Abstract
Background
Vascular closure devices (VCDs) are effective at reducing the time to ambulation for patients undergoing cardiac catheterization procedures, and in reducing the risk of vascular complications in selected patient cohorts. However, the frequency and consequence of failure of VCDs is not well defined.
Methods and Results
From a prospective registry of consecutive patients undergoing cardiac catheterization at our center, 9823 patients who received either a collagen-plug (Angio-Seal®) based or a suture-based (Perclose®) VCD were selected for the study. VCD failure was defined as unsuccessful deployment or failure to achieve hemostasis. Major vascular complication was defined as any retroperitoneal hemorrhage, limb ischemia, or any surgical repair. Minor vascular complication was defined as any groin bleeding, hematoma (≥ 5 cm), pseudoaneurysm or arteriovenous fistula. ‘Any’ vascular complication was defined as either a major or minor vascular complication.
Among the 9823 patients in the study, VCD failed in 268 (2.7%; 2.3% diagnostic vs. 3.0% PCI; P = 0.029) patients. Patients with VCD failure had significantly increased risk of any (6.7% vs. 1.4%; P < 0.0001), major (1.9% vs. 0.6%; P = 0.006) or minor (6.0% vs. 1.1%; P < 0.0001) vascular complication compared with the group with successful deployment of VCD. The increased risk of vascular complication was unchanged in a propensity score matched cohort.
Conclusions
In contemporary practice, VCD failure is rare but when it does fail, it is associated with significant increase in the risk of vascular complications. Patients with VCD failure should be closely monitored to prevent vascular complications.
Keywords: Angio-Seal, complications, Perclose, vascular closure devices
Introduction
Femoral arterial access is the most common method of vascular access for coronary angiography and percutaneous coronary intervention (PCI) in the United States. Vascular closure devices (VCD) have emerged as an effective alternative to traditional mechanical compression after cardiac catheterization since their introduction in the 1990’s. Angio-Seal® (St. Jude Medical, Inc., St. Paul, MN) and the Perclose® (Abbott Vascular, Santa Clara, CA) devices remain the most popular VCDs. These devices have the potential to reduce the time to hemostasis, facilitate early patient mobilization, decrease hospital length of stay and improve patient satisfaction. 1–6
However, the data on efficacy and safety of these devices is controversial. A number of meta-analyses and prospective randomized and non-randomized studies have shown variable results. Some studies have shown that these devices are as efficacious as manual compression, 7, 8 while others have shown superiority of these devices compared to manual compression both for efficacy 9 and cost-minimization despite the upfront cost of these devices, largely based on a lower complication rate with VCD. 10 However, other studies have expressed concern about increased risk of complications with these devices. 11–17 There are also concerns about excess vascular inflammation and scarring associated with VCDs.
The data on the frequency of VCD failure is not well defined and the consequence of such failure has not been fully established. Most of the prior data comes from smaller studies with significant variation in the results making robust conclusions from these studies difficult. The objective of the present study was two folds:
To evaluate the frequency of VCD failure in contemporary practice and the implications, with regard to vascular complications, of such a failure.
To evaluate the risk of VCD failure in the two most frequently used VCD-collagen-plug based device versus suture-based device.
Methods
Study Population
We prospectively evaluated consecutive patients undergoing cardiac catheterization (either diagnostic or PCI) via femoral access at the Brigham and Women’s Hospital between January 1, 2002, and December 31, 2005. Patients’ who received either a collagen-plug (Angio-Seal®) based or a suture-based (Perclose®) VCD were selected for the study, as these were the most frequently used closure devices in our center. Patients presenting with cardiogenic shock or those who required an intra aortic balloon pump placement during the cardiac catheterization procedure were excluded from the study. Informed written consent was obtained from all patients and the study was approved by the institution review board.
Data Collection
A prospective catheterization laboratory database, based on the American College of Cardiology–National Cardiovascular Data Registry definitions, was used to record clinical and procedural elements for each patient. 18 Patients were prospectively followed up for the occurrence of in-hospital vascular complications.
Cardiac Catheterization Protocol
Diagnostic coronary catheterization and PCI were performed according to standard guidelines. Unless contraindicated, all PCI patients received aspirin, clopidogrel, and weight-adjusted heparin therapy as per the standard American College of Cardiology/American Heart Association recommendations. Peri-procedural glycoprotein IIb/IIIa inhibitors and/or bivalirudin were used at the discretion of the treating physician. Anatomical landmarks were identified by pre-procedure fluoroscopy, and vascular access was obtained through single-wall common femoral arterial puncture.
Vascular Closure Device Protocol
Femoral angiography was performed in all patients prior to VCD deployment. The type of VCD use was left at the operator’s discretion. The collagen based (Angio-Seal®) device and the suture-based (Perclose®) VCD were deployed using standard technique as described by the food and drug administration packet insert. Briefly, the Angio-Seal device consists of an absorbable anchor and a collagen sponge connected by an absorbable positioning Dexon suture, whereas the Perclose device consists of a non absorbable suture. Ambulation was advised after 2 to 4 hours after closure device deployment. VCD failure was defined as unsuccessful deployment or failure to achieve hemostasis in the laboratory. In patients with VCD failure, hemostasis was achieved by manual compression followed by mechanical compression device usage (FemoStop®, Radi Medical Systems, Inc., Wilmington, MA) as needed. Local lidocaine with epinephrine injection was used as needed to stop oozing from the arteriotomy site. Bed rest was recommended for at least 2 hours after successful deployment of VCD, but in patients in whom VCD failed, bed rest was recommended for at least 6 hours (and depending on the size of the sheath).
Outcome Measures
Patients were followed up for the occurrence of in hospital vascular complications. Vascular complications included: groin bleeding (defined as blood loss at the access site resulting in blood transfusion, increased length of stay, or drop in hemoglobin >3 g/dL), hematoma (size ≥ 5 cm), pseudoaneurysm (confirmed by ultrasonography), arteriovenous fistula (confirmed by ultrasonography), retroperitoneal hemorrhage (confirmed by computed tomographic scan), limb ischemia (loss of peripheral pulse requiring vascular or surgical evaluation), or any case requiring vascular access–related surgical intervention. Major vascular complication was defined as any retroperitoneal hemorrhage, limb ischemia, or any vascular access–related surgical intervention. Minor vascular complication was defined as any groin bleeding, hematoma (≥ 5 cm), pseudoaneurysm or arteriovenous fistula. ‘Any’ vascular complication was defined as either a major or minor vascular complication. The analyses were adjusted for the operator’s experience. We defined a high-volume operator as an operator who had performed >100 VCD deployments.
Statistical Analysis
All analysis was carried out using standard statistical package (SPSS for Windows, Version 13.0, SPSS Inc., Chicago, Illinois). Continuous variables were reported as mean value ± SD. Patient groups were compared using Student t test (for normally distributed variable) or the Wilcoxon rank-sum test (for other variables) for continuous variables and Chi-square test or Fisher-exact tests for categorical variables. P value was considered significant at <0.05.
Univariate analysis was performed to determine the clinical and procedural characteristics associated with VCD failure. Univariate variables that were predictive of VCD failure were considered in the multivariate logistic regression analysis. The discriminatory power of the logistic models was measured using the area under the receiver-operator curve, and goodness of fit was measured with the Hosmer-Lemeshow C statistic.
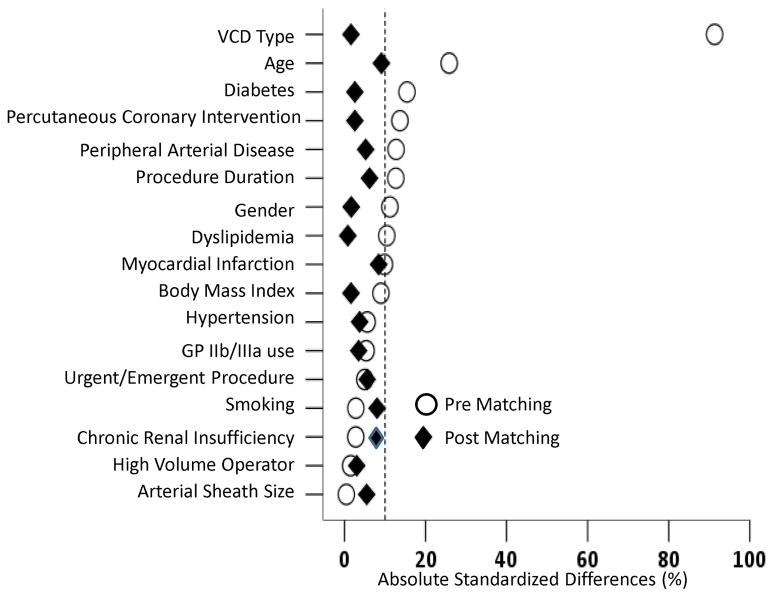
Because of significant differences in key baseline characteristics between participants with successful deployment of VCD and those with VCD failure (Table 1 and Figure 1), we used propensity score matching to assemble a cohort in which all the measured baseline covariates would be well balanced. Propensity score is the conditional probability of having an exposure given a set of measured baseline covariates. 19, 20 Propensity scores for VCD failure were estimated using a non-parsimonious multivariable logistic regression model. 21 In the model, VCD failure was used as the dependent variable, and the 17 baseline characteristics displayed in Figure 1 were entered as covariates. Using a greedy matching protocol, we were able to match 248 VCD failure patients (92% of the 268 VCD failure patients) with 248 patients with successful deployment of the VCD who had similar propensity scores. We estimated absolute standardized differences for all 17 of the covariates between participants with and without VCD failure to assess pre-match imbalance and post match balance. 22 Absolute standardized differences directly quantify balance in the means (or proportions) of covariates across the groups and are expressed as percentages of pooled SDs. An absolute standardized difference of 0% on a covariate indicates no between-group imbalance for that covariate, and values <10% indicate inconsequential imbalance. 22
Table 1.
Baseline Characteristics of Patients with and without Successful Deployment of a Vascular Closure Device before and after Propensity Score Matching.
| Parameter | Before Propensity Score Match | After Propensity Score Match | ||||
|---|---|---|---|---|---|---|
| VCD Success (N = 9555) | VCD Failure (N = 268) | P | VCD Success (N = 248) | VCD Failure (N = 248) | P | |
| Age, years (SD) | 64 ± 12 | 67 ± 13 | <0.0001 | 69 ± 12 | 68 ± 13 | 0.310 |
| Age >70 years, % | 3322 (33%) | 131 (49%) | <0.0001 | 123 (50%) | 125 (50%) | 0.857 |
| Men, % | 6567 (69%) | 170 (63%) | 0.065 | 159 (64%) | 157 (63%) | 0.852 |
| BMI (kg/m2) | 29 ± 5 | 29 ± 6 | 0.159 | 29 ± 6 | 29 ± 6 | 0.856 |
| BMI >30, % | 3420 (36%) | 108 (40%) | 0.129 | 90 (36%) | 94 (38%) | 0.710 |
| Hypertension, % | 7122 (74%) | 206 (77%) | 0.388 | 186 (75%) | 190 (77%) | 0.675 |
| Diabetes Mellitus, % | 2463 (26%) | 88 (33%) | 0.009 | 79 (32%) | 82 (33%) | 0.774 |
| Dyslipidemia, % | 6642 (69%) | 173 (65%) | 0.082 | 159 (64%) | 160 (64%) | 0.925 |
| Smoking, % | 4621 (48%) | 126 (47%) | 0.893 | 107 (43%) | 117 (47%) | 0.665 |
| Chronic Renal Insufficiency, % | 442 (5%) | 14 (5%) | 0.646 | 17 (7%) | 13 (5%) | 0.451 |
| Peripheral Arterial Disease, % | 721 (7%) | 30 (11%) | 0.027 | 24 (10%) | 28 (11%) | 0.558 |
| Percutaneous Coronary Intervention, % | 5676 (59%) | 177 (66%) | 0.029 | 169 (68%) | 166 (67%) | 0.774 |
| Urgent/Emergent Procedure, % | 4644 (49%) | 137 (51%) | 0.416 | 121 (49%) | 128 (52%) | 0.530 |
| Myocardial Infarction, % | 1557 (16%) | 54 (20%) | 0.093 | 38 (15%) | 52 (21%) | 0.103 |
| STEMI, % | 572 (6%) | 15 (6%) | 0.791 | 16 (6%) | 14 (6%) | 0.706 |
| GP IIb/IIIa Use, % | 2555 (27%) | 78 (29%) | 0.389 | 76 (31%) | 72 (29%) | 0.695 |
| Bivalirudin Use, % | 676 (7%) | 19 (7%) | 0.993 | 32 (13%) | 18 (7%) | 0.037 |
| Eptifibatide Use, % | 2420 (25%) | 73 (27%) | 0.478 | 75 (30%) | 67 (27%) | 0.427 |
| Mean Arterial Sheath Size, Fr (SD) | 6.1 ± 0.4 | 6.1 ± 0.4 | 0.942 | 6.1 ± 0.3 | 6.1 ± 0.4 | 0.542 |
| Arterial Sheath ≥7Fr | 488 (5%) | 17 (6%) | 0.366 | 12 (5%) | 17 (7%) | 0.339 |
| Arterial Sheath ≥8Fr | 238 (3%) | 6 (2%) | 0.762 | 5 (2%) | 6 (2%) | 0.760 |
| High Volume Operator, % | 9388 (98%) | 263 (98%) | 0.885 | 243 (98%) | 244 (98%) | 0.737 |
| Device Type | <0.0001 | 0.857 | ||||
| Angio-Seal,® % | 8045 (84%) | 119 (44%) | 112 (45%) | 110 (44%) | ||
| Perclose,® % | 1510 (16%) | 149 (56%) | 136 (55%) | 138 (56%) | ||
| Procedure Duration, mins | 67 ± 102 | 80 ± 134 | 0.053 | 78 ± 122 | 85 ± 108 | 0.510 |
| Length of Stay, days | 3.62 ± 6.71 | 4.09 ± 5.55 | 0.183 | 3.76 ± 4.95 | 4.11 ± 5.17 | 0.449 |
BMI = body mass index; STEMI = ST segment elevation myocardial infarction; VCD = vascular closure device
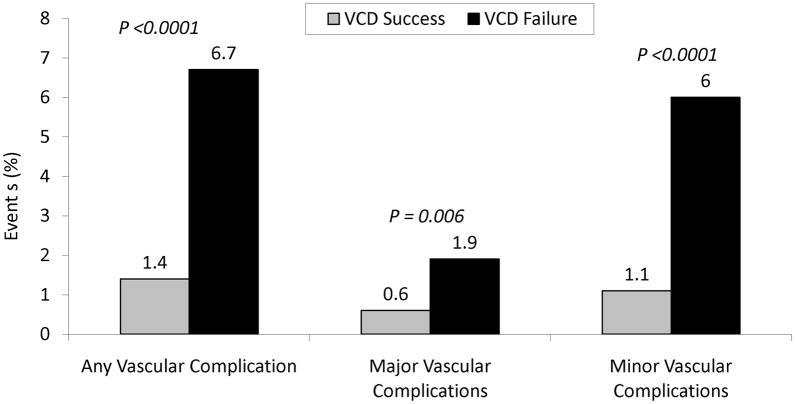
Figure 1.
Incidence of vascular complications in patients with VCD failure compared to those with successful deployment of VCD.
The incidence of any, major or minor vascular complication was substantially higher in the group with VCD failure compared to the group with successful deployment of VCD. VCD = vascular closure device.
In the overall cohort, the use of suture-based (Perclose®) VCD was associated with a significant increase in the risk of VCD failure compared to the collagen plug based (Angio-Seal®) device (Table 2). However, given significant differences in the baseline characteristics of the collagen plug based group compared to the suture-based group, we assembled a separate propensity score matched cohort to match the suture-based group with the collagen plug based group, to evaluate the risk of VCD failure.
Table 2.
Vascular Closure Device Failure and the Risk of Any Vascular Complications
| OR (95% CI) | P | ROC | |
|---|---|---|---|
| Overall (Unadjusted) | 4.99 (3.00–8.28) | <0.0001 | 0.546 |
| Adjusted for all significant covariates | 4.57 (2.71–7.69) | <0.0001 | 0.763 |
| Propensity score matched cohort | 5.63 (1.62–19.58) | 0.007 | 0.678 |
| Propensity score matched cohort adjusted for all significant covariates | 5.08 (1.44–17.86) | 0.011 | 0.788 |
RCO = receiver operating characteristic curve.
Results
Among the study cohort of 9823 patients, 8164 (83%) patients had a collagen plug based VCD and 1659 (17%) had a suture-based VCD deployment. 3970 (40%) patients underwent diagnostic cardiac catheterization while 5853 (60%) patients underwent PCI.
Baseline Characteristics
Among the study cohort of 9823 patients, VCD failed in 268 (2.7%) patients, 91 (2.3%) patients undergoing diagnostic catheterization and 177 (3.0%) patients undergoing PCI (P = 0.029). The baseline clinical and procedural characteristics of patients with successful deployment of VCD vs. those with VCD failure were imbalanced (Table 1). Patients with VCD failure were older, and were more likely to be diabetics, those with peripheral arterial disease, those undergoing PCI procedure, those with deployment of suture based VCD and those with a greater procedural duration compared to patients with successful deployment of VCD (Table 1).
VCD Failure and Vascular Complications
Compared to patients with successful deployment of VCD, patients with VCD failure had a 4.8 fold higher incidence of any vascular complications, 3.2 fold higher incidence of major vascular complications and a 5.4 fold increased risk of minor vascular complication (Figure 1). For the individual components of vascular complications, VCD failure was associated with an increased risk of vascular access related surgical repair (0.7% vs. 0.1%; P = 0.003), pseudoaneurysm (0.7% vs. 0.1%; P = 0.008), groin bleeding (0.7% vs. 0.3%; P = 0.185), arteriovenous fistula (0.4% vs. 0.0%; P = 0.006), hematoma (4.5% vs. 0.6%, P <0.0001), retroperitoneal bleeding (0.4% vs. 0.3%, P = 0.939) and limb ischemia (0.4% vs. 0.1%; P = 0.036).
In patients undergoing diagnostic catheterization, VCD failure was associated with a significant increase in the risk of any vascular complication (4.4% vs. 0.4%; P <0.0001) driven mainly by an increase in major vascular complication (3.3% vs. 0.2%; P <0.0001) and a trend towards increase in the risk of minor vascular complications (1.1% vs. 0.2%; P = 0.077). On the contrary, in patients undergoing PCI, VCD failure was associated with a significant increase in any vascular complication (7.9% vs. 2.1%; P <0.0001), driven mainly by an increase in minor vascular complication (7.3% vs. 1.4%; P <0.0001) with no substantial increase in major vascular complications (1.1% vs. 0.8%; P = 0.621).
To control for baseline difference in the VCD failure vs. VCD success group, propensity score matching was performed with matching of 248 VCD failure patients (92% of the 268 VCD failure patients) with 248 patients with successful deployment of the VCD. Post matching, all 17 variables showed excellent matching (100% of variables with absolute standard difference of <10%) (Figure 2). The baseline characteristic was well matched between the 2 groups (Table 1). In this matched cohort of patients, VCD failure was associated with a significant increase in any vascular complications (6.5% vs. 1.2%; P = 0.002), minor vascular complications (5.6% vs. 1.2%; P = 0.007) with a trend towards increased risk of major vascular complications (2.0% vs. 0.4%; P = 0.099).
Figure 2.
Absolute standardized differences in baseline covariates between patients with and without VCD failure, before and after propensity score matching (post-match standardized difference <10% indicates excellent covariate balance).
There was an increased risk of any vascular complication with VCD failure for the overall cohort, after adjusting for significant baseline variables, in the propensity score matched cohort and in the propensity matched cohort after adjusting for significant baseline variables (Table 2). Patients with any vascular complications had a significant increase in the length of stay (4.6 ± 5.1 days vs. 3.5 ± 6.8 days; P = 0.024). This was mainly due to increased length of stay in patients with major vascular complications (5.8± 6.8 days vs. 3.5 ± 6.8 days; P = 0.016) with no significant difference in patients with minor vascular complications (3.6 ± 2.9 days vs. 3.5 ± 6.8 days; P = 0.881).
Predictors of VCD Failure
The univariate predictors of VCD failure were older age group, patients with diabetics, those with peripheral arterial disease, those undergoing PCI, longer duration of the procedure and those with the usage of suture based VCD compared to the collagen-plug based device (Table 3). The multivariable predictors were an older age group, those undergoing PCI and those with the usage of suture based VCD. Suture-based VCD conferred a 6.6 fold higher risk of VCD failure compared to the collagen based VCD even after controlling for baseline variables (Table 3).
Table 3.
Univariate and Multivariable Predictors of Vascular Closure Device Failure
| Parameter | Odds Ratio | 95% CI | P |
|---|---|---|---|
| Univariate Predictors | |||
| Age | 1.02 | 1.01–1.03 | <0.0001 |
| Age >70 years | 1.79 | 1.41–2.29 | <0.0001 |
| Men | 1.27 | 0.98–1.63 | 0.066 |
| Diabetes Mellitus | 1.41 | 1.09–1.82 | 0.010 |
| Dyslipidemia | 1.25 | 0.97–1.61 | 0.083 |
| Peripheral Arterial Disease | 1.54 | 1.05–2.27 | 0.028 |
| Percutaneous Coronary Intervention | 1.33 | 1.03–1.72 | 0.029 |
| Myocardial Infarction | 1.30 | 0.96–1.76 | 0.094 |
| Perclose Device® | 6.67 | 5.21–8.54 | <0.0001 |
| Procedure Duration | 1.002 | 1.000–1.004 | 0.017 |
| Multivariable Predictors | |||
| Age | 1.02 | 1.01–1.03 | 0.001 |
| Perclose Device® | 6.56 | 5.04–8.54 | <0.0001 |
| Percutaneous Coronary Intervention | 1.34 | 1.01–1.79 | 0.045 |
| Myocardial Infarction | 1.38 | 0.99–1.92 | 0.060 |
| Diabetes Mellitus | 1.28 | 0.97–1.71 | 0.085 |
Collagen Plug Based (Angio-Seal) VCD vs. Suture Based (Perclose) VCD
Given the increased risk of VCD failure with suture based device, we performed a formal propensity score matching analysis to match for the propensity to use the suture-based device, to evaluate the risk of VCD failure in a matched cohort. The baseline characteristic of the unmatched cohort is described in table 4. Compared to the collagen plug based VCD cohort, the suture-based cohort were older, more likely to have peripheral arterial disease, but less likely to present with myocardial infarction, less likely to be treated with glycoprotein IIb/IIIa, had a smaller size arterial sheath and were less likely to undergo PCI. The use of suture based closure device was associated with a 6.7 folds increase in the risk of VCD failure compared to the collagen plug based cohort (Table 5). The increased risk of VCD failure with suture-based device was seen both in diagnostic cases (6.4% vs. 4.3%, P <0.0001) and PCI cases (11.1% vs. 1.5%, P <0.0001), when compared to collagen plug based device.
Table 4.
Baseline Characteristics of Patients Based on the type of Vascular Closure Device used before and after Propensity Score Matching.
| Parameter | Before Propensity Score Match | After Propensity Score Match | ||||
|---|---|---|---|---|---|---|
| Angio-Seal® (N = 8164) | Perclose® (N = 1659) | P | Angio-Seal® (N = 1473) | Perclose® (N = 1473) | P | |
| Age, years (SD) | 64 ± 12 | 65 ± 12 | 0.059 | 64 ± 13 | 64 ± 12 | 0.910 |
| Age >70 years, % | 2866 (35%) | 587 (35%) | 0.829 | 554 (38%) | 514 (35%) | 0.125 |
| Men, % | 5615 (69%) | 1122 (68%) | 0.359 | 976 (66%) | 997 (68%) | 0.411 |
| BMI (kg/m2) | 29 ± 5 | 28 ± 5 | 0.068 | 29 ± 5 | 28 ± 5 | 0.440 |
| BMI >30, % | 2959 (36%) | 569 (34%) | 0.132 | 503 (34%) | 460 (31%) | 0.091 |
| Hypertension, % | 6109 (75%) | 1219 (73%) | 0.249 | 1064 (72%) | 1083 (73%) | 0.431 |
| Diabetes Mellitus, % | 2100 (26%) | 451 (27%) | 0.216 | 394 (27%) | 393 (27%) | 0.967 |
| Dyslipidemia, % | 5738 (70%) | 1077 (65%) | <0.0001 | 944 (64%) | 971 (66%) | 0.297 |
| Smoking, % | 3954 (48%) | 793 (48%) | 0.638 | 702 (48%) | 717 (49%) | 0.580 |
| Chronic Renal Insufficiency, % | 371 (4%) | 85 (5%) | 0.307 | 27 (2%) | 44 (3%) | 0.041 |
| Peripheral Arterial Disease, % | 606 (7%) | 145 (9%) | 0.066 | 141 (10%) | 134 (9%) | 0.658 |
| Percutaneous Coronary Intervention, % | 4943 (60%) | 910 (55%) | <0.0001 | 802 (54%) | 809 (55%) | 0.796 |
| Urgent/Emergent Procedure, % | 4192 (51%) | 850 (51%) | 0.934 | 730 (50%) | 699 (47%) | 0.253 |
| Myocardial Infarction, % | 1377 (17%) | 234 (14%) | 0.006 | 216 (15%) | 196 (13%) | 0.288 |
| STEMI, % | 495 (6%) | 92 (5%) | 0.417 | 72 (5%) | 74 (5%) | 0.865 |
| GP IIb/IIIa Use, % | 2253 (28%) | 380 (23%) | <0.0001 | 325 (22%) | 339 (23%) | 0.537 |
| Bivalirudin Use, % | 571 (7%) | 124 (7%) | 0.487 | 120 (8%) | 113 (8%) | 0.633 |
| Eptifibatide Use, % | 2132 (26%) | 361 (22%) | <0.0001 | 306 (21%) | 320 (22%) | 0.528 |
| Venous Access, % | 3182 (39%) | 671 (40%) | 0.264 | 615 (42%) | 624 (42%) | 0.737 |
| Mean Arterial Sheath Size, Fr (SD) | 6.1 ± 0.4 | 6.0 ± 0.3 | <0.0001 | 6.0 ± 0.3 | 6.0 ± 0.2 | 0.607 |
| Arterial Sheath ≥7Fr | 446 (5%) | 59 (4%) | 0.001 | 44 (3%) | 55 (4%) | 0.261 |
| Arterial Sheath ≥8Fr | 234 (3%) | 10 (1%) | <0.0001 | 23 (2%) | 8 (1%) | 0.006 |
| High Volume Operator, % | 8013 (98%) | 1638 (99%) | 0.098 | 1453 (99%) | 1452 (99%) | 0.875 |
| Procedure Duration, mins | 67 ± 93 | 68 ± 141 | 0.735 | 72 ± 91 | 71 ± 126 | 0.975 |
| Length of Stay, days | 3.62 ± 6.79 | 4.19 ± 6.10 | <0.0001 | 3.76 ± 4.95 | 4.11 ± 5.17 | 0.449 |
BMI = body mass index; STEMI = ST segment elevation myocardial infarction; VCD = vascular closure device
Table 5.
Perclose vs. Angio-Seal and the Risk of Vascular Closure Device Failure.
| OR (95% CI) | P | ROC | |
|---|---|---|---|
| Overall (Unadjusted) | 6.67 (5.21–8.54) | <0.0001 | 0.699 |
| Adjusted for all significant covariates | 6.68 (5.14–8.69) | <0.0001 | 0.752 |
| Propensity score matched cohort | 1.25 (0.96–1.64) | 0.100 | 0.528 |
| Propensity score matched cohort adjusted for all significant covariates | 1.29 (0.98–1.70) | 0.065 | 0.663 |
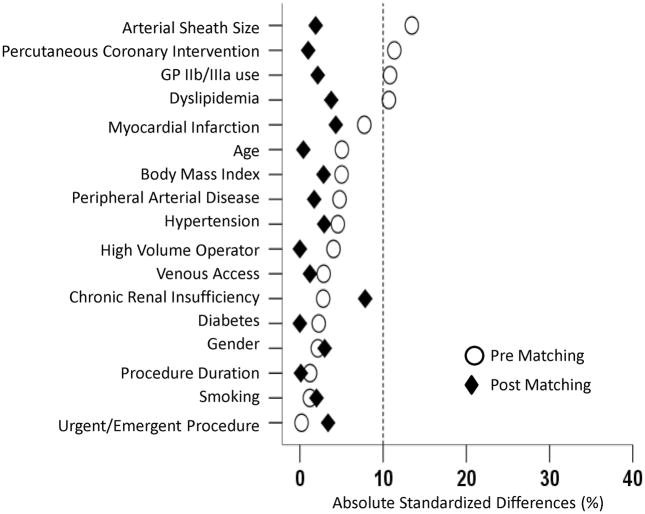
The propensity score matching resulted in matching of 1473 (89% of 1659 suture based VCD patients) suture-based VCD patients with 1473 patients in the collagen plug based device category (Table 4). Post matching, there was excellent balance in the baseline variables with 100% of the variables with an absolute standardized difference of <10% (Figure 3). Even in this matched cohort of patients, the use of suture based VCD device was associated with a trend (P = 0.06) towards a 29% increased risk of VCD failure compared to the collagen plug based device (Table 5).
Figure 3.
Absolute standardized differences in baseline covariates between the Perclose® group and the Angio-Seal® group, before and after propensity score matching (post-match standardized difference <10% indicates excellent covariate balance).
Discussion
This study assessed the frequency of VCD failure and the implications of such a failure. The results of the present study showed that the risk of VCD failure is rare in contemporary practice but when it does occur it is associated with significant increase in the risk of vascular complications. However, it is reassuring that in patients with successful deployment of VCD, the risk of vascular complications was very low.
Vascular Closure Device Failure
Vascular closure devices are being increasingly used in patients after cardiac catheterization. VCD reliably shortens the time to hemostasis compared with manual compression and thus allows earlier patient ambulation. 3 In addition, sheath removal via manual compression generally requires the operator to wait for the activated clotting time to reach a level of <180’s, 23 while VCD use allows immediate removal of the femoral sheath regardless of anticoagulation status. While length of stay for PCI patients will not necessarily be reduced by early ambulation (unless same-day PCI is adopted), it can be reduced for diagnostic patients. 24 In addition, for many patients, VCD can allow improved patient satisfaction and comfort related to the avoidance of prolonged sheath insertion and manual compression. 23, 25
Despite these advantages, a small proportion of these devices fail. The failure rate reported for these devices have ranged widely from 1.5% to 20% in contemporary studies. However, the consequence of such failure has not been formally evaluated.
In our large single center experience, the VCD failure rate was 2.7% and was greater in patients undergoing PCI compared to those undergoing diagnostic cardiac catheterization (3.0% vs. 2.3%). The results of the present studies shows that VCD failure is associated with up to a 5 fold increase in the risk of vascular complications compared to the group with successful deployment of these devices. This was true even in a propensity score adjusted cohort where patients were matched for known predictors of increased vascular complications including glycoprotein IIb/IIIa use, those presenting for urgent or emergent procedure, arterial sheath size and those undergoing PCI. Even after adjusting for 17 of these variables, VCD failure was associated with a substantial increase in the risk of vascular complications. In this study, we did not compare the risk of vascular complications with VCD failure with that of manual compression alone. However, extrapolating the results from our prior study suggests that the risk of vascular complications associated with VCD failure (4.4% for diagnostic and 7.9% for PCI) was substantially higher than the risk associated with manual compression alone (1.1% for diagnostic and 4.9% for PCI).
A number of factors might contribute to this excess risk with VCD failure. In patients with VCD failure, VCDs are deployed during full therapeutic procedural anticoagulation (in patients undergoing PCI) while manual compression is delayed until coagulation has returned to near normal function, thus increasing the severity of bleeding complications when these devices fail. Moreover, deployment of VCD might instill a false sense of security. It has to be noted that the risk of vascular complications associated with VCD failure was substantially higher compared to manual compression alone even in diagnostic cases as well. In our study, an older age group, those undergoing PCI and those in whom suture based VCD were used were at increased risk of VCD failure.
Collagen Plug Based Device Versus Suture Based Device
The collagen plug based and the suture-based device differs in many ways. Studies in canine model have shown that the collagen plug closure device produced greater vessel narrowing and peri-adventitial inflammation (extravascular scarring) compared to suture closure device. 26 The failure rates for these devices from prior studies have been heterogeneous. For the collagen-plug based device, the device failure rate has been reported to vary widely from 1.5% 15 to 15.4%. 27 However, these studies have been small, and the definition of outcomes has been heterogeneous. Similarly, for the suture based device, several small studies have reported the failure rate to vary between 1.5% 15 to 14.3%. 28 The current analyses extend the findings of several studies that have compared the failure rate of these two devices head to head. In the randomized trial comparing compression, Perclose Proglide and Angio-Seal VIP for arterial closure following percutaneous coronary intervention (CAP) trial, there was a significant higher risk of VCD failure with suture based device compared to collagen plug based device (15.9% vs. 0.0%; p <0.0001). 25 However, the study was limited by the small number of patients, 63, who were randomized to the Perclose group. In a large case series of 4,525 patients, Applegate et al. 29 showed a significantly higher risk of VCD failure with Perclose device compared to Angio-Seal device (5.9% vs. 2.9%; P <0.05). The current analysis extends these findings to larger cohorts of patients, and incorporates propensity score matching to adjust for baseline variables in an attempt to further reduce treatment assignment bias in the comparison of the devices utilized.
Study Limitations
The data is from a single, high volume center with an institutional policy of routine VCD use and hence the generalizability of our study to other centers is limited. Despite the institutional policy for deployment of VCDs and the propensity analysis reported, unadjusted biases may still remain in this observational data set. In this study we evaluated in hospital outcomes only, and hence late complications may be underestimated.
Conclusions
In contemporary practice, vascular closure device failure is rare both for diagnostic and for percutaneous coronary intervention, but when it occurs it is associated with a substantial increase in the risk of vascular complications, higher than the reported rates for manual compression alone. It is reassuring that in patients with successful deployment of VCD, the risk of vascular complications is very low (<1.5%).
In a propensity-adjusted cohort, there was a trend towards excess failure rate with suture-based device. Patients with VCD failure should be carefully monitored for vascular complications.
Acknowledgments
Sources of Funding:
This work was partially funded by partially funded by NIH R01-LM008142
Footnotes
This work was presented in part at the 2009 Annual Scientific Session of the American College of Cardiology, Orlando, Florida.
Disclosures:
No conflict of interest for any of the authors for this work.
References
- 1.Chevalier B, Lancelin B, Koning R, Henry M, Gommeaux A, Pilliere R, Elbaz M, Lefevre T, Boughalem K, Marco J, Dupouy P. Effect of a closure device on complication rates in high-local-risk patients: results of a randomized multicenter trial. Catheter Cardiovasc Interv. 2003;58:285–291. doi: 10.1002/ccd.10431. [DOI] [PubMed] [Google Scholar]
- 2.Gerckens U, Cattelaens N, Lampe EG, Grube E. Management of arterial puncture site after catheterization procedures: evaluating a suture-mediated closure device. Am J Cardiol. 1999;83:1658–1663. doi: 10.1016/s0002-9149(99)00174-5. [DOI] [PubMed] [Google Scholar]
- 3.Kussmaul WG, 3rd, Buchbinder M, Whitlow PL, Aker UT, Heuser RR, King SB, Kent KM, Leon MB, Kolansky DM, Sandza JG., Jr Rapid arterial hemostasis and decreased access site complications after cardiac catheterization and angioplasty: results of a randomized trial of a novel hemostatic device. J Am Coll Cardiol. 1995;25:1685–1692. doi: 10.1016/0735-1097(95)00101-9. [DOI] [PubMed] [Google Scholar]
- 4.Nasu K, Tsuchikane E, Sumitsuji S. Clinical effectiveness of the Prostar XL suture-mediated percutaneous vascular closure device following PCI: results of the Perclose Accele Rated Ambulation and DISchargE (PARADISE) Trial. J Invasive Cardiol. 2003;15:251–256. [PubMed] [Google Scholar]
- 5.Slaughter PM, Chetty R, Flintoft VF, Lewis S, Sykora K, Beattie DM, Schwartz L. A single center randomized trial assessing use of a vascular hemostasis device vs. conventional manual compression following PTCA: what are the potential resource savings? Cathet Cardiovasc Diagn. 1995;34:210–214. doi: 10.1002/ccd.1810340106. [DOI] [PubMed] [Google Scholar]
- 6.Ward SR, Casale P, Raymond R, Kussmaul WG, 3rd, Simpfendorfer C. Efficacy and safety of a hemostatic puncture closure device with early ambulation after coronary angiography. Angio-Seal Investigators. Am J Cardiol. 1998;81:569–572. doi: 10.1016/s0002-9149(97)00970-3. [DOI] [PubMed] [Google Scholar]
- 7.Applegate RJ, Sacrinty MT, Kutcher MA, Baki TT, Gandhi SK, Santos RM, Little WC. Propensity score analysis of vascular complications after diagnostic cardiac catheterization and percutaneous coronary intervention 1998–2003. Catheter Cardiovasc Interv. 2006;67:556–562. doi: 10.1002/ccd.20677. [DOI] [PubMed] [Google Scholar]
- 8.Nikolsky E, Mehran R, Halkin A, Aymong ED, Mintz GS, Lasic Z, Negoita M, Fahy M, Krieger S, Moussa I, Moses JW, Stone GW, Leon MB, Pocock SJ, Dangas G. Vascular complications associated with arteriotomy closure devices in patients undergoing percutaneous coronary procedures: a meta-analysis. J Am Coll Cardiol. 2004;44:1200–1209. doi: 10.1016/j.jacc.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 9.Arora N, Matheny ME, Sepke C, Resnic FS. A propensity analysis of the risk of vascular complications after cardiac catheterization procedures with the use of vascular closure devices. Am Heart J. 2007;153:606–611. doi: 10.1016/j.ahj.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Resnic FS, Arora N, Matheny M, Reynolds MR. A cost-minimization analysis of the angio-seal vascular closure device following percutaneous coronary intervention. Am J Cardiol. 2007;99:766–770. doi: 10.1016/j.amjcard.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koreny M, Riedmuller E, Nikfardjam M, Siostrzonek P, Mullner M. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization: systematic review and meta-analysis. JAMA. 2004;291:350–357. doi: 10.1001/jama.291.3.350. [DOI] [PubMed] [Google Scholar]
- 12.Wagner SC, Gonsalves CF, Eschelman DJ, Sullivan KL, Bonn J. Complications of a percutaneous suture-mediated closure device versus manual compression for arteriotomy closure: a case-controlled study. J Vasc Interv Radiol. 2003;14:735–741. doi: 10.1097/01.rvi.0000079982.80153.d9. [DOI] [PubMed] [Google Scholar]
- 13.Sohail MR, Khan AH, Holmes DR, Jr, Wilson WR, Steckelberg JM, Baddour LM. Infectious complications of percutaneous vascular closure devices. Mayo Clin Proc. 2005;80:1011–1015. doi: 10.4065/80.8.1011. [DOI] [PubMed] [Google Scholar]
- 14.Brown DB. Current status of suture-mediated closure: what is the cost of comfort? J Vasc Interv Radiol. 2003;14:677–681. doi: 10.1097/01.rvi.0000079977.80153.80. [DOI] [PubMed] [Google Scholar]
- 15.Cura FA, Kapadia SR, L’Allier PL, Schneider JP, Kreindel MS, Silver MJ, Yadav JS, Simpfendorfer CC, Raymond RR, Tuzcu EM, Franco I, Whitlow PL, Topol EJ, Ellis SG. Safety of femoral closure devices after percutaneous coronary interventions in the era of glycoprotein IIb/IIIa platelet blockade. Am J Cardiol. 2000;86:780–782. A789. doi: 10.1016/s0002-9149(00)01081-x. [DOI] [PubMed] [Google Scholar]
- 16.Dangas G, Mehran R, Kokolis S, Feldman D, Satler LF, Pichard AD, Kent KM, Lansky AJ, Stone GW, Leon MB. Vascular complications after percutaneous coronary interventions following hemostasis with manual compression versus arteriotomy closure devices. J Am Coll Cardiol. 2001;38:638–641. doi: 10.1016/s0735-1097(01)01449-8. [DOI] [PubMed] [Google Scholar]
- 17.Kahn ZM, Kumar M, Hollander G, Frankel R. Safety and efficacy of the Perclose suture-mediated closure device after diagnostic and interventional catheterizations in a large consecutive population. Catheter Cardiovasc Interv. 2002;55:8–13. doi: 10.1002/ccd.10045. [DOI] [PubMed] [Google Scholar]
- 18.Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS, Williams JF. The American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR): building a national clinical data repository. J Am Coll Cardiol. 2001;37:2240–2245. doi: 10.1016/s0735-1097(01)01372-9. [DOI] [PubMed] [Google Scholar]
- 19.Rosenbaum P, Rubin D. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 20.Rubin D. Using propensity score to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2:169–188. [Google Scholar]
- 21.Ahmed A, Husain A, Love TE, Gambassi G, Dell’Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 23.Duffin DC, Muhlestein JB, Allisson SB, Horne BD, Fowles RE, Sorensen SG, Revenaugh JR, Bair TL, Lappe DL. Femoral arterial puncture management after percutaneous coronary procedures: a comparison of clinical outcomes and patient satisfaction between manual compression and two different vascular closure devices. J Invasive Cardiol. 2001;13:354–362. [PubMed] [Google Scholar]
- 24.Hermiller J, Simonton C, Hinohara T, Lee D, Cannon L, Mooney M, O’Shaughnessy C, Carlson H, Fortuna R, Yarbrough CA, Zapien M, Chou T. Clinical experience with a circumferential clip-based vascular closure device in diagnostic catheterization. J Invasive Cardiol. 2005;17:504–510. [PubMed] [Google Scholar]
- 25.Martin JL, Pratsos A, Magargee E, Mayhew K, Pensyl C, Nunn M, Day F, Shapiro T. A randomized trial comparing compression, Perclose Proglide and Angio-Seal VIP for arterial closure following percutaneous coronary intervention: the CAP trial. Catheter Cardiovasc Interv. 2008;71:1–5. doi: 10.1002/ccd.21333. [DOI] [PubMed] [Google Scholar]
- 26.Gargiulo NJ, 3rd, Veith FJ, Ohki T, Scher LA, Berdejo GL, Lipsitz EC, Menegus M, Greenberg M. Histologic and duplex comparison of the perclose and angio-seal percutaneous closure devices. Vascular. 2007;15:24–29. doi: 10.2310/6670.2007.00004. [DOI] [PubMed] [Google Scholar]
- 27.Assali AR, Sdringola S, Moustapha A, Ghani M, Salloum J, Schroth G, Fujise K, Anderson HV, Smalling RW, Rosales OR. Outcome of access site in patients treated with platelet glycoprotein IIb/IIIa inhibitors in the era of closure devices. Catheter Cardiovasc Interv. 2003;58:1–5. doi: 10.1002/ccd.10384. [DOI] [PubMed] [Google Scholar]
- 28.Chamberlin JR, Lardi AB, McKeever LS, Wang MH, Ramadurai G, Grunenwald P, Towne WP, Grassman ED, Leya FS, Lewis BE, Stein LH. Use of vascular sealing devices (VasoSeal and Perclose) versus assisted manual compression (Femostop) in transcatheter coronary interventions requiring abciximab (ReoPro) Catheter Cardiovasc Interv. 1999;47:143–147. doi: 10.1002/(SICI)1522-726X(199906)47:2<143::AID-CCD1>3.0.CO;2-M. discussion 148. [DOI] [PubMed] [Google Scholar]
- 29.Applegate RJ, Grabarczyk MA, Little WC, Craven T, Walkup M, Kahl FR, Braden GA, Rankin KM, Kutcher MA. Vascular closure devices in patients treated with anticoagulation and IIb/IIIa receptor inhibitors during percutaneous revascularization. J Am Coll Cardiol. 2002;40:78–83. doi: 10.1016/s0735-1097(02)01924-1. [DOI] [PubMed] [Google Scholar]