Abstract
Sulindac, atorvastatin or prebiotic dietary fiber may reduce colorectal cancer (CRC) risk. However, clinical trial data are currently limited. We conducted a randomized, phase II chemoprevention trial involving subjects age ≥ 40 years, with previously resected colon cancer or multiple/advanced colorectal adenomas. Magnification chromoendoscopy (MCE) was performed to identify and characterize rectal aberrant crypt foci (ACF); eligibility criteria required ≥ 5 rectal ACF at baseline. Intervention assignments were: (A) atorvastatin 20 mg qd; (B) sulindac 150 mg bid; (C) oligofructose-enriched inulin (as ORAFTI®Synergy1) 6 gm bid; or (D) control (maltodextrin) 6 gm bid, for 6 months. Percent change in rectal ACF number (%ΔACF) within arm was the primary endpoint. Secondary endpoints included changes in proliferation (Ki67) and apoptosis (caspase-3), as measured from normal mucosa biopsy samples. Among 85 eligible randomized subjects, 76 (86%) completed the trial per protocol. The median (range) of rectal ACF was 9 (5–34) and 8 (0–37) at baseline and post-intervention, respectively. The median (standard deviation) for %ΔACF was 5.6 (−69–143%), −18.6 (−83–160%), −3.6 (−88-83%) and −10.0 (−100–117%) in the atorvastatin, sulindac, ORAFTI®Synergy1 and control arms, respectively. Neither within arm (p=0.12–0.59) nor between arm (p=0.30–0.92) comparisons of %ΔACF were statistically significant. The active and control interventions also appeared to have similar effects on mucosal proliferation and apoptosis (p > 0.05 for each comparison). Data from this multi-center, phase II trial do not provide convincing evidence of CRC risk reduction from six month interventions with atorvastatin, sulindac or ORAFTI®Synergy1, although statistical power was limited by the relatively small sample size.
Keywords: Colorectal cancer, chemoprevention, clinical trial, phase II
Introduction
Colorectal cancer (CRC) has the third highest incidence rate of all cancers in the United States, with over 145,000 new cases diagnosed each year (1). Early detection (and removal) of premalignant neoplasia remains the cornerstone of CRC prevention. Chemoprevention may provide adjunctive benefits in high-risk patient populations. Despite strong potential, chemoprevention has achieved relatively limited clinical impact to date, in large part because of reportedly unfavorable and/or incompletely defined risk:benefit ratios for many candidate agents (2, 3).
Existing data support further evaluation of nonsteroidal anti-inflammatory drugs (NSAIDs), statin drugs (a.k.a. 3-hydroxy-3methylgutaryl Coenzyme A reductase inhibitors) and prebiotic dietary fiber in early phase chemoprevention trials (4). Sulindac (a nonselective NSAID) has been shown to regress polyps among individuals genetically predisposed to developing CRC (5), although results from subsequent clinical trials of sulindac (alone or in combination with other candidate chemoprevention agents) involving subjects with sporadic colorectal neoplasia remain inconclusive (6–9). Atorvastatin, the most widely prescribed drug in the United States (www.imshealth.com; accessed 4/13/10), is a lipophilic statin and may have anti-inflammatory, pro-apoptotic or other growth inhibitory properties based on preclinical data (10). Some, but not all, prospective observational studies have reported an inverse association between statin use and CRC risk (11–14). However, to date, no statin-based CRC chemoprevention trials have been reported. ORAFTI®Synergy1 is a prebiotic fiber composed of oligofructose and polyfructose chains and has been shown to inhibit intestinal tumors in rodents (15). Favorable effects on several stool- and blood-based biomarkers were recently observed when ORAFTI®Synergy1 was administered in combination with two probiotic agents to subjects with a history of resected colorectal neoplasia (16).
To maximize efficiency, early phase chemoprevention trials should apply validated, modifiable, easy-to-measure surrogate endpoint biomarkers (SEBs) as the primary endpoint (17). Aberrant crypt foci (ACF), which are characterized by aggregated clusters of thickened and enlarged mucosal crypts, respond to chemopreventive interventions in animal models and have been correlated with synchronous colorectal neoplasia in some, but not all, observational studies as recently reviewed (18, 19). Rectal ACF can be readily quantified by magnification chromoendoscopy (MCE) and have been analyzed as primary endpoints in a limited number of previous CRC chemoprevention trials (17, 20). Using the infrastructure of the multi-center Cancer Prevention Network (CPN), we conducted a randomized, phase II clinical trial of six month interventions with sulindac, atorvastatin, ORAFTI®Synergy1, or control (maltodextrin) to assess the effects of these candidate agents on percent change in rectal ACF number (primary endpoint) and other tissue-based biomarkers (secondary endpoints) among subjects at increased risk for sporadic CRC.
Materials and Methods
All aspects of the study protocol were reviewed and approved by the appropriate Institutional Review Board for human research at each participating site. The Data and Safety Monitoring Board of the Mayo Clinic Cancer Center reviewed safety data every 6 months and efficacy data within 6 months of the planned interim analysis.
Baseline Evaluation
Subjects were enrolled through 10 CPN member organizations (Brigham and Women’s Hospital, Boston, MA; Fox Chase Cancer Center, Philadelphia, PA; Hines Veterans Administration, Hines, IL; Indiana University, Indianapolis, IN; Kaiser Permanente Medical Group, Sacramento, CA; Mayo Clinic Arizona, Scottsdale, AZ; Mayo Clinic Rochester, Rochester, MN; University of Connecticut, Farmington, CT; University of Illinois at Chicago, Chicago, IL; and University of Pittsburgh, Pittsburgh, PA), beginning in April 2006 and ending in August 2008. The target population was defined as patients ≥ 40 years of age with a history of previously resected colon cancer (excluding subjects with stage IV disease) or “advanced” colorectal adenomas (≥1 cm in maximal diameter, ≥ 3 in total number, villous morphology, or high-grade dysplasia). After informed consent was obtained, willing participants completed a focused interview, brief CRC risk factor survey, limited physical exam and peripheral blood draw. General eligibility criteria were: no current use of nonsteroidal anti-inflammatory drugs (NSAIDs; excluding low-dose aspirin, defined as ≤ 162.5 mg per day or ≤ 325 mg every other day), corticosteroids, statin drugs, anticoagulant/antiplatelet drugs, investigational drugs, or other compounds likely to interfere with the planned study interventions; and ECOG performance status ≤ 2. Women of childbearing potential were also required to document a negative pregnancy test prior to enrollment. Exclusion criteria were: history of invasive malignancy (other than colon cancer) within the preceding 5 years; rectal surgery; heritable cancer syndrome, inflammatory bowel disease, pelvic radiation therapy; endoscopically confirmed peptic ulcer disease; currently breastfeeding; allergy to compounds of similar chemical structure or biological composition as the study agents; or uncontrolled intercurrent illness that, in the investigator’s opinion, might limit compliance with the study requirements.
Trial enrollment further required ≥ 5 rectal ACF at the baseline MCE exam. Study endoscopists were provided with a reference set of endoscopic images (depicting rectal ACF and/or other mucosal abnormalities) and were asked to complete a short knowledge assessment test based on these images prior to conducting their first study-related procedure. MCE exams were performed using Olympus close focus prototype colonoscopes (model CF-Q160ALE). Pre-procedure bowel cleansing was achieved using oral purgatives (i.e., polyethylene glycol preparation), or rectal enemas for subjects who were unwilling or unable to comply with oral purgation. Following routine mucosal inspection, the distance from the anal verge to the middle valve of Houston was recorded to define the rectal segment (approximately 15 cm in length). Mucosal washings were performed until > 90% of the rectal mucosa was well visualized. All polyps ≥ 2 mm were removed from the rectal segment; adequate hemostasis was achieved and additional mucosal washings were performed, as needed, prior to proceeding. Using a spray catheter, the rectal mucosa was coated with at least 60 cc of 10% Mucomyst solution (left in place for 2 minutes), washed with 60 cc of water to clear any residual mucus, and dyed with at least 60 cc of 0.2% methylene blue (21) solution (left in place for 2 minutes). Adequate dye-spray application was defined as ≥ 90% of the rectal mucosa interpreted as well-stained by the study endoscopist. Rectal ACF were identified by a minimum of 5 aggregated crypts in a single grouping (maximum spacing between crypts no more than two times the average crypt diameter) and a crypt diameter at least 1.5 times the diameter of surrounding normal crypts (Figure 1). Recorded ACF characteristics included total count, tissue plane (flat, elevated, depressed), staining intensity (equal, lighter, darker), predominant lumenal shape (irregular, mixed, normal, oval/round, slit-like), crypt number (5–9, 10–19, ≥ 20) and distance from the anal verge (to allow comparison between pre- and post-intervention exams).
Figure 1.
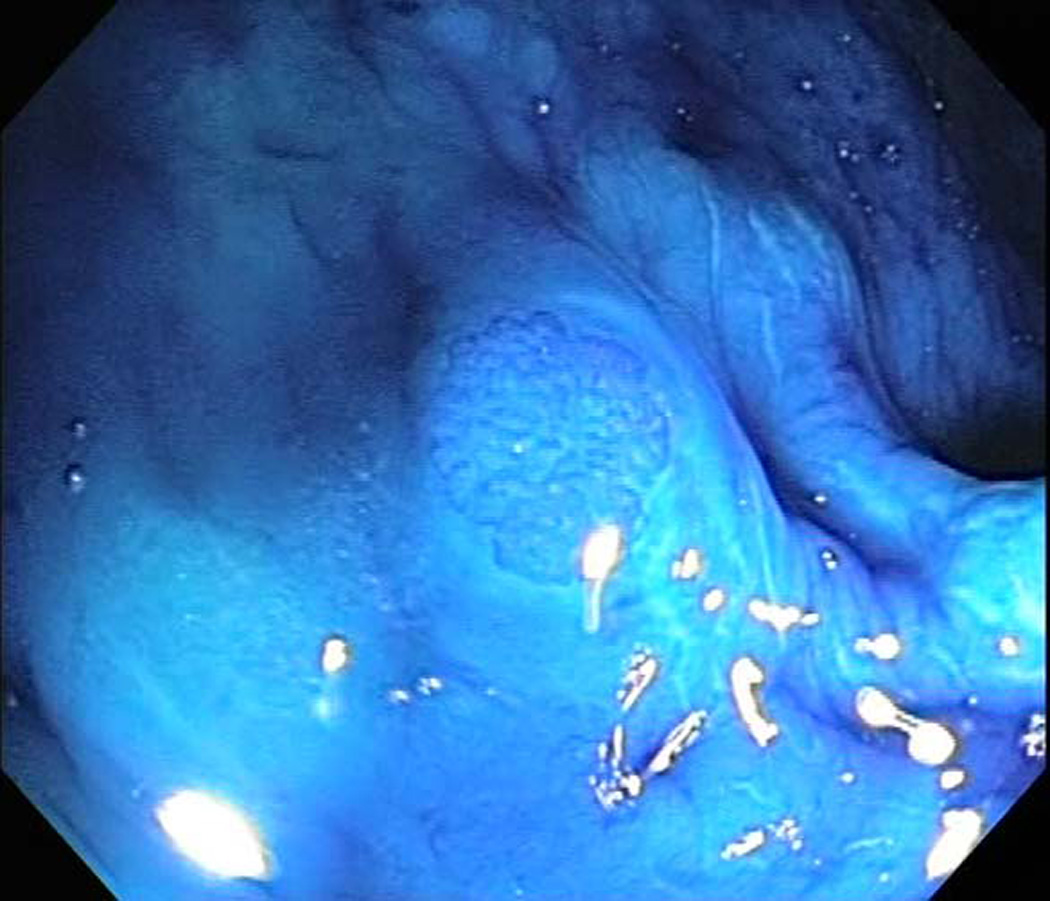
Representative image of rectal aberrant crypt foci (ACF) detected by magnification chromoendoscopy.
For subjects with ≥ 5 rectal ACF at the baseline MCE exam, up to 8 biopsies were obtained from normal-appearing rectal mucosa; no rectal ACF were biopsied at baseline for these subjects. Subjects with < 5 rectal ACF at the baseline MCE exam were not eligible for randomization, but up to 8 biopsies were obtained from normal-appearing rectal mucosa and up to 4 biopsies were obtained from rectal ACF for histologic assessment and other correlative studies. Biopsy samples were divided and fixed in 10% neutral buffered formalin followed by paraffin-embedding (odd numbered samples) or immediately frozen in liquid nitrogen (even numbered samples) for future biomarker analyses.
Intervention Assignments and On-Study Monitoring
Subjects were randomly assigned to one of four intervention arms for 6 months: (A) atorvastatin 20 mg tablet once per day; (B) sulindac 150 mg tablet twice per day; (C) ORAFTI®Synergy1 6 gm powder twice per day; or (D) control (maltodextrin powder twice per day; formulated to permit blinded comparison to the ORAFTI®Synergy1 intervention arm). Randomization was performed by CPN personnel using a dynamic allocation procedure (22) that balanced the intervention arms based on the following stratification factors: history of colon resection (yes vs. no), baseline ACF number (5–9 vs. ≥ 10) and participating site. Telephone interviews were conducted at months 1, 2, 4 and 5 post-randomization and an interim office visit was performed at month 3 (90 +/− 10 days) post-randomization (including a limited physical exam and a peripheral blood draw). Agent compliance and concomitant medication use were recorded. Adverse events were classified and graded using NCI Common Toxicity Criteria, version 3.0 (available at www.ctep.cancer.gov). Attribution of agent-related adverse events was performed by the designated medical monitor (GDZ).
Post-Intervention Evaluation
Subjects returned at 6 months (180 +/− 10 days) post-randomization to assess compliance, concomitant medication use and adverse events. A limited physical exam and peripheral blood draw were also performed. MCE exams were performed using the same standardized protocol applied at baseline. Rectal ACF were counted and characterized, including classification of incident (newly identified) versus prevalent (present at the baseline exam) lesions. For all subjects, up to 8 biopsies were obtained from normal-appearing rectal mucosa and up to 8 biopsies were obtained from rectal ACF (if > 8 rectal ACF were identified, the largest lesions were targeted for biopsy). Biopsy samples were divided and fixed in 10% neutral buffered formalin followed by paraffin-embedding (odd numbered samples) or immediately frozen in liquid nitrogen (even numbered samples) for future biomarker analyses.
Tissue Biomarker Assays
Formalin-fixed, paraffin-embedded rectal biopsy samples were processed on site and shipped to Mayo Clinic Rochester for further analyses. Results were reported by an experienced gastrointestinal pathologist (TCS), without prior knowledge of the intervention assignment or endoscopic findings. General histology was classified as normal, hyperplastic, dysplastic, or other. When present, dysplasia was graded as low-grade, high-grade, invasive carcinoma or indeterminate. Mucosal proliferation and apoptosis were measured from normal-appearing rectal biopsy samples using a dual immunohistochemistry (IHC) staining protocol to label Ki67 and cleaved caspase-3, respectively. IHC results were recorded as the percent of positively stained cells per visualized field.
Statistical Analyses
The primary trial endpoint was defined as the percent change in number of rectal ACF (%ΔACF = [post-intervention ACF number - baseline ACF number] / baseline ACF number). Trial participants were considered evaluable for the primary endpoint if ACF data were available from both the baseline and post-intervention MCE exams, applying the intent to treat principle. The original sample size was powered to detect a difference of at least one standard deviation (SD) in the mean %ΔACF, based on 3 pair-wise comparisons (i.e., each active intervention arm versus the control [maltodextrin] arm), with a planned variability assessment (without halting accrual) of the %ΔACF to be conducted after data from 40 subjects were available (n = 10 per intervention arm). The SD in the mean %ΔACF was conservatively estimated as the range of possible values (from 0 to 1, divided by four; SD = 25%); 25 subjects per intervention arm yielded 92% power using the two-sample t-test, with a two-sided α= 0.016 (adjusted for multiple comparisons).
Due to bi-directionality in the observed %ΔACF data at the planned variability assessment, the estimated SD was revised to include a wider range of possible values (from −1 to 1, divided by four; SD = 50%). Under these considerations, 17 subjects in each of the sulindac and control arms yielded 76% power to detect a difference of at least one SD in the mean %ΔACF, with a two-sided α = 0.05 (one-sample Wilcoxon test, unadjusted for multiple comparisons). Given emerging controversy regarding the application of rectal ACF as an intermediate endpoint biomarker for CRC chemoprevention trials (23, 24), as well as slower than anticipated accrual, a decision was made to proceed with this revised, albeit moderately powered, sample size estimate.
Summary statistics and frequency tables were used to describe baseline patient characteristics and adverse event rates, and compared between intervention arms using a Chi-square (or Fisher’s exact) test or Kruskal Wallis test for categorical and continuous variables, respectively. Descriptive statistics were used to summarize the baseline and post-intervention levels of the proliferation (Ki67) and apoptosis (caspase-3) biomarkers. %ΔACF and percent change in the biomarker levels were compared within each arm using the Wilcoxon signed rank procedure. The between arm comparisons (both between the active intervention arms and each active intervention arm and control) were carried out using the Wilcoxon rank-sum test. Adverse events were reported as maximum severity per subject and type, across the duration of intervention. All attributions collected for adverse events were reported and all statistical tests are two-sided, unless otherwise noted. Data analyses were conducted using SAS version 9.1.3 (SAS Institute, Inc., Cary, NC).
Results
Cohort Description
A CONSORT overview of subject recruitment is shown in Figure 2. One hundred forty-two unique subjects provided informed consent and were pre-registered for the baseline evaluation. Baseline MCE exams were completed for 129 subjects, of whom 33 had < 5 rectal ACF. Eight additional subjects were excluded based on failure to meet other eligibility criteria. Randomization was performed for 88 subjects. Three subjects were subsequently withdrawn post-randomization after further review of complete baseline evaluation data. Therefore, the intervention cohort included 85 subjects. Baseline demographics are provided in Table 1, by trial participation status. In general, subjects in the intervention cohort (n=85) had similar baseline characteristics as ineligible/withdrawn subjects (except with respect to characteristics that directly influenced trial participation status).
Figure 2.
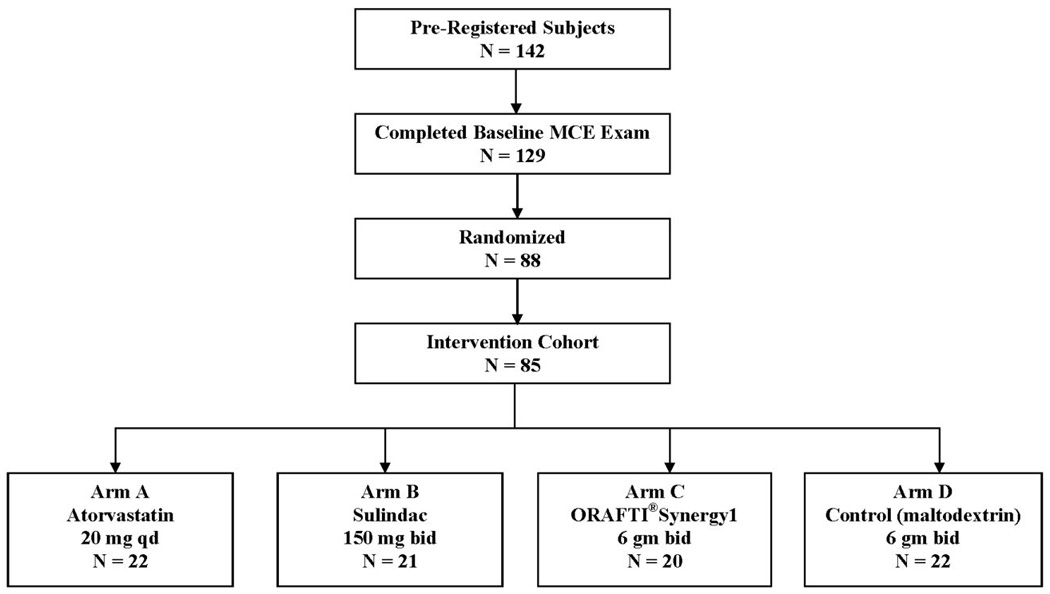
CONSORT overview of subject recruitment.
TABLE 1.
Baseline Demographics, by Trial Participation Status
| All Registered (n=142) |
Ineligible or Withdrawn (n=57) |
Intervention Cohort (n=85) |
p-value1 | |
|---|---|---|---|---|
| Characteristic2 | ||||
| Age, years | 0.49 | |||
| Median | 58 | 59 | 58 | |
| Range | 41–78 | 41–76 | 41–78 | |
| Gender, N (%) | 0.22 | |||
| Women | 55 (38.7) | 26 (45.6) | 29 (34.1) | |
| Men | 87 (61.3) | 31 (54.4) | 56 (65.9) | |
| BMI, kg/m2 | 0.81 | |||
| Median | 28.3 | 28.2 | 28.3 | |
| Range | 15.5–51.3 | 22.5–47.7 | 15.5–51.3 | |
| Cigarette Smoking Status, N (%) | 0.82 | |||
| Never | 56 (42.4) | 21 (44.7) | 35 (41.2) | |
| Current | 18 (13.6) | 5 (10.6) | 13 (15.3) | |
| Former | 58 (43.9) | 21 (44.7) | 37 (43.5) | |
| Alcohol, Drinks per Day, N (%) | 0.85 | |||
| None | 50 (37.9) | 17 (36.2) | 33 (38.8) | |
| < 1 | 69 (52.3) | 24 (51.1) | 45 (52.9) | |
| 2–3 | 10 (7.6) | 5 (10.6) | 5 (5.9) | |
| ≥ 4 | 3 (2.3) | 1 (2.1) | 2 (2.4) | |
| Family History of CRC, N (%)3 | 0.12 | |||
| Yes | 27 (20.4) | 6 (12.8) | 21 (24.7) | |
| No | 105 (79.6) | 41 (87.2) | 64 (75.3) |
p-value for comparison between ineligible or withdrawn subjects versus intervention cohort subjects using Fisher’s Exact test for categorical responses and Rank Sum test for continuous responses;
data for one or more characteristics were missing for n=10 ineligible/withdrawn subjects;
defined as one or more first degree, biological (non-adopted) relative(s) with colorectal cancer.
Baseline ACF
Mucosal dye spray allowed for adequate inspection in 127/129 (99%) subjects, including 85/85 (100%) subjects in the intervention cohort. Nine subjects had no rectal ACF identified. In the remaining 120 subjects, a total of 998 rectal ACF were found (Table 2). The median number (range) of rectal ACF was 2 (0–12) and 9 (5–34) for ineligible/withdrawn and intervention cohort subjects, respectively (p< 0.001). Other baseline ACF characteristics that differed by trial participation status included lumen shape (p=0.005), tissue plane (p<0.001), and staining intensity (p<0.001).
TABLE 2.
Baseline Rectal Aberrant Crypt Foci (ACF), by Trial Participation Status
| All Registered (n=129) |
Ineligible or Withdrawn (n=44) |
Intervention Cohort (n=85) |
p-value1 | |
|---|---|---|---|---|
| ACF Number, total | 998 | 127 | 871 | - |
| ACF Number, per subject2 | < 0.001 | |||
| Median (Range) | 7 (0–34) | 2 (0–12) | 9 (5–34) | |
| Crypts per ACF, N (%) | 0.97 | |||
| 5–9 | 178 (17.8) | 22 (17.3) | 156 (17.9) | |
| 10–19 | 345 (34.6) | 43 (33.9) | 302 (34.7) | |
| ≥20 | 475 (47.6) | 62 (48.8) | 413 (47.4) | |
| Lumen Shape, N (%) | 0.005 | |||
| Irregular | 138 (13.8) | 15 (11.8) | 123 (14.1) | |
| Mixed | 101 (10.1) | 26 (20.5) | 75 (8.6) | |
| Normal | 15 (1.5) | 3 (2.4) | 12 (1.4) | |
| Oval/Round | 609 (61.0) | 70 (55.1) | 539 (61.9) | |
| Slit-like | 132 (13.2) | 13 (10.2) | 119 (13.7) | |
| Othera | 3 (0.3) | 0 (0.0) | 3 (0.3) | |
| Maximum diameter, mm2 | 0.09 | |||
| Median (Range) | 1.0 (0.1–10.0) | 1.0 (0.3–4.0) | 1.0 (0.1–10.0) | |
| Tissue Plane, N (%)3 | < 0.001 | |||
| Depressed | 2 (0.2) | 1 (0.8) | 1 (0.1) | |
| Flat | 575 (57.6) | 53 (41.7) | 522 (59.9) | |
| Elevated | 421 (42.2) | 73 (57.5) | 348 (40.0) | |
| Staining Intensity, N (%)3 | < 0.001 | |||
| Equal | 336 (33.7) | 50 (39.4) | 286 (32.8) | |
| Lighter | 122 (12.2) | 27 (21.3) | 95 (10.9) | |
| Darker | 540 (54.1) | 50 (39.4) | 490 (56.3) |
p-value for comparison between ineligible or withdrawn subjects versus intervention cohort subjects using Fisher’s Exact test for categorical responses and Rank Sum test for continuous responses;
median (range);
relative to the surrounding rectal mucosa.
Intervention Arms, Agent Compliance and Adverse Events
By design, the intervention arms were evenly balanced with respect to history of colon resection (yes vs. no; p=1.00), baseline ACF number (5–9 vs. ≥ 10; p=0.98) and participating site (p=0.97) (Table 3). Baseline age, gender, BMI, cigarette smoking status, daily alcohol consumption, family history of CRC and history of chronic NSAID/analgesic use were also similarly distributed across intervention arms (p > 0.05 for each variable). Agent compliance for at least 3 months was reported by 77/85 (91%) subjects; 74/85 (87%) subjects were compliant with the full 6 month intervention. The median (range) of planned agent doses received was 96.7% (1.4, 108.1%) overall. Reported compliance was lowest in the control arm (median [range] of 86.4% [1.4, 103.9]; p=0.02 for comparison across intervention arms). At least one adverse event, regardless of attribution, was reported by 60/85 (71%) subjects (Table 3). The large majority of these adverse events were classified as grade 1 (56%) or grade 2 (31%), with fewer grade 3 (6%) and no grade 4 adverse events reported. Based on the medical monitor’s adjudication, none of the grade 3 adverse events were related to the study agent.
TABLE 3.
Baseline Demographics, Intervention Compliance and Adverse Events (AEs), by Randomization Arm
| Overall (n=85) |
Atorvastatin (n=22) |
Sulindac (n=21) |
ORAFTI® Synergy1 (n=20) |
Control (n=22) |
p value1 | |
|---|---|---|---|---|---|---|
| Characteristic | ||||||
| Age, Years | 0.07 | |||||
| Median | 58 | 61 | 55 | 64 | 57 | |
| Range | 41–78 | 44–78 | 41–74 | 48–78 | 44–72 | |
| Gender, N (%) | 0.51 | |||||
| Women | 29 (34.1) | 5 (22.7) | 9 (42.9) | 8 (40.0) | 7 (31.8) | |
| Men | 56 (65.9) | 17 (77.3) | 12 (57.1) | 12 (60.0) | 15 (68.2) | |
| BMI, kg/m2 | 0.78 | |||||
| Median | 28.3 | 27.2 | 29.0 | 27.9 | 29.4 | |
| Range | 15.5–51.3 | 15.5–42.0 | 18.4–51.0 | 19.8–39.6 | 22.5–51.3 | |
| Cigarette Smoking Status, N (%) | 0.42 | |||||
| Never | 35 (41.2) | 9 (40.9) | 9 (42.9) | 11 (55.0) | 6 (27.3) | |
| Current | 13 (15.3) | 5 (22.7) | 4 (19.1) | 1 (5.0) | 3 (13.6) | |
| Former | 37 (43.5) | 8 (36.4) | 8 (38.1) | 8 (40.0) | 13 (59.1) | |
| Alcohol, Drinks per Day, N (%) | 0.16 | |||||
| None | 33 (38.8) | 9 (40.9) | 6 (28.6) | 12 (60.0) | 6 (27.3) | |
| ≤1 | 45 (52.9) | 10 (45.5) | 14 (66.7) | 7 (35.0) | 14 (63.6) | |
| 2–3 | 5 (5.6) | 3 (13.6) | 0 (0.0) | 1 (5.0) | 1 (4.6) | |
| ≥ 4 | 2 (2.4) | 0 (0.0) | 1 (4.8) | 0 (0.0) | 1 (4.6) | |
| Family History of CRC, N (%) | 0.42 | |||||
| Yes | 21 (24.7) | 5 (22.7) | 6 (28.6) | 7 (35.0) | 3 (13.6) | |
| No | 64 (75.3) | 17 (77.3) | 15 (71.4) | 13 (65.0) | 19 (86.4) | |
| History of Surgical Resection, N (%) | 1.00 | |||||
| Yes | 25 (29.4) | 7 (31.8) | 6 (28.6) | 6 (30.0) | 6 (27.3) | |
| No | 60 (70.6) | 15 (68.2) | 15 (71.4) | 14 (70.0) | 16 (72.7) | |
| Intervention Compliance | ||||||
| Planned Doses Received, % | 0.02 | |||||
| Median | 96.7 | 99.3 | 96.4 | 97.4 | 86.4 | |
| Range | 1.4–108.1 | 28.9–101.7 | 5.6–108.1 | 50.0–103.1 | 1.4–103.9 | |
| Adverse Events, N (%)2 | ||||||
| Any Grade | 60 (70.6) | 15 (68.2) | 14 (66.7) | 12 (60.0) | 19 (86.4) | 0.25 |
| Grade 1 | 48 (56.5) | 11 (50.0) | 12 (57.1) | 10 (50.0) | 15 (68.2) | 0.60 |
| Grade 2 | 26 (30.6) | 5 (22.7) | 4 (19.0) | 4 (20.0) | 13 (59.1) | 0.02 |
| Grade 3 | 5 (5.9) | 2 (9.1) | 2 (9.5) | 0 (0.0) | 1 (4.5) | 0.71 |
p value for comparison across randomization arms, using Fisher’s Exact test;
maximum severity AE per participant.
Post-Intervention ACF
Complete post-intervention MCE data were available for 77/85 (91%) subjects. Reasons for incomplete data on the remaining 8 subjects were: lost to follow-up (n=3), consent withdrawn (n=2), medical contraindication to the study-related procedures (n=1) or other (n=2). Among the 77 subjects who completed the post-intervention MCE exam, rectal ACF were identified in 76 subjects (n=766 rectal ACF total), with a median (range) of 8 (0–37) (Table 4). Per the examining endoscopist, 518 ACF (68%) were categorized as incident (i.e., newly identified) and 248 ACF (32%) were categorized as prevalent (i.e., present at the baseline evaluation). Compared to prevalent ACF, incident ACF had fewer crypts (p< 0.001), were more likely to be elevated (p=0.002) and stained more intensely relative to the surrounding rectal mucosa (p<0.001).
TABLE 4.
Change in Rectal ACF Number, by Randomization Arm
| Overall (n=77) |
Atorvastatin (n=20) |
Sulindac (n=18) |
ORAFTI®Synergy1 (n=20) |
Control (n=19) |
|
|---|---|---|---|---|---|
| Baseline | |||||
| Median | 9.0 | 9.0 | 9.5 | 8.5 | 9.0 |
| Range | 5–34 | 5–24 | 5–34 | 5–28 | 6–17 |
| Mean | 10.3 | 10.1 | 11.1 | 10.2 | 9.7 |
| Std Deviation | 5.2 | 5.0 | 6.9 | 5.5 | 3.1 |
| Post-Intervention | |||||
| Median | 8.0 | 8.0 | 7.5 | 8.5 | 8.0 |
| Range | 0–41 | 3–41 | 1–27 | 1–34 | 0–20 |
| Mean | 9.9 | 11.0 | 9.9 | 10.3 | 8.5 |
| Std Deviation | 7.6 | 8.6 | 8.0 | 8.7 | 4.9 |
| % Change1 | |||||
| Median | −7.1 | 5.6 | −18.6 | −3.6 | −10.0 |
| Range | −100–160 | −69–143 | −83–160 | −88–83 | −100–117 |
| Mean | −5.3 | 8.4 | −13.3 | −8.8 | −8.6 |
| Std Deviation | 50.3 | 49.1 | 55.4 | 49.2 | 48.8 |
| p value2 | n/a | 0.30 | 0.60 | 0.92 | n/a |
| p value3 | 0.20 | 0.59 | 0.12 | 0.54 | 0.41 |
(post-intervention ACF number – baseline ACF number) / baseline ACF number;
Wilcoxon Rank Sum p-value for comparison of % change in ACF between each active intervention (atorvastatin, sulindac, or ORAFTI®Synergy1) versus control;
Signed Rank p-value for comparison of % change in ACF within each randomization arm.
Data regarding %ΔACF are shown in Table 4, overall and by intervention arm. Subjects assigned to the control arm exhibited no change in rectal ACF number (median value [range] of %ΔACF = −10.0% [−100, 117%]) between the post-intervention and baseline MCE exams. For subjects in the atorvastatin, sulindac and ORAFTI®Synergy1 active arms, the median value (range) of %ΔACF was 5.6% (−69, 143%); −18.6% (−83, 160%); and −3.6% (−88, 83%), respectively. The observed %ΔACF values were not statistically significant when each active intervention arm was independently compared to the control arm (p=0.30, 0.60 and 0.92) or when the post-intervention versus baseline ACF counts were compared within each active intervention arm (p=0.59, 0.12 and 0.54).
Proliferation and Apoptosis
Ki67 and caspase-3 assay data were measured from baseline and post-intervention biopsy samples of normal-appearing rectal mucosa, as secondary endpoints. Paired results were available for 61/85 (72%) and 55/85 (65%) subjects, respectively. For Ki67, the mean (SD) percent change was 13.6 (44.1) for subjects assigned to the control arm (Figure 3). The corresponding values were 6.5 (62.3), 12.2 (52.8) and 34.7 (70.6) for subjects assigned to receive atorvastatin, sulindac, or ORAFTI®Synergy1, respectively. None of the within arm or between arm comparisons were statistically significant (p > 0.05 for each comparison). For caspase-3, the mean (SD) percent change was 130.6 (81.8) for subjects assigned to the control arm (Figure 4). The corresponding values were 106.0 (140.3), 131.9 (154.1) and 120.5 (195.6) for subjects assigned to receive atorvastatin, sulindac, or ORAFTI®Synergy1, respectively. Caspase-3 levels increased significantly when compared within each intervention arm (atorvastatin, p=0.008; sulindac, p=0.005; ORAFTI®Synergy1, p=0.05; control, p=0.001). However, comparisons of change in caspase-3 level between each active arm and the control arm were not statistically significant (p > 0.05 for each comparison).
Figure 3.
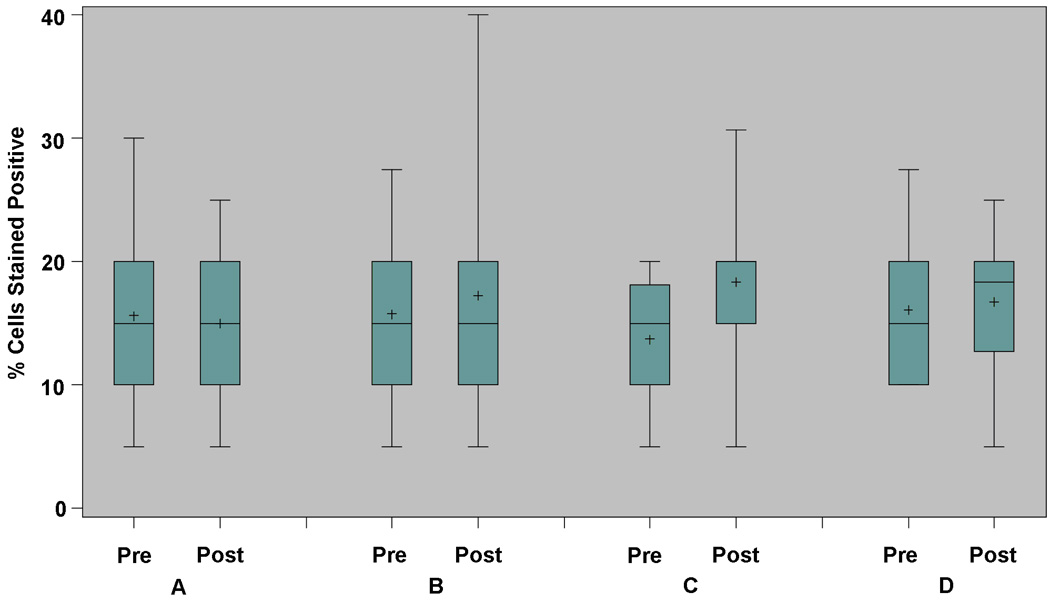
Change in proliferation (Ki67), by intervention arm. Based on biopsy samples from normal-appearing rectal mucosa obtained during the pre-intervention (baseline) and post-intervention evaluations. Box and whisker plot, demonstrating median, interquartile range, and extreme values; mean value also represented (+). Arm A: atorvastatin 20 mg tablet once per day; Arm B: sulindac 150 mg tablet twice per day; Arm C: ORAFTI®Synergy1 6 gm powder twice per day; Arm D: control (maltodextrin powder) twice per day.
Figure 4.
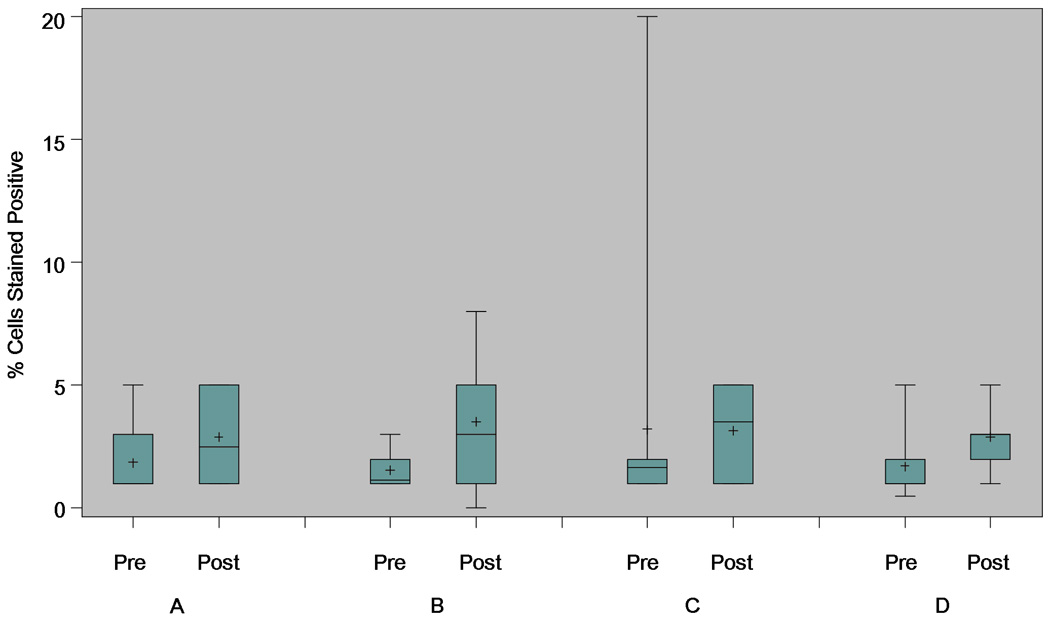
Change in apoptosis (caspase-3), by intervention arm. Based on biopsy samples from normal-appearing rectal mucosa obtained during the pre-intervention (baseline) and post-intervention evaluations. Box and whisker plot, demonstrating median, interquartile range, and extreme values; mean value also represented (+). Arm A: atorvastatin 20 mg tablet once per day; Arm B: sulindac 150 mg tablet twice per day; Arm C: ORAFTI®Synergy1 6 gm powder twice per day; Arm D: control (maltodextrin powder) twice per day.
Histologic Assessment
Histology findings were compared to MCE findings in secondary data analyses. Normal mucosa biopsy samples were available for review from 98 (34 ineligible/withdrawn, 64 randomized) subjects (median [range] = 2 [1–8] biopsies per subject), including 144 biopsies from the baseline evaluation and 56 biopsies from the post-intervention evaluation. Histology findings from the endoscopically normal-appearing biopsy samples included normal mucosa (n=180; 90%), hyperplasia (n=5; 3%), low-grade dysplasia (n=2; 1%) and other (n=13; 7%). Rectal ACF biopsies were available for review from 68 subjects (median [range] = 3 [1–8] biopsies per subject), including 28 biopsies from the baseline evaluation and 164 biopsies from the post-intervention evaluation. Histology findings from the rectal ACF biopsy samples included normal mucosa (n=113; 59%), hyperplasia (n=72; 38%), low-grade dysplasia (n=4; 2%) and other (n=3; 2%).
Discussion
In this prospective, randomized clinical trial, six-month interventions with sulindac, atorvastatin and ORAFTI®Synergy1 did not yield significant reductions in rectal ACF number, as compared to control (maltodextrin), among subjects at increased risk for sporadic CRC. To our knowledge, this study represents the largest ACF-based CRC chemoprevention trial reported to date, and the first such trial to be conducted in stand-alone fashion, rather than ancillary to or embedded within a larger parent study. Further, secondary analyses of tissue-based biomarkers (assessed from normal-appearing rectal mucosa) in our study revealed that none of the interventions had a statistically significant effect on cellular proliferation, while all of interventions (including the maltodextrin control) were associated with increased apoptosis. Yet, since the observed pro-apoptotic effects did not differ appreciably between the active and control interventions, the relevance of these latter data to defining the CRC chemopreventive potential of sulindac, atorvastatin and ORAFTI®Synergy1 remains indeterminate.
Only two other prospective CRC chemoprevention trials have been reported with change in rectal ACF number as the primary endpoint (17, 20). Takayama, et al. initially described the results of an open-label trial of sulindac 100 mg tid conducted among a subset of subjects (n=20) enrolled in a MCE study designed to evaluate ACF prevalence across CRC risk groups (20). Compared to 9 untreated subjects, 11 subjects who received sulindac for 8–12 months had significantly fewer rectal ACF at the post-treatment MCE exam (p <0.001 for difference between groups). More recently, Cho and colleagues (17) used a substudy design to assess change in ACF number among 45 participants enrolled in the Adenoma Prevention with Celecoxib (APC) trial. Following an 8–12 month intervention period, celecoxib (200 mg or 400 mg bid) did not appear to beneficially modulate rectal ACF number, with average +/− SE values for pre- to postintervention change in ACF number of 1.8 +/− 1.1 and 2.3 +/−1.7 among subjects assigned to the active versus placebo arms, respectively (p=0.77). Up to 5 rectal ACF per subject were biopsied at baseline; however, accounting for baseline ACF removal did not meaningfully alter the primary endpoint comparison (p=0.60).
Although rectal ACF have been widely discussed as a putative precursor to colorectal adenoma and cancer (20, 25), concerns have been recently raised regarding measurement of these lesions as an intermediate endpoint for CRC chemoprevention trials (23), due in large part to the low prevalence of histologically-confirmed dysplasia. Indeed, in the aforementioned studies by Takayama (20) and Cho (17), dysplastic ACF comprised only 5% (161/3155) and 0% (0/70), respectively, of the microscopically analyzed lesions. In our trial, dysplastic ACF were similarly infrequent (2%; based on histologic review of post-intervention ACF biopsy samples). Previous observational studies have reported a somewhat wider range of dysplastic ACF (0–23%), albeit from variably defined subject populations (26–30). Nonetheless, further investigation seems prudent to clarify the validity of rectal ACF as a surrogate marker for CRC risk prior to incorporating these lesions as intermediate endpoints in future prevention studies.
Emerging data suggest that the natural history of rectal ACF could also influence the results of short-term CRC chemoprevention trials. In an ancillary study to the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial, Schoen and colleagues performed follow-up MCE exams at one year on 64 subjects with rectal ACF identified (mean = 2.1 per subject), but not removed, at the baseline evaluation (31). Forty-three percent of the baseline ACF were re-identified at follow-up. Moreover, 56% of the subjects had newly-identified rectal ACF. A subsequent analysis of subjects who underwent rectal ACF removal at baseline and returned for follow-up MCE exam one year later showed relatively little change in the mean number of lesions per subject (2.25 vs. 1.93)(32), indicating a dynamic change in ACF progression, regression and/or detection rates over the lifespan of a typical phase II CRC chemoprevention trial. To explore this possibility in our intervention cohort, we conducted sensitivity analyses based on incident versus prevalent ACF and observed no appreciable differences in the agent-specific effects (data not shown).
Potential challenges related to the MCE exam should also be considered when interpreting the results of our phase II CRC chemoprevention trial. Most notably, based again on data from the PLCO ancillary ACF study, the interrater agreement for most endoscopic criteria used to identify rectal ACF appears to be low (33). In addition, up to 55% of endoscopically-identified ACF have not been confirmed by histology in other North American studies (27), which is consistent with the “false positive” rate observed in our trial. Thus, variability in the endpoint assessment might have contributed to our generally null results.
Major strengths of our study include the prospective, multi-center trial design that afforded successful achievement of our revised accrual target. We also utilized a standardized MCE exam protocol, which incorporated pre-trial ACF knowledge assessment and uniform, prototype endoscopy equipment across study sites, to minimize potential influence from technical challenges on the primary endpoint evaluation. Yet, even with this rigorous approach, striking standard deviations were observed in the %ΔACF data. Several additional factors might have affected our ability to detect a significant benefit from sulindac, atorvastatin or ORAFTI®Synergy1. First, polymorphisms in flavin monooxygenase 3 have been shown to influence sulindac efficacy in other high-risk populations (34), but were not measured in our study cohort. Second, based on a recently published meta-analysis of data from 18 studies and involving more than 1.5 million subjects (35), the magnitude of CRC risk reduction associated with statin use may be much lower (8%) than anticipated during the design phase of our trial. In fact, observational data from the APC trial cohort imply that statin drug use may be associated with increased adenoma risk among subjects with previously resected colorectal neoplasia (36). Third, while prebiotic dietary fiber alone might be anticipated to provide a somewhat lesser degree of CRC risk reduction than candidate pharmaceutical agents, limited available data suggest that ORAFTI®Synergy1 could have greater anti-cancer potential if given in combination with probiotic agents (16, 37). Fourth, despite being larger than any previously reported CRC chemoprevention trial with an ACF endpoint (17, 20), our study was only moderately powered to detect the defined intervention effect. Lastly, since the assessed biomarkers (endoscopic and tissue-based) were measured from the rectum only, it remains conceivable that chemopreventive effects confined to the colon could have gone undetected.
In summary, data from this phase II randomized, partially-blinded chemoprevention trial do not provide convincing evidence of CRC risk reduction from atorvastatin, sulindac, or ORAFTI®Synergy1. Larger sample size, longer intervention period, and/or alternate endpoints should be considered if further evaluation of these candidate agents is pursued. Ongoing investigation of the endoscopic, histologic and molecular characteristics of rectal ACF that accurately reflect CRC risk may also serve to clarify if, or how, these lesions can be effectively applied as surrogate markers in future prevention studies.
Acknowledgments
The authors gratefully acknowledge the staff of the Mayo Clinic Clinical Research Unit (supported by grant M01-RR00585); Drs. Vandana Nehra, Wilma Lingle, Charles Erlichman and Frank Sincrope; and Elsa (Hope) Carlson, Colleen Garvey, and Sharon Kaufman for their assistance with study design, administration and manuscript preparation. This work was sponsored by the National Cancer Institute, Division of Cancer Prevention, contract N01-CN-35000.
Research Support: Supported by a contract from the National Cancer Institute (N01CN35000). Research support (study supplies, equipment and/or meeting support) was also provided by the Beneo Group and Olympus America.
Footnotes
Disclosures: Dr. Limburg served as a consultant for Genomic Health, Inc. from 8/12/08–4/19/10. Mayo Clinic has licensed Dr. Limburg's intellectual property to Exact Sciences and he and Mayo Clinic have contractual rights to receive royalties through this agreement.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Herszenyi L, Farinati F, Miheller P, et al. Chemoprevention of colorectal cancer: feasibility in everyday practice? Eur J Cancer Prev. 2008;17:502–514. doi: 10.1097/CEJ.0b013e3282f0c080. [DOI] [PubMed] [Google Scholar]
- 3.Das D, Arber N, Jankowski JA. Chemoprevention of colorectal cancer. Digestion. 2007;76:51–67. doi: 10.1159/000108394. [DOI] [PubMed] [Google Scholar]
- 4.Hawk ET, Umar A, Viner JL. Colorectal cancer chemoprevention--an overview of the science. Gastroenterology. 2004;126:1423–1447. doi: 10.1053/j.gastro.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 6.Ladenheim J, Garcia G, Titzer D, et al. Effect of sulindac on sporadic colonic polyps. Gastroenterology. 1995;108:1083–1087. doi: 10.1016/0016-5085(95)90206-6. [DOI] [PubMed] [Google Scholar]
- 7.Meyskens FL, McLaren CE, Pelot D, et al. Difluoromethylornithine Plus Sulindac for the Prevention of Sporadic Colorectal Adenomas: A Randomized Placebo-Controlled, Double-Blind Trial. Cancer Prev Res Phila Pa. 2008;1:9–11. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuhashi N, Nakajima A, Fukushima Y, et al. Effects of sulindac on sporadic colorectal adenomatous polyps. Gut. 1997;40:344–349. doi: 10.1136/gut.40.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hixson LJ, Earnest DL, Fennerty MB, et al. NSAID effect on sporadic colon polyps. Am J Gastroenterol. 1993;88:1652–1656. [PubMed] [Google Scholar]
- 10.Sassano A, Platanias LC. Statins in tumor suppression. Cancer Lett. 2008;260:11–19. doi: 10.1016/j.canlet.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 11.Singh H, Mahmud SM, Turner D, et al. Long-term use of statins and risk of colorectal cancer: a population-based study. Am J Gastroenterol. 2009;104:3015–3023. doi: 10.1038/ajg.2009.574. [DOI] [PubMed] [Google Scholar]
- 12.Yang YX, Hennessy S, Propert K, et al. Chronic statin therapy and the risk of colorectal cancer. Pharmacoepidemiol Drug Saf. 2008;17:869–876. doi: 10.1002/pds.1599. [DOI] [PubMed] [Google Scholar]
- 13.Vinogradova Y, Hippisley-Cox J, Coupland C, et al. Risk of colorectal cancer in patients prescribed statins, nonsteroidal anti-inflammatory drugs, and cyclooxygenase-2 inhibitors: nested case-control study. Gastroenterology. 2007;133:393–402. doi: 10.1053/j.gastro.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs EJ, Rodriguez C, Brady KA, et al. Cholesterol-lowering drugs and colorectal cancer incidence in a large United States cohort. J Natl Cancer Inst. 2006;98:69–72. doi: 10.1093/jnci/djj006. [DOI] [PubMed] [Google Scholar]
- 15.Pool-Zobel B, van Loo J, Rowland I, et al. Experimental evidences on the potential of prebiotic fructans to reduce the risk of colon cancer. Br J Nutr. 2002;87 Suppl 2:S273–S281. doi: 10.1079/BJNBJN/2002548. [DOI] [PubMed] [Google Scholar]
- 16.Rafter J, Bennett M, Caderni G, et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am J Clin Nutr. 2007;85:488–496. doi: 10.1093/ajcn/85.2.488. [DOI] [PubMed] [Google Scholar]
- 17.Cho NL, Redston M, Zauber AG, et al. Aberrant crypt foci in the adenoma prevention with celecoxib trial. Cancer Prev Res (Phila Pa) 2008;1:21–31. doi: 10.1158/1940-6207.CAPR-07-0011. [DOI] [PubMed] [Google Scholar]
- 18.Corpet DE, Pierre F. How good are rodent models of carcinogenesis in predicting efficacy in humans? A systematic review and meta-analysis of colon chemoprevention in rats, mice and men. Eur J Cancer. 2005;41:1911–1922. doi: 10.1016/j.ejca.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Stevens RG, Swede H, Rosenberg DW. Epidemiology of colonic aberrant crypt foci: review and analysis of existing studies. Cancer Lett. 2007;252:171–183. doi: 10.1016/j.canlet.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takayama T, Katsuki S, Takahashi Y, et al. Aberrant crypt foci of the colon as precursors of adenoma and cancer. N Engl J Med. 1998;339:1277–1284. doi: 10.1056/NEJM199810293391803. [DOI] [PubMed] [Google Scholar]
- 21.Wong Kee Song LM, Adler DG, Chand B, et al. Chromoendoscopy. Gastrointest Endosc. 2007;66:639–649. doi: 10.1016/j.gie.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 22.Pocock SL, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 23.Lance P, Hamilton SR. Sporadic aberrant crypt foci are not a surrogate endpoint for colorectal adenoma prevention. Cancer Prev Res (Phila Pa) 2008;1:4–8. doi: 10.1158/1940-6207.CAPR-08-0043. [DOI] [PubMed] [Google Scholar]
- 24.Gupta AK, Schoen RE. Aberrant crypt foci: are they intermediate endpoints of colon carcinogenesis in humans? Curr Opin Gastroenterol. 2009;25:59–65. doi: 10.1097/MOG.0b013e32831db286. [DOI] [PubMed] [Google Scholar]
- 25.Gupta AK, Pretlow TP, Schoen RE. Aberrant crypt foci: what we know and what we need to know. Clin Gastroenterol Hepatol. 2007;5:526–533. doi: 10.1016/j.cgh.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Hurlstone DP, Karajeh M, Sanders DS, et al. Rectal aberrant crypt foci identified using high-magnification-chromoscopic colonoscopy: biomarkers for flat and depressed neoplasia. Am J Gastroenterol. 2005;100:1283–1289. doi: 10.1111/j.1572-0241.2005.40891.x. [DOI] [PubMed] [Google Scholar]
- 27.Rudolph RE, Dominitz JA, Lampe JW, et al. Risk factors for colorectal cancer in relation to number and size of aberrant crypt foci in humans. Cancer Epidemiol Biomarkers Prev. 2005;14:605–608. doi: 10.1158/1055-9965.EPI-04-0058. [DOI] [PubMed] [Google Scholar]
- 28.Seike K, Koda K, Oda K, et al. Assessment of rectal aberrant crypt foci by standard chromoscopy and its predictive value for colonic advanced neoplasms. Am J Gastroenterol. 2006;101:1362–1369. doi: 10.1111/j.1572-0241.2006.00578.x. [DOI] [PubMed] [Google Scholar]
- 29.Stevens RG, Swede H, Heinen CD, et al. Aberrant crypt foci in patients with a positive family history of sporadic colorectal cancer. Cancer Lett. 2007;248:262–268. doi: 10.1016/j.canlet.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Mutch MG, Schoen RE, Fleshman JW, et al. A multicenter study of prevalence and risk factors for aberrant crypt foci. Clin Gastroenterol Hepatol. 2009;7:568–574. doi: 10.1016/j.cgh.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Schoen RE, Mutch M, Rall C, et al. The natural history of aberrant crypt foci. Gastrointest Endosc. 2008;67:1097–1102. doi: 10.1016/j.gie.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 32.Pinsky PF, Fleshman J, Mutch M, et al. One year recurrence of aberrant crypt foci. Cancer Prev Res (Phila Pa) 3:839–843. doi: 10.1158/1940-6207.CAPR-09-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta AK, Pinsky P, Rall C, et al. Reliability and accuracy of the endoscopic appearance in the identification of aberrant crypt foci. Gastrointest Endosc. 2009;70:322–330. doi: 10.1016/j.gie.2008.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hisamuddin IM, Wehbi MA, Schmotzer B, et al. Genetic polymorphisms of flavin monooxygenase 3 in sulindac-induced regression of colorectal adenomas in familial adenomatous polyposis. Cancer Epidemiol Biomarkers Prev. 2005;14:2366–2369. doi: 10.1158/1055-9965.EPI-05-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonovas S, Filioussi K, Flordellis CS, et al. Statins and the risk of colorectal cancer: a meta-analysis of 18 studies involving more than 1.5 million patients. J Clin Oncol. 2007;25:3462–3468. doi: 10.1200/JCO.2007.10.8936. [DOI] [PubMed] [Google Scholar]
- 36.Bertagnolli MM, Hsu M, Hawk ET, et al. Statin use and colorectal adenoma risk: results from the adenoma prevention with celecoxib trial. Cancer Prev Res (Phila Pa) 3:588–596. doi: 10.1158/1940-6207.CAPR-09-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rowland IR, Rumney CJ, Coutts JT, et al. Effect of Bifidobacterium longum and inulin on gut bacterial metabolism and carcinogen-induced aberrant crypt foci in rats. Carcinogenesis. 1998;19:281–285. doi: 10.1093/carcin/19.2.281. [DOI] [PubMed] [Google Scholar]


