Abstract
A lack of regulatory T (TReg) cells that express CD4, CD25 and forkhead box P3 (FOXP3) results in severe autoimmunity in both mice and humans. Since the discovery of TReg cells, there has been intense investigation aimed at determining how they protect an organism from autoimmunity and whether defects in their number or function contribute to the development of autoimmunity in model systems. The next phase of investigation — that is, to define the role that defects in TReg cells have in human autoimmunity — is now underway. This Review summarizes our progress so far towards understanding the role of CD4+CD25+FOXP3+ TReg cells in human autoimmune diseases and the impact that this knowledge might have on the diagnosis and treatment of these diseases.
Regulatory T (TReg) cells, defined by the expression of CD4, CD25 and the transcription factor forkhead box P3 (FOXP3), have a central role in protecting an individual from autoimmunity. This role was first identified in mice in which the absence of TReg cells, or the depletion of TReg cells, resulted in the development of autoimmune gastritis, thyroiditis, diabetes and inflammatory bowel disease (IBD)1,2. Subsequently, numerous studies in animal models of autoimmunity showed that defects in CD4+CD25+FOXP3+ TReg cells can contribute to the development of autoimmunity and that the disease could be reversed by the adoptive transfer of TReg cells (reviewed in REF. 3). This was followed by studies identifying the presence of TReg cells in human peripheral blood and their ability to suppress T cell proliferation in vitro4–6. The importance of T cell regulation in human disease is highlighted by the severe inflammation and autoimmunity that occurs in individuals who suffer from immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX). These individuals develop a broad range of autoantibodies, insulin-dependent diabetes, thyroiditis, eczema, haemolytic anaemia and IBD, and in the absence of a bone marrow transplant, these patients die at an early age (reviewed in REF. 1). These observations have driven a search for mechanisms of defective T cell regulation in human autoimmunity. In this Review, I discuss the evidence supporting the involvement of impaired T cell regulation in auto-immunity and our current understanding of the source of these regulatory defects. This is considered in the context of several autoimmune and inflammatory diseases: type 1 diabetes, multiple sclerosis, systemic lupus erythematosus (SLE), rheumatoid arthritis, IBD and psoriasis. Although multiple types of regulatory T cell have been described7, I focus on the CD4+CD25+FOXP3+ TReg cell subset in this Review.
Mechanisms of impaired T cell regulation
To address the question of whether immune suppression by TReg cells is impaired in the setting of human auto-immune disease, it is important to recognize the potential means by which such a defect may occur. As shown in FIG. 1 and described in BOX 1, defects in the number and function of TReg cells, as well as a resistance of effector T cells to TReg cell-mediated suppression, could each contribute to failed T cell regulation. Each of these defects has been shown to contribute to the development of autoimmunity in various model systems. In these models, the underlying mechanisms by which these defects in regulation occur have also been investigated. Such studies indicate that cell-intrinsic defects in effector T cells, CD4+FOXP3+ T cells and antigen-presenting cells (APCs), as well as alterations in the composition of the inflammatory milieu, can contribute to failed tolerance to self. Here I address how each of these potential breaches in regulation can be assessed in humans, the current evidence that defects in these pathways are present and the challenges that must be overcome to further define the mechanisms of impaired tolerance in human autoimmune disease.
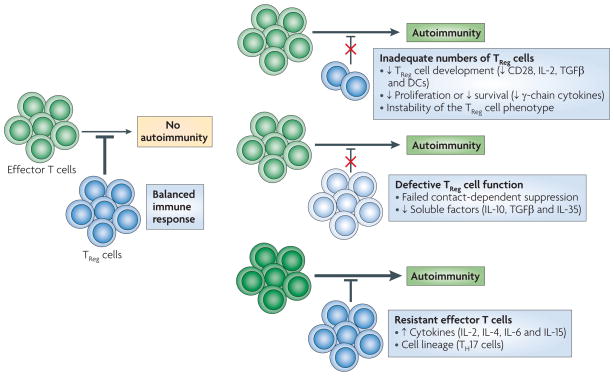
Figure 1. Causes of impaired TReg cell-mediated suppression in autoimmunity.
Autoimmunity can result from a loss of regulation of autoreactive T cells. Failures of regulatory T (TReg) cell-mediated regulation include: inadequate numbers of TReg cells owing to their inadequate development, proliferation or survival; defects in TReg cell function that is intrinsic to TReg cells; and resistance of pathogenic effector T cells to suppression by TReg cells owing to factors that are intrinsic to the effector cells or factors that are present in the inflammatory milieu and that support effector T cell resistance. DC, dendritic cell; IL, interleukin; TGFβ, transforming growth factor-β; TH17, T helper 17.
Box 1. Factors that affect T cell regulation in autoimmunity.
The number of regulatory T (TReg) cells found in individuals with autoimmune disease is influenced by TReg cell development, persistence and proliferation in the periphery and homing to the site of inflammation. Genetic factors are likely to have the strongest impact on thymic output of TReg cells. Maintenance of TReg cells in the periphery is a dynamic process, influenced in part by conditions that favour the induction of TReg cells in the periphery and support their proliferation and survival. Factors that favour the thymic development, peripheral growth and survival of TReg cells have been shown to have an effect on forkhead box P3 (FOXP3) expression11,12,110. Such factors include CD28, transforming growth factor-β (TGFβ), dendritic cells and the common cytokine receptor γ-chain (γc) cytokines (interleukin-2 (IL-2), IL-4, IL-7 and IL-15), which signal through signal transducer and activator of transcription 5 (STAT5).
TReg cell dysfunction in autoimmune disease may be due to a defect in one of the many mechanisms through which TReg cells function (reviewed in REF. 22). This could occur through inadequate expression of cell surface molecules that are known to be involved in contact-dependent suppression (such as cytotoxic T lymphocyte antigen 4 (CTLA4), CD39, lymphocyte activation gene 3 (LAG3), granzyme A and CD95 (also known as FAS)) or as a result of a failure to produce the soluble factors (such as TGFβ, IL-10 and IL-35) that are involved in some aspects of suppression. Underlying genetic factors may influence these mechanisms. In addition, the composition of the local milieu, including the types of antigen-presenting cells and cytokines (such as tumour necrosis factor (TNF)29,111, IL-4 (REF. 112), IL-6 (REF. 29), IL-12 (REF. 113), IL-7, IL-15 (REF. 114) and IL-21 (REF. 28)), can influence TReg cell function.
Multiple mechanisms by which effector T cells resist TReg cells have been proposed (reviewed in REF. 31). Cell-intrinsic resistance to suppression has been shown to occur in CD4+ memory T cells and T helper 17 (TH17) cells115. The cytokines IL-2, IL-4, IL-7 and IL-15 support the proliferation of effector T cells in the presence of TReg cells, indicating that despite the favourable roles of these cytokines in TReg cell homeostasis110, the presence of these cytokines in the short term allows effector T cells to bypass suppression by TReg cells. In addition, several members of the TNF receptor family have been implicated in this process: antibody specific for OX40 (also known as TNFRSF4) abrogates suppression when bound to effector T cells116, and ligation of 4-1BB (also known as TNFRSF9) results in suppression-resistant effector T cells117.
Inadequate numbers of TReg cells
In mouse models, the concept that inadequate numbers of TReg cells may contribute to autoimmunity is supported by the occurrence of aggressive autoimmunity in scurfy mice and is indirectly implied by the successful treatment of autoimmunity in mice through the adoptive transfer of wild-type TReg cells8,9. In addition, there is evidence from mouse models that, under the appropriate conditions, TReg cells can be induced in the periphery, and these TReg cells may protect from the development of autoimmunity8–10. Multiple factors influence the homeostasis and induction of TReg cells in the periphery, including CD28, interleukin-2 (IL-2), transforming growth factor-β (TGFβ)11 and dendritic cells (DCs)12.
Evidence that an inadequate number of TReg cells leads to autoimmunity in humans is most clearly shown in patients with IPEX, who completely lack TReg cells as a result of a mutation in FOXP3 (REF. 13). However, most patients with autoimmune disease probably have a more modest reduction in TReg cells. In these common diseases, the challenge is to determine whether the number of TReg cells is inadequate at the site of inflammation and whether this is due to systemic factors or factors in the local tissue milieu. In human disease, the task of enumerating TReg cells has been complicated by two main issues. The first issue is deciding which cells to count. This is complicated by the lack of a cell marker that is unique to TReg cells and the multiplicity of TReg cell subsets (BOX 2). The second issue is the extent to which the peripheral blood reflects the global number of TReg cells in the body and, more specifically, their number in inflamed tissues.
Box 2. Regulatory T cell subsets: origins, phenotypes and functions.
Multiple T cell subsets with suppressive functions have been identified. The CD4+ T cell subsets are defined by origin, function, and the expression of cell surface markers and the transcription factor forkhead box P3 (FOXP3).
Type 1 regulatory T (TR1) cells are induced in the periphery, suppress T cell proliferation through the production of interleukin-10 (IL-10) and transforming growth factor-β (TGFβ)118 and do not have a unique cell marker but are identified by their production of IL-10 and not pro-inflammatory cytokines.
T helper 3 (TH3) cells are a regulatory T cell population that originates in the periphery and mediates suppression through the secretion of TGFβ; similar to TR1 cells, they do not have a unique cell surface marker119.
CD4+CD25+FOXP3+ regulatory T (TReg) cells can be divided into two groups: thymus-derived natural TReg cells and periphery-induced adaptive TReg cells. Both populations express FOXP3 and suppress immune responses through contact-dependent mechanisms and the production of soluble factors, including the cytokines TGFβ, IL-10 and IL-35 (REFS 18,22). Thymus-derived CD4+CD25+FOXP3+ TReg cells are stable with respect to retaining regulatory function and FOXP3 expression in the periphery. They are unique in that their FOXP3 locus is demethylated120 and they express the transcription factor Helios20. Adaptive TReg cells can be induced in the periphery from a CD4+FOXP3− T cell population following T cell receptor stimulation in the presence of TGFβ. These cells express the same cell surface markers as natural TReg cells and suppress immune responses through cytokines and contact-dependent mechanisms. They can be distinguished from natural TReg cells based on FOXP3 DNA methylation patterns and their lack of Helios expression.
It has now become clear that the FOXP3+ T cell population is composed of several populations that are defined by the expression of CD25, CD45RA and FOXP3. Miyara et al.21 defined these populations as a naive TReg cell population that is CD25hiCD45RA+FOXP3hi, an effector TReg cell population that is CD25hiCD45RA−FOXP3hi and a non-regulatory FOXP3+ population that is CD25hiCD45RA−FOXP3low. Our growing understanding of the complexity of TReg cells indicates that we must continue to consider how alterations in the composition, function and stability of the TReg cell pool may contribute to autoimmunity. When examining the literature, understanding how TReg cells are identified and isolated has important implications for interpretation of the data. New markers will improve these studies in the future.
TReg cells were first defined on the basis of their expression of CD25, which forms part of the high-affinity IL-2 receptor. Among the CD4+CD25+ T cell population is a subset of cells that express CD25 at a high level — approximately 4% of the CD4+ population in human blood — and most of this population has regulatory function. Unfortunately, the definition of TReg cells based on the level of CD25 expression has not been consistently reported in the literature, and this makes comparisons between studies difficult. Furthermore, CD25 is also expressed by recently activated T cells, resulting in the inclusion of CD4+CD25+ effector T cells in the TReg cell population. With the discovery that expression of FOXP3 has a central role in the differentiation and maintenance of TReg cells14,15, the use of flow cytometry-based analysis of FOXP3 expression in T cells became the gold standard for defining TReg cells. However, it then became evident that FOXP3 can also be expressed by effector T cells following activation16, raising the possibility that any assessment of TReg cell number or function may include recently activated effector T cells in the TReg cell population. Furthermore, as FOXP3 is a nuclear protein, assessment of its expression in T cells requires fixation and permeabilization of the cells, resulting in an inability to obtain viable cells for further functional analysis. In the past few years, additional markers, such as CD127 (also known as IL-7Rα)17, have been identified that assist in the distinction of effector T cells from TReg cells and facilitate the experimental purification of TReg cells18.
More recently, the ability to distinguish thymus-derived natural TReg cells from those that are induced in the periphery (BOX 2) has been facilitated by the discovery that the presence of specific demethylated sites in the FOXP3 promoter19 and the expression of the nuclear protein Helios are unique to thymus-derived natural TReg cells20. The use of these markers, as well as the use of intracellular cytokine staining, has led to the discovery of discrete TReg cell subsets that have unique functional characteristics21,22. Defining these TReg cell subsets may be of central importance when enumerating TReg cells in autoimmunity.
Defining defects in TReg cell function
Identifying defects in the function of TReg cells is made difficult both by the multiple mechanisms used by TReg cells to suppress inflammation (reviewed in REFS 23,24) and by the manner in which suppression is measured. In addition, assessment of TReg cell function in humans requires the use of in vitro assays that, owing to the rarity of TReg cells in the peripheral blood, must be carried out with low cell numbers, limiting the type and quality of assays that can be done. Currently, assays of TReg cell function address the ability of TReg cells to inhibit the proliferation of, or cytokine production by, co-cultured effector T cells (BOX 3). Most co-culture assays are carried out with autologous responder T cells and APCs; such studies can define defects in suppression but do not specifi cally test the function of TReg cells. To determine whether the source of impaired suppression is intrinsic to the TReg cells, investigators have used assays that examine the suppressive function of an individual’s TReg cells using effector T cell and/or APC populations that are isolated from healthy controls. Although these assays provide insight into potential TReg cell defects, they cannot account for the impact of the local milieu on TReg cell function.
Box 3. In vitro assays of suppression.
Studies of regulatory T (TReg) cell function in human autoimmune diseases have examined the proliferation of responder cells in response to polyclonal activation in co-cultures with TReg cells isolated from populations of peripheral blood mononuclear cells. Measurements are based on the incorporation of 3H or on the dilution of the fluorescent label 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE). Evaluation of cytokine production by the responder cells can also be carried out in conjunction with these assays. CFSE dilution is in several ways a superior approach for examining TReg cell-mediated suppression, as it reflects the number of cell divisions throughout the culture period, whereas 3H incorporation assays only reflect the level of proliferation that occurs during the period in which the 3H is present in the culture (typically 12–16 hours). The use of CFSE also has the advantage of allowing simultaneous analysis of cell surface markers and cytokines to better define the character of the proliferating responder cells. However, the number of cells that is required for CFSE assays is much greater than that needed for 3H incorporation assays, making CFSE assays less useful when only small amounts of blood are available. The outcome of these studies is influenced by the type and strength of the stimulus used, and interpretation of any of these studies should take this into account41. In addition, the manner by which the TReg cells are isolated can influence the composition of the TReg cell pool and therefore the degree of inhibition. The type of selection method (bead based or flow cytometry based) and the stringency of selection (based on the level of CD25 expression and the use of additional TReg cell markers) can lead to large differences in the percentage of isolated cells that express forkhead box P3 (FOXP3) and in the level of suppression. Alternative approaches that measure antigen-specific or pathogenic T cell responses in the presence or absence of TReg cells have also been used. The limitation of these assays is typically the weak response of CD4+ T cells to a self antigen owing to low precursor frequency, which is further complicated by the inability to control for the TReg cell/effector T cell ratio in the culture.
Measuring resistance of effector T cells to suppression
The resistance of effector T cells to TReg cells has been observed in several animal models of autoimmunity. In these models, inflammation and tissue destruction progress despite the presence of functional TReg cells at the site of inflammation. Such findings suggest that a resistance of effector T cells to TReg cells may contribute to disease progression. This phenomenon has been described in two mouse models of diabetes (non-obese diabetic mice (NOD mice)25–27 and DO11.10 RIP-mOVA mice28), in experimental autoimmune encephalomyelitis (EAE)29 and in the MRL–lpr mouse model of SLE30.
There seem to be multiple ways in which effector T cells can become resistant to suppression. The mechanisms by which this occurs include T cell-intrinsic defects, alterations in the strength of T cell activation and exposure to T cell growth factors (reviewed in REF. 31). To determine whether this phenomenon occurs in humans, an assay system that allows for the assessment of effector T cell suppression by allogeneic TReg cells was required. Such assays have been established and have provided evidence of effector T cell resistance to suppression in several human autoimmune diseases (see below).
Each of the defects in regulation described above may contribute, either in isolation or in combination, to the development of human autoimmune disease. The task of determining the extent and character of these defects in human disease is challenging. Despite these limitations, when studies of human subjects are carried out with a consistent approach to analysis and a well-matched control population, it is possible to determine the potential defects in T cell regulation in an individual. This knowledge then allows the development of hypotheses and the determination of disease mechanisms that are relevant to human disease.
TReg cells and type 1 diabetes
Type 1 diabetes is an immune-mediated disease that results in inflammation and destruction of the pancreatic islet cells, resulting in a lifelong need for insulin. A role for TReg cells in autoimmune diabetes is evident in individuals with IPEX, in which the absence of TReg cells results in enhanced susceptibility to diabetes. In addition, in NOD mice (a spontaneous model of type 1 diabetes), TReg cell numbers and FOXP3 expression decrease with the age of the animals despite ongoing autoimmunity32, and over time the pathogenic effector T cells in the pancreatic islets become resistant to suppression26–28. Adoptive transfer of TReg cells ameliorates disease in NOD mice9, as does treatment with the growth factor IL-2, which increases FOXP3 expression and TReg cell number32.
Whether the number of TReg cells is reduced or normal in patients with type 1 diabetes remains an issue of controversy. The initial analyses of TReg cell numbers (using CD25 expression to define TReg cells) reported a significant decrease in CD4+CD25+ TReg cell numbers in individuals with newly diagnosed or established disease33. This study was followed by a series of other studies that found no difference in the number of CD4+CD25+ or CD4+CD25hi T cells in the peripheral blood of patients with type 1 diabetes compared with controls34–37. Later studies using nuclear staining of FOXP3 as a marker of TReg cells also did not observe differences between healthy controls and patients35. Moreover, a recent study38 found no significant difference in the level of demethylation at the TReg cell-specific demethylated region (TSDR) of FOXP3 in TReg cells from patients with type 1 diabetes compared with controls and, furthermore, the authors did not find differences in the distribution of TReg cells between the CD4+FOXP3+CD45RA+ and CD4+FOXP3+CD45RO+ TReg cell subsets. Another recent study examining these TReg cell subsets in individuals with new-onset diabetes reports a relative increase in the CD45RA−FOXP3low T cell population compared with controls and shows that these cells produce IL-17 and are non-regulatory. Thus, in early disease there may be an inadequate number of functional TReg cells39.
Although the total number of circulating TReg cells seems to be normal in patients with type 1 diabetes, it is possible that the persistence and function of TReg cells are impaired at sites of inflammation. A recent study showed that TReg cells from patients with type 1 diabetes had a reduced capacity to respond to IL-2, and the authors correlated this defect in IL-2-induced signalling with a loss of FOXP3 expression38. This finding mirrors the loss of FOXP3 expression by TReg cells infiltrating the islets in NOD mice owing to a decrease in the levels of available IL-2 (REF. 32). A histological study of islets that were isolated from patients with new-onset type 1 diabetes immediately post-mortem found that FOXP3+ T cells were only rarely present in the islets; this is consistent with the idea that there are inadequate numbers of TReg cells in the islets of individuals with type 1 diabetes40. Currently, additional studies that are designed to enumerate TReg cells at the sites of inflammation — the pancreatic islets and draining lymph nodes — are underway and may shed more light on this issue.
A functional defect in TReg cells isolated from individuals with type 1 diabetes has been shown by several investigators (reviewed in REF. 41). These studies assessed the impact of CD4+CD25+ TReg cells, isolated by beads or flow cytometry, on autologous responder CD4+ T cells following nonspecific activation. Although the results have been mixed, most of these studies showed a decrease in the degree of TReg cell-mediated inhibition34,36,37. All of the suppression assays were carried out using autologous effector T cells, so they do not specifically define the defect as being intrinsic to the TReg cells. More recently, defects that are intrinsic to a TReg cell population in type 1 diabetes have been identified. In these studies42,43, the TReg cells of several individuals with type 1 diabetes were unable to suppress effector T cells from a healthy control.
The role of effector T cell resistance in type 1 diabetes has also been assessed by two independent groups. Studies by my group showed effector T cell resistance to suppression in type 1 diabetes, using a 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE)-based assay. We found that the resistance was intrinsic to the effector T cells and occurred irrespective of the source of TReg cells (natural TReg cells or inducible TReg cells)42. Similar resistance to suppression was also shown by Lawson et al.43 using a 3H-based assay system. Both of these investigations studied individuals with long-standing type 1 diabetes, raising the important issue of whether the defect is a cause or an effect of disease.
In summary, the current evidence does not indicate a global deficiency in TReg cell numbers in type 1 diabetes but does not rule out a more focal deficiency in the TReg cell compartment, with respect to either a subset of TReg cells or the specificity of TReg cells for islet antigens. Multiple studies show that suppression is impaired and indicate that this is, in part, due to the resistance of effector T cells to suppression by TReg cells.
TReg cells and multiple sclerosis
Multiple sclerosis is an autoimmune disease characterized by inflammatory lesions and degeneration in the central nervous system (CNS). The pathogenic CD4+ T cells in multiple sclerosis target myelin-based antigens, have the ability to migrate to the CNS and have unique effector functions44. Although the inflammatory components involved in multiple sclerosis are numerous, defects in TReg cells as a component of this disease are implicated by both the mouse model of multiple sclerosis (EAE) and the human disease.
Animals that express a transgenic T cell receptor specific for myelin basic protein (MBP) and lack recombination-activating genes (RAGs) spontaneously develop EAE owing to an absence of TReg cells14, indicating a role for TReg cells in the prevention of EAE. Further studies have shown an increase in susceptibility to EAE following depletion of TReg cells using CD25-specific antibody, and adoptive transfer of TReg cells reduces the incidence of EAE14,45. However, after the disease is established, the ability of TReg cells to control inflammation in the CNS is controversial, despite their ability to migrate to the CNS. One explanation for this is the resistance of effector T cells in the CNS to TReg cell-mediated suppression during active disease owing to the production of IL-6 and tumour necrosis factor (TNF)45.
As for other human autoimmune diseases, the number of TReg cells in the peripheral blood is the most extensively investigated aspect of T cell regulation in multiple sclerosis. Studies have assessed TReg cell number in patients with active or inactive disease and those receiving therapy. In most of these studies, no differences were observed in the percentage of TReg cells (defined as CD4+CD25hi cells) in the blood of healthy control individuals or subjects with multiple sclerosis, irrespective of disease activity46–52. However, the number of TReg cells has been found to be increased in the cerebrospinal fluid compared with the number in the peripheral blood in patients with multiple sclerosis49,53. In two studies54,55, the number of TReg cells (defined as CD4+CD25+FOXP3+ cells) was found to be increased in patients with multiple sclerosis; in the study by de Andrés et al.55, this increase normalized after 6 months of therapy with interferon-β (IFNβ). Only one study found a decrease in CD4+CD25+FOXP3+ T cell numbers compared with healthy controls53; in this case, the decrease was unique to subjects with relapsing–remitting multiple sclerosis and normal cell numbers were restored following treatment with IFNβ. An important aspect of this study was that the authors observed that the percentage of FOXP3+ cells was stable over a 1-year period in a subset of untreated patients with relapsing–remitting multiple sclerosis53.
More recent studies have identified alterations in the composition of the TReg cell pool in multiple sclerosis. Papers by Haas et al.48 and Venken et al.51 both reported a decrease in the naive or recent thymic immigrant TReg cells, a population of TReg cells that has enhanced suppressive function. A decrease in the percentage of CD4+CD25hi cells that express CD39, a molecule that has been linked to TReg cell function in mice, has also been described52. In addition, subjects with multiple sclerosis have been reported to have an increase in the CD127+ population of FOXP3+ TReg cells, a subset that is not suppressive50. One interesting finding that also questions the importance of TReg cell numbers in multiple sclerosis was made in patients with multiple sclerosis who received daclizumab (Zenapax; Hoffmann-La Roche), a monoclonal antibody that targets CD25 and results in a reduction in FOXP3+ cell numbers. The authors of this study did not observe a correlation between the clinical response to the treatment and the decrease in FOXP3+ cells in these patients56. Overall, there is no evidence for inadequate numbers of TReg cells in established multiple sclerosis, but more recent studies suggest that alterations in the composition of the TReg cell population may have a role in the disease.
There has also been extensive study of TReg cell function in multiple sclerosis. Such studies consistently show impaired suppression when TReg cells are incubated with autologous responder T cells, using co-cultures in which proliferation is measured by either 3H incorporation or CFSE dilution46,50,57–59. In two of these studies46,57, the suppression of secretion of IFNγ by effector T cells has also been shown to be diminished in patients with multiple sclerosis. In addition, two studies have examined the suppression of myelin-specific T cells and found a decrease in the suppression of myelin oligodendrocyte glycoprotein (MOG)- and MBP-specific responses in patients with multiple sclerosis compared with controls54,57. However, it is worth noting that no correlation between TReg cell function and disease activity has been identified.
Several sources of regulatory defects have been observed in multiple sclerosis. Contamination of the studied TReg cell population with non-suppressive cells has been described by two groups50,58. Michel et al.50 found that the level of suppression did not differ between multiple sclerosis subjects and control subjects if only the top 2% of CD4+CD25+ cells was used in suppression assays, thereby excluding a subset of CD127+ cells that produce pro-inflammatory cytokines. A defect in the production of the regulatory cytokine IL-10 by TReg cells has also been implicated by Astier et al.59, who found that following activation of CD4+ T cells from patients with multiple sclerosis using CD3-specific antibody and CD46-specific antibody, IL-10 production is reduced. However, this was not seen when the cells were activated with CD3-specific antibody and CD28-specific antibody and thus it does not explain the impaired suppression seen in other studies but suggests yet another mechanism by which suppression might be impaired in multiple sclerosis59.
Three studies have addressed whether effector T cells are resistant to suppression in multiple sclerosis46,48,57. None of these studies observed defective suppression of effector T cells from patients with multiple sclerosis by TReg cells from controls. However, these experiments were carried out with a very limited number of samples (one to three individuals were studied in each investigation). Given that effector T cell resistance has been observed in EAE45, that multiple sclerosis is a heterogeneous disease and that recent studies have described effector T cell resistance in other autoimmune diseases42,60,61, this area would benefit from further investigation.
Overall, TReg cells do not seem to be reduced in number in multiple sclerosis, but clear defects in suppression have been identified. These defects may, in part, be due to the composition and function of the TReg cell pool.
TReg cells and SLE
SLE is a systemic autoimmune disease that is characterized by the presence of autoantibodies and immune complexes that target multiple organ systems, including the skin, joints, kidneys and CNS. Unlike the autoimmune diseases discussed earlier, there are many target tissues in SLE, not just one, and it is thought to be largely a B cell-mediated disease. Nevertheless, deficiency of TReg cells results in the development of lupus-like characteristics, including glomerulonephritis and the development of DNA-specific antibodies. These findings indicate that failure of TReg cell-mediated suppression may have a role in human SLE8.
Many studies have assayed the number of TReg cells in the peripheral blood of individuals with SLE (reviewed in REF. 62). Most, but not all, of these studies have shown that the percentage of CD4+CD25hi cells is decreased in patients with SLE. This decrease in CD4+CD25hi TReg cells was found to be inversely correlated with disease activity in several studies63–67, no correlation was reported in one study68 and, in a few cases, an increase in TReg cells has been observed60. However, no increase was observed when FOXP3 was used as a marker of TReg cells60, and an increase in TReg cell numbers following treatment with corticosteroids has been observed by two groups69,70.
The function of TReg cells in SLE has been assessed by multiple groups. Several of these groups have found no defect in function when very stringent methods of isolation and selection of TReg cells were used21,66. However, most studies of function, irrespective of the method of TReg cell selection, have shown a defect in their suppressive activity, mainly based on measures of effector T cell proliferation, although measures of IFNγ production by effector T cells have also confirmed these findings60,71,72. In several studies, the defect in suppression correlates with disease activity60,71. The source of defective suppression has been attributed to both the APCs and the TReg cells. Yan et al.73 showed that suppression was defective only in the presence of APCs and was linked to their production of IFNα. TReg cell-intrinsic defects have also been linked to increased sensitivity of these cells to cell death mediated by the death receptor CD95 (also known as FAS)21 and to a diminished expression of FOXP3 due to a relative lack of IL-2 production in SLE72.
The MRL–lpr mouse model of SLE has been shown to be associated with effector T cells that are resistant to suppression by TReg cells30. Several early TReg cell studies that addressed the issue of effector T cell resistance in SLE could not detect this defect72–74, but two recent studies have shown that effector T cells can evade suppression in SLE. Venigalla et al.60 observed a resistance to suppression in a cohort of patients with active SLE, whereas Vargas-Rojas et al.61 found effector T cell resistance in SLE irrespective of disease activity. The replication of these findings by two groups indicates that effector T cell resistance is a probable component of the loss of tolerance in SLE.
In SLE, defects in the number and function of TReg cells and in the resistance of effector T cells to suppression have been established. The observations of numerous possible regulatory defects may reflect the systemic character of this disease or a more significant role for TReg cells in SLE compared with their role in other autoimmune diseases.
TReg cells and rheumatoid arthritis
As in other autoimmune diseases, the experimental model of rheumatoid arthritis (collagen-induced arthritis) is exacerbated by depletion of TReg cells75. However, unlike the diseases discussed earlier, the target tissue in rheumatoid arthritis — the synovium — can be obtained from patients with disease. This has allowed investigators to analyse the number and function of TReg cells not only in the peripheral blood of these patients but also in the diseased tissue. Several analyses of TReg cell numbers in the peripheral blood of subjects with rheumatoid arthritis have produced differing results. In established disease, the CD4+CD25hi population has been shown to be no different from that of controls76,77, whereas a modest decrease in TReg cells was reported for untreated patients with early stage rheumatoid arthritis78. These findings contrast with observations by Han et al.79, who reported an increase in the relative and absolute numbers of TReg cells (based on CD4+CD25hiFOXP3+ staining) in the peripheral blood of patients with rheumatoid arthritis compared with numbers in controls. Despite these differences, there is general agreement that the percentage of TReg cells is higher in the synovial fluid in patients with rheumatoid arthritis than in controls76–78.
Initial studies of the suppressive function of TReg cells isolated from both the peripheral blood and the synovium found no defects in suppression76–78. However, in later studies Ehrenstein et al.80 identified a focal defect in TReg cell function in rheumatoid arthritis with respect to the cells’ ability to suppress the production of IFNγ and TNF in co-culture assays. They further established that this defect is intrinsic to the TReg cells of subjects with rheumatoid arthritis. Subsequent studies by this group have shown a defect in cytotoxic T lymphocyte antigen 4 (CTLA4)-mediated inhibition of T cell receptor signalling in TReg cells from patients with rheumatoid arthritis. This defect can be reversed by overexpression of CTLA4 in these TReg cells81.
Resistance of effector T cells to suppression has not been tested exhaustively in rheumatoid arthritis and was not the reason for the impaired suppression observed by Ehrenstein et al.80. However, it has been shown that synovial macrophages may influence the responsiveness of effector T cells to TReg cells through their increased expression of MHC class II molecules and CD86 and increased production of TNF, IL-6 and IL-7, thereby altering the cytokine milieu and the stimulatory conditions in which suppression occurs in the joint82.
In addition to being able to sample the target tissue, studies of rheumatoid arthritis benefit from the existence of well-established biological therapies, allowing the impact of these therapies on TReg cell number and function to be studied. This has been done in the context of the TNF-specific agent infliximab (Remicade; Centocor/Merck). Patients treated with this biological therapy were found to have an increase in the number of peripheral TReg cells, and this correlated with changes in the level of C-reactive protein, a marker of disease activity and inflammation80. This increase in TReg cells was not a result of expansion of the natural TReg cell population but was due to the induction of TGFβ-producing TReg cells80.
In summary, defects in immune regulation in rheumatoid arthritis do occur and are probably due to both TReg cell-intrinsic defects and the inflammatory milieu that is present in the rheumatoid joint.
TReg cells and IBD
The term IBD refers to two diseases — Crohn’s disease and ulcerative colitis — that are distinguished by their underlying pathology. Despite pathological differences, both diseases are thought to be T cell-driven diseases and to result from a loss of immune tolerance in the gut. TReg cells have a central role in the maintenance of tolerance in the gut, which is exemplified by the wasting disease and gastritis that develop in mice lacking TReg cells and by the reversal of disease by adoptive transfer of TReg cells (reviewed in REF. 83). A role for TReg cells in the regulation of inflammatory disease of the gut in humans is further supported by the finding that individuals with IPEX develop severe bowel inflammation as a component of their illness13.
Studies of TReg cells in ulcerative colitis and Crohn’s disease have also benefited from the ability to examine the number and function of TReg cells not only in the peripheral blood but also in the target organ. Studies of peripheral blood TReg cell numbers have given mixed results. Saruta et al.84 described an increase in CD4+CD25+FOXP3+ T cells among individuals with Crohn’s disease, as also described by Takahashi et al.85. However, the same study85 described an inverse correlation between CD4+CD25+ cells and disease activity among patients with ulcerative colitis. Maul et al.86 found that the number of CD4+CD25hiFOXP3+ T cells was lower in patients with active IBD (a combination of subjects with Crohn’s disease and ulcerative colitis were studied) and higher in patients with inactive disease. More recent studies have described a decrease in TReg cells in subjects with active ulcerative colitis when subjects with irritable bowel syndrome were used as controls87. Furthermore, a relative and absolute decrease in TReg cell numbers was also described by Eastaff-Leung et al.88.
Despite these differences in results from studies of the peripheral blood, observations in the gut consistently show an increase in the percentage of FOXP3+ cells in inflamed lamina propria and in mesenteric lymph nodes, particularly in and near inflamed tissue86–91. However, the increase in TReg cells found in patients with IBD raises the question of how many TReg cells are sufficient to control inflammation. To address this question, Maul et al.86 compared colonic biopsies of inflamed tissue from subjects with ulcerative colitis, Crohn’s disease, diverticulitis and infectious enteritis and found that TReg cells were similarly increased in all of these inflammatory diseases. Similar findings were reported by Uhlig et al.90, indicating that in IBD the increase in TReg cells seems to be similar to that accompanying all types of inflammation.
In addition to defining TReg cell numbers in the periphery and the colon, several studies have looked at the impact of therapy on TReg cell numbers. An increase in CD4+CD25+ T cells was seen in subjects with ulcerative colitis after standard treatment, correlating with their disease activity level85, whereas TReg cell numbers were decreased among patients with Crohn’s disease who were treated with thiopurines (purine antimetabolites that are widely used in the treatment of autoimmune disorders)84. The results from studies of infliximab treatment have been mixed: no impact of the therapy on TReg cell number was reported by one group92, whereas two groups described an increase in TReg cells in the peripheral blood and lamina propria with therapy87,93.
The functional studies of the TReg cells isolated from the peripheral blood, mesenteric lymph nodes or lamina propria of individuals with IBD all show that the suppression by these TReg cells is similar to that achieved by TReg cells from control individuals84,86,89,94–97. Although limited in scope, three studies have examined the question of the responsiveness of effector T cells in IBD. No defect was found by two groups89,94, whereas Fantini et al.98 found effector T cells to be resistant to suppression in Crohn’s disease. The limitation of each of these studies of TReg cell function is the inability to examine all of the factors that may influence their function in the lamina propria, including the cytokine milieu and the character of the APCs.
TReg cells and psoriasis
Psoriasis is a skin disorder that is characterized by erythematous scaling plaques, which are the result of inflammatory infiltrates. Psoriasis is thought to be a T cell-mediated disease of autoimmune origin, based on histological findings99, mouse models100 and the therapeutic efficacy of TNF-targeted therapies.
The importance of TReg cells in this disease has been examined in the peripheral blood and the inflamed skin of patients. Unlike in IBD, the number of TReg cells (defined by expression of FOXP3) in the peripheral blood of individuals with psoriasis is increased, and this increase is positively correlated with the disease activity index101. CD4+CD25+FOXP3+ TReg cells are also present in psoriatic lesions102 and, similar to the peripheral blood, are higher in lesional skin biopsies than in control or uninvolved skin biopsies101,103. However, an analysis by Chen et al.104 found that a relative imbalance favouring effector T cells was present in both the peripheral blood and psoriatic skin lesions. Additional studies of TReg cells in patients treated with infliximab showed that TReg cell numbers were increased105 and a more diverse T cell receptor repertoire was present in the TReg cell population106.
The functional capacity of TReg cells in both the peripheral blood and lesional skin of patients with psoriasis is impaired with respect to their ability to suppress both autologous and control effector T cells. In addition, the effector T cells of patients with psoriasis have an enhanced proliferative capacity compared with control cells107. Goodman et al.108 have extended these findings by identifying a mechanism that may contribute to this failure in regulation. Levels of IL-6 are increased in lesional skin, and both the effector T cell and TReg cell populations located in the skin have increased cell surface expression of the IL-6 receptor. Furthermore, IL-6-specific antibody can reverse the impairment in suppression that is observed in co-cultures of TReg cells and effector T cells from patients with psoriasis. IL-6 is known to enhance the resistance of effector T cells to TReg cell-mediated suppression109, but Goodman et al. speculate that it may also inhibit TReg cell function. These studies of TReg cell function raise two potential causes of impaired TReg cell-mediated suppression in psoriasis: impaired TReg cell function and resistance of effector T cells to suppression (in part due to increased production of IL-6 at the site of inflammation, and potentially due to an increased capacity to respond to IL-6 owing to upregulated receptor expression).
Conclusion and implications
It is now clear from studies of animal models of autoimmunity that defects in TReg cell number or function can contribute to disease and that therapies directed at these defects have the potential to prevent and also cure these diseases. This knowledge is now being applied to human autoimmune disease and a picture is emerging that also implicates defects in suppression by TReg cells in these diseases (TABLE 1). Despite the example of IPEX, there is currently no clear evidence that a global deficiency in the number of TReg cells is the source of failed regulation in the more common forms of autoimmunity. Instead, the presence of increased numbers of TReg cells in the affected tissues of patients with rheumatoid arthritis, IBD and psoriasis suggests that the reason for failed regulation in the inflamed tissue may be insufficient or defective TReg cell function due to either cell-intrinsic or cell-extrinsic factors. This hypothesis is supported by the predominance of studies that find an impairment in the suppressive capacity of TReg cells from individuals with autoimmune disease, and also by our expanding understanding of how the cytokine milieu and local APCs may modify TReg cell function or contribute to a resistance of the pathogenic T cell populations to suppression. Such involvement of the local environment has been implicated in the pathogenesis of type 1 diabetes, SLE and possibly psoriasis.
Table 1.
Overview of TReg cells in autoimmunity
| Disease | TReg cell number (percentage of CD4+CD25hi or CD4+CD25+FoXP3+ cells) | TReg cell function | Effector T cell resistance | Response to therapy | |
|---|---|---|---|---|---|
| Peripheral blood | Tissue | ||||
| Type 1 diabetes | Normal | ND | Decreased | Increased | ND |
| Multiple sclerosis | Normal; altered subsets of TReg cells | Increased in the CNS | Decreased | Normal | Increased TReg cell numbers with IFNβ therapy53 |
| Systemic lupus erythematosus | Decreased | ND | Decreased | Increased | Increased TReg cell numbers with corticosteroids |
| Rheumatoid arthritis | Increased | Increased in the synovial fluid of active disease | Decreased | Normal | Increased TReg cell numbers with infliximab therapy correlating with change in C-reactive protein |
| Inflammatory bowel disease | Decreased in active ulcerative colitis; normal in Crohn’s disease | Increased in the lamina propria and mesenteric lymph nodes | Normal | Normal | ND |
| Psoriasis | Increased | Increased in the skin | Decreased | Increased | ND |
CNS, central nervous system; FOXP3, forkhead box P3; IFNβ, interferon-β; ND, not determined; TReg, regulatory T.
Animal models suggest that an increase in TReg cell number at the site of inflammation is likely to be therapeutic in autoimmunity. This could be achieved in humans through adoptive transfer of in vitro-expanded autologous TReg cells or by the use of agents that promote TReg cell proliferation, survival and induction. However, these approaches will not target the site of inflammation; approaches that hold promise in this area include the use of antigen-specific TReg cells and/or TReg cells that express tissue-specific homing receptors. However, our current understanding of TReg cells in human autoimmune disease indicates that functional defects probably have the greatest impact on disease. The causes of these functional defects are multiple and include intrinsic defects in TReg cells and effector T cells, as well as extrinsic factors present at the site of inflammation. The mechanisms that underlie these defects are just being uncovered in human autoimmune disease, and an understanding of these mechanisms is likely to direct the development of new approaches for treating autoimmunity.
To achieve the goal of restoring tolerance in autoimmunity, the field must move forward in several ways. First, the character of TReg cell and effector T cell function during the course of an autoimmune disease must be defined. This will allow us to determine the point at which specific defects in regulation occur and help us to identify when an intervention would provide therapeutic benefit. Second, the mechanisms that cause impaired suppression by TReg cells in human disease must be determined. In some cases these mechanisms of impaired tolerance may be of genetic origin; it will be useful to define these mechanisms using data from genome-wide association studies, which would allow the assessment of how genetic variants in pathways related to TReg cells and effector T cells influence regulation. In addition, the identity of factors that influence not only cell number and function but also the plasticity of TReg cells is a newly emerging area of TReg cell biology that will need to be incorporated into these studies. Although the in vitro assays of human TReg cell function fail to completely mimic the in vivo milieu, they can help us to define the differences between cells obtained from healthy subjects and from individuals with autoimmune disease. The development of new assays that more closely replicate the in vivo environment should help to further resolve these issues in the future. Such approaches may require an assessment of the quality of TReg cell–effector T cell interactions, and this assessment can be carried out in short-term cultures and on a small scale using novel imaging or molecular approaches. Such approaches could then be applied to the identification of the mechanisms that underlie failed suppression and new targets for therapy.
Acknowledgments
J.H.B. is supported by grants from the US National Institutes of Health, the Juvenile Diabetes Research Foundation and the Alliance for Lupus Research.
- Immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome
(IPEX). A disease caused by mutations in the transcription factor forkhead box P3 (FOXP3) and characterized by refractory enteritis and, in some patients, autoimmune endocrinopathies, autoimmune diabetes and thyroiditis. Unlike scurfy mice, peripheral blood mononuclear cells from patients with IPEX fail to produce cytokines after in vitro stimulation
- Scurfy mice
A mouse strain with a spontaneous mutation in the transcription factor forkhead box P3 (FOXP3; also known as scurfin), which leads to a rapidly fatal lymphoproliferative disease, causing death by about 4 weeks of age. FOXP3-deficient mice lack regulatory T cells
- Non-obese diabetic mice
(NOD mice). NOD mice spontaneously develop type 1 diabetes mellitus as a result of autoreactive T cell-mediated destruction of pancreatic islet β-cells
- DO11.10 RIP-mOVA mice
A transgenic mouse model of type 1 diabetes in which a transgene encoding membrane-bound ovalbumin (mOVA) is expressed in the pancreas under the control of the rat insulin promoter (RIP) and therefore acts as a self antigen. Co-expression of a transgenic T cell receptor (DO11.10) in these mice leads to the development of spontaneous diabetes
- Experimental autoimmune encephalomyelitis
(EAE). An experimental mouse model of multiple sclerosis that is induced in susceptible animals by immunization with central nervous system antigens. EAE is an autoimmune disease that is mediated by CD4+ T helper 1 (TH1) cells and interleukin-17-producing TH17 cells that are reactive to components of the myelin sheath. The cells infiltrate the nervous parenchyma, release pro-inflammatory cytokines and chemokines, promote leukocyte infiltration and contribute to demyelination
- MRL–lpr mice
A mouse strain that spontaneously develops glomerulonephritis and other symptoms of systemic lupus erythematosus. The lpr mutation causes a defect in CD95 (also known as FAS), preventing apoptosis of activated lymphocytes. The MRL strain contributes disease-associated mutations that have yet to be identified
- Crohn’s disease
A form of chronic inflammatory bowel disease that can affect the entire gastrointestinal tract but is most common in the colon and terminal ileum. It is characterized by transmural inflammation, strictures and granuloma formation and is thought to result from an abnormal T cell-mediated response to commensal bacteria
- Ulcerative colitis
A mucosal inflammation involving the rectum and extending for a variable distance along the colon
Footnotes
Competing interests statement
The author declares no competing financial interests.
FURTHER INFORMATION
Jane Hoyt Buckner’s homepage: http://www.benaroyaresearch.org/our-research/faculty/jane-buckner
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Ochs HD, Gambineri E, Torgerson TR. IPEX, FOXP3 and regulatory T-cells: a model for autoimmunity. Immunol Res. 2007;38:112–121. doi: 10.1007/s12026-007-0022-2. [DOI] [PubMed] [Google Scholar]
- 2.Brunkow ME, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nature Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 4.Baecher-Allan C, et al. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. One of the first papers to describe human CD4+CD25+ TReg cells and to investigate their function. [DOI] [PubMed] [Google Scholar]
- 5.Stephens LA, et al. Human CD4+CD25+ thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol. 2001;31:1247–1254. doi: 10.1002/1521-4141(200104)31:4<1247::aid-immu1247>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 6.Taams LS, et al. Antigen-specific T cell suppression by human CD4+CD25+ regulatory T cells. Eur J Immunol. 2002;32:1621–1630. doi: 10.1002/1521-4141(200206)32:6<1621::AID-IMMU1621>3.0.CO;2-Q. This paper describes the antigen-specific nature of human TReg cells. [DOI] [PubMed] [Google Scholar]
- 7.Jiang S, et al. Regulatory T cells and transplantation tolerance. Hum Immunol. 2006;67:765–776. doi: 10.1016/j.humimm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi S, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. This paper describes the ability of CD4+CD25+ T cells to suppress autoimmune disease. [PubMed] [Google Scholar]
- 9.Tang Q, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. This paper shows the enhanced ability of antigen-specific TReg cells to cure diabetes in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. This paper shows that TReg cells can cure colitis in a mouse model. [DOI] [PubMed] [Google Scholar]
- 11.Liston A, Rudensky AY. Thymic development and peripheral homeostasis of regulatory T cells. Curr Opin Immunol. 2007;19:176–185. doi: 10.1016/j.coi.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Darrasse-Jeze G, et al. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;39:537–545. doi: 10.1136/jmg.39.8.537. This paper describes the syndrome IPEX and its relationship to loss of FOXP3 expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 15.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 16.Walker MR, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25− T cells. J Clin Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu W, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakaguchi S, et al. FOXP3+ regulatory T cells in the human immune system. Nature Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 19.Floess S, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thornton AM, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyara M, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. This paper refines our understanding of the subsets of CD4+FOXP3+ T cells, and has important implications with respect to the suppressive and proliferative capacity of TReg cells. [DOI] [PubMed] [Google Scholar]
- 22.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nature Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bluestone JA, Boehmer H. Regulatory T cells. Semin Immunol. 2006;18:77. doi: 10.1016/j.smim.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Workman CJ, et al. The development and function of regulatory T cells. Cell Mol Life Sci. 2009;66:2603–2622. doi: 10.1007/s00018-009-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gregori S, et al. Dynamics of pathogenic and suppressor T cells in autoimmune diabetes development. J Immunol. 2003;171:4040–4047. doi: 10.4049/jimmunol.171.8.4040. [DOI] [PubMed] [Google Scholar]
- 26.You S, et al. Autoimmune diabetes onset results from qualitative rather than quantitative age-dependent changes in pathogenic T-cells. Diabetes. 2005;54:1415–1422. doi: 10.2337/diabetes.54.5.1415. [DOI] [PubMed] [Google Scholar]
- 27.D’Alise AM, et al. The defect in T-cell regulation in NOD mice is an effect on the T-cell effectors. Proc Natl Acad Sci USA. 2008;105:19857–19862. doi: 10.1073/pnas.0810713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clough LE, et al. Release from regulatory T cell-mediated suppression during the onset of tissue-specific autoimmunity is associated with elevated IL-21. J Immunol. 2008;180:5393–5401. doi: 10.4049/jimmunol.180.8.5393. [DOI] [PubMed] [Google Scholar]
- 29.Korn T, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nature Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monk CR, et al. MRL/Mp CD4+, CD25− T cells show reduced sensitivity to suppression by CD4+, CD25+ regulatory T cells in vitro: a novel defect of T cell regulation in systemic lupus erythematosus. Arthritis Rheum. 2005;52:1180–1184. doi: 10.1002/art.20976. [DOI] [PubMed] [Google Scholar]
- 31.Walker LS. Regulatory T cells overturned: the effectors fight back. Immunology. 2009;126:466–474. doi: 10.1111/j.1365-2567.2009.03053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang Q, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kukreja A, et al. Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest. 2002;109:131–140. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Putnam AL, et al. CD4+CD25high regulatory T cells in human autoimmune diabetes. J Autoimmun. 2005;24:55–62. doi: 10.1016/j.jaut.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Brusko T, et al. No alterations in the frequency of FOXP3+ regulatory T-cells in type 1 diabetes. Diabetes. 2007;56:604–612. doi: 10.2337/db06-1248. [DOI] [PubMed] [Google Scholar]
- 36.Brusko TM, et al. Functional defects and the influence of age on the frequency of CD4+ CD25+ T-cells in type 1 diabetes. Diabetes. 2005;54:1407–1414. doi: 10.2337/diabetes.54.5.1407. [DOI] [PubMed] [Google Scholar]
- 37.Lindley S, et al. Defective suppressor function in CD4+CD25+ T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–99. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 38.Long SA, et al. Defects in IL-2R signaling contribute to diminished maintenance of FOXP3 expression in CD4+CD25+ regulatory T cells of type 1 diabetic subjects. Diabetes. 2010;59:407–415. doi: 10.2337/db09-0694. Based on the knowledge that genes involved in the IL-2 receptor signalling pathway are associated with susceptibility to type 1 diabetes, this paper identifies a defect in IL-2 receptor signalling in CD4+CD25+ T cells from patients with type 1 diabetes and, further, links this to a lack of TReg cell persistence in vitro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marwaha AK, et al. Cutting edge: Increased IL-17-secreting T cells in children with new-onset type 1 diabetes. J Immunol. 2010;185:3814–3818. doi: 10.4049/jimmunol.1001860. [DOI] [PubMed] [Google Scholar]
- 40.Willcox A, et al. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009;155:173–181. doi: 10.1111/j.1365-2249.2008.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tree TI, Roep BO, Peakman M. A mini meta-analysis of studies on CD4+CD25+ T cells in human type 1 diabetes: report of the Immunology of Diabetes Society T Cell Workshop. Ann NY Acad Sci. 2006;1079:9–18. doi: 10.1196/annals.1375.002. This paper reviews the early studies of TReg cells in type 1 diabetes and discusses issues surrounding the assays used to assess TReg cell function. [DOI] [PubMed] [Google Scholar]
- 42.Schneider A, et al. The effector T cells of diabetic subjects are resistant to regulation via CD4+ FOXP3+ regulatory T cells. J Immunol. 2008;181:7350–7355. doi: 10.4049/jimmunol.181.10.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lawson JM, et al. Increased resistance to CD4+CD25hi regulatory T cell-mediated suppression in patients with type 1 diabetes. Clin Exp Immunol. 2008;154:353–359. doi: 10.1111/j.1365-2249.2008.03810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nature Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 45.Kohm AP, et al. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 46.Viglietta V, et al. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Putheti P, et al. Circulating CD4+CD25+ T regulatory cells are not altered in multiple sclerosis and unaffected by disease-modulating drugs. J Clin Immunol. 2004;24:155–161. doi: 10.1023/B:JOCI.0000019780.93817.82. [DOI] [PubMed] [Google Scholar]
- 48.Haas J, et al. Prevalence of newly generated naive regulatory T cells (Treg) is critical for Treg suppressive function and determines Treg dysfunction in multiple sclerosis. J Immunol. 2007;179:1322–1330. doi: 10.4049/jimmunol.179.2.1322. [DOI] [PubMed] [Google Scholar]
- 49.Feger U, et al. Increased frequency of CD4+ CD25+ regulatory T cells in the cerebrospinal fluid but not in the blood of multiple sclerosis patients. Clin Exp Immunol. 2007;147:412–418. doi: 10.1111/j.1365-2249.2006.03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Michel L, et al. Patients with relapsing-remitting multiple sclerosis have normal Treg function when cells expressing IL-7 receptor α-chain are excluded from the analysis. J Clin Invest. 2008;118:3411–3419. doi: 10.1172/JCI35365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Venken K, et al. Natural naive CD4+CD25+CD127low regulatory T cell (Treg) development and function are disturbed in multiple sclerosis patients: recovery of memory Treg homeostasis during disease progression. J Immunol. 2008;180:6411–6420. doi: 10.4049/jimmunol.180.9.6411. [DOI] [PubMed] [Google Scholar]
- 52.Borsellino G, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 53.Venken K, et al. Compromised CD4+ CD25high regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology. 2007;123:79–89. doi: 10.1111/j.1365-2567.2007.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar M, et al. CD4+CD25+FoxP3+ T lymphocytes fail to suppress myelin basic protein-induced proliferation in patients with multiple sclerosis. J Neuroimmunol. 2006;180:178–184. doi: 10.1016/j.jneuroim.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 55.de Andrés C, et al. Interferon β-1a therapy enhances CD4+ regulatory T-cell function: an ex vivo and in vitro longitudinal study in relapsing-remitting multiple sclerosis. J Neuroimmunol. 2007;182:204–211. doi: 10.1016/j.jneuroim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 56.Oh U, et al. Regulatory T cells are reduced during anti-CD25 antibody treatment of multiple sclerosis. Arch Neurol. 2009;66:471–479. doi: 10.1001/archneurol.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haas J, et al. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol. 2005;35:3343–3352. doi: 10.1002/eji.200526065. [DOI] [PubMed] [Google Scholar]
- 58.Huan J, et al. Decreased FOXP3 levels in multiple sclerosis patients. J Neurosci Res. 2005;81:45–52. doi: 10.1002/jnr.20522. [DOI] [PubMed] [Google Scholar]
- 59.Astier AL, et al. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J Clin Invest. 2006;116:3252–3257. doi: 10.1172/JCI29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venigalla RK, et al. Reduced CD4+, CD25− T cell sensitivity to the suppressive function of CD4+, CD25high, CD127−/low regulatory T cells in patients with active systemic lupus erythematosus. Arthritis Rheum. 2008;58:2120–2130. doi: 10.1002/art.23556. [DOI] [PubMed] [Google Scholar]
- 61.Vargas-Rojas MI, et al. Quantitative and qualitative normal regulatory T cells are not capable of inducing suppression in SLE patients due to T-cell resistance. Lupus. 2008;17:289–294. doi: 10.1177/0961203307088307. References 60 and 61 were the first studies to clearly show effector T cell resistance in patients with autoimmune disease. [DOI] [PubMed] [Google Scholar]
- 62.Gerli R, et al. Identification of regulatory T cells in systemic lupus erythematosus. Autoimmun Rev. 2009;8:426–430. doi: 10.1016/j.autrev.2009.01.004. An excellent review of the literature on TReg cells and SLE. [DOI] [PubMed] [Google Scholar]
- 63.Crispin JC, Martinez A, Alcocer-Varela J. Quantification of regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2003;21:273–276. doi: 10.1016/s0896-8411(03)00121-5. [DOI] [PubMed] [Google Scholar]
- 64.Miyara M, et al. Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol. 2005;175:8392–8400. doi: 10.4049/jimmunol.175.12.8392. [DOI] [PubMed] [Google Scholar]
- 65.Mellor-Pita S, et al. Decrease of regulatory T cells in patients with systemic lupus erythematosus. Ann Rheum Dis. 2006;65:553–554. doi: 10.1136/ard.2005.044974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suen JL, et al. Altered homeostasis of CD4+ FoxP3+ regulatory T-cell subpopulations in systemic lupus erythematosus. Immunology. 2009;127:196–205. doi: 10.1111/j.1365-2567.2008.02937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee JH, et al. Inverse correlation between CD4+ regulatory T-cell population and autoantibody levels in paediatric patients with systemic lupus erythematosus. Immunology. 2006;117:280–286. doi: 10.1111/j.1365-2567.2005.02306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu MF, et al. Decreased CD4+CD25+ T cells in peripheral blood of patients with systemic lupus erythematosus. Scand J Immunol. 2004;59:198–202. doi: 10.1111/j.0300-9475.2004.01370.x. [DOI] [PubMed] [Google Scholar]
- 69.Suarez A, et al. Enrichment of CD4+ CD25high T cell population in patients with systemic lupus erythematosus treated with glucocorticoids. Ann Rheum Dis. 2006;65:1512–1517. doi: 10.1136/ard.2005.049924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang B, et al. Reduction of forkhead box P3 levels in CD4+CD25high T cells in patients with new-onset systemic lupus erythematosus. Clin Exp Immunol. 2008;153:182–187. doi: 10.1111/j.1365-2249.2008.03686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alvarado-Sanchez B, et al. Regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2006;27:110–118. doi: 10.1016/j.jaut.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 72.Valencia X, et al. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 73.Yan B, et al. Dysfunctional CD4+, CD25+ regulatory T cells in untreated active systemic lupus erythematosus secondary to interferon-α-producing antigen-presenting cells. Arthritis Rheum. 2008;58:801–812. doi: 10.1002/art.23268. [DOI] [PubMed] [Google Scholar]
- 74.Yates J, et al. Natural regulatory T cells: number and function are normal in the majority of patients with lupus nephritis. Clin Exp Immunol. 2008;153:44–55. doi: 10.1111/j.1365-2249.2008.03665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morgan ME, et al. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003;48:1452–1460. doi: 10.1002/art.11063. [DOI] [PubMed] [Google Scholar]
- 76.Cao D, et al. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol. 2003;33:215–223. doi: 10.1002/immu.200390024. [DOI] [PubMed] [Google Scholar]
- 77.Mottonen M, et al. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 2005;140:360–367. doi: 10.1111/j.1365-2249.2005.02754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lawson CA, et al. Early rheumatoid arthritis is associated with a deficit in the CD4+CD25high regulatory T cell population in peripheral blood. Rheumatology. 2006;45:1210–1217. doi: 10.1093/rheumatology/kel089. [DOI] [PubMed] [Google Scholar]
- 79.Han GM, et al. CD4+CD25high T cell numbers are enriched in the peripheral blood of patients with rheumatoid arthritis. Cell Immunol. 2008;253:92–101. doi: 10.1016/j.cellimm.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ehrenstein MR, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFα therapy. J Exp Med. 2004;200:277–285. doi: 10.1084/jem.20040165. A thorough study of TReg cell function in rheumatoid arthritis and during TNF-targeted therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Flores-Borja F, et al. Defects in CTLA-4 are associated with abnormal regulatory T cell function in rheumatoid arthritis. Proc Natl Acad Sci USA. 2008;105:19396–19401. doi: 10.1073/pnas.0806855105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Amelsfort JM, et al. Proinflammatory mediator-induced reversal of CD4+, CD25+ regulatory T cell-mediated suppression in rheumatoid arthritis. Arthritis Rheum. 2007;56:732–742. doi: 10.1002/art.22414. [DOI] [PubMed] [Google Scholar]
- 83.Izcue A, Powrie F. Special regulatory T-cell review: regulatory T cells and the intestinal tract — patrolling the frontier. Immunology. 2008;123:6–10. doi: 10.1111/j.1365-2567.2007.02778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saruta M, et al. Characterization of FOXP3+CD4+ regulatory T cells in Crohn’s disease. Clin Immunol. 2007;125:281–290. doi: 10.1016/j.clim.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 85.Takahashi M, et al. An inverse correlation of human peripheral blood regulatory T cell frequency with the disease activity of ulcerative colitis. Dig Dis Sci. 2006;51:677–686. doi: 10.1007/s10620-006-3191-2. [DOI] [PubMed] [Google Scholar]
- 86.Maul J, et al. Peripheral and intestinal regulatory CD4+ CD25high T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–1878. doi: 10.1053/j.gastro.2005.03.043. This is a well-controlled study of TReg cell numbers in IBD that is notable for its use of inflammatory controls. [DOI] [PubMed] [Google Scholar]
- 87.Li Z, et al. Reciprocal changes of Foxp3 expression in blood and intestinal mucosa in IBD patients responding to infliximab. Inflamm Bowel Dis. 2010;16:1299–1310. doi: 10.1002/ibd.21229. [DOI] [PubMed] [Google Scholar]
- 88.Eastaff-Leung N, et al. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol. 2010;30:80–89. doi: 10.1007/s10875-009-9345-1. [DOI] [PubMed] [Google Scholar]
- 89.Rahman MK, et al. The pathogen recognition receptor NOD2 regulates human FOXP3+ T cell survival. J Immunol. 2010;184:7247–7256. doi: 10.4049/jimmunol.0901479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Uhlig HH, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sitohy B, et al. Basal lymphoid aggregates in ulcerative colitis colon: a site for regulatory T cell action. Clin Exp Immunol. 2008;151:326–333. doi: 10.1111/j.1365-2249.2007.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hvas CL, et al. Discrete changes in circulating regulatory T cells during infliximab treatment of Crohn’s disease. Autoimmunity. 2010;43:325–333. doi: 10.3109/08916930903509064. [DOI] [PubMed] [Google Scholar]
- 93.Ricciardelli I, et al. Anti tumour necrosis-α therapy increases the number of FOXP3+ regulatory T cells in children affected by Crohn’s disease. Immunology. 2008;125:178–183. doi: 10.1111/j.1365-2567.2008.02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Holmen N, et al. Functional CD4+CD25high regulatory T cells are enriched in the colonic mucosa of patients with active ulcerative colitis and increase with disease activity. Inflamm Bowel Dis. 2006;12:447–456. doi: 10.1097/00054725-200606000-00003. [DOI] [PubMed] [Google Scholar]
- 95.Yu QT, et al. Expression and functional characterization of FOXP3+ CD4+ regulatory T cells in ulcerative colitis. Inflamm Bowel Dis. 2007;13:191–199. doi: 10.1002/ibd.20053. [DOI] [PubMed] [Google Scholar]
- 96.Kelsen J, et al. FoxP3+CD4+CD25+ T cells with regulatory properties can be cultured from colonic mucosa of patients with Crohn’s disease. Clin Exp Immunol. 2005;141:549–557. doi: 10.1111/j.1365-2249.2005.02876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Makita S, et al. Intestinal lamina propria retaining CD4+CD25+ regulatory T cells is a suppressive site of intestinal inflammation. J Immunol. 2007;178:4937–4946. doi: 10.4049/jimmunol.178.8.4937. [DOI] [PubMed] [Google Scholar]
- 98.Fantini MC, et al. Smad7 controls resistance of colitogenic T cells to regulatory T cell-mediated suppression. Gastroenterology. 2009;136:1308–1316. doi: 10.1053/j.gastro.2008.12.053. [DOI] [PubMed] [Google Scholar]
- 99.Baadsgaard O, et al. The role of the immune system in the pathogenesis of psoriasis. J Invest Dermatol. 1990;95:S32–S34. doi: 10.1111/1523-1747.ep12505715. [DOI] [PubMed] [Google Scholar]
- 100.Bata-Csorgo Z, et al. Kinetics and regulation of human keratinocyte stem cell growth in short-term primary ex vivo culture. Cooperative growth factors from psoriatic lesional T lymphocytes stimulate proliferation among psoriatic uninvolved, but not normal, stem keratinocytes. J Clin Invest. 1995;95:317–327. doi: 10.1172/JCI117659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yan KX, et al. Foxp3+ regulatory T cells and related cytokines differentially expressed in plaque vs. guttate psoriasis vulgaris. Br J Dermatol. 2010;163:48–56. doi: 10.1111/j.1365-2133.2010.09742.x. [DOI] [PubMed] [Google Scholar]
- 102.Bovenschen HJ, et al. Identification of lesional CD4+ CD25+ Foxp3+ regulatory T cells in psoriasis. Dermatology. 2006;213:111–117. doi: 10.1159/000093849. [DOI] [PubMed] [Google Scholar]
- 103.Zhang L, et al. Increased Th17 cells are accompanied by FoxP3+ Treg cell accumulation and correlated with psoriasis disease severity. Clin Immunol. 2010;135:108–117. doi: 10.1016/j.clim.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 104.Chen L, et al. Dynamic frequency of CD4+CD25+Foxp3+ Treg cells in psoriasis vulgaris. J Dermatol Sci. 2008;51:200–203. doi: 10.1016/j.jdermsci.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 105.Quaglino P, et al. Circulating CD4+CD25brightFOXP3+ T cells are up-regulated by biological therapies and correlate with the clinical response in psoriasis patients. Dermatology. 2009;219:250–258. doi: 10.1159/000238305. [DOI] [PubMed] [Google Scholar]
- 106.Diluvio L, et al. Infliximab therapy induces increased polyclonality of CD4+CD25+ regulatory T cells in psoriasis. Br J Dermatol. 2010;162:895–897. doi: 10.1111/j.1365-2133.2010.09650.x. [DOI] [PubMed] [Google Scholar]
- 107.Sugiyama H, et al. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol. 2005;174:164–173. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goodman WA, et al. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J Immunol. 2009;183:3170–3176. doi: 10.4049/jimmunol.0803721. References 107 and 108 address the function of lesional TReg cells in psoriasis and link these findings to TReg cell- and tissue-specific mechanisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 110.Yates J, et al. The maintenance of human CD4+ CD25+ regulatory T cell function: IL-2, IL-4, IL-7 and IL-15 preserve optimal suppressive potency in vitro. Int Immunol. 2007;19:785–799. doi: 10.1093/intimm/dxm047. [DOI] [PubMed] [Google Scholar]
- 111.Valencia X, et al. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thornton AM, et al. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519–6523. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 113.King IL, Segal BM. Cutting edge: IL-12 induces CD4+CD25− T cell activation in the presence of T regulatory cells. J Immunol. 2005;175:641–645. doi: 10.4049/jimmunol.175.2.641. [DOI] [PubMed] [Google Scholar]
- 114.Ben Ahmed M, et al. IL-15 renders conventional lymphocytes resistant to suppressive functions of regulatory T cells through activation of the phosphatidylinositol 3-kinase pathway. J Immunol. 2009;182:6763–6770. doi: 10.4049/jimmunol.0801792. [DOI] [PubMed] [Google Scholar]
- 115.Yang J, et al. Allograft rejection mediated by memory T cells is resistant to regulation. Proc Natl Acad Sci USA. 2007;104:19954–19959. doi: 10.1073/pnas.0704397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Takeda I, et al. Distinct roles for the OX40–OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. 2004;172:3580–3589. doi: 10.4049/jimmunol.172.6.3580. [DOI] [PubMed] [Google Scholar]
- 117.Choi BK, et al. 4-1BB-dependent inhibition of immunosuppression by activated CD4+CD25+ T cells. J Leukoc Biol. 2004;75:785–791. doi: 10.1189/jlb.1003491. [DOI] [PubMed] [Google Scholar]
- 118.Groux H, et al. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Weiner HL. Induction and mechanism of action of transforming growth factor-β-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–214. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 120.Janson PC, et al. FOXP3 promoter demethylation reveals the committed Treg population in humans. PLoS ONE. 2008;3:e1612. doi: 10.1371/journal.pone.0001612. [DOI] [PMC free article] [PubMed] [Google Scholar]