Abstract
We synthesized a series of octapeptide analogs of somatostatin, containing N-terminal tryptophan or another amino acid followed by the hexapeptide sequences Cys-Phe-D-Trp-Lys-Thr-Cys or Cys-Tyr-D-Trp-Lys-Val-Cys and a C-terminal threoninamide or tryptophanamide. After purification by HPLC, the inhibitory activities of these analogs on the release of growth hormone (somatotropin) in rats were determined in vivo. The eight octapeptides with an N-terminal tryptophan residue were found to have a greater inhibitory effect than somatostatin. The most potent of these analogs, D-Trp-Cys-Phe-D-Trp-Lys-Thr-Cys-Thr-NH2, was 94.3 times more active than somatostatin. The other analogs, in order of decreasing potency, were Ac-Trp-Cys-Phe-D-Trp-Lys-Thr-Cys-Thr-NH2, D-Trp(For)-Cys-Phe-D-Trp-Lys-Thr-Cys-Thr-NH2, D-Trp-Cys-Tyr-D-Trp-Lys-Val-Cys-Thr-NH2, Ac-Trp(For)-Cys-Phe-D-Trp-Lys-Thr-Cys-Thr-NH2, Ac-Trp-Cys-Tyr-D-Trp-Lys-Val-Cys-Thr-NH2, D-Trp-Cys-Phe-D-Trp-Lys-Thr-Cys-Trp-NH2, and D-Trp-Cys-Tyr-D-Trp-Lys-Val-Cys-Trp-NH2. The growth hormone inhibitory activity of these analogs was from 53.7 to 11.6 times greater than that of somatostatin. The octapeptides containing D- or L-tryptophan at the N-terminus, phenylalanine at position 3, and threonine at position 6 exhibited a greater inhibitory effect on growth hormone release than that of the analogs with tyrosine and valine at positions 3 and 6, respectively. Substitution of D-tryptophan for D-phenylalanine at the N-terminus in the octapeptide containing phenylalanine in the third, threonine in the sixth, and threoninamide in the C-terminal position also increased the growth hormone-release inhibitory activity. Time-course assay showed that D-Trp-Cys-Phe-D-Trp-Lys-Thr-Cys-Thr-NH2 (RC-98-I), in a dose of 1 microgram/kg of body weight, inhibited the release of growth hormone for at least 3 hr. In view of their high activity and prolonged duration of action, some of these analogs could be useful clinically.
Full text
PDF
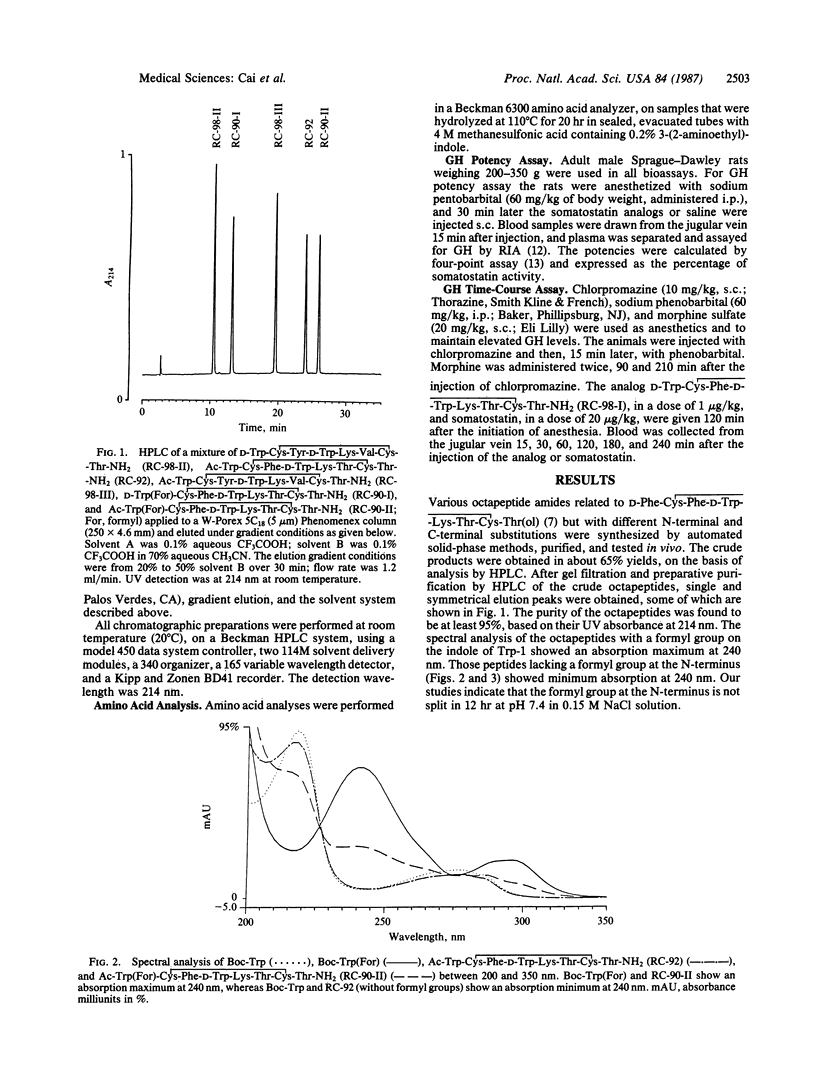
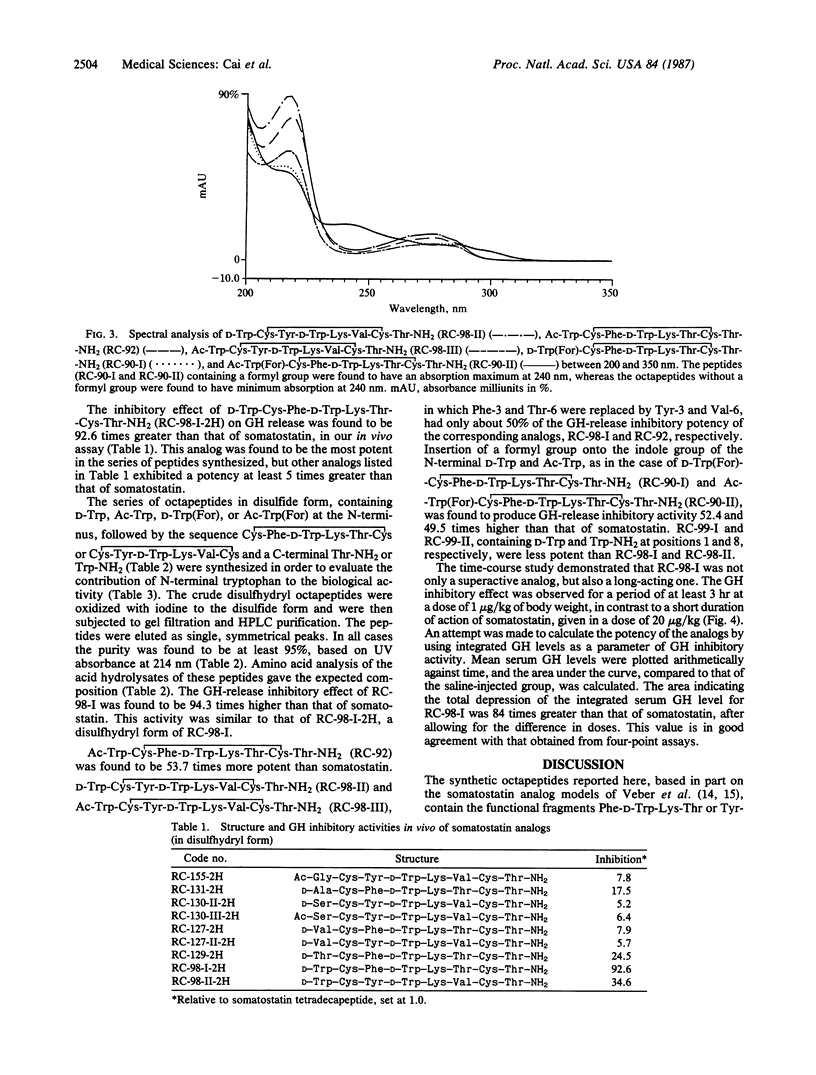
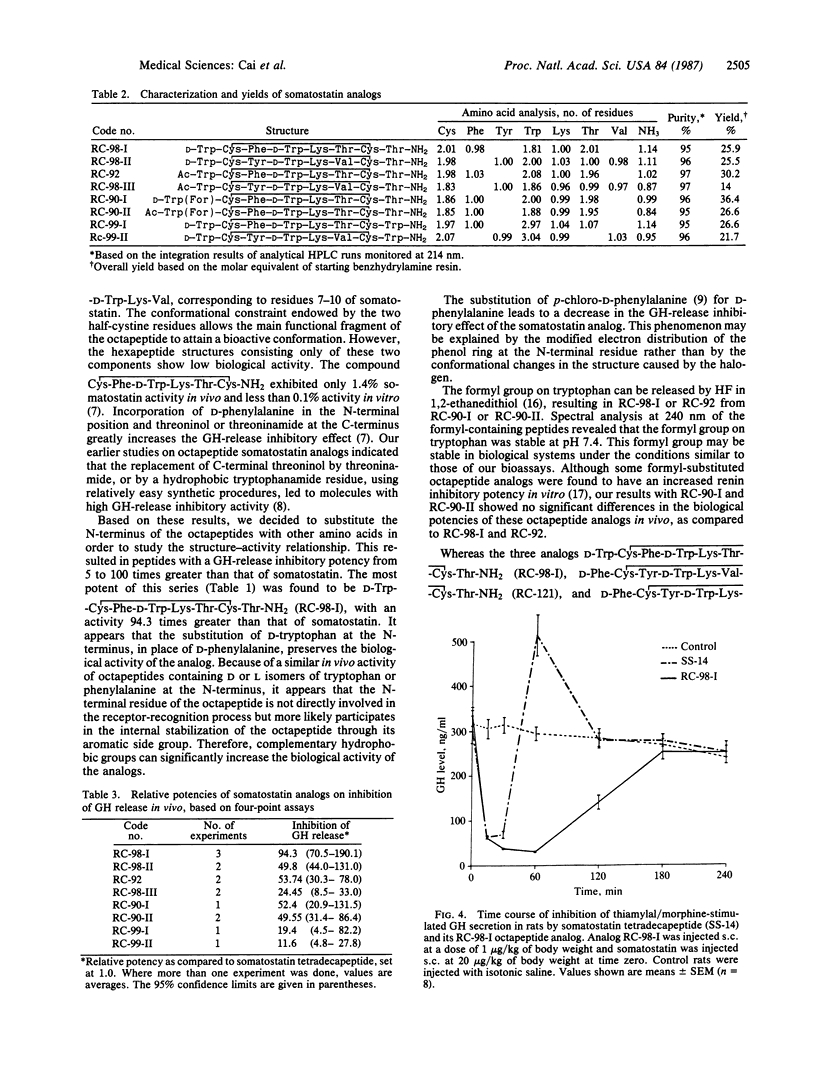

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W., Briner U., Doepfner W., Haller R., Huguenin R., Marbach P., Petcher T. J., Pless SMS 201-995: a very potent and selective octapeptide analogue of somatostatin with prolonged action. Life Sci. 1982 Sep 13;31(11):1133–1140. doi: 10.1016/0024-3205(82)90087-x. [DOI] [PubMed] [Google Scholar]
- Brown M., Rivier J., Vale W. Somatostatin: analogs with selected biological activities. Science. 1977 Jun 24;196(4297):1467–1469. doi: 10.1126/science.867045. [DOI] [PubMed] [Google Scholar]
- Cai R. Z., Szoke B., Lu R., Fu D., Redding T. W., Schally A. V. Synthesis and biological activity of highly potent octapeptide analogs of somatostatin. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1896–1900. doi: 10.1073/pnas.83.6.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers C. A., Murphy W. A., Redding T. W., Coy D. H., Schally A. V. Synthesis and biological actions of prosomatostatin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6171–6174. doi: 10.1073/pnas.77.10.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W. A., Heiman M. L., Lance V. A., Mezo I., Coy D. H. Octapeptide analogs of somatostatin exhibiting greatly enhanced in vivo and in vitro inhibition of growth hormone secretion in the rat. Biochem Biophys Res Commun. 1985 Nov 15;132(3):922–928. doi: 10.1016/0006-291x(85)91895-9. [DOI] [PubMed] [Google Scholar]
- Murphy W. A., Meyers C. A., Coy D. H. Potent, highly selective inhibition of growth hormone secretion by position 4 somatostatin analogs. Endocrinology. 1981 Aug;109(2):491–495. doi: 10.1210/endo-109-2-491. [DOI] [PubMed] [Google Scholar]
- Rivier J., Brazeau P., Vale W., Guillemin R. Somatostatin analogs. Relative importance of the disulfide bridge and of the Ala-Gly side chain for biological activity. J Med Chem. 1975 Feb;18(2):123–126. doi: 10.1021/jm00236a001. [DOI] [PubMed] [Google Scholar]
- Schally A. V., Coy D. H., Meyers C. A. Hypothalamic regulatory hormones. Annu Rev Biochem. 1978;47:89–128. doi: 10.1146/annurev.bi.47.070178.000513. [DOI] [PubMed] [Google Scholar]
- Veber D. F., Freidlinger R. M., Perlow D. S., Paleveda W. J., Jr, Holly F. W., Strachan R. G., Nutt R. F., Arison B. H., Homnick C., Randall W. C. A potent cyclic hexapeptide analogue of somatostatin. Nature. 1981 Jul 2;292(5818):55–58. doi: 10.1038/292055a0. [DOI] [PubMed] [Google Scholar]
- Veber D. F., Saperstein R., Nutt R. F., Freidinger R. M., Brady S. F., Curley P., Perlow D. S., Paleveda W. J., Colton C. D., Zacchei A. G. A super active cyclic hexapeptide analog of somatostatin. Life Sci. 1984 Apr 2;34(14):1371–1378. doi: 10.1016/0024-3205(84)90009-2. [DOI] [PubMed] [Google Scholar]


