakanishi S. Tissue distribution of rat angiotensinogen mRNA and structural analysis of its heterogeneity. J Biol Chem. 1986 Jan 5;261(1):319–323. [PubMed] [Google Scholar]
- Schelling P., Ganten U., Sponer G., Unger T., Ganten D. Components of the renin-angiotensin system in the cerebrospinal fluid of rats and dogs with special consideration of the origin and the fate of angiotensin II. Neuroendocrinology. 1980 Nov;31(5):297–308. doi: 10.1159/000123092. [DOI] [PubMed] [Google Scholar]
- Schirar A., Capponi A., Catt K. J. Regulation of uterine angiotensin II receptors by estrogen and progesterone. Endocrinology. 1980 Jan;106(1):5–12. doi: 10.1210/endo-106-1-5. [DOI] [PubMed] [Google Scholar]
- Speth R. C., Bumpus F. M., Husain A. Identification of angiotensin II receptors in the rat ovary. Eur J Pharmacol. 1986 Nov 4;130(3):351–352. doi: 10.1016/0014-2999(86)90293-1. [DOI] [PubMed] [Google Scholar]
- Speth R. C., Singh R., Smeby R. R., Ferrario C. M., Husain A. Restricted dietary sodium intake alters peripheral but not central angiotensin II receptors. Neuroendocrinology. 1984 May;38(5):387–392. doi: 10.1159/000123922. [DOI] [PubMed] [Google Scholar]
- Steele M. K., Gallo R. V., Ganong W. F. A possible role for the brain renin-angiotensin system in the regulation of LH secretion. Am J Physiol. 1983 Dec;245(6):R805–R810. doi: 10.1152/ajpregu.1983.245.6.R805. [DOI] [PubMed] [Google Scholar]