Abstract
Background
Peer substance involvement (PSI) is a robust correlate of adolescent substance use. A small number of genetically informative studies suggest that shared genetic and environmental factors contribute to this association but have not clarified our understanding of the mechanisms by which PSI influences the etiology of regular substance involvement (RSI), particularly in women.
Methods
We use data on 2,176 twin women, who were part of a population-based cohort from U.S. Midwest, to examine the relationship between self-reported PSI during adolescence and a composite regular substance involvement factor (RSI) representing regular tobacco, alcohol and cannabis use during young adulthood, using genetically informative correlation, moderation and joint correlation-moderation models.
Results
There was evidence for a significant additive genetic X environment interaction. PSI was moderately heritable (h2=0.25). Genetic, shared and non-shared influences on RSI overlapped with influences on PSI, with common genetic factors accounting for 18.5% of the genetic influences on RSI. Even after controlling for these shared genetic influences, RSI was more heritable in those reporting greater PSI.
Conclusions
While young women may select peers based on certain dispositional traits (e.g. permissiveness towards substance use), the social milieu constructed by PSI does modify the architecture of increased RSI in those individuals with increasing levels of PSI being associated with stronger expression of heritable influences.
The extent to which an individual affiliates with substance using or delinquent peers has consistently emerged as one of the strongest correlates of adolescent onset substance use and substance use problems (1–10). Despite these frequently reported associations, controversy remains regarding the mechanisms underlying them: while peer affiliations are most commonly conceptualized as risk factors for the development of substance use, via processes of socialization, it has also been observed that these associations may reflect processes of selection whereby individuals with a heightened propensity for substance use select and affiliate with peers who share a liability to substance use (11–15). Thus, the observed associations may reflect the combined influences of both selection and socialization processes (16).
A further issue to consider is that, while peer affiliations have traditionally been conceptualized as an environmental factor, there is an emerging literature suggesting that, with rare exception (17), peer factors are influenced by heritable factors (18). For example, Gillespie et al. (14) reported that 23–37% of the variation in liability to affiliating with substance using peers could be attributed to additive genetic influences while Kendler et al. (18) reported that 30–50% of the variation in liability to affiliate with substance using peers could be attributed to additive genetic factors with the relative importance of such influences increasing with increasing age. An intriguing new study (19) of peer networks shows heritable influences on attributes of peer networks, such as how often someone is named as a friend and how frequently they connect different individuals as peers Parallel evidence supports the existence of moderate to strong heritable influences on adolescent substance use (20–24) and substance related problems (25–27).
While the literature supports the existence of genetic influences on both peer affiliations and on liability to substance use, the nature of the relationship between peer affiliations and substance involvement, particularly from a genetic perspective, remains less certain. Several possible mechanisms have been posited, however, the most commonly tested hypothesis for the relationship between PSI and substance involvement is of correlated influences. For instance, Walden et al. (17) found that peer deviance was associated with early substance use via overlapping shared environmental factors. In a recent twin study of the association between peer deviance and cannabis use, Gillespie and colleagues (14) reported substantial overlap of shared environmental factors (25–73%) but also highlighted the role of overlapping genetic (50–78%) and non-shared environmental (21–26%) influences. Other reports have also supported the role of overlapping risk factors on substance use and PSI (28–31).
An alternative hypothesis, now garnering much attention in this exciting era of gene discovery, is that of (additive) gene x environment interaction (32), whereby vulnerability to substance-related outcomes is ameliorated or exacerbated by the environmental milieu associated with substance-using peers. Particularly, if the potent influence of PSI extends into later stages of substance involvement, such as regular use, then investigating mechanisms by which PSI modifies gene expression for regular substance involvement (RSI) will be critical, not only from a public health standpoint, but also for genomic studies that seek to maximize their ability to identify genes for substance use disorders (e.g.(33–35)).
We also focus on a well-characterized sample of women – from a behavior genetic standpoint, it is possible that gene - environment interplay (correlations and interactions) as they relate to peer affiliations may be of particular importance in adolescent women as substance use and affiliating with delinquent peers is considerably more non-normative in women compared to their male counterparts (38).
In an effort to delineate the relationship underlying PSI and RSI, from the perspective of (additive) gene x environment interaction, we apply a series of advanced twin models to extensive longitudinal interview and questionnaire data from a sample of young adult women to:
investigate the extent to which additive genetic, shared environmental and non-shared environmental factors influence peer substance involvement(PSI);
estimate the extent to which overlapping genetic, shared and non-shared environmental factors influence PSI and regular substance involvement (RSI);
examine whether PSI moderates the magnitude of genetic and environmental influences on RSI via (additive) gene x environment (and environment x environment) interactions.
examine whether PSI continues to modify genetic and environmental influences on RSI when influences that contribute to their overlap are accounted for.
MATERIALS AND METHODS
Sample
Data for this study are drawn from the Missouri Adolescent Female Twin Study (MOAFTS). MOAFTS consists of a cohort of female same-sex twin pairs born between July 1st 1975 – June 30th 1985 who were identified from birth records (39). Further details regarding the initial sample recruitment and the baseline interview (conducted 1994–1999), response rates and characteristics of the baseline data, which are not utilized in the current study, as they do not contain detailed assessments of substance use, are given elsewhere (40;41). Subsequently, the twins were invited to complete a mailed questionnaire and participate in the five-year follow-up telephone interview. The questionnaire was mailed one year (1996–1999) after the baseline interviews and included a series of questions on peer substance involvement. 2788 twins, aged 14–24 years, returned completed questionnaires. The five-year follow-up interview was conducted from 2002–2004 and included a detailed assessment of substance involvement. All eligible twins, including those who may not have completed a baseline assessment, were invited to participate in the follow-up, provided that they, or their parents, had not previously indicated an unwillingness to participate in future studies. A total of 3,787 twins, aged 18–29 years, completed follow-up interviews, however of those interviewed, only 2,484 twins also had data on PSI from the questionnaire. Of these subjects with interview and questionnaire data, we retained those who reported European-American ancestry and had a valid zygosity assignment as the member of a monozygotic or dizygotic twin pair – this final sample consisted of 2176 European-American women, from 574 MZ and 439 DZ pairs as well as 150 twins whose co-twin did not participate.
Measures
Two measures were used in these analyses:
Regular substance involvement (RSI)
This measure was created by factor analyzing 3 dichotomous items representing regular alcohol use, regular cigarette smoking and repeated cannabis use. Data for this measure were lifetime and were obtained from the five-year follow-up interview.
Regular alcohol use was defined as a lifetime history of drinking a drink per month for a period of 6 months in a row. In this sample, 53.5% of the women reported regular alcohol use.
Regular cigarette smoking was defined as a lifetime history of either (a) smoking 100 or more cigarettes (yes/no) or (b) smoking 21–99 cigarettes during the lifetime but also reporting smoking daily or weekly for at least 2 months in a row (yes/no). If either condition was satisfied, then the respondent was classified as a regular smoker − 36% of the women reported smoking cigarettes regularly.
Repeated cannabis use was defined as a lifetime history of using cannabis 11 or more times and endorsed by 18% of the sample. There is currently no standardized measure of ‘regular’ cannabis use, however those using cannabis less frequently are assumed to not be at risk for dependence.
These measures represent regular/repeated substance use. They were also selected as, in our interview, individuals endorsing these items only were subsequently queried about substance abuse/dependence, making these items ‘thresholds’ to vulnerability to problematic substance involvement.
Peer substance involvement (PSI)
This measure was based on factor analysis of 9 questionnaire items where the twins reported on their peers’ substance use behaviors yielded a factor score representing PSI. Twins were asked to endorse ‘how many of your friends would you guess’: (a) smoked cigarettes regularly, (b) used smokeless tobacco, (c) drank alcoholic beverages regularly, (d) got drunk at least once a week, (e) smoked marijuana or hashish, (f) used crack cocaine, (g) used cocaine in powder form, (h) used heroin or (i) sniffed glue, gases or spray. Responses were provided on a 5-point Likert scale ranging from ‘None’ to ‘All’. The pattern of endorsement of the PSI items is shown in Table 1.
Table 1.
Prevalence (N=2,176) and factor loadings of measures contributing to the regular substance involvement (RSI) and peer substance involvement (PSI) factors in the Missouri Adolescent Female Twin Study (MOAFTS)
| % endorse | Factor loading | |||||
|---|---|---|---|---|---|---|
|
Regular substance involvement (RSI) | ||||||
| Regular alcohol use | 53.5 | 0.40 | ||||
| Regular cigarette smoking | 36.0 | 0.68 | ||||
| Repeated cannabis use | 18.1 | 0.65 | ||||
|
Peer substance involvement (PSI) | ||||||
| None | A Few | Some | Most | All | Factor Loading | |
| Smoked cigarettes regularly | 21.9 | 32.0 | 21.5 | 21.8 | 2.8 | 0.74 |
| Use smokeless tobacco | 59.1 | 27.3 | 11.3 | 2.1 | 0.2 | 0.47 |
| Drink alcoholic beverages regularly | 25.7 | 25.8 | 23.8 | 20.5 | 4.3 | 0.71 |
| Get drunk at least once a week | 38.1 | 27.6 | 19.9 | 12.2 | 2.1 | 0.75 |
| Smoke marijuana/hashish | 52.9 | 24.9 | 13.0 | 7.6 | 1.6 | 0.74 |
| Use crack cocaine | 89.0 | 8.9 | 1.5 | 0.3 | 0.2 | 0.56 |
| Use powder cocaine | 88.9 | 8.7 | 2.0 | 0.3 | 0.1 | 0.61 |
| Use heroin | 95.9 | 3.4 | 0.5 | 0.1 | 0.1 | 0.41 |
| Sniff glue, gas or sprays | 91.4 | 7.3 | 1.1 | 0.1 | 0.2 | 0.36 |
1st 3 Eigenvalues for RSI: 0.56, −0.05, −0.25; Proportion explained by 1st factor: 100%. 1st 3 Eigenvalues for PSI: 8.34, 3.07, 0.27; Proportion explained by 1st factor: 80%.
Factor analysis
Maximum likelihood factor analysis was conducted in SAS (42), with tests for validity in MPlus (43). The number of factors necessary to explain covariance across the items assessing RSI and PSI, which were factor-analyzed separately, was assessed using Eigenvalues and visual inspection of scree plots. A single factor satisfactorily explained the data on regular alcohol use, regular cigarette smoking and repeated cannabis use. Factor loadings ranged from0.40–0.65 (Table 1) – the factor score resulting from this analysis constituted the RSI measure. For peer substance use, 2 factors were initially extracted. However, the 1st factor explained 80% of the covariance across items, with factor loadings ranging from 0.36 to 0.75 (Table 1) – we used this factor to represent PSI. The generated factor scores for PSI and RSI were extracted and PROC REG was used to regress out the effects of age (defined as 3 dummy measures representing age at questionnaire: age less than 16 years, between 16 and 17 years and between 18 and 20 years with age greater than 21 years as the reference). The corresponding residuals, representing the RSI and PSI factor scores without these confounding effects of age, were used for all subsequent analyses including twin modeling. Compared with those aged 21 and older, those aged 14–17 years at the time of the questionnaires, had lower PSI scores (p < .0001 for those aged 14–16 years and p< .001 for those aged 16–17 years) suggesting fewer friends who used substances. All twins were 18 years and older at the time of assessment of RSI using interviews. However, younger individuals, aged 18–20 years, were considerably less likely (p < .0001) to report RSI than those aged 21 years and older.
Our decision to use residualized scores, instead of explicitly modeling dummies for age in the twin models, was primarily related to feasibility (and comparability) of testing the various twin models. While there are limitations to using residualized scores (e.g. the impact of age cannot be simultaneously modeled), there are challenges to including age as a covariate in the some of the more complex models, due to power.
Twin models
Classical twin models (44) that draw data from monozygotic (MZ) and dizygotic (DZ) twin pairs can be used to disentangle the extent to which additive genetic (A), shared environmental (C) and non-shared environmental (E) factors influence individual differences in PSI and RSI. Additive genetic influences refer to the aggregate variance attributable to segregating genes – members of MZ twin pairs share 100% of their genes identical-by-descent while, on average, members of DZ twin pairs share 50% of their genes identical-by-descent. Under the equal environments assumptions, shared environmental factors are shared equally, at 100%, across members of MZ and DZ twin pairs. Non-shared environmental factors are uncorrelated across members of twin pairs.
A series of twin models (shown in Figure 1) were fitted to the data to test mechanisms by which PSI might associate with individual differences in RSI. Model-fitting was conducted using raw data and Full Information Maximum Likelihood in the software package Mx (45).
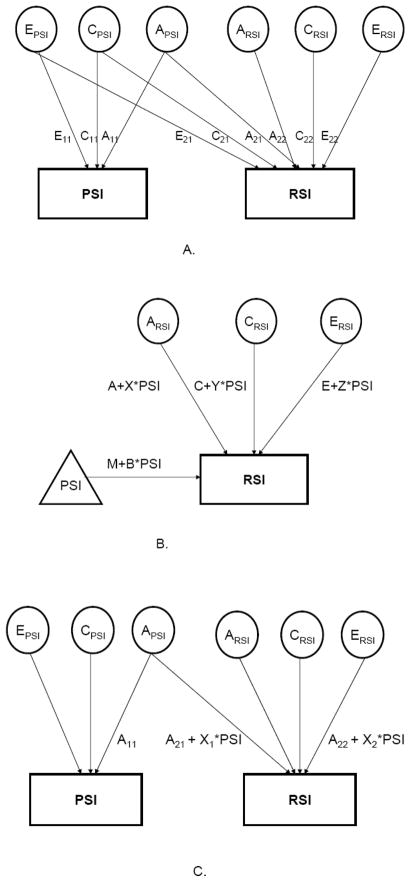
Figure 1.
The correlated influences (Panel A), moderating influences (Panel B) and joint correlation-moderating influence (Panel C) twin models used to test the relationship between peer substance involvement (PSI) and regular substance involvement (RSI). For Panel C, while moderation of only additive genetic (A) influences is shown, C (shared environment) and E (non-shared environment) were similarly overlapping and moderated by PSI. For instance, total shared environmental influences on RSI were represented by (C21+Y1*PSI)2 + (C22+Y2*PSI)2.
Correlated Influences
The first hypothesis of correlated influences was tested using a Cholesky decomposition (44;46). In this model, A, C and E influence RSI and PSI. Influences on RSI (from Figure 1, Panel A) are those that are overlapping with influences on PSI (A21, C21 and E21) and those that are specific to RSI (A22, C22 and E22). For instance, the total genetic variance in RSI is equal to genetic factors that are specific to RSI and those shared between RSI and PSI ((A21)2 + (A22)2).
Moderation
The second hypothesis tested whether PSI moderated the A, C and E influences on RSI. Here, PSI potentially moderates the extent to which A, C and E influence RSI – the moderation of the A component by PSI represents a latent genetic x measured environment interaction – thus, as shown in Panel B of Figure 1, total additive genetic influences on RSI are modified from A to A+X*PSI, where X represents the extent to which PSI moderates the magnitude of genetic influences on RSI (47–49) – therefore, the statistical significance of X represents (additive) gene x environment interaction.
Joint correlation-moderation
This model (Panel C of Figure 1) jointly tests the extent to which correlated and moderating influences shape the association between RSI and PSI (49). Drawing from the correlated influences hypothesis, the joint correlation-moderation model tests the extent to which overlapping and specific A, C and E factors influence liability to RSI. To incorporate moderation influences, both the overlapping and specific A,C and E influences on RSI are then moderated by PSI. For instance, the genetic influences on RSI can now be visualized as those overlapping with PSI (A21) and those specific to RSI (A22). Each of these genetic parameters are moderated by PSI such that the net genetic influences on RSI are (A21+X1*PSI) and (A22+X2*PSI). Here, both X1 and X2 support gene x environment interaction whereby the former represents G x E for additive genetic influences on RSI that are shared with PSI while the latter represents G x E for additive genetic influences specific to RSI.
RESULTS
Association between PSI and RSI
Table 2 shows the within-person association between levels of reported PSI (divided into quartiles for display purposes) and the individual measures that were used to construct the RSI measure. Rates of regular alcohol use, regular cigarette smoking and repeated cannabis use increased with increasing PSI indicating significant association between PSI and RSI in this young adult female sample.
Table 2.
Association between measures constituting the regular substance involvement (RSI) factor and varying levels (represented by the 1st, 2nd, 3rd and 4th quartile) of peer substance involvement (PSI)
| Prevalence in each quartile of PSI | χ2df=3 | ||||
|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | ||
| Regular alcohol use | 40.6% | 40.3% | 65.6% | 69.7% | 163.1 |
| Regular cigarette smoking | 20.1% | 19.4% | 40.0% | 63.3% | 314.0 |
| Repeated cannabis use | 1.7% | 1.8% | 5.0% | 14.8% | 114.0 |
Twin models
Correlated influences
Results from the correlated influences model are shown in Table 3. Additive genetic (25%), shared (33%) and non-shared environmental influences were responsible for individual differences in PSI and for RSI (A=42%, C=24%, E=34%). Composite genetic influences in RSI were attributable to those overlapping with PSI (genetic correlation of 0.43) and those specific to it. Shared environmental influences on RSI were completely overlapping with PSI (Δχ2=0.07 for 1 df constraining specific C on RSI to zero). Non-shared environmental influences on RSI were largely specific, however, a modest yet significant correlation of 0.11 (Δχ2=6.61 for 1 df constraining overlapping E to zero) was noted. Thus, under the correlated influences model, total covariation between RSI and PSI was attributable to overlapping genetic, shared and non-shared environmental factors.
Table 3.
Parameter estimates from a series of twin models fitted to data on peer substance involvement (PSI) and regular substance involvement (RSI) in young adult female twins (N=2,176)
| Additive Genetic | Shared Environment | Non-shared Environment | |
|---|---|---|---|
|
Correlated Influences Model | |||
| PSI | 0.25 [0.09–0.42] | 0.33 [0.18–0.47] | 0.42 [0.37–0.48] |
| RSI | 0.42 [0.27–0.56] | 0.24 [0.10–0.37] | 0.34 [0.30–0.39] |
| Correlation | 0.43 [0.13–0.80] | 0.95 [0.63–1.00] | 0.11 [0.03–0.19] |
|
Moderating Model** | |||
| RSI main | 0.47 [0.35–0.54] | 0.18 [−0.34–0.36] | −0.48 [−0.51–0.45] |
| PSI moderator | 0.11 [0.02–0.17] | 0.04 [−0.16–0.18] | −0.13 [−0.17–0.09] |
|
Joint Correlation-Moderation Model** | |||
| PSI | 0.42 [0.23–0.55] | 0.52 [0.40–0.62] | 0.57 [0.54–0.61] |
| RSI (common) | 0.15 [−0.04–0.33] | 0.43 [0.29–0.52] | 0.12 [0.07–0.16] |
| RSI (specific) | 0.45 [0.33–0.50] | 0.01 [−0.31–0.33] | 0.46 [0.44–0.50] |
| PSI moderator (common path) | −0.01 [−0.09–0.09] | 0.01 [−0.07–0.08] | −0.05 [−0.09–0.01] |
| PSI moderator (specific path) | 0.13 [0.05–0.19] | 0.00 [−0.14–0.13] | 0.10 [0.06–0.14] |
Un-standardized un-squared estimates presented. Unlike the results for Correlated Influences Model where path estimates were squared, summed and extracted as proportions of variance, the moderation models cannot be standardized – at each level of the moderator, the total variance varies. Therefore, we present raw path estimates for these models. To view relative contributions of A, C and E, within a standardized framework, view Figure 1.
NOTE: AIC for each model is 961.2, −86.5 and 616.6
For all analyses, residualized PSI and RSI scores were used. However, to determine whether using residual scores instead of modeling dummies for age within the twin model influenced our analyses, we redid the correlated influences model with raw RSI and PSI scores and dummy-coded age as covariates in the twin model. Results were unchanged.
Moderating Influences
Results from the moderation model are presented in Table 3 and are graphically depicted in Figure 2 (Panel A). There was significant evidence for G x E additive genetic influences on RSI increased as levels of peer deviance increased. There was no evidence for shared environmental factors nor for any moderating influence on them (Δχ2=0.58 for 2 df). However, non-shared environmental factors on RSI were significantly moderated by PSI – as peer deviance increased, the role of non-shared environmental influences on RSI also increased. This implies an overall increase in RSI variance in those with higher PSI scores. Finally, it was also noted that PSI increased overall levels of RSI (B=0.39, 95% C.I. 0.34–0.43).
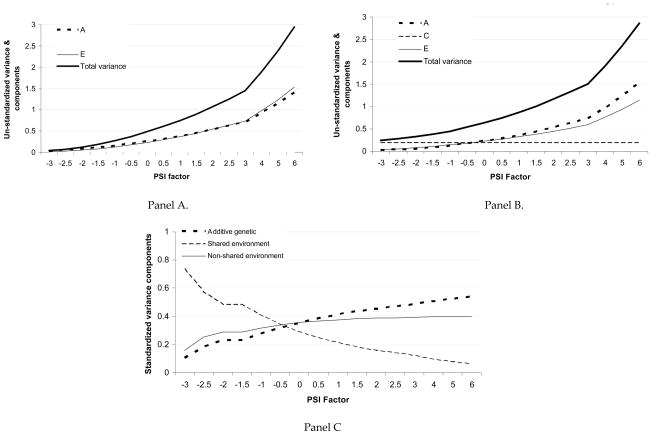
Figure 2.
Panel A represents results from the moderating influences model. The total variance as well as the contributions of additive genetic (A) and non-shared environmental factors (E) are shown – as noted in Table 3, shared environmental influences (C) were not statistically significant here and hence, are not shown – estimates are un-standardized, such that at each level of PSI, total variance = A+E.
Panel B and C represent results from the joint correlation-moderating influences models. Panel B presents unstandardized results while panel C presents standardized variance components.
Joint correlation-moderation influences
Results from the joint correlation-moderation model are presented in Table 3 and graphically represented in Panels B and C of Figure 2. First, when correlated influences and moderation were jointly modeled between RSI and PSI, there was no evidence for common genetic influences between RSI and PSI – instead (Δχ2=3.42 for 2 df), PSI was found to moderate the specific genetic influences on RSI. Second, there continued to be no evidence for moderation of shared environmental influences on RSI and all shared environmental influences on RSI were overlapping with PSI. Third, there was evidence for overlapping non-shared environmental influences on PSI and RSI. Furthermore, PSI moderated both these overlapping and the specific non-shared environmental influences on RSI. As shown in Panel B of Figure 2, similar to the Moderating Influences model, total variance (heavy solid line), as well as variance attributable to additive genetic (dotted line) and non-shared environmental (light solid line) factors increased with increasing PSI – however, by jointly modeling PSI with RSI, we were able to capture shared environmental factors that overlap between PSI and RSI – as shown by the dashed line in Panel B (Figure 2), these remained constant across the range of PSI. Panel C (Figure 2) shows standardized results – the relative magnitude of genetic influences in Panel C refer to the proportion of the total variance in RSI attributable to moderated additive genetic factors. As shown here, when standardized, the magnitude of additive genetic (and of non-shared environmental) influence relative to other factors (i.e. C) increases with increasing PSI while the magnitude of shared environmental factors becomes less important with increasing PSI. It is noteworthy that while the effects of C on RSI, all of which are overlapping with PSI, are not moderated, due to moderation of A and E, their relative contribution to the total variance does change with changing PSI. Accordingly, in young women with lower levels of PSI, individual differences in RSI are largely attributable to shared environmental factors – with increasing exposure to substance-using peers, individual differences in RSI can be attributed to additive genetic and non-shared environmental factors.
Peer socialization
Peer socialization was also examined as an alternate mechanism contributing to peer similarity. A direction-of-causation model, where the relationship between PSI and RSI was quantified by causal paths (14) was fitted to these data. This socialization model was virtually indistinguishable from the correlational/selection model (Δχ2=2.17 for 1 df). Both causal paths (PSI→RSI, β1=−0.68; RSI→PSI, β2=1.0) were significant (Δχ2=68.6 and 90.8 for 1 df) and similar to those reported previously (14).
DISCUSSION
Moderation/Genetic x Environment Interaction
The concept of moderation of the etiology of RSI via PSI has been infrequently tested within a genetically informative framework (50). By relative fit (Akaike’s Information Criterion or AIC), the moderating influences model most parsimoniously explained the relationship between RSI and PSI (AIC of −86.5, compared with AIC=961.2 for correlated influences and AIC=616.6 for the joint model). Moderation of heritable influences (e.g. genes influencing RSI) by PSI represents a (additive) gene x environment interaction (G x E). Such interactions are responsible for variations in the etiology of substance use behaviors – for instance, Dick and colleagues (29) have found that as peer alcohol use increased, heritable factors associated with an adolescent’s own alcohol involvement also increased. Harden et al. (31) provided evidence for both gene by environment correlation – whereby individuals with increased genetic liability for substance use are more likely to affiliate with substance using peers – and for gene by environment interaction, or that the influence of substance using peers was stronger in those individuals who had a high genetic liability to recent alcohol and tobacco use. Most recently, Button and colleagues (15) used a similar series of models to report that genetic influences were more prominent at both very low and at very high levels of peer delinquency. In that study, no evidence for moderation of genetic factors common to RSI and PSI was noted.
G x E for Peer Similarity
We find evidence for a unique model of peer similarity which may be particularly relevant to the etiology of substance use. First, we noted heritable influences on PSI – while this is consistent with the literature, it highlights the importance of studying the etiology of measures presumed to be ‘environmental’. Second, individual differences in PSI and RSI were attributable to partially overlapping genetic and completely overlapping environmental factors – the genetic overlap represents gene-environment correlation (rGE)(51;52), or those genetic influences that increase the likelihood of RSI and of exposure to substance-using peers. It is vital to control for rGE when studying GxE – for instance, measured gene studies may wish to examine if having a certain genotype increases an individual’s likelihood of both RSI and of exposure to PSI prior to testing whether PSI modifies the action of that genotype on risk for RSI.
There are some limitations to the present analyses. First, the study was conducted on a sample of women. While similar evidence has been seen for males, there is also support for variations in the diversity and patterns of substance involvement across sexes (53). Further, the effect of GxE may be more attenuated in males (54) with a prior investigation finding greater evidence for positive peer assortment in girls (38). Thus, whether the same factor structure or genetic findings would be obtained in males remains unknown. Second, PSI reports were extracted from the twin respondent‘s reports from a questionnaire that preceded the interview. While there is some evidence that respondent reports of peer behavior may augment estimates of peer similarity, there is also growing evidence that, within the context of risk influences on the respondent’s behavior, perceived measures may be more predictive of respondent behavior (55). The questionnaire for PSI preceded the interview for RSI - we consider this to be a strength of our study – twins reported objectively on PSI as adolescents and at a later time point, when they were past the period of risk for initiation of alcohol, cigarettes and cannabis, were interviewed about their own substance use. Third, we excluded 199 African-American women from the present analyses – variation in patterns of RSI may be attributable to ethnic differences – for instance, when African-American subjects were included, factor loadings for PSI were markedly different (for e.g. the loading for smoking cigarettes regularly dropped from 0.74 to 0.42 with generally lower loadings for licit substances) suggesting differences in the factorial structure. While it would be important to study the effects of PSI on RSI in African-American women, the small sample size precluded us from formally testing these differences.
In conclusion, important genetic links exist between PSI and RSI. While individuals may select peers based on certain dispositional traits (e.g. permissiveness towards substance use), the social milieu constructed by PSI does modify the architecture of increased RSI in those individuals with increasing levels of PSI being associated with stronger expression of heritable influences.
Acknowledgments
Funding: Research supported by AA07728 & AA09022 (ACH), DA23668 (AA) & DA18660 & DA18267 (MTL).
Reference List
- 1.Adrados JL. The influence of family, school, and peers on adolescent drug misuse. Int J Addict. 1995 Sep;30(11):1407–23. doi: 10.3109/10826089509055840. [DOI] [PubMed] [Google Scholar]
- 2.Bahr SJ, Marcos AC, Maughan SL. Family, educational and peer influences on the alcohol use of female and male adolescents. J Stud Alcohol. 1995 Jul;56(4):457–69. doi: 10.15288/jsa.1995.56.457. [DOI] [PubMed] [Google Scholar]
- 3.Brook JS, Brook DW, De La RM, Duque LF, Rodriguez E, Montoya ID, et al. Pathways to marijuana use among adolescents: cultural/ecological, family, peer, and personality influences. J Am Acad Child Adolesc Psychiatry. 1998 Jul;37(7):759–66. [PubMed] [Google Scholar]
- 4.Bauman KE, Ennett ST. On the importance of peer influence for adolescent drug use: commonly neglected considerations. Addiction. 1996 Feb;91(2):185–98. [PubMed] [Google Scholar]
- 5.Ellickson PL, Tucker JS, Klein DJ, Saner H. Antecedents and outcomes of marijuana use initiation during adolescence. Prev Med. 2004 Nov;39(5):976–84. doi: 10.1016/j.ypmed.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Fergusson DM, Swain-Campbell NR, Horwood LJ. Deviant peer affiliations, crime and substance use: a fixed effects regression analysis. J Abnorm Child Psychol. 2002 Aug;30(4):419–30. doi: 10.1023/a:1015774125952. [DOI] [PubMed] [Google Scholar]
- 7.Henry KL. Low prosocial attachment, involvement with drug-using peers, and adolescent drug use: a longitudinal examination of mediational mechanisms. Psychol Addict Behav. 2008 Jun;22(2):302–8. doi: 10.1037/0893-164X.22.2.302. [DOI] [PubMed] [Google Scholar]
- 8.Simons-Morton B, Chen R, Abroms L, Haynie DL. Latent growth curve analyses of peer and parent influences on smoking progression among early adolescents. Health Psychol. 2004 Nov;23(6):612–21. doi: 10.1037/0278-6133.23.6.612. [DOI] [PubMed] [Google Scholar]
- 9.Simons-Morton B, Chen RS. Over time relationships between early adolescent and peer substance use. Addict Behav. 2005 Oct 14; doi: 10.1016/j.addbeh.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Korhonen T, Huizink AC, Dick DM, Pulkkinen L, Rose RJ, Kaprio J. Role of individual, peer and family factors in the use of cannabis and other illicit drugs: a longitudinal analysis among Finnish adolescent twins. Drug Alcohol Depend. 2008 Sep 1;97(1–2):33–43. doi: 10.1016/j.drugalcdep.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fergusson DM, Horwood LJ. Prospective childhood predictors of deviant peer affiliations in adolescence. J Child Psychol Psychiatry. 1999 May;40(4):581–92. [PubMed] [Google Scholar]
- 12.Fergusson DM, Woodward LJ, Horwood LJ. Childhood peer relationship problems and young people’s involvement with deviant peers in adolescence. J Abnorm Child Psychol. 1999 Oct;27(5):357–69. doi: 10.1023/a:1021923917494. [DOI] [PubMed] [Google Scholar]
- 13.Lacourse E, Nagin DS, Vitaro F, Cote S, Arseneault L, Tremblay RE. Prediction of early-onset deviant peer group affiliation: a 12-year longitudinal study. Arch Gen Psychiatry. 2006 May;63(5):562–8. doi: 10.1001/archpsyc.63.5.562. [DOI] [PubMed] [Google Scholar]
- 14.Gillespie NA, Neale MC, Jacobson K, Kendler KS. Modeling the genetic and environmental association between peer group deviance and cannabis use in male twins. Addiction. 2009 Mar;104(3):420–9. doi: 10.1111/j.1360-0443.2008.02457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Button TM, Stallings MC, Rhee SH, Corley RP, Boardman JD, Hewitt JK. Perceived peer delinquency and the genetic predisposition for substance dependence vulnerability. Drug Alcohol Depend. 2009 Feb 1;100(1–2):1–8. doi: 10.1016/j.drugalcdep.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kandel DB. Homophily, Selection, and Socialization in Adolescent Friendships. The American Journal of Sociology. 1978;84(2):427–36. [Google Scholar]
- 17.Walden B, McGue M, Lacono WG, Burt SA, Elkins I. Identifying shared environmental contributions to early substance use: the respective roles of peers and parents. J Abnorm Psychol. 2004 Aug;113(3):440–50. doi: 10.1037/0021-843X.113.3.440. [DOI] [PubMed] [Google Scholar]
- 18.Kendler KS, Jacobson KC, Gardner CO, Gillespie N, Aggen SA, Prescott CA. Creating a social world: a developmental twin study of peer-group deviance. Arch Gen Psychiatry. 2007 Aug;64(8):958–65. doi: 10.1001/archpsyc.64.8.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fowler JH, Dawes CT, Christakis NA. Model of genetic variation in human social networks. Proc Natl Acad Sci U S A. 2009 Feb 10;106(6):1720–4. doi: 10.1073/pnas.0806746106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heath AC, Lynskey MT, Waldron M. Substance Use. In: Rutter M, Taylor E, editors. Handbook of Child and Adolescent Psychiatry. London: Blackwell; 2007. [Google Scholar]
- 21.Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Arch Gen Psychiatry. 2003 Dec;60(12):1256–64. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- 22.McGue M, Elkins I, Iacono WG. Genetic and environmental influences on adolescent substance use and abuse. Am J Med Genet. 2000 Oct 9;96(5):671–7. doi: 10.1002/1096-8628(20001009)96:5<671::aid-ajmg14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 23.Maes HH, Woodard CE, Murrelle L, Meyer JM, Silberg JL, Hewitt JK, et al. Tobacco, alcohol and drug use in eight- to sixteen-year-old twins: the Virginia Twin Study of Adolescent Behavioral Development. J Stud Alcohol. 1999 May;60(3):293–305. doi: 10.15288/jsa.1999.60.293. [DOI] [PubMed] [Google Scholar]
- 24.Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Arch Gen Psychiatry. 2008 Jun;65(6):674–82. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agrawal A, Lynskey MT. Are there genetic influences on addiction: evidence from family, adoption and twin studies. Addiction. 2008 Jul;103(7):1069–81. doi: 10.1111/j.1360-0443.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- 26.Kendler KS, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Arch Gen Psychiatry. 2007 Nov;64(11):1313–20. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- 27.Tsuang MT, Bar JL, Harley RM, Lyons MJ. The Harvard Twin Study of Substance Abuse: what we have learned. Harv Rev Psychiatry. 2001 Nov;9(6):267–79. [PubMed] [Google Scholar]
- 28.Dick DM, Pagan JL, Holliday C, Viken R, Pulkkinen L, Kaprio J, et al. Gender differences in friends’ influences on adolescent drinking: a genetic epidemiological study. Alcohol Clin Exp Res. 2007 Dec;31(12):2012–9. doi: 10.1111/j.1530-0277.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- 29.Dick DM, Pagan JL, Viken R, Purcell S, Kaprio J, Pulkkinen L, et al. Changing environmental influences on substance use across development. Twin Res Hum Genet. 2007 Apr;10(2):315–26. doi: 10.1375/twin.10.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fowler T, Lifford K, Shelton K, Rice F, Thapar A, Neale MC, et al. Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. Addiction. 2007 Mar;102(3):413–22. doi: 10.1111/j.1360-0443.2006.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harden KP, Hill JE, Turkheimer E, Emery RE. Gene-environment correlation and interaction in peer effects on adolescent alcohol and tobacco use. Behav Genet. 2008 Jul;38(4):339–47. doi: 10.1007/s10519-008-9202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: multiple varieties but real effects. J Child Psychol Psychiatry. 2006 Mar;47(3–4):226–61. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- 33.Treutlein J, Cichon S, Ridinger M, Wodarz N, Soyka M, Zill P, et al. Genome-wide association study of alcohol dependence. Arch Gen Psychiatry. 2009 Jul;66(7):773–84. doi: 10.1001/archgenpsychiatry.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dick DM, Latendresse SJ, Lansford JE, Budde JP, Goate A, Dodge KA, et al. Role of GABRA2 in trajectories of externalizing behavior across development and evidence of moderation by parental monitoring. Arch Gen Psychiatry. 2009 Jun;66(6):649–57. doi: 10.1001/archgenpsychiatry.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hicks BM, South SC, Dirago AC, Iacono WG, McGue M. Environmental adversity and increasing genetic risk for externalizing disorders. Arch Gen Psychiatry. 2009 Jun;66(6):640–8. doi: 10.1001/archgenpsychiatry.2008.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maher B. The Search for the Genome ‘Dark Matter’ Moves Closer. Nature News. 2008:1235. [Google Scholar]
- 37.Maher B. Personal genomes: The case of the missing heritability. Nature. 2008 Nov 6;456(7218):18–21. doi: 10.1038/456018a. [DOI] [PubMed] [Google Scholar]
- 38.Rose RJ. How do adolescents select their friends? A behavior-genetic perspective. In: Pulkkinen L, Caspi A, editors. Paths to Successful Development: Personality in the Life Course. Cambridge, UK: Cambridge University Press; 2002. pp. 106–28. [Google Scholar]
- 39.Heath AC, Howells W, Bucholz KK, Glowinski AL, Nelson EC, Madden PA. Ascertainment of a mid-western US female adolescent twin cohort for alcohol studies: assessment of sample representativeness using birth record data. Twin Res. 2002 Apr;5(2):107–12. doi: 10.1375/1369052022974. [DOI] [PubMed] [Google Scholar]
- 40.Heath AC, Madden PA, Grant JD, McLaughlin TL, Todorov AA, Bucholz KK. Resiliency factors protecting against teenage alcohol use and smoking: influences of religion, religious involvement and values, and ethnicity in the Missouri Adolescent Female Twin Study. Twin Res. 1999 Jun;2(2):145–55. doi: 10.1375/136905299320566013. [DOI] [PubMed] [Google Scholar]
- 41.Knopik VS, Sparrow EP, Madden PA, Bucholz KK, Hudziak JJ, Reich W, et al. Contributions of parental alcoholism, prenatal substance exposure, and genetic transmission to child ADHD risk: a female twin study. Psychol Med. 2005 May;35(5):625–35. doi: 10.1017/s0033291704004155. [DOI] [PubMed] [Google Scholar]
- 42.SAS Institute. SAS User Guide, Version 8.2. Cary, NC: SAS Institute Inc; 1999. [Google Scholar]
- 43.Muthen LK, Muthen BO. Mplus User’s Guide. 5. Los Angeles, CA: Muthen & Muthen; 2007. [Google Scholar]
- 44.Neale MC, Cardon LR. Methodology for Genetic studies of Twins and Families. Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- 45.Neale MC. Statistical Modeling with Mx. Dept of Psychiatry; Box # 980710, Richmond VA 23298: 2004. [Google Scholar]
- 46.Commandant Benoit. Note sur une méthode de résolution des équations normales provenant de l’application de la méthode des moindres carrés à un systéme d’équations linéaires en nombre inférieur à celui des inconnues (Procédé du Commandant Cholesky) Bulletin géodésique. 1924;2:67–77. [Google Scholar]
- 47.Martin NG, Eaves LJ, Heath AC. Prospects for detecting genotype X environment interactions in twins with breast cancer. Acta Genet Med Gemellol (Roma ) 1987;36(1):5–20. doi: 10.1017/s0001566000004542. [DOI] [PubMed] [Google Scholar]
- 48.Dick DM, Rose RJ, Viken RJ, Kaprio J, Koskenvuo M. Exploring gene-environment interactions: socioregional moderation of alcohol use. J Abnorm Psychol. 2001 Nov;110(4):625–32. doi: 10.1037//0021-843x.110.4.625. [DOI] [PubMed] [Google Scholar]
- 49.Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Res. 2002 Dec;5(6):554–71. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- 50.Madden PA, Bucholz KK, Todorov AA, Grant JD, Heath AC. The assessment of peer selection and peer environmental influences on behavior using pairs of siblings or twins. Twin Res. 2002 Feb;5(1):38–43. doi: 10.1375/1369052022884. [DOI] [PubMed] [Google Scholar]
- 51.Scarr S, McCartney J. How people make their own environment: A theory of genotype greater than environmental effects. Child Development. 1983;54:424–35. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- 52.Plomin R, DeFries JC, Loehlin JC. Genotype-environment interaction and correlation in the analysis of human behavior. Psychol Bull. 1977 Mar;84(2):309–22. [PubMed] [Google Scholar]
- 53.Derringer J, Krueger RF, McGue M, Iacono WG. Genetic and environmental contributions to the diversity of substances used in adolescent twins: a longitudinal study of age and sex effects. Addiction. 2008 Oct;103(10):1744–51. doi: 10.1111/j.1360-0443.2008.02305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dick DM, Bernard M, Aliev F, Viken R, Pulkkinen L, Kaprio J, et al. The role of socioregional factors in moderating genetic influences on early adolescent behavior problems and alcohol use. Alcohol Clin Exp Res. 2009 Oct;33(10):1739–48. doi: 10.1111/j.1530-0277.2009.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Latendresse SJ, Rose RJ, Viken RJ, Pulkkinen L, Kaprio J, Dick DM. Parental socialization and adolescents’ alcohol use behaviors: predictive disparities in parents’ versus adolescents’ perceptions of the parenting environment. J Clin Child Adolesc Psychol. 2009 Mar;38(2):232–44. doi: 10.1080/15374410802698404. [DOI] [PMC free article] [PubMed] [Google Scholar]