Abstract
Background
When antiretroviral therapy does not fully suppress HIV replication, suboptimal levels of antiretrovirals can select for antiretroviral resistant variants of HIV. These variants may exhibit reduced replication capacity and result in lower viral loads in blood. Our study evaluated whether antiretroviral resistance was associated with viral loads in the cerebrospinal fluid (CSF) and better neuropsychological (NP) performance.
Methods
We enrolled ninety-four participants and each participant underwent a comprehensive neuromedical evaluation that used structured clinical assessments of medical history, ART and other medication use, comprehensive NP testing and neurological and general physical signs of disease. Blood was collected by venipuncture and all participants were offered lumbar puncture. Univariate and multivariate statistical methods were used to analyze the relationship between antiretroviral resistance, blood and CSF HIV RNA levels, substance use, and NP performance.
Results
Antiretroviral resistance, detected in blood, was associated with lower CSF viral loads (p<0.01) and better NP performance (p=0.04) in multivariate analyses, independent of past and current ARV use and blood viral loads (Model: p< 0.01). However, HIV RNA levels in CSF did not independently correlate with NP performance. Low viral loads in the CSF limited our ability to investigate the relationship between antiretroviral resistance detected in CSF and NP performance.
Conclusions
Even in the absence of ART, antiretroviral resistance-associated mutations correlate with better NP performance possibly because these mutations reflect reduced neurovirulence compared with wild-type HIV.
Background
HIV associated neurocognitive disorders (HAND) range in severity from disabling dementia to asymptomatic cognitive, motor and behavioral changes. With the widespread use of antiretroviral therapy (ART) in economically privileged countries, the incidence of HIV-associated dementia (HAD), characterized by severe neuropsychological (NP) impairment and inability to perform activities of daily living, has significantly decreased (reviewed in Deutsch 2001, Sacktor 2002). Despite a decrease in incident HAD, less severe forms of HAND have persisted (Antinori 2002, Giancola 2006, Antinori 2007, Tozzi 2007) and may actually be increasing as HIV-infected individuals live longer (reviewed in MacArthur 2004, Ances 2007). Comorbidities that are common in individuals infected with HIV, like hepatitis C virus (HCV) infection and methamphetamine abuse, are also associated with NP impairment and may make it difficult to distinguish the contribution of each to NP impairment (Rippeth 2004, Clifford 2005, Letendre 2005, Cherner 2005, Richardson 2005, Letendre 2007).
Effective ART, as assessed by suppression of blood plasma viral load, is considered the standard of care for HAND; however, poor penetration into the central nervous system (CNS) by some antiretroviral drugs suggests that suppression of blood plasma viral load may not be an adequate guide when selecting treatment options for HAND and raises the concern that suboptimal antiretroviral concentrations could select for resistance-associated mutations (Letendre 2004, Antinori 2005). Studies that have examined CSF viral loads in the setting of ART and antiretroviral resistance suggest ART lowers CSF viral loads even in the setting of antiretroviral resistance; however, the clinical significance of these findings remains unclear (Antinori 2005, Spudich 2006)
In addition, resistance associated mutations can affect HIV replication and fitness in the presence or absence of ART. Studies have examined the relationship between resistance-associated mutations, in vivo viral load and HIV disease (Samri 2000, Schmitt 2000, Antinori 2001, Deeks 2001, Barbour 2002, Campbell 2003, Paredes 2009). These studies, however, were limited to blood viral loads and focused on indicators of HIV disease in the blood, like CD4+ cell counts. Considerably less is known about the impact of antiretroviral resistance on cerebrospinal fluid (CSF) viral load and the brain. To this end, we investigated the relationships between resistance-associated mutations, viral loads in blood and CSF, and NP performance.
Methods
Eligibility
Our study consisted of 94 participants enrolled in a research study at the University of California San Diego’s HIV Neurobehavioral Research Center. Participant blood was collected by venipuncture and for sequencing purposes only participants with at least 500 HIV RNA copies/ml in blood plasma were included in this study. All participants were offered lumbar puncture with 69 consenting and having a successful procedure. Participants were excluded if they had significant head trauma, brain surgery, cerebral palsy, a seizure disorder, history of CNS opportunistic infection or received treatment with interferon-alpha. All subjects provided informed consent according to a protocol approved by the UCSD Human Research Protections Program.
Study Design and Statistical Analyses
To investigate the relationship between antiretroviral resistance detected in blood and HIV RNA levels in blood and CSF, we utilized univariate and multivariate analyses. First, we examined demographic and medical characteristics of study participants in association with the presence or absence of resistance-associated mutations. HIV RNA levels in blood and CSF were log transformed to stabilize variances. Fisher’s exact tests were used to compare categorical or binary measures and Wilcoxon rank sum tests were used to compare continuous measures. Multiple regressions were performed using CSF HIV RNA or bood HIV RNA as the continuous outcome and the detection of antiretroviral resistance in blood as the main predictor of interest. Other variables used in this model included past and current ART use, methamphetamine dependence, estimated duration of infection and plasma HIV RNA levels. Analyses of CSF HIV RNA levels were limited to the sixty-nine participants with successfully completed lumbar punctures. These analyses included the use of Tobit analyses to adjust for censored data because HIV RNA levels are subject to limit of detection censoring (Tobin 1958). In addition, the demographic and medical characteristics of study participants for whom CSF HIV RNA were available were compared to the other study participants to assess the informativeness of the missing data.
To examine the impact of antiretroviral resistance on the relationship between CSF HIV RNA levels and NP performance, we utilized both univariate and multivariate approaches. The univariate approach consisted of a series of univariate analyses with participants grouped by the presence or absence of antiretroviral resistance. The first examined the relationship between CSF HIV RNA levels and NP performance and the second examined the relationship between blood HIV RNA levels and NP performance. Because ongoing ART use can significantly alter CSF and blood HIV RNA levels (Spudich 2006, Marra 2009), we excluded participants currently receiving ART. This univariate analysis was limited by the number of participants (i.e. power) and it does not control for other potentially confounding variables. To more effectively address these concerns, we performed additional multivariate analyses which included all available data and incorporated models that allowed us to address current and past ART and a number of other important factors that may influence the relationship between CSF viral load and NP performance including: methamphetamine dependence, current and nadir CD4 count and estimated duration of infection. Using this multivariate approach, we were able to examine if resistance had an impact on NP performance independent of current and past ART use; and if resistance had an impact on NP performance independent of CSF HIV RNA levels.
In exploratory analysis, we examined genotypic discordance between blood and CSF among those with blood and CSF resistance data available. Resistance discordance was defined as the presence of one or more resistance-associated mutation(s) in blood not present in the CSF or vice-versa. We limited the analysis of resistance discordance to amino acids with a Stanford HIV Resistance Database “mutation score” of 30 or greater (http://hivdb.stanford.edu, March 2009). In further exploratory analyses of viral genetic discordance, Fisher’s exact tests were used to compare categorical or binary measures and the Wilcoxon rank sum tests to examine if resistance discordance altered HIV RNA levels. As univariate analyses were not informative, multivariate analysis was not performed. Statistical analyses were performed using JMP (version 5.0 for Mac, SAS Institute, Cary, NC, USA) and R version 2.3.1 (R Development Core Team 2006).
Neuromedical and Neuropsychological Testing
Participants underwent standardized NP assessments of seven ability domains (learning, delayed recall, verbal fluency, processing speed, attention/working memory, abstraction/executive functioning, motor speed), as previously described (Heaton 1994, Rippeth 2004). All NP tests were administered and scored by trained psychometrists using demographically corrected normative data. Results were summarized by a neuropsychologist using global ratings that range from 1 (above average) to 9 (severely impaired), based on the demographically adjusted test scores in the seven ability domains. A global score of 5 or higher denotes NP impairment that is present in at least two ability areas (Woods 2004).
At the time of their NP testing and clinical sample collection, the urine of each participant was screened with a point-of-care test for common recreational drugs, including amphetamines, cocaine, barbiturates, tetrahydrocannabinol, opiates, benzodiazepines, and phencyclidines (Rapid Response; Biotechnostix, Inc., Markham, Ontario, Canada). In addition, participants received a Breathalyzer test to evaluate alcohol intoxication (Alcohol Countermeasure Systems, Toronto, Ontario, Canada). NP testing was rescheduled if alcohol was detected or if the participant’s urine was positive for non-prescribed substances, with the exception of cannabis, given its long elimination period. Likewise, participants were not tested if they appeared to be intoxicated or in withdrawal.
Methamphetamine dependence was determined with the Structured Clinical Interview from the Diagnostic and Statistical Manual of Mental Disorders version IV (Spitzer 1995). Inclusion in the parent study from which the methamphetamine users were drawn, required lifetime dependence and evidence of use within the previous eighteen months, as well as a minimum of ten days of abstinence prior to NP testing.
Laboratory Measures and Antiretroviral Resistance Genotyping
Blood was collected by venipuncture and CSF was collected by lumbar puncture. HIV and HCV infections were diagnosed by serology. HIV RNA levels in blood plasma and CSF were measured (Amplicor HIV-1 Monitor; Roche Diagnostics, Branchburg, NJ). The ultrasensitive assay was used for CSF (lower limit level of detection of 50 copies/ml) and the standard assay was used for blood (lower limit level of detection of 400 copies/ml). A fluorescence-activated cell sorter quantified CD4 lymphocytes. The ViroSeq HIV genotyping system (Applied Biosystems, Alameda, CA) was used for population-based pol sequencing of HIV RNA extracted from blood plasma per manufacturer instructions (Smith 2007). Genotyping of the reverse trancriptase coding region of CSF-derived HIV RNA included cDNA synthesis with RETROscript kit (Applied Biosystems, Alameda, CA) using random decamers according to manufacture’s protocol. Followed by two rounds of amplification with Taq polymerase (Invitrogen, Carlsbad, California) as previously described (Koelsch 2003), using primers CI-Pol 1 and 3RT at cycling parameters: 95 °C × 2 min; 95 °C × 30 s, 50 °C × I min, 72 °C × 1 min for 35 cycles; 72 °C × 10 min and primers 5RT and 3RT at cycling parameters: 95 °C × 2 min; 95 °C × 30 s, 50 °C × I min, 72 °C × 1 min for 35 cycles; 72 °C × 10 min. All assays included negative controls with PCR products visualized by agarose gel electrophoresis, and were conducted in conditions to minimize PCR contamination. Sequencing was performed on an ABI 3100 Genetic and sequences were manually reviewed using BioEdit and ViroSeq genotyping software (version 2.4.2; Applied Biosystems, Alameda, CA). The Stanford HIV Resistance Database (http://hivdb.stanford.edu, March 2009) was used to interpret antiretroviral resistance from genotypic data.
Results
Study Participants and Antiretroviral Resistance
Participants were mostly Caucasian men in their mid 30s (median 35 years). The median HIV RNA levels were 4.7 (blood) and 2.9 (CSF) log10 copies/ml. The median blood CD4+ cell count was 319/μl, and 25% of participants had a positive serology for HCV. As expected, methamphetamine use was common in the cohort with 57% of participants reporting a history of abuse or dependence (Table 1). Lumbar punctures were successfully performed on 73% of participants (69 of 94). Clinical and demographic characteristics of participants with successful lumbar punctures resembled participants without lumbar punctures (data not shown), except that participants who did not undergo lumbar puncture had longer estimated durations of HIV infection (mean 8.5 years vs. 5.5 years, p = 0.02).
Table 1.
Participant Demographics and Clinical Characteristics
| Overall | AR+ (range) | AR− (range) | p-value | |
|---|---|---|---|---|
| Sample Size | 94 | 48 | 46 | |
| Age (years) | 35 | 34 (23–39) | 35 (21–51) | > 0.10 |
| Sex (male) | 92% | 96% | 88% | > 0.10 |
| Ethnicity (non-Caucasian) | 37% | 38% | 37% | > 0.10 |
| Education (years) | 12 | 12 (9–20) | 12 (6–18) | > 0.10 |
| Duration of HIV (years) | 5.3 | 8.1 (.052–16.4) | 4.1 (0–16.9) | < 0.01 |
| HIV RNA, CSF (log10 c/mL)* | 2.9 | 2.6 (1.7–4.4) | 3.3 (1.7–6.2) | < 0.01 |
| HIV RNA, Plasma (log10 c/mL) | 4.7 | 4.4 (2.8–6) | 4.8 (3.3–6.3) | < 0.05 |
| CD4 Count, Current (/μL) | 319 | 340 (4–1188) | 308 (3–1296) | > 0.10 |
| CD4 Count, Nadir (/μL) | 216 | 200 (0–772) | 269 (0–1296) | > 0.10 |
| AIDS Diagnosis | 47% | 50% | 43% | > 0.10 |
| Past ARV Use | 63% | 73% | 51% | < 0.05 |
| Current ARV Use | 29% | 50% | 7% | < 0.001 |
| −Adherence (<95% in 4 weeks) | 53% | 50% | 75% | > 0.10 |
| HCV Seropositive | 25% | 25% | 24% | > 0.10 |
| Methamphetamine Abuse Ever | 57% | 51% | 63% | > 0.10 |
Values are medians or proportions; n = 94;
subgroup analysis
P-values are based on univariate analyses. Individuals with drug resistance (AR+) differed from those with no drug resistance (AR−) in duration of HIV infection, CSF HIV RNA, plasma HIV RNA, past antiretroviral (ARV) use, and current ARV use.
At the time of study evaluation, 63% had a past history of ART use and 29% were receiving ART at the time of participation and sampling. One or more resistance-associated mutations were detected in the blood plasma of 48 of the 94 study participants (51%). The most common mutations, M184V and K103N, were detected in (22%) and (16%) of participants respectively (Supplementary Table 1). Univariate analyses demonstrated that individuals with antiretroviral resistance (AR+) differed from those with no antiretroviral resistance (AR−) in duration of HIV infection, levels of CSF and blood plasma HIV RNA, past ART use, and current ART use (Table 1). Participants with resistant virus did not differ in current CD4+ cell count, CD4+ cell nadir, HCV serostatus, or diagnosis of AIDS (Table 1).
Resistance profiles for the reverse transcriptase coding region derived from HIV RNA from CSF were obtained for twenty-six participants. Median CSF viral loads for these participants were higher than for those participants for whom resistance profiles could not be obtained (3.16 log10 copies/ml vs. 2.6 log10 copies/ml, p=.013), but did not differ with regard to demographic characteristics, current CD4+ cell count, CD4+ cell nadir, HCV serostatus, or diagnosis of AIDS (data not shown). Resistance-associated mutations in the reverse transcriptase coding region from HIV RNA populations in the CSF were identified in four of these twenty-six (15%) participants. Genotypic discordance between blood and CSF, defined as the presence of one or more resistance-associated mutation in blood not present in CSF or vice-versa, was found in one of these four participants (25%) (Supplementary Table 2). In all but one case, participants with a major resistance associated mutation(s) in blood also had the mutation(s) in the CSF.
Antiretroviral Resistance and CSF Viral Loads
In univariate analyses, lower CSF viral loads were associated with the presence of antiretroviral resistance in blood-derived virus, lower HIV blood viral load, and current ARV use. Multivariate analyses, which included adjustments for current and past ART use and blood viral load, demonstrated that lower CSF HIV RNA levels were associated with the presence of antiretroviral resistance in blood (Model: p< 0.01). This relationship was particularly strong for participants with the M184V mutation (Model: p< 0.01). Among the 26 participants with completed CSF resistance profiles of reverse transcriptase, univariate analysis did not indicate differences in CSF viral loads between individuals with and without evidence of resistance in CSF.
Antiretroviral Resistance, Methamphetamine Dependence and Neuropsychological Performance
To examine the impact of antiretroviral resistance on the relationship between CSF viral load and NP performance, we conducted univariate analyses with participants stratified by the presence or absence of antiretroviral resistance. The first examined the relationship between CSF viral load and NP performance and second the relationship between blood viral load and NP performance. Because ART use can significantly alter CSF and blood viral load, we excluded participants currently receiving ART. In these analyses, the correlation between global rating and CSF viral load was stronger in individuals without antiretroviral resistance than in individuals with antiretroviral resistance, although this difference did not reach statistical significance (Figure 1). The correlation between global rating and blood viral load was also stronger in individuals without antiretroviral resistance (AR−) than in individuals with antiretroviral resistance (Figure 2). Multivariate analysis demonstrated that antiretroviral resistance in blood-derived virus (β =−0.88; p=0.024) was associated with better NP performance (Model: Adjusted R2=0.12, p=0.017) (Table 2). The opposite was observed for methamphetamine dependence (β = 0.76; p=0.031) and duration of HIV infection (β =0.073; p=0.057): each was independently associated with worse NP performance (Table 2). Similar results were observed when log-transformed CSF viral load was added to the analysis as a predictor. In multivariate analyses, antiretroviral use, blood viral load and CSF viral load did not explain additional variance in NP performance. Antiretroviral resistance was found to be associated with both CSF HIV RNA levels and NP performance, although no significant relationship between CSF HIV RNA and NP performance was observed.
Figure 1. Univariate Analysis of CSF HIV RNA Levels and Neuropsychological Performance.
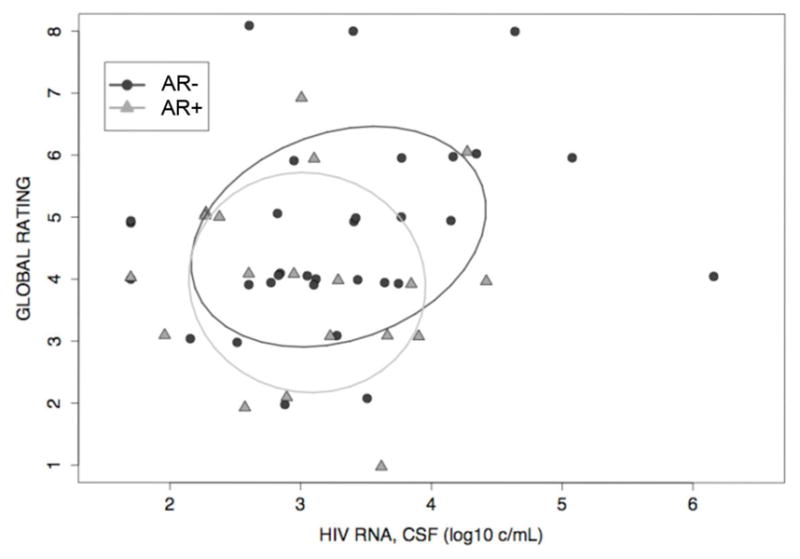
Confidence Ellipses (Global Rating vs. CSF Viral Load) for individuals without antiretroviral resistance (AR−) (rho = 0.27, p = 0.14) and individuals with antiretroviral resistance (AR+) (rho = −0.07, p = 0.78) demonstrate that the correlation between global rating and CSF viral load is stronger in individuals without antiretroviral resistance than in individuals with antiretroviral resistance, although this did not reach statistical significance. To exclude any direct ART effect, this analysis excluded participants on ART at the time of the study.
Figure 2. Univariate Analysis of Blood HIV RNA Levels and Neuropsychological Performance.
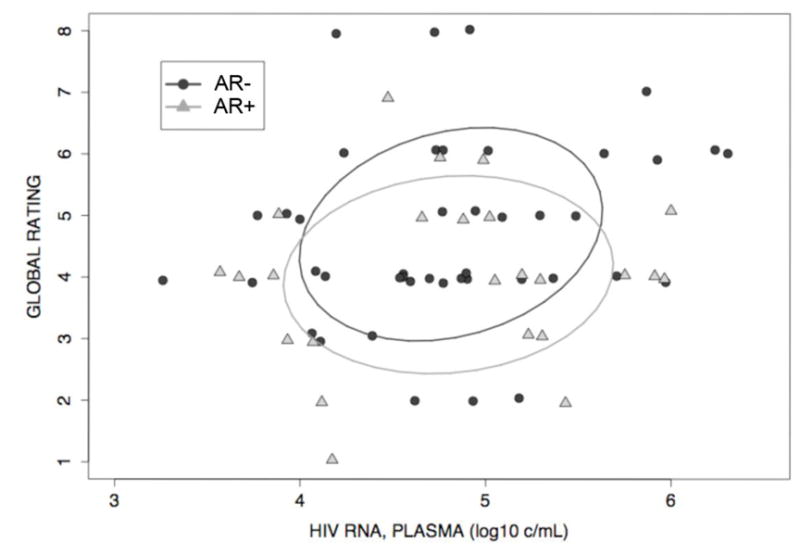
Confidence Ellipses (Global Rating vs. Plasma Viral Load) for individuals without antiretroviral resistance (AR−) (rho = 0.25, p = 0.10) and individuals with antiretroviral resistance (AR+) (rho = −0.16, p = 0.29) demonstrate that although not statistically significant, the correlation between global rating and plasma viral load is stronger in individuals without antiretroviral resistance than in individuals with antiretroviral resistance. To exclude any direct ART effect, this analysis excluded participants on ART at the time of the study.
Table 2.
Summary of Multivariate Regressions Modeling Neuropsychological Performance
| Model A. NP performance as a function of antiretroviral resistance, meth abuse, HIV infection duration, and ARV use | ||
|---|---|---|
| Coefficient | p-value | |
| Intercept | 3.96 | <0.0001 |
| Antiretroviral Resistance (blood) | −0.88 | 0.02 |
| Duration of HIV (years) | 0.07 | 0.06 |
| Methamphetamine Abuse Ever | 0.76 | 0.03 |
| ARV Use | 0.38 | 0.40 |
| Adjusted R2=0.12 | ||
| Model B. NP performance as a function of antiretroviral resistance, meth abuse, HIV infection duration, and ARV use and CSF HIV RNA levels | ||
|---|---|---|
| Coefficient | p-value | |
| Intercept | 3.31 | <0.0001 |
| Antiretroviral Resistance (blood) | −0.79 | 0.05 |
| Duration of HIV (years) | 0.07 | 0.06 |
| Methamphetamine Abuse Ever | 0.83 | 0.02 |
| ARV Use | 0.47 | 0.31 |
| HIV RNA, CSF (log10 c/mL) | 0.18 | 0.35 |
| Adjusted R2=0.12 | ||
| Model C. NP performance as a function of antiretroviral resistance, meth abuse, HIV infection duration, and ARV use and plasma HIV RNA levels | ||
|---|---|---|
| Coefficient | p-value | |
| Intercept | 3.33 | <0.0001 |
| Antiretroviral Resistance (blood) | −0.67 | 0.04 |
| Duration of HIV (years) | 0.04 | 0.23 |
| Methamphetamine Abuse Ever | 0.76 | 0.01 |
| ARV Use | 0.56 | 0.16 |
| HIV RNA, Plasma (log10 c/mL) | 0.150 | 0.45 |
| Adjusted R2=.09 | ||
In multivariate analysis, antiretroviral resistance was found to be associated with NP performance, although no significant relationship between CSF HIV RNA and NP performance was observed. Antiretroviral use, blood viral load and CSF viral load did not explain additional variance in NP performance.
Discussion
We found that the detection of antiretroviral resistance in HIV populations in blood was significantly associated with both lower CSF HIV RNA levels and better NP performance. These findings may help to explain what appears to be a weakening relationship between CSF viral load and NP performance in the modern treatment era. Thus in our study and in other more recent reports (Sevigny 2004) CSF HIV RNA levels were not independently associated with better NP performance. Taken together, these studies provide evidence that HIV variants harboring resistance-associated mutations may be less replication competent and less neurovirulent than wild-type HIV.
Single mutations within pol can result in reduced replication capacity in vitro (Goudsmit 1996, Harrigan 1998, White 2002, Collins 2004, Cong 2007). Single amino acid substitutions in clade B virus that result in the greatest reduction in replication capacity include nucleoside reverse transcriptase inhibitor (NRTI) associated mutations (K65R, T215Y and M184V) and non-nucleoside reverse transcriptase inhibitor (NNRTI) associated mutations (V106A, Y188H and G190S) (Iglesias-Ussel 2002, Collins 2004, Johnson 2007, Martinez-Picado 2008). Our study extends these observations by demonstrating that resistance-associated mutations known to reduce replication capacity in vitro, particularly M184V, are associated with lower CSF viral loads, independent of past and current ART use. In contrast, the NNRTI associated mutation K103N, was not associated with lower viral loads, as might be predicted from its negligible reduction in replication capacity in vitro (Nicrasti 2003, Koval 2006, Johnson 2007).
Suboptimal ART may select for HIV that is less replication competent and less neuropathogenic, but ongoing HIV replication regardless of phenotype leads to brain injury in a substantial proportion of untreated individuals. Although this investigation suggests that resistance-associated mutations may benefit the nervous system, this benefit is unlikely to match that from virologic suppression. In addition, compensatory mutations may accumulate that restore replication capacity while maintaining antiretroviral resistance. Understanding how individual resistance-associated mutations contribute to HAND is difficult, given no standard in vitro method to characterize HIV phenotypes by neurovirulence exists. One approach that might yield additional insight is to use an in vitro assay that utilizes microglia or brain macrophages, the likely cell types productively infected by HIV in the brain (Reviewed in Fischer-Smith 2008), to assess the replication capacity of specific resistance associated mutations (Perez-Bercoff 2007).
Some limitations of this study should be acknowledged. It analyzed retrospectively an existing cohort with specimens only from participants with detectable blood viral loads. In addition, analyses of CSF viral load were based on a subset of participants with available lumbar punctures. While detailed analysis demonstrated that this subset did not significantly differ from the other participants in the study, selection bias is possible. Analyses of antiretroviral resistance also were primarily based on HIV RNA extracted from blood. We did investigate CSF resistance profiles for twenty-six participants, but genotypic assessment was limited because viral loads were low in the CSF, which limited sequencing of the reverse transcriptase coding region. In all but one case, participants with a major resistance associated mutation(s) in blood also had the mutation(s) in the CSF. This degree of concordance in CSF and blood resistance-associated mutations is likely explained in part by regimen stability, as the median time on current ART regimen for these participants was 10 months. Although current ART therapy was relatively stable, different ART regimens among participants and a wide range in time on and off ART, made it difficult to employ a more rigorous assessment of ART use. Even though our analysis was nested in a well-characterized cohort and is one of largest studies of its kind, these limitations dictate that our observations should be validated in larger prospective studies with more frequent sampling.
In conclusion, our findings suggest that antiretroviral resistance alters the relationship between CSF viral loads and better NP performance. In particular, the presence of antiretroviral resistance appears to be associated with better NP performance. This may be mediated by impaired viral fitness and is likely less beneficial than complete viral suppression by ART. If antiretroviral resistance alters CSF viral loads this may in part explain what appears to be a weakening relationship between CSF viral load and NP performance in the modern treatment era. Further study of antiretroviral resistance and HIV neuropathogenesis may contribute to an improved understanding of HIV disease in the CNS and perhaps improvements in treatment options for HAND.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants DA12065, MH083552, AI077304, AI69432, MH62512, AI27670, AI38858, AI43638, AI43752, AI047745, NS51132, UCSD Centers for AIDS Research Viral Pathogenesis Core (AI36214), AI29164, AI47745, AI 064086, AI57167 and the San Diego Veterans Affairs Healthcare System (10-92-035).
Footnotes
Potential conflicts of interest: None
Presented in part: 15th Conference on Retroviruses and Opportunistic Infections, Boston, MA February 3-6 2008 (abstract 394).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27(1):86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- 2.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antinori A, Giancola ML, Grisetti S, Soldani F, Alba L, Liuzzi G, Amendola A, Capobianchi M, Tozzi V, Perno CF. Factors influencing virological response to antiretroviral drugs in cerebrospinal fluid of advanced HIV-1-infected patients. Aids. 2002;16(14):1867–76. doi: 10.1097/00002030-200209270-00003. [DOI] [PubMed] [Google Scholar]
- 4.Antinori A, Liuzzi G, Cingolani A, Bertoli A, Di Giambenedetto S, Trotta MP, Rizzo MG, Girardi E, De Luca A, Perno CF. Drug-resistant mutants of HIV-1 in patients exhibiting increasing CD4 cell count despite virological failure of highly active antiretroviral therapy. Aids. 2001;15(17):2325–7. doi: 10.1097/00002030-200111230-00017. [DOI] [PubMed] [Google Scholar]
- 5.Antinori A, Perno CF, Giancola ML, Forbici F, Ippolito G, Hoetelmans RM, Piscitelli SC. Efficacy of cerebrospinal fluid (CSF)-penetrating antiretroviral drugs against HIV in the neurological compartment: different patterns of phenotypic resistance in CSF and plasma. Clin Infect Dis. 2005;41(12):1787–93. doi: 10.1086/498310. [DOI] [PubMed] [Google Scholar]
- 6.Back NK, Nijhuis M, Keulen W, Boucher CA, Oude Essink BO, van Kuilenburg AB, van Gennip AH, Berkhout B. Reduced replication of 3TC-resistant HIV-1 variants in primary cells due to a processivity defect of the reverse transcriptase enzyme. Embo J. 1996;15(15):4040–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Barbour JD, Wrin T, Grant RM, Martin JN, Segal MR, Petropoulos CJ, Deeks SG. Evolution of phenotypic drug susceptibility and viral replication capacity during long-term virologic failure of protease inhibitor therapy in human immunodeficiency virus-infected adults. J Virol. 2002;76(21):11104–12. doi: 10.1128/JVI.76.21.11104-11112.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brew BJ, Pemberton L, Cunningham P, Law MG. Levels of human immunodeficiency virus type 1 RNA in cerebrospinal fluid correlate with AIDS dementia stage. J Infect Dis. 1997;175(4):963–6. doi: 10.1086/514001. [DOI] [PubMed] [Google Scholar]
- 9.Campbell TB, Schneider K, Wrin T, Petropoulos CJ, Connick E. Relationship between in vitro human immunodeficiency virus type 1 replication rate and virus load in plasma. J Virol. 2003;77(22):12105–12. doi: 10.1128/JVI.77.22.12105-12112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherner M, Letendre S, Heaton RK, Durelle J, Marquie-Beck J, Gragg B, Grant I. Hepatitis C augments cognitive deficits associated with HIV infection and methamphetamine. Neurology. 2005;64(8):1343–7. doi: 10.1212/01.WNL.0000158328.26897.0D. [DOI] [PubMed] [Google Scholar]
- 11.Clifford DB, Yang Y, Evans S. Neurologic consequences of hepatitis C and human immunodeficiency virus coinfection. J Neurovirol. 2005;11(Suppl 3):67–71. doi: 10.1080/13550280500513762. [DOI] [PubMed] [Google Scholar]
- 12.Collins JA, Thompson MG, Paintsil E, Ricketts M, Gedzior J, Alexander L. Competitive fitness of nevirapine-resistant human immunodeficiency virus type 1 mutants. J Virol. 2004;78(2):603–11. doi: 10.1128/JVI.78.2.603-611.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cong ME, Heneine W, Garcia-Lerma JG. The fitness cost of mutations associated with human immunodeficiency virus type 1 drug resistance is modulated by mutational interactions. J Virol. 2007;81(6):3037–41. doi: 10.1128/JVI.02712-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deeks SG, Wrin T, Liegler T, Hoh R, Hayden M, Barbour JD, Hellmann NS, Petropoulos CJ, McCune JM, Hellerstein MK, Grant RM. Virologic and immunologic consequences of discontinuing combination antiretroviral-drug therapy in HIV-infected patients with detectable viremia. N Engl J Med. 2001;344(7):472–80. doi: 10.1056/NEJM200102153440702. [DOI] [PubMed] [Google Scholar]
- 15.Deutsch R, Ellis RJ, McCutchan JA, Marcotte TD, Letendre S, Grant I. AIDS-associated mild neurocognitive impairment is delayed in the era of highly active antiretroviral therapy. Aids. 2001;15(14):1898–9. doi: 10.1097/00002030-200109280-00027. [DOI] [PubMed] [Google Scholar]
- 16.Ellis RJ, Hsia K, Spector SA, Nelson JA, Heaton RK, Wallace MR, Abramson I, Atkinson JH, Grant I, McCutchan JA. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. HIV Neurobehavioral Research Center Group. Ann Neurol. 1997;42(5):679–88. doi: 10.1002/ana.410420503. [DOI] [PubMed] [Google Scholar]
- 17.Fischer-Smith T, Bell C, Croul S, Lewis M, Rappaport J. Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies. J Neurovirol. 2008;14(4):318–26. doi: 10.1080/13550280802132857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giancola ML, Lorenzini P, Balestra P, Larussa D, Baldini F, Corpolongo A, Narciso P, Bellagamba R, Tozzi V, Antinori A. Neuroactive antiretroviral drugs do not influence neurocognitive performance in less advanced HIV-infected patients responding to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;41(3):332–7. doi: 10.1097/01.qai.0000197077.64021.07. [DOI] [PubMed] [Google Scholar]
- 19.Harrigan PR, Bloor S, Larder BA. Relative replicative fitness of zidovudine-resistant human immunodeficiency virus type 1 isolates in vitro. J Virol. 1998;72(5):3773–8. doi: 10.1128/jvi.72.5.3773-3778.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heaton RK, Velin RA, McCutchan JA, Gulevich SJ, Atkinson JH, Wallace MR, Godfrey HP, Kirson DA, Grant I. Neuropsychological impairment in human immunodeficiency virus-infection: implications for employment. HNRC Group. HIV Neurobehavioral Research Center. Psychosom Med. 1994;56(1):8–17. doi: 10.1097/00006842-199401000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Iglesias-Ussel MD, Casado C, Yuste E, Olivares I, Lopez-Galindez C. In vitro analysis of human immunodeficiency virus type 1 resistance to nevirapine and fitness determination of resistant variants. J Gen Virol. 2002;83(Pt 1):93–101. doi: 10.1099/0022-1317-83-1-93. [DOI] [PubMed] [Google Scholar]
- 22.Koelsch KK, Smith DM, Little SJ, Ignacio CC, Macaranas TR, Brown AJ, Petropoulos CJ, Richman DD, Wong JK. Clade B HIV-1 superinfection with wild-type virus after primary infection with drug-resistant clade B virus. Aids. 2003;17(7):F11–6. doi: 10.1097/00002030-200305020-00001. [DOI] [PubMed] [Google Scholar]
- 23.Koval CE, Dykes C, Wang J, Demeter LM. Relative replication fitness of efavirenz-resistant mutants of HIV-1: correlation with frequency during clinical therapy and evidence of compensation for the reduced fitness of K103N + L100I by the nucleoside resistance mutation L74V. Virology. 2006;353(1):184–92. doi: 10.1016/j.virol.2006.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Letendre S, Paulino AD, Rockenstein E, Adame A, Crews L, Cherner M, Heaton R, Ellis R, Everall IP, Grant I, Masliah E. Pathogenesis of Hepatitis C Virus Coinfection in the Brains of Patients Infected with HIV. J Infect Dis. 2007;196(3):361–70. doi: 10.1086/519285. [DOI] [PubMed] [Google Scholar]
- 25.Letendre SL, Cherner M, Ellis RJ, Marquie-Beck J, Gragg B, Marcotte T, Heaton RK, McCutchan JA, Grant I. The effects of hepatitis C, HIV, and methamphetamine dependence on neuropsychological performance: biological correlates of disease. Aids. 2005;19(Suppl 3):S72–8. doi: 10.1097/01.aids.0000192073.18691.ff. [DOI] [PubMed] [Google Scholar]
- 26.Letendre SL, McCutchan JA, Childers ME, Woods SP, Lazzaretto D, Heaton RK, Grant I, Ellis RJ. Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Ann Neurol. 2004;56(3):416–23. doi: 10.1002/ana.20198. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Picado J, Martinez MA. HIV-1 reverse transcriptase inhibitor resistance mutations and fitness: a view from the clinic and ex vivo. Virus Res. 2008;134(1–2):104–23. doi: 10.1016/j.virusres.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 28.McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol. 2004;157(1–2):3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 29.McArthur JC, McClernon DR, Cronin MF, Nance-Sproson TE, Saah AJ, St Clair M, Lanier ER. Relationship between human immunodeficiency virus-associated dementia and viral load in cerebrospinal fluid and brain. Ann Neurol. 1997;42(5):689–98. doi: 10.1002/ana.410420504. [DOI] [PubMed] [Google Scholar]
- 30.Paredes R, Sagar M, Marconi VC, Hoh R, Martin JN, Parkin NT, Petropoulos CJ, Deeks SG, Kuritzkes DR. In vivo fitness cost of the M184V mutation in multidrug-resistant human immunodeficiency virus type 1 in the absence of lamivudine. J Virol. 2009;83(4):2038–43. doi: 10.1128/JVI.02154-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez-Bercoff D, Wurtzer S, Compain S, Benech H, Clavel F. Human immunodeficiency virus type 1: resistance to nucleoside analogues and replicative capacity in primary human macrophages. J Virol. 2007;81(9):4540–50. doi: 10.1128/JVI.01620-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson JL, Nowicki M, Danley K, Martin EM, Cohen MH, Gonzalez R, Vassileva J, Levine AM. Neuropsychological functioning in a cohort of HIV− and hepatitis C virus-infected women. Aids. 2005;19(15):1659–67. doi: 10.1097/01.aids.0000186824.53359.62. [DOI] [PubMed] [Google Scholar]
- 33.Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10(1):1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- 34.Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol. 2002;8(Suppl 2):115–21. doi: 10.1080/13550280290101094. [DOI] [PubMed] [Google Scholar]
- 35.Sevigny JJ, Albert SM, McDermott MP, McArthur JC, Sacktor N, Conant K, Schifitto G, Selnes OA, Stern Y, McClernon DR, Palumbo D, Kieburtz K, Riggs G, Cohen B, Epstein LG, Marder K. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology. 2004;63(11):2084–90. doi: 10.1212/01.wnl.0000145763.68284.15. [DOI] [PubMed] [Google Scholar]
- 36.Smith DM, Wong JK, Shao H, Hightower GK, Mai SH, Moreno JM, Ignacio CC, Frost SD, Richman DD, Little SJ. Long-term persistence of transmitted HIV drug resistance in male genital tract secretions: implications for secondary transmission. J Infect Dis. 2007;196(3):356–60. doi: 10.1086/519164. [DOI] [PubMed] [Google Scholar]
- 37.Spudich S, Lollo N, Liegler T, Deeks SG, Price RW. Treatment benefit on cerebrospinal fluid HIV-1 levels in the setting of systemic virological suppression and failure. J Infect Dis. 2006;194(12):1686–96. doi: 10.1086/508750. [DOI] [PubMed] [Google Scholar]
- 38.Spitzer Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders version IV. 1995 [Google Scholar]
- 39.Tobin J. Estimation of relationships for limited dependent variables. Econometrica. 1958;v26:24–36. [Google Scholar]
- 40.Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, Vlassi C, Giulianelli M, Galgani S, Antinori A, Narciso P. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45(2):174–82. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- 41.Vitiello B, Goodkin K, Ashtana D, Shapshak P, Atkinson JH, Heseltine PN, Eaton E, Heaton R, Lyman WD. HIV-1 RNA concentration and cognitive performance in a cohort of HIV-positive people. Aids. 2007;21(11):1415–1422. doi: 10.1097/QAD.0b013e328220e71a. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Dykes C, Domaoal RA, Koval CE, Bambara RA, Demeter LM. The HIV-1 reverse transcriptase mutants G190S and G190A, which confer resistance to non-nucleoside reverse transcriptase inhibitors, demonstrate reductions in RNase H activity and DNA synthesis from tRNA(Lys, 3) that correlate with reductions in replication efficiency. Virology. 2006;348(2):462–74. doi: 10.1016/j.virol.2006.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber J, Chakraborty B, Weberova J, Miller MD, Quinones-Mateu ME. Diminished replicative fitness of primary human immunodeficiency virus type 1 isolates harboring the K65R mutation. J Clin Microbiol. 2005;43(3):1395–400. doi: 10.1128/JCM.43.3.1395-1400.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei X, Liang C, Gotte M, Wainberg MA. Negative effect of the M184V mutation in HIV-1 reverse transcriptase on initiation of viral DNA synthesis. Virology. 2003;311(1):202–12. doi: 10.1016/s0042-6822(03)00173-9. [DOI] [PubMed] [Google Scholar]
- 45.White KL, Margot NA, Wrin T, Petropoulos CJ, Miller MD, Naeger LK. Molecular mechanisms of resistance to human immunodeficiency virus type 1 with reverse transcriptase mutations K65R and K65R+M184V and their effects on enzyme function and viral replication capacity. Antimicrob Agents Chemother. 2002;46(11):3437–46. doi: 10.1128/AAC.46.11.3437-3446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, Hinkin CH, Lazzaretto D, Cherner M, Marcotte TD, Gelman BB, Morgello S, Singer EJ, Grant I, Heaton RK. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004;26(6):759–78. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


