Abstract
Although adoptive T-cell therapy has shown clinical success, efficacy is limited by low levels of T-cell trafficking to, and survival in, the immunosuppressive environment of an established tumor. Oncolytic virotherapy has recently emerged as a promising approach to induce both direct tumor cell killing and local proinflammatory environments within tumors. However, inefficient systemic delivery of oncolytic viruses remains a barrier to use of these agents against metastatic disease that is not directly accessible to the end of a needle. Here we show that the ability of antigen-specific T cells to circulate freely, and to localize to tumors, can be exploited to achieve the systemic delivery of replication-competent, oncolytic vesicular stomatitis virus (VSV). Thus, VSV loaded onto OT-I T cells, specific for the SIINFEKL epitope of the ovalbumin antigen, was efficiently delivered to established B16ova tumors in the lungs of fully immune-competent C57Bl/6 mice leading to significant increases in therapy compared to the use of virus, or T cells, alone. Although OT-I T-cell-mediated delivery of VSV led to viral replication within tumors and direct viral oncolysis, therapy was also dependent upon an intact host immune system. Moreover, VSV loading onto the T cells increased both T-cell activation in vitro and T-cell trafficking in vivo. The combination of adoptive T-cell transfer of antigen-specific T cells, along with oncolytic virotherapy, can, therefore, increase the therapeutic utility of both approaches through multiple mechanisms and should be of direct translational value.
Keywords: VSV, oncolytic viruses, immunotherapy, adoptive T-cell transfer
Introduction
Oncolytic virotherapy, based upon the observed ability of several different viruses, either unmodified or genetically engineered, to replicate preferentially in tumor, as opposed to normal, cells has emerged as a promising novel strategy for cancer therapy.1–3 However, a major barrier to the widespread use of these viral agents to treat metastatic disease remains the inability to deliver them in a truly systemic manner to established tumors which are not directly accessible to direct injection.4,5 This stems, in part at least, from virus neutralization, nonspecific adhesion of the virus particles to multiple host cell types and from active sequestration of particles.4,6–9 A further problem is the inability to localize viruses specifically to tumors and to promote their extravasation from the circulation.10
In this latter respect, extensive preclinical and clinical studies have shown that tumors are often infiltrated with T cells with specificity for antigens expressed by the tumor cells themselves.11–14 These antigen-specific T cells can be recovered from tumors, expanded in vitro and adoptively transferred back to the patient where there is convincing evidence that they traffic, at least to some degree, to tumors expressing the cognate antigen.12,14 Exploiting this adoptive T-cell transfer has been used successfully as an antitumor therapy with increasingly encouraging results, based upon the intrinsic ability of the T cells to recognize and kill tumor directly.12,14,15
We, and others, hypothesized that it might be possible to exploit the natural tumor homing ability of antigen-specific T cells to increase the tumor localization of intravenously (i.v.) injected viral particles.16–23 Moreover, by associating virus with such T cells it may also be possible to ameliorate some the problems of virus neutralization, sequestration and nonspecific adhesion.5 Indeed, we and others have shown that antigen-specific T cells can be used very effectively, in both immune-competent and immune-deficient mice, to chaperone virus particles to tumors, to induce their local release at the tumor site and to promote increased therapy over and above the therapy induced by the cytolytic activity of the T cells alone.16–23
In addition to T cells, several other types of cells have been used as carriers of genes/viruses to protect the virus from the multiple neutralizing and sequestering antiviral systems which the host normally uses to remove unwanted viral infections.24–27 It is now clear that although the type of carrier and virus can differ it is possible to use cells to chaperone therapeutic viral particles to sites of tumor growth, either on,21,23 or inside16,17,24 the cells, and to generate potent antitumor therapy even in the presence of a fully intact immune system.
We recently showed that it is possible to use T cells with specificity for an antigen expressed only by the tumor cells to deliver replication-incompetent retroviral particles to tumors.21,23 This approach utilized the observation that retroviral particles will nonspecifically adhere to the surface of many different cells6 and that these adhered viruses can then be released from the cell surface for productive infection of neighboring cells.21,23,28 Moreover, virus release is promoted within the protease rich environment of an established tumor. 21,23 When the retrovirus encoded either a suicide gene,21 or a chemokine,23 significant antitumor therapy was achieved over and above that possible with the virus or T cells alone. In addition, loading the retroviral particles onto antigen-specific T cells conferred a significant degree of immune privilege by protecting virus from complement and antibody neutralization.21
Having shown significant therapy with the release of replication-incompetent retroviral vectors from the surface of antigen-specific T cells, we reasoned that the release of replication-competent viruses may be more beneficial owing to their potential for increased spread through the tumor.1,3,29 Since we have shown that intratumoral injection of vesicular stomatitis virus (VSV) not only induces oncolysis of the local tumor30–32 but also primes antitumor CD8+ T-cell responses,33 here we have combined adoptive T-cell therapy with VSV oncolytic virotherapy to treat a murine model of metastatic melanoma. As a murine model of adoptive T-cell therapy, we used OT-I CD8+ T cells that express a transgenic T-cell receptor specific for the SIINFEKL epitope of the ovalbumin protein presented in the context of the H-2Kb major histocompatibility complex (MHC) class I molecule expressed by B16ova tumor cells.21,34 We also used VSV-δ51, in which the methionine 51 of the matrix protein is deleted. This virus can no longer block the nuclear export of interferon (IFN)-encoding mRNAs. Therefore, VSV-δ51 enhances the expression of antiviral IFNs in normal cells, which prevents viral replication and spread; however, many cancer cells remain insensitive to the effects of IFN-α and -β, making the virus highly tumor specific for replication and oncolysis.31,35 We show here that adoptive transfer of OT-I cells preloaded with VSV-δ51 was significantly better than T-cell therapy, or i.v. virus therapy alone, against B16ova tumors growing in C57Bl/6 mice. We could not detect appreciable replication of VSV within the OT-I cells but adherent virus was released and effectively infected, replicated in and killed tumor cells following coculture of loaded T cells with B16ova cells. In addition, VSV loading on OT-I cells activated the T cells in vitro (both in terms of direct killing activity and cytokine release) and increased their trafficking to B16ova tumors in vivo. Finally, an intact immune system was necessary for maximal therapeutic effects and mice cured of tumor through this combination of adoptive T-cell therapy and oncolytic virotherapy were protected long term against tumor challenge. Thus, although the preparation of antigen-specific T cells from patients can be time consuming and requires considerable expertise, simple loading of these cells with oncolytic virus could provide a next step in enhancing the efficacy of adoptive T-cell therapy for the treatment of metastatic disease.
Results
OT-I T cells are not productively infected by VSV-δ51
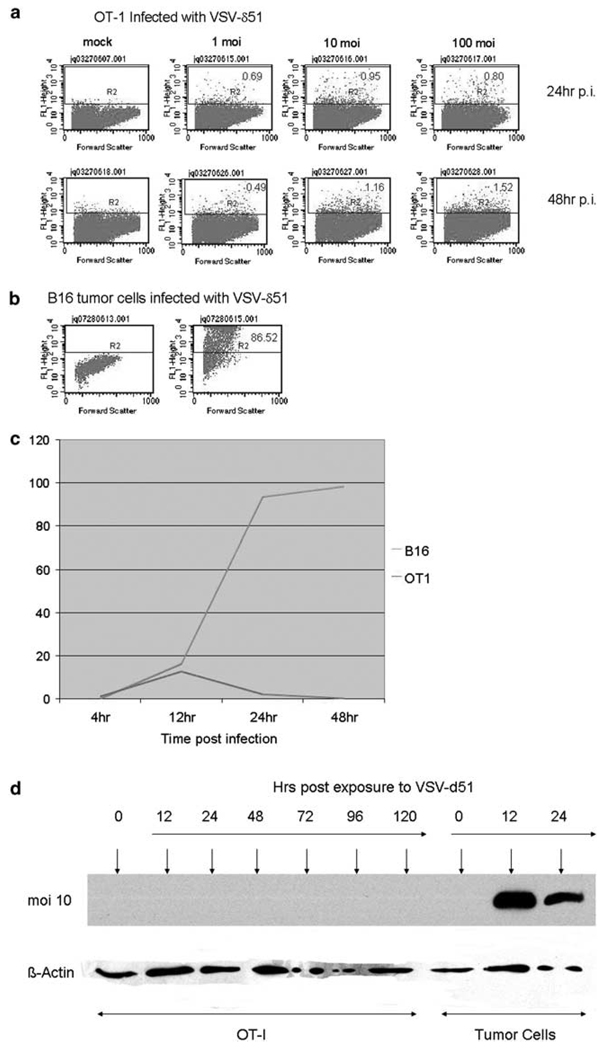
Even at a multiplicity of infection (MOI) of 100, only a maximum of 4% of OT-I T cells exposed to VSV-δ51 were infected 48 h after exposure to virus (Figure 1a). In contrast, an MOI of 1 was sufficient to infect >80% of B16 tumor cells at 24 h after exposure to virus (Figure 1b). A time course of transduction of the normal T cells, compared to B16 tumor cells, as assessed by green fluorescent protein (GFP) expression, indicated a low peak of transduction of OT-I T cells at about 12 h after exposure to VSV-δ51, which rapidly declined, suggesting an abortive viral infection and lack of spread. This was in sharp contrast to the kinetics of infection of the tumor cells (MOI 1), where the cells were transduced and initiated a rapidly spreading infection in culture (approaching 100% of cells expressing GFP by 48 h after exposure to virus) (Figure 1c). Similarly, although robust de novo expression of the VSV surface glycoprotein (VSV-G) glycoprotein was readily detectable following infection of B16, or other tumor cell lines, in vitro, no VSV-G expression was detected in OT-I T cells exposed to VSV-δ51 (Figure 1d). These data suggest that although VSV-δ51 enters both T cells and tumor cells (GFP expression) viral infection and spread is aborted in the normal cells but proceeds efficiently in the tumor cells (de novo VSV-G synthesis).
Figure 1.
Vesicular stomatitis virus (VSV)-δ51 infects OT-I T cells at very low efficiencies. (a, b) Three-day-activated OT-I cells, or (b) B16 tumor cells were exposed to cell-free stocks of VSV-δ51, which encodes a green fluorescent protein (GFP) reporter gene, at MOI of 1, 10 or 100 as shown. Cells were analyzed 24 or 48 h later for GFP expression. (c) Three-day-activated OT-I, or B16 tumor, cells were exposed to cell-free stocks of VSV-δ51 at an MOI of 1. Infected cultures were analyzed at time points shown using flow cytometry for viral-derived GFP expression. The percentage of cells in each culture that were GFP+ are plotted with time. (d) Three-day activated OT-I, or tumor, cells were exposed to cell-free stocks of VSV-δ51 at an MOI of 1. Cell lysates were prepared at the times indicated and probed for expression of VSV-G or β-actin using western blotting.
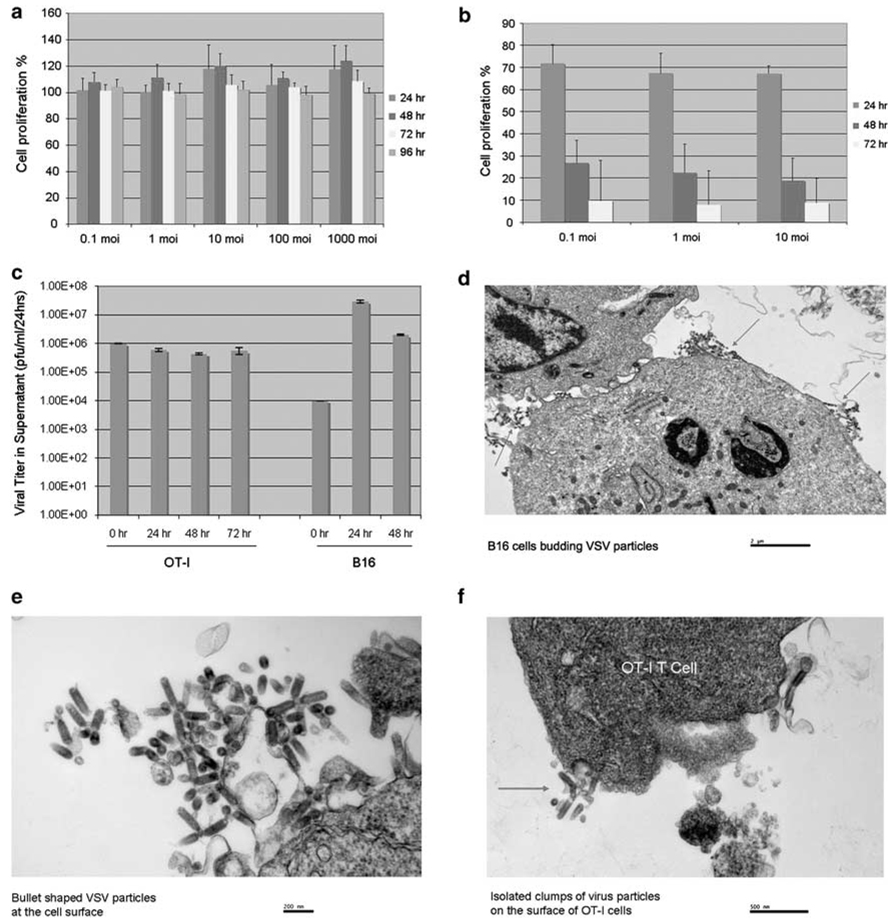
Consistent with a lack of replication of VSV-δ51 in OT-I T cells, no significant cytotoxicity was observed following exposure of the T cells to VSV-δ51 even at remarkably high MOI (Figure 2a). In fact, we observed a small, but consistent increase in the number of OT-I treated with VSV-δ51 compared to unloaded T cells. In contrast, VSV-δ51 was potently cytotoxic to tumor cell lines as expected (Figure 2b). Similarly, although infection of B16 tumor cells with VSV-δ51 led to increases of 3–4 logs in virus yield (over the input virus) as little as 24 h following exposure to virus, virus released into the supernatant from OT-I cells did not exceed the amount of input virus (Figure 2c). Interestingly, however, virus could be detected consistently in the supernatants of OT-I exposed to VSV-δ51 at significant levels through 72 h after exposure (Figure 2c). These observations suggested that either OT-I T cells are supporting very low levels of virus replication or that input virus may initially adhere to the cell surface and then dissociate with time. Consistent with this hypothesis, electron microscopy showed that 24 h after exposure to virus B16 cells were producing plentiful levels of newly synthesized viral particles budding off at all parts of the cell surface (Figures 2d and e); in contrast, OT-I T cells exposed to VSV-δ51 did not show evidence of productive infection since cells were not covered with large amounts of newly budding particles at the surface (Figure 2f) as was the case for B16 cells (Figure 2d); however, localized clusters of bullet-shaped viral particles were still seen at the surface of OT-I cells at higher magnifications (Figure 2f), which may represent either cell-adherent virus from the input stock or low levels of newly generated virus.
Figure 2.
Vesicular stomatitis virus (VSV)-δ51 replicates very poorly, if at all, in OT-I T cells. (a) Three-day-activated OT-I cells were infected at MOI ranging from 0 (control) to 1000. Cell proliferation was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and expressed as a percentage of the proliferation of unloaded OT-I cells. Data shown are the average of six values ± s.d. (b) B16 tumor cells were infected at MOI ranging from 0 (control) to 10. Cell proliferation was measured by MTT assay and expressed as a percentage of the proliferation of uninfected cells. Data shown are the average of six values ± s.d. (c) A total of 106 3-day-activated OT-I, or 104 B16 tumor, cells were infected with VSV-δ51 at an MOI of 1 at t = 0 h. Every 24 h, cell-free supernatants were removed and assayed for viral titers. (d–f) B16 (d, e), or 3-day activated OT-I tumor (f), cells were infected with VSV-δ51 at an MOI of 1. After 24 h, cells were harvested, washed 3 × in phosphate-buffered saline (PBS) at 4 °C and examined by electron microscopy. Red arrows show sites of virus budding (all around the cell surface on B16 cells (d), or virus clusters on OT-I cells (f).
Taken together, these data suggest that although VSV-δ51 does not replicate efficiently, or at all, in OT-I T cells, virus adhered to the cell surface may be retained for several hours before dissociating and becoming available, potentially, for infection of additional cell types.
VSV-δ51 adhered to/released from OT-I T cells can be released to infect tumor cells in vitro
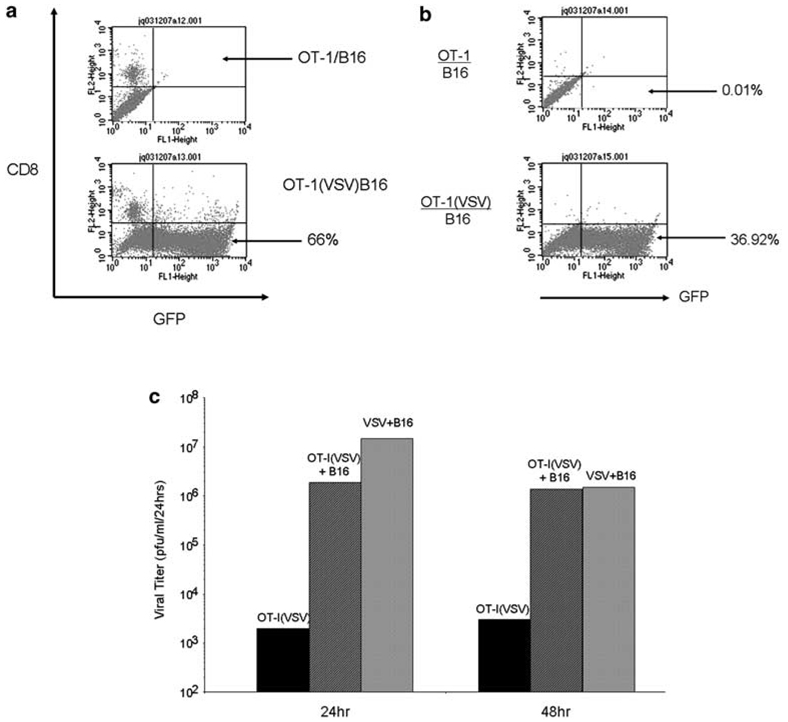
When VSV-δ51-loaded OT-I T cells were cocultured with B16 tumor cells, even at low T-cell:tumor cell ratios, the tumor cells in these cocultures (CD8– cells) became selectively transduced to high levels as assessed by GFP expression from the VSV-δ51 (Figure 3a). In addition, extensive cytotoxicity was induced in B16 cells cocultured with OT-I loaded with VSV-δ51, to levels similar to that produced by cell-free infection with VSV-δ51 (data not shown). Infection of B16 tumor cells was still observed (although less efficiently) if the VSV-δ51-loaded OT-I were separated from the tumor cells by a transwell membrane indicating that tumor cell infection is, at least in part, through the release of virus from the OT-I cells (Figure 3b). Consistent with the results of Figure 2c, incubation of OT-I T cells with VSV-δ51 did not significantly amplify the levels of input virus over time whereas incubation of virus with B16 tumor cells induced a 2–3 log increase in virus produced from the cultures relative to the initial inoculum dose (Figure 3c). However, when virus-loaded OT-I T cells were cocultured with B16 tumor cells, significant levels of virus were produced over and above the levels of input virus, indicating high levels of virus replication in the B16 tumor cells (Figure 3c). Taken together, these data indicate that virus adhered to, or produced from, the OT-I T cells can be released and lead to productive, oncolytic infection of tumor cells brought into close association with the T cells.
Figure 3.
Vesicular stomatitis virus (VSV)-δ51 is passed from OT-I T cells to tumor cells where it replicates freely. (a) OT-I cells, or OT-I cells loaded by VSV at an MOI of 1 (OT-I(VSV)), were cocultured with B16 cells at a ratio of 1:10. B16 cells were used in these experiments instead of B16ova to avoid any confounding results from cytotoxicity of OT-I against B16ova cells. After 24 h, cocultures were harvested and analyzed by flow cytometry for expression of green fluorescent protein (GFP) and CD8. CD8–GFP+ cells indicate expression of GFP from VSV-δ51 in B16, as opposed to OT-I, cells. (b) The experiment of (a) above was repeated except that the B16 cells were plated in the bottom chamber of a transwell plate and the OT-I, or OT-I(VSV), cells were plated in the top chamber (pore size 0.45 µm). After 24 h, the B16 cultures in the bottom chamber were harvested and analyzed by flow cytometry for expression of GFP and CD8. (c) OT-I cells loaded by VSV at an MOI of 1 were cultured alone (OT-I(VSV)) or were cocultured with B16 at a ratio of 1:20 (OT-I(VSV)+B16). Simultaneously, B16 cells were infected with VSV-δ51 at an MOI of 1 (VSV+B16) at t = 0 h. Every 24 h, cell-free supernatants were removed and assayed for viral titers. Data shown are representative of five separate experiments.
VSV loading augments T-cell function and activity in vitro
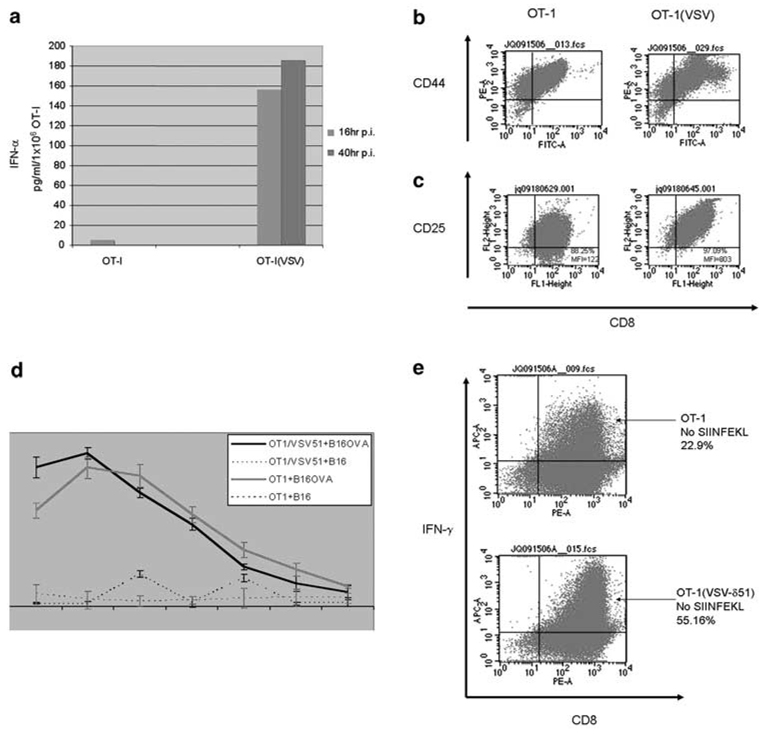
Exposure to VSV-δ51 induced large amounts of both IFN-α (Figure 4a) and IFN-β (data not shown), characteristic of a typical antiviral response to infection of normal cells with VSV. Even though 3-day activated OT-I T cells were already highly activated (grown for 3 days in interleukin (IL)-2 and SIINFEKL antigen), OT-I T cells exposed to VSV-δ51 showed significant further increased expression of T-cell activation markers such as CD44 and CD25 (Figures 4b and c). In vitro studies suggested that VSV-δ51 loading may also enhance the survival of OT-I T cells in culture, as evidenced by modest increases in expression of T-cell survival factors such as BCL-2 (data not shown).
Figure 4.
Vesicular stomatitis virus (VSV)-δ51 loading activates OT-I. (a) A total of 1 × 106 OT-I, or VSV-δ51-loaded OT1 (OT-I(VSV)), were plated in six-well plates. Culture supernatants were harvested at 16 and 40 h postinfection (p.i.), and were assayed by enzyme-linked immunosorbent assay (ELISA) for interferon (IFN)-α. (b, c) OT-I cells were loaded at an MOI of 1 with VSV-δ51 or left unloaded. After 24 h, cells were analyzed by flow cytometry for expression the T-cell activation markers CD44 (b) or CD25 (c) along with CD8. (d) Three-day-activated OT-I, or OT-I cells loaded with VSV-δ51 (MOI 1) were used as effectors against B16 or B16ova targets loaded with Cr51. OT-I cells (effectors) were incubated for only short periods of time with their targets (B16 or B16ova) at the ratios shown, supernatants were harvested and analyzed for Cr51 release. Levels of lysis were relatively low in these assays because of the short period of time over which the assay needed to be conducted to exclude tumor cell lysis as a result of infection by VSV-δ51 released from the loaded OT-I T cells. Thus, control experiments demonstrated that cytotoxicity to B16 or B16ova tumor cells as a result of infection with VSV-δ51 is only significant after 6 h of virus incubation (not shown). Data shown are representative of three separate experiments; bars, s.d. *P<0.05 (VSV/OT1+B16 versus OT1+B16). (e) Three-day activated OT-I cells were loaded at an MOI of 1 with VSV-δ51 or left unloaded. The OT-I cells were grown for a further 24 h in the absence of added SIINFEKL peptide and were analyzed by flow cytometry for intracellular expression of IFN-γ along with CD8.
This increased activation also translated into increased effector function both at the level of direct cytolysis of antigen-expressing B16ova target cells (Figure 4d) and increased expression of IFN-γ over and above that of already activated T cells (Figure 4e). Thus, as expected, OT-I cells grown for short periods of time with B16ova targets induced significantly more killing than with antigen-negative B16 cells (Figure 4d). However, if the OT-I cells were preloaded with VSV-δ51, highly significantly increased killing of B16ova targets was observed relative to that produced by unloaded OT-I, even in the short time periods of this assay as necessitated by the need to exclude any cytotoxicity induced by VSV-mediated tumor cell killing (Figure 4d). In addition, when 3-day activated OT-I cells were grown for a further 24 h in the absence of their cognate-activating antigen (supplied as SIINFEKL peptide in the culture medium), exposure to VSV-δ51 induced even greater levels of intracellular IFN-γ expression compared to OT-I grown without SIINFEKL or VSV-δ51 (Figure 4e). Taken together, these data show that loading of OT-I T cells with VSV-δ51 significantly enhances markers of T-cell activation and effector function, suggesting that they may be more efficient at clearing tumor cells in vivo.
Combination of adoptive T-cell therapy with oncolytic virotherapy in vivo
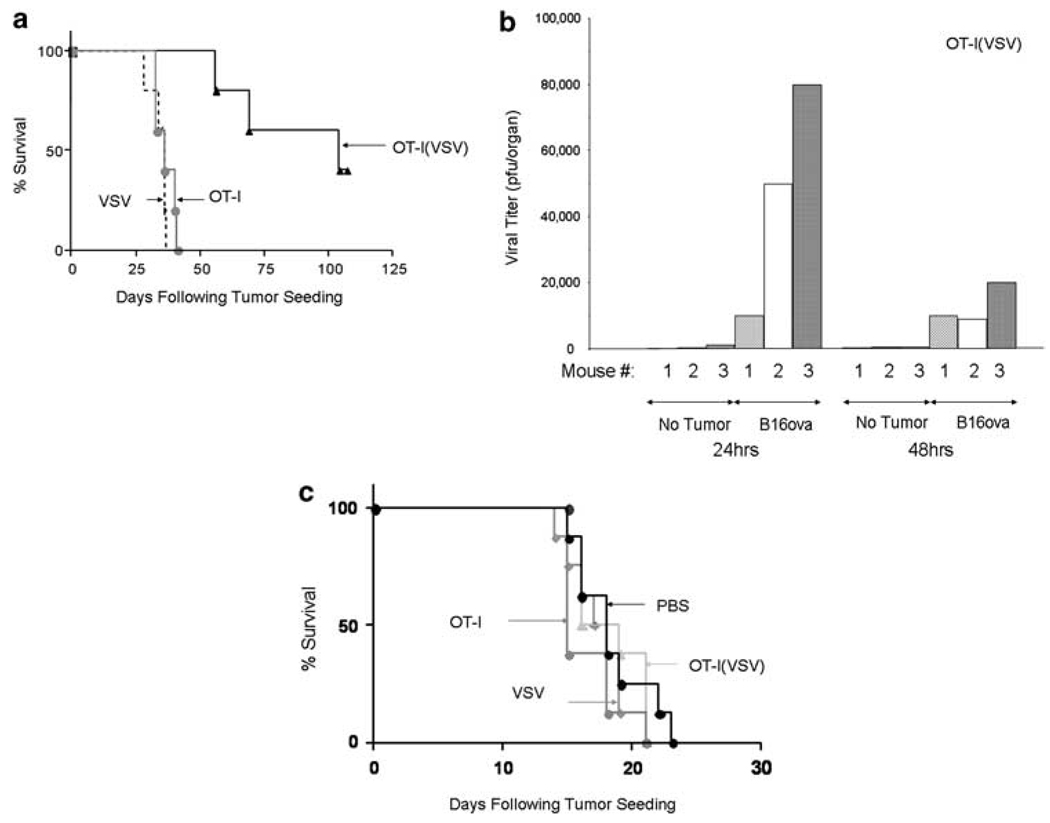
These data were consistent with our hypothesis that it may be possible to use adoptive transfer of antigen-specific T cells to carry oncolytic virus to the site of established tumor growth. To investigate whether such an approach would have therapeutic efficacy in vivo, mice bearing 6-day-established, lung metastases of B16ova were treated with subtherapeutic levels of adoptive T-cell therapy, or i.v. oncolytic virotherapy, alone, which we have previously shown to be completely therapeutically ineffective and equivalent to phosphate-buffered saline (PBS) treatment alone in both C57Bl/6 and nude mice. Alternatively, mice were treated with a combination in which OT-I T cells were loaded with VSV-δ51. Highly significant increases in survival were observed in mice treated with OT-I(VSV) compared to any of the other groups (P = 0.002 compared to OT-I or to VSV alone) (Figure 5a). These therapeutic effects were heavily dependent upon the dose of OT-I(VSV) used, as adoptive transfer of one log fewer VSV-δ51-loaded T cells gave no therapeutic benefit over OT-I alone (not shown). Moreover, 100% of the long-term survivors from this treatment were protected against a rechallenge with a dose of B16ova tumor cells which is lethal to naive mice (data not shown), indicating that the combination of adoptive T-cell-, and oncolytic virotherapy is sufficient to activate antitumor T-cell memory responses (Figure 5a).
Figure 5.
OT-I loaded with vesicular stomatitis virus (VSV)-δ51 treats metastatic B16ova tumors. (a) A total of 3 × 105 B16ova cells were injected i.v. into C57Bl/6 mice (five mice per group). After 6 days, mice were treated i.v. with 2 × 105 untreated OT-I (OT-I), 2 × 105 OT-I loaded with VSV-δ51 at an MOI of 1 (VSV/OT1) or with 2 × 106 PFU of cell-free VSV-δ51 (VSV). Survival with time following tumor seeding is shown. **P = 0.002. Data are representative of three separate experiments. Long-term survivors cured of their tumor burden by treatment with OT-I(VSV) were rechallenged 107 days following the initial tumor challenge with 5 × 105 B16ova tumor cells subcutaneously along with a group of naive C57Bl/6 mice. Whereas all of the naive mice succumbed to tumor by day 28 after challenge, all the long-term survivors remained tumor free. (b, c) C57Bl/6 mice were injected i.v. with either 3 × 105 B16ova cells or with phosphate-buffered saline (PBS). After 2 weeks, mice were treated i.v. with 3 × 106 OT-I loaded with VSV-δ51 at an MOI of 1 (b) or with 3 × 106 PFU of cell-free VSV-δ51 (VSV) (not shown). (c) The experiment of (a) above was repeated except that C57Bl/6 mice (eight per group) were injected i.v. with B16, instead of B16ova, tumor cells to seed tumors that do not express the cognate ova antigen recognized by the adoptively transferred OT-I T cells.
To understand the mechanisms of this therapy, lungs recovered from mice treated 24 h previously with OT-I(VSV) T cells consistently contained 1–2 logs higher levels of VSV-δ51 if they contained preseeded lung tumors, compared to lungs from mice with no tumor (Figure 5b). In addition, virus could still be recovered at high levels 48 h after adoptive T-cell transfer only in lungs of tumor-bearing mice (Figure 5b). We also examined the distribution of VSV-δ51 following T-cell-mediated delivery. Of the organs examined (spleen, brain, liver, kidney, heart, blood, bone marrow), VSV could only be recovered 24 h following adoptive T-cell transfer from two of three spleens of nontumor-bearing mice (mean titer of 7 × 102 PFU). VSV was also recovered from spleens of three of three tumor-bearing mice at a higher mean titer (mean titer of 9 × 103 PFU). We have shown that B16ova tumors seed in the spleen of C57Bl/6 mice using PCR (data not shown and Qiao et al., submitted); therefore, we believe that the increased spleen titers of OT-I-delivered VSV-δ51 are due to viral replication in these splenic tumors that provide a substrate for VSV replication delivered from the OT-I T cells. Interestingly, VSV-δ51 was also detected at titers of less than 103 PFU in the heart and blood of one of three animals with preseeded tumors. We are currently investigating whether these titers also represent replication from tumors seeded in these organs.
When the same experiment was performed with simple i.v. injection of (cell-free) VSV-δ51 virus, there were no significant differences in the amounts of virus that could be recovered from the lungs of tumor-bearing, or tumor-free mice (data not shown), virus could only be recovered from the lungs of one of three tumor-bearing mice and the level of virus in any of these lungs was significantly lower than those obtained following OT-I-mediated delivery of VSV-δ51 to tumor-bearing lungs (data not shown). These data indicate that B16ova tumors provide an effective substrate for viral replication following OT-I-mediated delivery of VSV-δ51 and that the amount of virus that can access these tumors is significantly higher using T-cell-mediated delivery compared to simple i.v. injection. Finally, the therapeutic effects of adoptive transfer of OT-I(VSV) against B16ova tumors (as seen in Figure 5a) were completely lost when adoptive transfer was used to treat B16 target tumors, which do not express the cognate antigen for the T cells (Figure 5c). These data show that tumor antigen-specific virus delivery is achieved using OT-I T cells to carry VSV-δ51.
VSV loading enhances T-cell trafficking
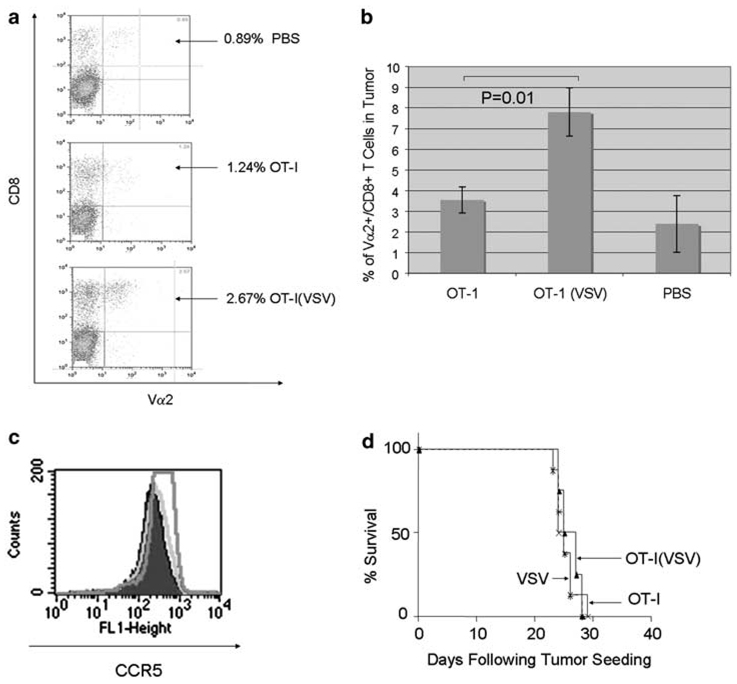
We hypothesized that the combination of the increased T-cell activation induced by the VSV-δ51 loading of the T cells (Figure 4) coupled with initiation of intratumoral viral oncolysis (Figure 5) would establish a proinflammatory environment at the tumor site which might enhance trafficking to the tumor not only of adoptively transferred OT-I T cells but also of endogenous immune effectors.33,36 Previously, we have observed that levels of trafficking of OT-I T cells to antigen-positive (and antigen-negative) tumors were initially very low (upto 24 h postadoptive transfer) but that antigen-specific T cells accumulated over time only in antigen-positive tumors (72–96 h).37 Consistent with these findings, within 24 h of adoptive transfer only small numbers of OT-I T cells (CD8+, Vα-2+) over background were detected within B16ova tumors (Figure 6a). However, more OT-I T cells were consistently detected in the B16ova tumor if those T cells had previously been exposed to VSV-δ51 in vitro (Figure 6a). We also observed very significant increased levels of accumulation of OT-I T cells in tumors at longer periods postadoptive transfer if the OT-I cells had initially been loaded with VSV-δ51 compared to unloaded OT-I T cells (P = 0.01; Figure 6b). Intratumoral accumulation at these later time points most likely reflects increased T-cell survival and proliferation of the adoptively transferred T cells, rather than initial homing efficiencies21,33,37—effects that may be associated with the local environment induced by the ‘hyperactivated’ OT-I cells which are expressing high levels of IFN-α/β and -γ.
Figure 6.
OT-I(VSV) induces increased T-cell trafficking and primes host immunity. (a) B16ova tumors were established subcutaneously in C57Bl/6 mice. After 10 days, mice were treated i.v. with phosphate-buffered saline (PBS), 5 × 106 unloaded OT-I or 5 × 106 OT-I loaded with vesicular stomatitis virus (VSV)-δ51 (MOI 1). Tumors were harvested 24 (a) or 96 h (b) later, dissociated and analyzed by flow cytometry for OT-I T cells, expressed as the percentage of the lymphocytes from the tumors which labeled double positive for both CD8 and the Vα2 T-cell receptor expressed by OT-I transgenic mice. Data shown are representative of three experiments. (c) Three-day activated OT-I (green trace), or OT-I loaded with VSV-δ51 (MOI 1) (pink trace), were analyzed by flow cytometry 24 h following viral loading for expression of CCR5 (pink). Isotype control is the purple trace. (d) A total of 3 × 105 B16ova cells were injected i.v. into nude mice (eight per group). After 6 days, mice were treated i.v. with 2 × 105 untreated OT-I (OT-I), 2 × 105 OT-I loaded with VSV-δ51 at an MOI of 1 (VSV/OT1) or with 2 × 106 PFU of cell-free VSV-δ51 (VSV). Survival with time following tumor seeding is shown. Data are representative of two separate experiments.
Increased tumor trafficking in the short term (24 h) following adoptive T-cell transfer could be explained, at least in part, by the observation that in vitro loading with VSV-δ51 induced higher levels of expression of CCR5, the receptor for CCL5, which has been shown to promote the ability of activated CD8+ effector T cells to infiltrate tumors38 (Figure 6c). However, we did not observe any changes in the levels of CD62L, downregulation of which would also contribute to keeping adoptively transferred T cells out of the lymph nodes and allow for increased tumor homing (data not shown).
OT-I(VSV) adoptive T-cell therapy activates potent endogenous antitumor T-cell responses
Previously, we have shown that activation of endogenous T cells against tumor-associated antigens is enhanced by the establishment of a proinflammatory environment at the tumor site by the initiation of intratumoral viral oncolysis.33 In addition, the high levels of increased effector functions induced by VSV-δ51 loading of OT-I in vitro (increased levels of IFN-α/β, IFN-γ and cytotoxic T lymphocytes (CTL) activity) suggested that intratumoral localization of these ‘hyperactivated’ T cells would further contribute to a proinflammatory environment that activates an endogenous antitumor immune response. To test whether such effects are important in the therapy seen in Figure 5a, we repeated the experiments of Figure 5a in nude mice. We deliberately selected the maximal dose of OT-I T cells that would not give therapy in either immunocompetent C57Bl/6, or nude, mice to be able to observe additive therapeutic benefits from the carriage of VSV by the T cells. Because the minimal therapeutic dose of OT-I that achieves 50% tumor cures of B16ova is about one log higher in C57Bl/6 mice than in nude mice (5 × 105 OT-I), we selected the dose of 2 × 105 OT-I cells which, from our previous studies, shows no efficacy in the nude mouse model. Whereas significant OT-I-mediated delivery of VSV-δ51 in C57Bl/6 immune-competent mice led to very significant increased survival of tumor-bearing mice (Figure 5a), the therapy was completely lost in nude mice lacking T cells (Figure 6d). This result indicates that the host-derived T cells that are activated against tumor by local oncolysis,33 and provide long-term immune memory (Figure 5a), are also critical for the efficacy of direct OT-I(VSV) T-cell-mediated antitumor therapy. Therefore, antitumor therapy induced by OT-I-mediated carriage of VSV (Figure 5a) is clearly not solely due to direct intratumoral virus delivery, replication and oncolysis but also requires multiple interacting factors derived from the host to contribute to tumor clearance.
Discussion
We show here that the tumor homing, and direct tumor cell killing, associated with adoptive transfer of antigen-specific CD8+ T cells13 can be successfully combined with the direct tumor oncolysis1,3,29 and immune stimulation33 associated with oncolytic virotherapy. Our data show that loading VSV-δ51 onto T cells enhances the efficiency of the virotherapy component of the combined therapy by allowing delivery of the virus to systemic tumors at levels that cannot be reached by simple i.v. injection of cell-free virus. However, in addition, it is also clear that virus loading on the T cells enhances the efficacy of the adoptive T-cell therapy component by increasing the effectiveness of the T cells themselves in terms of increased killing activity, effector cytokine production and tumor trafficking. Therefore, the combination of adoptive T-cell therapy with oncolytic virotherapy represents a truly symbiotic relationship in terms of generating improved therapy compared to either approach alone.
Our initial hypothesis underpinning these studies was that OT-I T cells could serve as effective carriers of VSV-δ51 to the tumor site and, following intratumoral T-cell localization, virus would be released to establish a spreading, productive infection of the B16ova tumor cells leading to viral oncolysis and tumor cures. This arose from our previous studies where we showed that activated T cells can carry retroviral particles to tumors through a process of cell surface adhesion of the viral particles.21–23 Here, we have extended that work by replacing the replication-defective retrovirus by oncolytic, replication-competent VSV-δ51.35 Our initial rationale for this change was that an oncolytic, replication-competent virus released at the tumor site in vivo would spread more efficiently through the tumor, and generate higher levels of tumor cell killing than a replication-defective retrovirus encoding a suicide gene.29 We postulated that VSV-δ51 would not replicate well in OT-I cells due to the intact type I IFN response of these cells, which was confirmed by our in vitro studies (Figure 1–Figure 3). This approach differs from that used by others where viruses such as adenovirus,16 measles,18 vaccinia virus17 or VSV-δ51 itself24 have been loaded into cells which are themselves fully permissive for viral replication. In the time that it takes the cell carriers to circulate and localize to tumors, the virus can undergo a replicative cycle leading to high levels of virus release at the tumor site. However, we investigated whether VSV-δ51 would adhere to the cell surface of OT-I T cells in the same way that retroviral particles do21 and, if so, whether it would be released and become available for infection of more permissive tumor cells into which it would be brought in contact with by the trafficking of the T cells in vivo. Both our in vitro (Figure 2) and in vivo (Figure 5) studies confirm that OT-I T-cell carriers can release sufficient VSV-δ51 to initiate spreading viral infections of tumor cells. We cannot exclude that the source of this released virus is in fact very low levels of de novo synthesized VSV particles released by the OT-I, as opposed to cell surface-adhered virus that is released upon the T cells reaching the tumor. However, our in vitro studies clearly show that if OT-I cells do make new virus it is at very low levels. Therefore, we have shown that OT-I T cells can act as effective cell carriers for oncolytic VSV-δ51 particles and that these cells allow significantly higher levels of virus delivery to metastatic tumors in a fully immune-competent host than is achievable by simple i.v. injection of cell-free virus. The importance of virus delivery and replication to the overall therapeutic effects is underscored by the observation that heat-inactivated VSV-δ51, which does not replicate in tumor cells, loaded onto OT-I was ineffective at stimulating tumor cures in the experiments of Figure 5. Therefore, carriage of VSV-δ51 directly to tumors by the OT-I cells forms a major part of the success of this combined therapy.
While we confirmed that the VSV-δ51 released from OT-I cells can eradicate many more tumor cells than the retroviral vector we used in our previous studies,21 we also gained an additional unexpected advantage over the use of retroviral particles in that VSV-δ51 induced very significant increased activation of the OT-I T cells. Our data show that simple loading of OT-I with VSV-δ51 induced expression of proinflammatory cytokines associated with both an antiviral response (IFN-α/β) and T-cell effector functions (IFN-γ). This was accompanied by increased cytolytic function and phenotypic changes associated with better trafficking to tumors and T-cell activation. It seems probable that the increased activation status of these T cells plays a significant role in the enhanced therapy of the OT-I(VSV) treatment group compared to the OT-I treatment alone through multiple pathways including increased trafficking of the T cells to the tumor site (Figure 5), higher levels of direct tumor cell killing by the T cells (Figure 4), enhanced indirect tumor cell killing through cytokine production (Figure 4) and through priming of antitumor host immune responses that are critical for the overall efficacy of the therapy (Figure 6), consistent with our previous studies demonstrating that CD8+ T cells mediate effective antitumor therapy following direct intratumoral injection of VSV into B16 tumors.33
Studies are currently underway in our laboratory to define the molecular mechanisms by which VSV-δ51 activates the OT-I T cells. For example, VSV-G can bind to Toll-like receptors, some of which are expressed by T cells, and that this can lead directly to cell activation.39 In addition, however, we also believe that some of the VSV-δ51 enters the OT-I cell since such induction of the IFN-α/β response (Figure 4) is typical of a cellular antiviral response upon cell infection. We are currently investigating the link between this ongoing antiviral response and the induction of increased T-cell activation (CTL activity and IFN-γ) as a further explanation of the ability of VSV-δ51 to activate T-cell function.
Taken together, our data demonstrate that the loading of antigen-specific T cells with VSV-δ51 leads to enhanced antitumor therapy, compared to T-cell therapy or virotherapy alone. Moreover, we have identified multiple mechanisms for these effects in which the adoptive T-cell therapy augments the efficiency of systemic virotherapy and by which the virus significantly augments the potency of the T cells. Thus, we have shown that T cells chaperone significant levels of virus into tumors leading to productive viral infection replication and oncolysis. In addition to direct tumor cell killing, oncolysis also generates a proinflammatory environment that helps to reverse the immunosuppressive environment of the tumor.33,36 In addition, our data show that viral loading activates the T cells and enhances their ability to traffic to tumors, to kill tumor cells once there and to secrete proinflammatory cytokines that further activate host immune responses against the tumor. Finally, our in vivo data show that all of these factors contribute to the generation of host-derived T-cell responses that are responsible for both ongoing tumor clearance and for the generation of long-term antitumor immune memory. It is highly likely that all of these mechanisms play an important role in generating the significant tumor therapy associated with this combination adoptive T cell and virotherapy, and studies are underway in our laboratory to dissect the roles of each component using mice genetically deficient in various aspects of the antiviral response and T-cell-mediated antitumor effector mechanisms. Overall, our results support a model in which VSV-mediated tumor cell oncolysis is critical to initiate the priming of antitumor host immunity. The results of Figure 6d clearly suggest that it is this immunity that is central to the overall therapeutic efficacy of this approach rather than direct viral oncolysis. Nonetheless, viral oncolysis is still essential to initiate tumor cell killing and the subsequent immune priming that results in tumor clearance.
A major issue for the systemic delivery of oncolytic viruses is the ability to deliver in the presence of preexisting, neutralizing antibody-mediated immunity. Most patients will initially have no preexisting immunity to VSV but following the first treatment with virus such immunity might be expected to develop rapidly. In separate studies, we have shown that VSV particles loaded onto (murine) CD8+ T cells exhibit a ‘threshold of immunological visibility’ (Qiao et al., submitted). Thus, if the viral particles are loaded at high MOI (>10) on the T cells, delivery and antitumor therapy is inhibited in preimmune mice and naive mice are very effectively immunized against VSV following T-cell (VSV) administration. In contrast, if the viral particles are loaded at low MOI (<1) onto the T cells, antitumor therapy is not inhibited and the loaded T cells are not effective at raising anti-VSV immunity in naive mice. These results suggest that repeat administrations of VSV-loaded T-cell therapy may be possible in patients in which the level of viral loading is optimized to balance effective therapy with minimal immune stimulation/neutralization by the T-cell carriers.
From a clinical perspective, we have used the B16ova/OT-I model in these studies as a model of adoptive T-cell therapy. It is clear that this is an idealized model, especially from the point of view of the transgenic source of the T cells, and the excellent specificity of the OT-I T cells for their target antigen. Indeed, the recovery, expansion and adoptive transfer of T cells with widely varying tumor affinities, and specificities, from patients remains a procedure that is limited to a few centers with great expertise in the area.13,14 Nonetheless, protocols using adoptive transfer have shown great promise in the treatment of malignant melanoma,12,14 and novel technologies are being developed to expand these treatments both using T cells with more specific and better antigen specificities and into other tumor types.14,40 The addition of a simple preincubation step with an oncolytic virus, such as VSV-δ51, would be clinically simple and, from the data reported here, would recruit the advantages of oncolytic virotherapy, as well as improved T-cell therapy, to an already potentially therapeutic treatment. Further studies on the systemic distribution/toxicity of VSV-δ51 following adoptive T-cell transfer are needed, although our data are encouraging that T-cell-associated virus does not cause overt toxicities.
In summary, we have shown that adoptive T-cell therapy can be combined with oncolytic virotherapy, in a manner in which both individual modalities significantly enhance the efficacy of the other, to generate highly significant therapeutic benefits over either alone. Despite the technical difficulties associated with the isolation, expansion and use of antigen-specific T cells for cancer immunotherapy, simple preincubation of oncolytic virus with these cells offers potential for improved adoptive T-cell therapies, as well as provides a potential outlet for the use of oncolytic virotherapy in a truly systemic context.
Materials and methods
Cells and viruses
B16 cells are murine melanoma cells described previously. 21,41 B16ova (H-2Kb) were derived from B16 cells stably transduced by a cDNA encoding the chicken ovalbumin gene.41 HT1080 cells are human fibrosarcoma (ATCC CCL-121). The cells were cultured with Dulbecco’s modified Eagle’s minimal essential medium (Life Technologies, Rockville, MD, USA) supplemented with 10% fetal calf serum (Life Technologies). B16ova cells were cultured in the presence of G418 (5 mg ml−1) as described previously.41 All cell lines were monitored routinely and found to be free of Mycoplasma infection.
VSV-δ51 (VSV) is a recombinant VSV with the deletion of methionine residue at position 51 of the viral matrix protein, with the GFP gene inserted between G and L sequences as previously described.31 Viruses were grown on Vero cell monolayers. Supernatants containing progeny virus were harvested, concentrated and purified by sucrose gradient centrifugation. Viruses were titrated by standard plaque assays of serially diluted samples on BHK cells, and aliquot viruses were stored at −80 °C as described in Diaz et al.33
Mice
Athymic nu/nu mice and C57Bl/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) at 6–8 weeks of age. The OT-I mouse strain was on a C57Bl background (H-2Kb) and expressed a transgenic T-cell receptor Vα2 specific for the SIINFEKL peptide of ovalbumin in the context of MHC class I, H2-Kb.34
Preparation of activated OT-I cells
Preparation of activated OT-I cells was as described previously.21 Briefly, naive OT-I cells were isolated from spleen and lymph nodes. Red blood cells were lysed by ACK buffer (0.15 m NH4Cl, 1.0 mm KHCO3, 0.1 mm EDTA, pH 7.2–7.4), and the dissociated single cell suspension was grown in Iscove’s modified Dulbecco’s medium plus 5% fetal bovine serum in the presence of 1 µg ml−1 SIINFEKL peptide, 50 µm β-mercaptoethanol, 1% Pen/Strep and 50 IU rIL-2 ml−1. Cells were harvested and purified through the centrifugation in lympholyte-M density gradient (Cedarlane, Burlington, NC, USA), 3 days after activation. The cells were used for in vivo injection or for in vitro assays. These populations consisted typically of >98% CD8+ T cells, of which >90% of the cells expressed the Vα2 chain of the transgenic OT-I T-cell receptor.
In vitro loading of OT-I T cells with VSV
Activated OT-I T cells were infected by VSV at the relevant MOI for 3 h at 37 °C. Virus-loaded OT-I cells were washed 3 × in PBS at 4 °C and used for in vivo transfer or plated in culture for in vitro assays at the times indicated.
Viral transfer assay in vitro
Activated OT-I cells were loaded as described above. After 3 h, OT-I T cells were cultured with B16, or other types of tumor, cells at ratios indicated. At the times indicated, culture supernatants were harvested and centrifuged to discard cell debris for viral titration. In parallel, viral-loaded OT-I or infected tumor cells, or unloaded OT-I cocultured with tumor cells, were incubated as controls of viral transfer, vector expression and replication in the tumor cells.
Antibodies, reagents and flow cytometry
Anti-CD8b phycoerythrin (PE), Allophycocyanin (APC), and fluorescein isothiocyanate (FITC), anti-CD44 PE, anti-CD25 PE, anti-CCR5 FITC, anti-Vα2 PE, anti-Bcl2 FITC, anti-IFN-γ and the isotype controls were purchased from BD Pharmingen (San Diego, CA, USA). ELISA Kit for mouse IFN-α was purchased from PBL Biomedical Laboratories (Piscataway, NJ, USA). BD Cytofix/Cytoperm Kit was purchased from BD Pharmingen. MTT Kit for cell proliferation was purchased from Roche Applied Science (Indianapolis, IN, USA). The immunodominant SIINFEKL epitope of the ova antigen was synthesized at the Mayo Molecular Biology Core Facility. For flow cytometry, fluorescence data were collected on FACScan apparatus and analyzed using CellQuest software.
Intracellular staining for Bcl2 or IFN-γ
OT-I or VSV-loaded OT-I cells were first stained by anti-CD8b PE antibody. Cells were then permeabilized and washed, and then stained with anti-Bcl2 FITC or anti-IFN-γ APC antibodies according to Cytofix/Cytoperm Kit instructions. Cells were analyzed by flow cytometry as described above.
Chromium51 release assay
A total of 5 × 106 B16, or B16ova, targets were labeled with 100 µCi of Cr51 for 90 min at 37 °C, washed three times with media and resuspended at 5 × 105 cells per ml. Targets were plated at 5 × 104 cells per well with effector cells (OT-I or OT-I(VSV)) at different ratios in 96-well U-bottom plates. Cocultures were incubated for 4 h at 37 °C in 5% CO2, as control experiments had previously demonstrated that cytotoxicity to B16 or B16ova tumor cells as a result of infection with VSV-δ51 is only significant after 6 h of virus incubation (not shown); 35 µl of coculture supernatants were then collected and added to LumaPlates (PerkinElmer, Shelton, CT, USA). Plates were allowed to dry at room temperature overnight before reading. Percentage of lysis was determined as: % lysis = ((sample lysis–spontaneous)/(maximum lysis–spontaneous)) × 100.
In vivo studies
All procedures were approved by the Mayo Foundation Institutional Animal Care and Use Committee. Mice were age and sex matched. To establish subcutaneous tumors, 5 × 105 B16ova cells in 100 µl of PBS were injected into the flank of mice. To establish systemic lung metastatic disease, C57Bl/6 mice or nude mice were injected i.v. with 3 × 105 B16ova cells in 100 µl of PBS. Animals were randomly assigned to different groups before treatments. Tumors were measured with a caliper three times weekly. Mice with metastatic lung metastases were examined daily, and were killed at the first sign of any distress.
Statistics
Survival data from the animal studies were analyzed by the log-rank test, and two-sample unequal variance Student’s t-test analysis was applied for in vitro assays and adoptively transferred OT-I cell trafficking analysis. Statistical significance was determined at the level of P<0.05.
Acknowledgements
We thank Toni L Higgins for expert secretarial assistance. This work was supported by the Mayo Foundation and by NIH Grant CA RO1107082-02. JQ is supported by a grant from the Susan G Komen Breast Cancer Foundation.
References
- 1.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15:651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 2.Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 3.Bell JC. Oncolytic viruses: what’s next? Curr Cancer Drug Targets. 2007;7:127–131. doi: 10.2174/156800907780058844. [DOI] [PubMed] [Google Scholar]
- 4.Fisher K. Striking out at disseminated metastases: the systemic delivery of oncolytic viruses. Curr Opin Mol Ther. 2006;8:301–313. [PubMed] [Google Scholar]
- 5.Harrington K, Vile R. Virus smuggling, tax evasion and tumor assassination. Nat Med. 2006;12:507–509. doi: 10.1038/nm0506-507. [DOI] [PubMed] [Google Scholar]
- 6.Pizzato M, Blair ED, Fling M, Kopf J, Tomassetti A, Weiss RA, et al. Evidence for nonspecific adsorption of targeted retrovirus vector particles to cells. Gene Therapy. 2001;8:1088–1096. doi: 10.1038/sj.gt.3301494. [DOI] [PubMed] [Google Scholar]
- 7.Alemany R, Suzuki K, Curiel DT. Blood clearance rates of adenovirus type 5 in mice. J Gen Virol. 2000;81(Part 11):2605–2609. doi: 10.1099/0022-1317-81-11-2605. [DOI] [PubMed] [Google Scholar]
- 8.Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci USA. 2006;103:12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol. 2005;79:7478–7491. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrington K, Alvarez-Vallina L, Crittenden M, Gough M, Chong H, Diaz RM, et al. Cells as vehicles for cancer gene therapy: the missing link between targeted vectors and systemic delivery? Hum Gene Ther. 2002;13:1263–1280. doi: 10.1089/104303402760128504. [DOI] [PubMed] [Google Scholar]
- 11.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–676. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yotnda P, Savoldo B, Charlet-Berguerand N, Rooney C, Brenner M. Targeted delivery of adenoviral vectors by cytotoxic T cells. Blood. 2004;104:2272–2280. doi: 10.1182/blood-2003-11-3803. [DOI] [PubMed] [Google Scholar]
- 17.Thorne SH, Negrin RS, Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy. Science. 2006;311:1780–1784. doi: 10.1126/science.1121411. [DOI] [PubMed] [Google Scholar]
- 18.Ong HT, Hasegawa K, Dietz AB, Russell SJ, Peng KW. Evaluation of T cells as carriers for systemic measles virotherapy in the presence of antiviral antibodies. Gene Therapy. 2007;14:324–333. doi: 10.1038/sj.gt.3302880. [DOI] [PubMed] [Google Scholar]
- 19.Crittenden M, Gough M, Chester J, Kottke T, Thompson J, Ruchatz A, et al. Pharmacologically regulated production of targeted retrovirus from T cells for systemic anti-tumor gene therapy. Can Res. 2003;63:3178–3180. [PubMed] [Google Scholar]
- 20.Chester J, Ruchatz A, Gough M, Crittenden M, Chong H, Loic-Cosset F, et al. Tumor antigen-specific induction of transcriptionally targeted retroviral vectors from chimeric immune receptor-modified T cells. Nat Biotechnol. 2002;20:256–263. doi: 10.1038/nbt0302-256. [DOI] [PubMed] [Google Scholar]
- 21.Cole C, Qiao J, Kottke T, Diaz RM, Ahmed A, Sanchez-Perez L, et al. Tumor-targeted, systemic delivery of therapeutic viral vectors using hitchhiking on antigen-specific T cells. Nat Med. 2005;11:1073–1081. doi: 10.1038/nm1297. [DOI] [PubMed] [Google Scholar]
- 22.Kottke T, Qiao J, Diaz RM, Ahmed A, Vroman B, Thompson J, et al. The perforin-dependent immunological synapse allows T-cell activation-dependent tumor targeting by MLV vector particles. Gene Therapy. 2006;13:1166–1177. doi: 10.1038/sj.gt.3302722. [DOI] [PubMed] [Google Scholar]
- 23.Thanarajasingam U, Sanz L, Diaz RM, Qiao J, Sanchez-Perez L, Kottke T, et al. Delivery of CCL-21 to metastatic disease improves the efficacy of adoptive T-cell therapy. Can Res. 2007;67:300–308. doi: 10.1158/0008-5472.CAN-06-1017. [DOI] [PubMed] [Google Scholar]
- 24.Power AT, Wang J, Falls TJ, Paterson JM, Parato KA, Lichty BD, et al. Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity. Mol Ther. 2007;15:123–130. doi: 10.1038/sj.mt.6300039. [DOI] [PubMed] [Google Scholar]
- 25.Jevremovic D, Gulati R, Hennig I, Diaz RM, Cole C, Kleppe LS, et al. Use of blood outgrowth endothelial cells as virus-producing vectors for gene delivery to tumors. Am J Physiology. 2004;287:H494–H500. doi: 10.1152/ajpheart.00064.2004. [DOI] [PubMed] [Google Scholar]
- 26.Gomez-Navarro J, Contreras JL, Arafat W, Jiang XL, Krisky D, Oligino T, et al. Genetically modified CD34+ cells as cellular vehicles for gene delivery into areas of angiogenesis in a rhesus model. Gene Therapy. 2000;7:43–52. doi: 10.1038/sj.gt.3301054. [DOI] [PubMed] [Google Scholar]
- 27.Komarova S, Kawakami Y, Stoff-Khalili MA, Curiel DT, Pereboeva L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol Canc Ther. 2006;5:755–766. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- 28.Rooney CM. Hitchhiker’s guide to the T cell. Nat Med. 2005;11:1051–1052. doi: 10.1038/nm1005-1051. [DOI] [PubMed] [Google Scholar]
- 29.Kirn D, Martuza RL, Zwiebel J. Replication-selective virotherapy for cancer: biological principles, risk management and future directions. Nat Med. 2001;7:781–787. doi: 10.1038/89901. [DOI] [PubMed] [Google Scholar]
- 30.Stojdl DF, Lichty BD, Knowles S, Marius R, Atkins H, Sonenberg N, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 31.Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 32.Lichty BD, Stojdl DF, Taylor RA, Miller L, Frenkel I, Atkins H, et al. Vesicular stomatitis virus: a potential therapeutic virus for the treatment of hematologic malignancy. Human Gene Ther. 2004;15:821–831. doi: 10.1089/hum.2004.15.821. [DOI] [PubMed] [Google Scholar]
- 33.Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J, Thompson J, et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- 34.Hogquist KA, Jameson SC, Health WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonistic peptides induce positive selection. Cell. 1994;76:17. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 35.Lichty BD, Power AT, Stojdl DF, Bell JC. Vesicular stomatitis virus: re-inventing the bullet. Trends Mol Med. 2004;10:210–216. doi: 10.1016/j.molmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Breitbach CJ, Paterson JM, Lemay CG, Falls TJ, McGuire A, Parato KA, et al. Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol Ther. 2007;15:1686–1693. doi: 10.1038/sj.mt.6300215. [DOI] [PubMed] [Google Scholar]
- 37.Sanchez-Perez L, Gough M, Qiao J, Thanarajasingam U, Kottke T, Ahmed A, et al. Synergy of adoptive T-cell therapy with intratumoral suicide gene therapy is mediated by host NK cells. Gene Therapy. 2007;14:998–1009. doi: 10.1038/sj.gt.3302935. [DOI] [PubMed] [Google Scholar]
- 38.Lavergne E, Combadiere C, Iga M, Boissonnas A, Bonduelle O, Maho M, et al. Intratumoral CC chemokine ligand 5 over-expression delays tumor growth and increases tumor cell infiltration. J Immunol. 2004;173:3755–3762. doi: 10.4049/jimmunol.173.6.3755. [DOI] [PubMed] [Google Scholar]
- 39.Georgel P, Jiang Z, Kunz S, Janssen E, Mols J, Hoebe K, et al. Vesicular stomatitis virus glycoprotein G activates a specific antiviral Toll-like receptor 4-dependent pathway. Virology. 2007;362:304–313. doi: 10.1016/j.virol.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 40.Cohen CJ, Zheng Z, Bray R, Zhao Y, Sherman LA, Rosenberg SA, et al. Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR. J Immunol Methods. 2005;175:5799–5808. doi: 10.4049/jimmunol.175.9.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linardakis E, Bateman A, Phan V, Ahmed A, Gough M, Olivier K, et al. Enhancing the efficacy of a weak allogeneic melanoma vaccine by viral fusogenic membrane glycoprotein-mediated tumor cell-tumor cell fusion. Can Res. 2002;62:5495–5504. [PubMed] [Google Scholar]