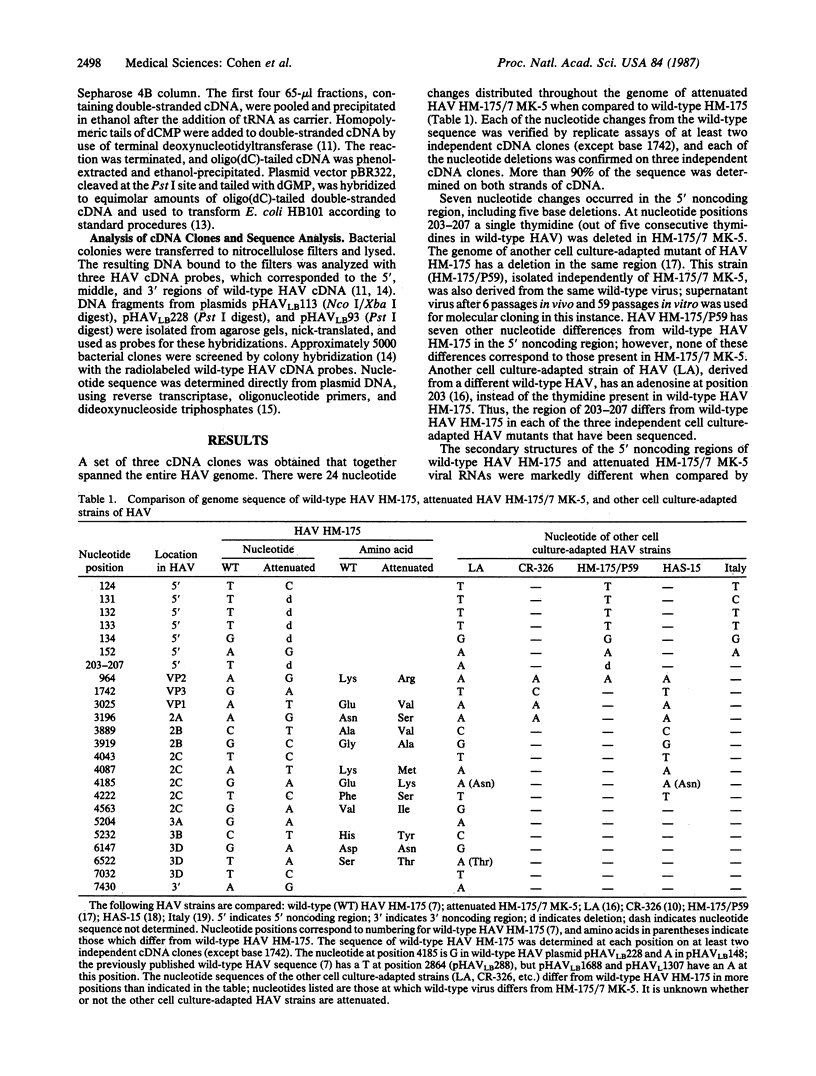
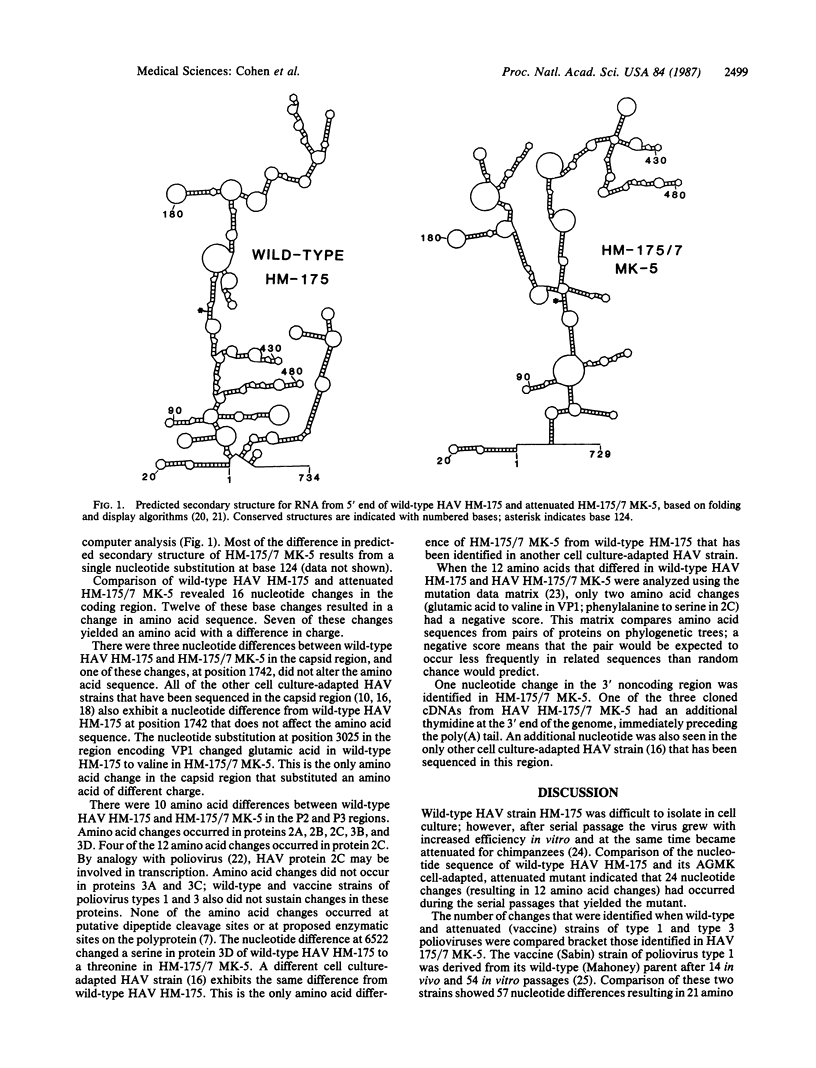
Abstract
The complete nucleotide sequence of an attenuated hepatitis A virus, HAV HM-175/7 MK-5, was determined from cloned cDNA. This virus was derived from wild-type HAV HM-175 after 32 passages in African green monkey kidney cells. The resultant cell culture-adapted virus is attenuated for chimpanzees. This virus was passaged an additional three times in monkey kidney cells to obtain sufficient virus for molecular cloning and was designated HM-175/7 MK-5. Three overlapping cDNA clones were obtained that together spanned the entire genome. Comparison of the nucleotide sequence of cDNA from wild-type virus (propagated in marmoset liver in vivo) with attenuated virus (grown in cell culture) showed 24 nucleotide changes distributed throughout the genome. Five base deletions occurred in the 5' noncoding region, and 12 of the 16 base substitutions in the coding region resulted in amino acid changes. Amino acid changes occurred in viral capsid proteins VP1 and VP2 and several of the nonstructural proteins. Thus, a small number of nucleotide changes are responsible for adaptation to cell culture and attenuation of HAV strain HM-175.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binn L. N., Bancroft W. H., Lemon S. M., Marchwicki R. H., LeDuc J. W., Trahan C. J., Staley E. C., Keenan C. M. Preparation of a prototype inactivated hepatitis A virus vaccine from infected cell cultures. J Infect Dis. 1986 Apr;153(4):749–756. doi: 10.1093/infdis/153.4.749. [DOI] [PubMed] [Google Scholar]
- Cohen J. I., Ticehurst J. R., Purcell R. H., Buckler-White A., Baroudy B. M. Complete nucleotide sequence of wild-type hepatitis A virus: comparison with different strains of hepatitis A virus and other picornaviruses. J Virol. 1987 Jan;61(1):50–59. doi: 10.1128/jvi.61.1.50-59.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daemer R. J., Feinstone S. M., Gust I. D., Purcell R. H. Propagation of human hepatitis A virus in African green monkey kidney cell culture: primary isolation and serial passage. Infect Immun. 1981 Apr;32(1):388–393. doi: 10.1128/iai.32.1.388-393.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. M., Dunn G., Minor P. D., Schild G. C., Cann A. J., Stanway G., Almond J. W., Currey K., Maizel J. V., Jr Increased neurovirulence associated with a single nucleotide change in a noncoding region of the Sabin type 3 poliovaccine genome. Nature. 1985 Apr 11;314(6011):548–550. doi: 10.1038/314548a0. [DOI] [PubMed] [Google Scholar]
- Feinstone S. M., Daemer R. J., Gust I. D., Purcell R. H. Live attenuated vaccine for hepatitis A. Dev Biol Stand. 1983;54:429–432. [PubMed] [Google Scholar]
- Francis D. P., Hadler S. C., Prendergast T. J., Peterson E., Ginsberg M. M., Lookabaugh C., Holmes J. R., Maynard J. E. Occurrence of hepatitis A, B, and non-A/non-B in the United States. CDC sentinel county hepatitis study I. Am J Med. 1984 Jan;76(1):69–74. doi: 10.1016/0002-9343(84)90752-6. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogle J. M., Chow M., Filman D. J. Three-dimensional structure of poliovirus at 2.9 A resolution. Science. 1985 Sep 27;229(4720):1358–1365. doi: 10.1126/science.2994218. [DOI] [PubMed] [Google Scholar]
- Jacobson A. B., Good L., Simonetti J., Zuker M. Some simple computational methods to improve the folding of large RNAs. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):45–52. doi: 10.1093/nar/12.1part1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandolf R., Hofschneider P. H. Molecular cloning of the genome of a cardiotropic Coxsackie B3 virus: full-length reverse-transcribed recombinant cDNA generates infectious virus in mammalian cells. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4818–4822. doi: 10.1073/pnas.82.14.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Linemeyer D. L., Menke J. G., Martin-Gallardo A., Hughes J. V., Young A., Mitra S. W. Molecular cloning and partial sequencing of hepatitis A viral cDNA. J Virol. 1985 May;54(2):247–255. doi: 10.1128/jvi.54.2.247-255.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto A., Omata T., Toyoda H., Kuge S., Horie H., Kataoka Y., Genba Y., Nakano Y., Imura N. Complete nucleotide sequence of the attenuated poliovirus Sabin 1 strain genome. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5793–5797. doi: 10.1073/pnas.79.19.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata T., Kohara M., Kuge S., Komatsu T., Abe S., Semler B. L., Kameda A., Itoh H., Arita M., Wimmer E. Genetic analysis of the attenuation phenotype of poliovirus type 1. J Virol. 1986 May;58(2):348–358. doi: 10.1128/jvi.58.2.348-358.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provost P. J., Bishop R. P., Gerety R. J., Hilleman M. R., McAleer W. J., Scolnick E. M., Stevens C. E. New findings in live, attenuated hepatitis A vaccine development. J Med Virol. 1986 Oct;20(2):165–175. doi: 10.1002/jmv.1890200208. [DOI] [PubMed] [Google Scholar]
- Provost P. J., Hughes J. V., Miller W. J., Giesa P. A., Banker F. S., Emini E. A. An inactivated hepatitis A viral vaccine of cell culture origin. J Med Virol. 1986 May;19(1):23–31. doi: 10.1002/jmv.1890190105. [DOI] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Molecular cloning of poliovirus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4887–4891. doi: 10.1073/pnas.78.8.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B. C., Anderson B. N., Coulepis A. G., Chenoweth M. P., Gust I. D. Molecular cloning of cDNA from hepatitis A virus strain HM-175 after multiple passages in vivo and in vitro. J Gen Virol. 1986 Aug;67(Pt 8):1741–1744. doi: 10.1099/0022-1317-67-8-1741. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Arnold E., Erickson J. W., Frankenberger E. A., Griffith J. P., Hecht H. J., Johnson J. E., Kamer G., Luo M., Mosser A. G. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985 Sep 12;317(6033):145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- Shapiro B. A., Maizel J., Lipkin L. E., Currey K., Whitney C. Generating non-overlapping displays of nucleic acid secondary structure. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):75–88. doi: 10.1093/nar/12.1part1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanway G., Cann A. J., Hauptmann R., Hughes P., Clarke L. D., Mountford R. C., Minor P. D., Schild G. C., Almond J. W. The nucleotide sequence of poliovirus type 3 leon 12 a1b: comparison with poliovirus type 1. Nucleic Acids Res. 1983 Aug 25;11(16):5629–5643. doi: 10.1093/nar/11.16.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanway G., Hughes P. J., Mountford R. C., Reeve P., Minor P. D., Schild G. C., Almond J. W. Comparison of the complete nucleotide sequences of the genomes of the neurovirulent poliovirus P3/Leon/37 and its attenuated Sabin vaccine derivative P3/Leon 12a1b. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1539–1543. doi: 10.1073/pnas.81.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton J. T., Jansen R., Lemon S. M. Neutralizing antibody to hepatitis A virus in immune serum globulin and in the sera of human recipients of immune serum globulin. Gastroenterology. 1985 Sep;89(3):637–642. doi: 10.1016/0016-5085(85)90462-7. [DOI] [PubMed] [Google Scholar]
- Takegami T., Semler B. L., Anderson C. W., Wimmer E. Membrane fractions active in poliovirus RNA replication contain VPg precursor polypeptides. Virology. 1983 Jul 15;128(1):33–47. doi: 10.1016/0042-6822(83)90316-1. [DOI] [PubMed] [Google Scholar]
- Ticehurst J. R., Racaniello V. R., Baroudy B. M., Baltimore D., Purcell R. H., Feinstone S. M. Molecular cloning and characterization of hepatitis A virus cDNA. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5885–5889. doi: 10.1073/pnas.80.19.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venuti A., Di Russo C., del Grosso N., Patti A. M., Ruggeri F., De Stasio P. R., Martiniello M. G., Pagnotti P., Degener A. M., Midulla M. Isolation and molecular cloning of a fast-growing strain of human hepatitis A virus from its double-stranded replicative form. J Virol. 1985 Nov;56(2):579–588. doi: 10.1128/jvi.56.2.579-588.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



