Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) is known to broadly regulate the cellular stress response. In contrast, it is unclear if the PACAP/PAC1 receptor pathway has a role in human psychological stress responses, such as posttraumatic stress disorder (PTSD). In heavily traumatized subjects, we find a sex-specific association of PACAP blood levels with fear physiology, PTSD diagnosis and symptoms in females (N=64, replication N=74, p<0.005). Using a tag-SNP genetic approach (44 single nucleotide polymorphisms, SNPs) spanning the PACAP (ADCYAP1) and PAC1 (ADCYAP1R1) genes, we find a sex-specific association with PTSD. rs2267735, a SNP in a putative estrogen response element within ADCYAP1R1, predicts PTSD diagnosis and symptoms in females only (combined initial and replication samples: N=1237; p<2x10−5). This SNP also associates with fear discrimination and with ADCYAP1R1 mRNA expression. Methylation of ADCYAP1R1 is also associated with PTSD (p < 0.001). Complementing these human data, ADCYAP1R1 mRNA is induced with fear conditioning or estrogen replacement in rodent models. These data suggest that perturbations in the PACAP/PAC1 pathway are involved in abnormal stress responses underlying PTSD. These sex-specific effects may occur via estrogen regulation of ADCYAP1R1. PACAP levels and ADCYAP1R1 SNPs may serve as useful biomarkers to further our mechanistic understanding of PTSD.
PACAP was first isolated from ovine hypothalamic extracts based on its ability to stimulate cAMP production in anterior pituitary cells1. It is a highly conserved member of the VIP/secretin/glucagon peptide family with pleiotropic functions in development, cell signaling, metabolism, homeostasis and cell protection2–6. Among these myriad functions are studies demonstrating 1) high expression of PACAP peptide and its selective PAC1 receptor in hypothalamic and limbic structures, 2) PACAP regulation of corticotropin releasing hormone (CRH) and autonomic function, 3) actions of PACAP in stress-related behavior, 4) reduced anxiety-like phenotypes in PACAP and PAC1 receptor null mice, and 5) blunted corticosterone response in knockout animals after emotional stressors. Thus, PACAP/PAC1 receptor signaling is integrally involved in stress mechanisms7,8. We hypothesized that PACAPergic systems may be important mediators of abnormal stress responses following psychological trauma contributing to PTSD, an extreme maladaptive and debilitating psychiatric disorder affecting up to 40% of individuals over lifetime exposure to traumatic events9,10.
Little is known about the biological processes regulating PTSD and other stress-related responses. To examine whether the PACAP/PAC1 pathway is associated with PTSD in a high risk, heavily traumatized population, we analyzed blood levels of PACAP, and genetic variation and methylation of the PACAP (ADCYAP1) and PAC1 receptor (ADCYAP1R1) genes in a cohort of over 1200 highly traumatized subjects with and without PTSD (See Suppl. Tables 1–2 for demographic information).
PACAP levels associated with PTSD in females
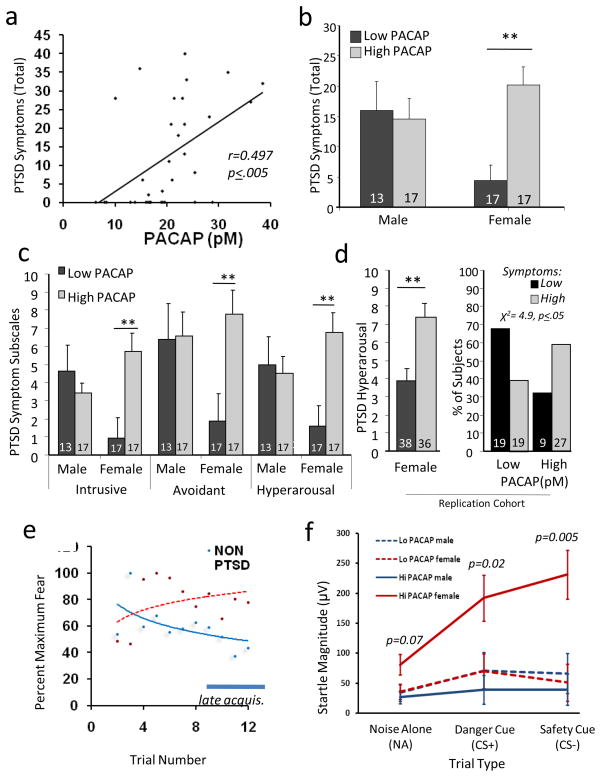
Using radioimmunoassay, we first examined PACAP peptide levels in peripheral blood samples from a previously described, highly traumatized, at risk population11–13 that had been matched on age, sex, and trauma histories (n = 64, see Suppl. Tables 1–3 for demographics). We found that PTSD symptoms (PTSD Symptom Scale14) were significantly correlated with PACAP38 (PACAP peptide containing 38 residues) blood levels in females (p < 0.005, r = 0.497, Fig 1A), but not in males (p>0.5). Also in females, PTSD diagnosis was associated with PACAP38 levels (p ≤ 0.001), with higher PACAP38 found in the PTSD cohort. Furthermore, PACAP levels (median split, low vs. high) were differentially associated with PTSD symptoms in females (Fig 1B). PACAP38 levels also predicted differential response on all three symptom clusters necessary to fulfill diagnostic criteria for PTSD (intrusive re-experiencing (e.g., trauma flashbacks), avoidance (e.g., avoidance of trauma reminders) and hyperarousal (e.g., increased startle response)) in females but not males (Fig 1C). These analyses were repeated in a second, all female cohort (N=74) with similar findings (Fig 1D; high vs. low PACAP38 levels, controlling for age, substance abuse and total trauma exposure, one-tailed t-tests: total symptoms, p ≤ 0.05, hyperarousal symptoms, p ≤ 0.001; and % with clinically significant symptoms, χ2=4.9, p<0.05). These observations were especially notable since females may be at twice the risk for PTSD as compared to males 9,11, implicating roles for sex hormones, especially estrogen, in the disorder15–17. When we controlled for common stress-related phenomena (depression and history of substance abuse), the effect of PACAP level on PTSD remained (p<0.05). In contrast, there was no effect of PACAP level on depression symptoms or substance abuse when controlling for PTSD.
Figure 1. PACAP blood levels predict PTSD symptoms in females.
A) PTSD Symptoms (scale range 0–51), relative to Plasma PACAP38 blood levels (pM); (N=34 females; r = 0.497, p ≤ 0.005). B) Total PTSD symptoms graphed relative to sex and levels of plasma PACAP38 (N=64, low: < 20pM, high: > 20pM); females with high PACAP blood levels have increased symptoms (**, p<0.0005). C) PACAP levels (low vs. high) are also differentially associated with PTSD Intrusive, Avoidance, and Hyperarousal symptoms in females (N = 64,** p < 0.005). D) PACAP levels (low vs. high) were examined in a replication sample of highly traumatized women, with differential association in hyperarousal symptoms (left, N=74,** p=0.002) and in percent of subjects with significant symptoms (right, χ2=4.9, p<0.05). E) Acoustic startle reflex (EMG) relative to the fear conditioning trial in subjects without (non-PTSD) vs with PTSD. Habituation is seen in non-PTSD subjects during late acquisition (bar). F) Startle magnitude during the late acquisition period vs. trial type (noise alone, CS+, and CS−), showing that females with high PACAP levels show enhanced startle responses to both fear cues (CS+, p = 0.02) and safety cues (CS−, p = 0.005) (N=27; 16 male, 11 female). Bars represent mean ± SEM., N’s for each group at bottom of bar graphs.
In addition to the psychological symptoms that define the syndrome, subjects with PTSD have been found to have abnormally high conditioned fear responses. This high level of fear may result from a combination of an inability to habituate to aversive stimuli, a decreased ability to extinguish (learn to inhibit) fear memories, and possibly an over-consolidation of the original fear memory18–22. Hence, we determined the physiological (electromyographic) levels of conditioned fear for 27 participants (16 male, 11 female) with PACAP blood levels. Fear potentiation was determined by measuring the acoustic startle reflex in the presence of startle cues alone, or startle cues combined with stimuli paired (conditioned stimulus, CS+) or unpaired (CS−) with an aversive airblast (Fig 1E). Female (but not male) subjects with high PACAP38 levels demonstrated markedly increased startle reflex responses to both CS+ and CS− cues. This was particularly pronounced during the late acquisition phase when normal subjects had habituated to the fearful stimuli (Fig 1F). In aggregate, these data suggest that PACAP38 peptide is strongly associated with the psychological and physiological symptoms of PTSD in women with a history of trauma.
ADCYAP1R1 associated with PTSD in females
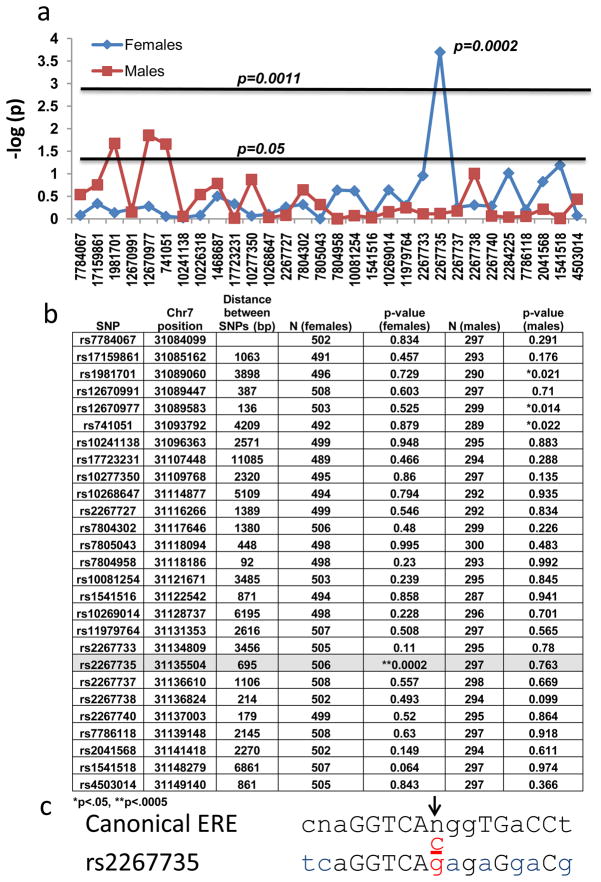
To assess whether there may be a genetic association of PTSD with polymorphisms in either the PACAP (ADCYAP1) or PAC1 receptor (ADCYAP1R1) locus, we performed a tag-SNP analysis (r2 = 0.8; MAF = 0.1) across both genes with a total of 44 SNPs (14 ADCYAP1 and 30 ADCYAP1R1 SNPs). Using logistic regression, we examined whether each SNP was associated with PTSD diagnosis in this cohort of highly traumatized urban civilian subjects (n = 798)11,12,23, in total, or stratified by gender (females: n = 503; males: n = 295). Only the ADCYAP1R1 receptor SNP rs2267735 (p = 0.0002 in females; NS in males) remained significant after experiment-wide multiple correction for sex and 44 independent tests (Figure 2A–B and Suppl. Fig. 1). No SNPs in the peptide ADCYAP1 gene met experiment-wide criteria for association (Suppl. Fig. 2). Given these striking gender differences and recent data demonstrating that ADCYAP1R1 gene expression may be dynamically modulated by estrogen24, the distribution of estrogen response elements (EREs) within ADCYAP1R1 gene was examined (Suppl. Table 4). We found that rs2267735 was within a predicted ERE (Fig 2C, Genomatix; matrix similarity = 0.877, core similarity = 1.0). Because rs2267735 is positioned within the central variable region of the consensus sequence, in silico analyses do not currently allow us to predict how the ‘C’ vs ‘G’ allele may differentially alter ERE function and further in vitro analyses are warranted.
Figure 2. Genetic Association of PAC1 Receptor (ADCYAP1R1) with PTSD.
A) 30 single nucleotide polymorphisms (SNPs) spanning the ADCYAP1R1 gene (x-axis), with the–log(p-value) of logistic regression for each SNP association with PTSD (diagnosis based on DSM-IV criteria from PTSD Symptom Scale). Subjects were analyzed with logistic regression in females only (N=503) or males only (N=295). Horizontal lines represent the nominal p = 0.05 or the corrected p-value, p = 0.0011 (44 SNPs, correcting for 30 ADCYAP1R1 SNPs and 14 ADCYAP1 SNPS (Supp. Fig. 1)). rs2267735 is the only SNP remaining significant after multiple corrections (p = 0.0002). B) Table of p-values resulting from the association of each genotyped, ADCYAP1R1 SNP with PTSD diagnosis (by gender). The location on Chromosome 7 (GRCh37) for each SNP including the distance (bp) between the SNPs is given. The average distance between SNPs is 2.2 kb. SNP rs2267735 is located in an intron of ADCYAP1R1, and is not in LD with other SNPS (for African Americans in our population, data not shown). C) rs2267735 (C/G), in red, is located within a canonical estrogen response element (ERE) binding site (Capitol letters – conserved canonical ERE nucleotides; blue letters – mismatches with the ADCYAP1R1 gene and canonical ERE; Reverse strand shown).
We next determined if the association between rs2267735 and PTSD diagnosis could be replicated in an additional 439 subjects. These subjects were from the same overall study, but were interviewed and had DNA collected after the original discovery population. Thus they served as a replication source from the same population but distinct in time and with different interviewing staff. The table in Figure 3A shows the logistic regression results for males and females separately in the initial population described in the tag-SNP analysis, the replication sample from the same population, and the combined sample of 1237 individuals. The main effect of the SNP on PTSD diagnosis could be replicated in women (p<0.05) and combining both samples increased the significance of the association (N = 763, p< 0.00002). As in the discovery sample, no effects were observed in males (male combined sample N = 474, p = 0.7).
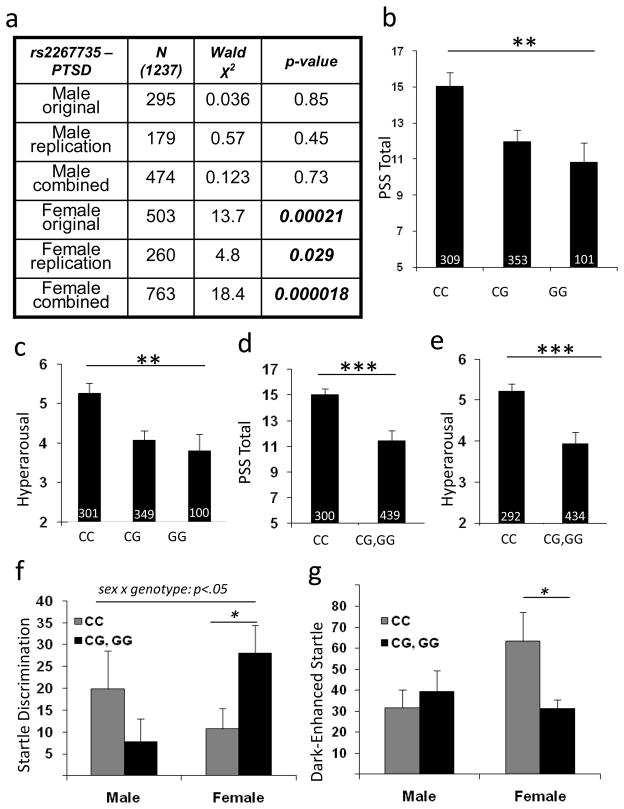
Figure 3. Association of ADCYAP1R1 rs2267735 with PTSD symptoms and physiological fear responses.
A) Table demonstrating the N, Wald χ2, and p-value, in males and females, in the original, replication, and combined samples for logistic regression of rs2267735 with PTSD diagnosis. B) Total PTSD symptoms (PSS total) are differentially associated with rs2267735 genotype in females (p ≤ 0.001). C) Hyperarousal is the most robustly associated symptom with rs2267735 genotype (p = 0.0009). D) In a dominant/recessive model, even after controlling for childhood trauma, adult trauma, and age, genotype predicts total PTSD symptoms (p ≤ 0.001) and (E) hyperarousal symptoms (p ≤ 0.0001). F) Fear Discrimination (CS+ Startle minus CS− Startle) is impaired in females with rs2267735 ‘CC’ genotype. G) Dark enhanced startle (StartleDark – StartleLight) is significantly increased in females with rs2267735 ‘CC’ genotype. (N’s are shown at base of each bar, bars represent mean ± SEM. N’s are slightly different across analyses due to differences in number of subjects across measures. * p<0.05; ** p<0.001; *** p<0.0002)
To further examine ADCYAP1R1 rs2267735 SNP associations with continuous PTSD symptom levels in females, we analyzed both an additive and a dominant model with total PTSD symptoms and symptom subscales using the combined samples (Fig 3B – E). The ‘CC’ allele was most robustly associated with total PTSD symptoms and among subscales, hyperarousal symptoms were the most strongly associated with rs2267735. Notably, even after controlling for childhood trauma history and adult trauma, age and race, (which slightly reduces total N due to missing data) the rs2267735 ‘CC’ allele was associated with higher levels of PTSD hyperarousal symptoms compared to ‘G’ carriers in women (p = 0.0008, Fig 3E), but not men (p=0.51).
We repeated the above analyses with Beck Depression Inventory (BDI) symptoms and history of lifetime substance abuse, and found no associations with these measures and rs2267735 (Suppl. Fig. 3), suggesting that this association may be relatively specific to PTSD. To address whether rs2267735 might be associated with other severe psychiatric illnesses, we performed analyses using bipolar disorder, schizophrenia, and Alzheimer’s disease samples. From the data of the Genetic Association Information Network (GAIN) publicly accessible database (http://www.ncbi.nlm.nih.gov/projects/gap), we analyzed the association of rs2267735 (included on the Affymetrix 6.0 SNP array) with bipolar disorder as well as schizophrenia. We did not observe a significant association of this SNP with these two disorders in subjects with African American (954 cases, 1195 controls) or European (1378 cases, 1351 controls) ancestry. Specifically, we found that all pre-computed p-values for associations of rs2267735 with schizophrenia or bipolar disorder were higher than the multiple-testing correction p-value of 0.01, indicating no major contribution of this variant.
Additionally, we examined the association of rs2267735 and Alzheimer’s in a previously characterized Alzheimer’s disease sample25. In this cohort of 342 subjects, we found no association with rs226735 and Alzheimer’s disease diagnosis using either the additive genetic model (p=0.19) or the dominant/recessive model (p=0.89). These data suggest that we find robust associations with rs2267735 in women, but not men, with PTSD. In contrast, we find no association with depression symptoms, substance abuse, Alzheimer’s disease, bipolar disorder, and schizophrenia across different samples. Note that for all of these negative results, due to the limited sample sizes, we cannot rule out the possibility that rs2267735 may be associated with PTSD in men or with other disorders with a smaller effect size than we see with PTSD in women.
To parallel our results with plasma PACAP38 levels, we next examined whether physiological measures of fear are differentially associated with the ADCYAP1R1 rs2267735 SNP. In PTSD, but not depression18, fear response to an inhibitory CS−, or ‘safety signal’, is exaggerated. The discrimination between CS+ and CS− improves across the training procedure in controls, but not in those with PTSD. We examined whether rs2267735 was associated with impaired fear discrimination late in conditioned acquisition, during the same period noted in Figure 1E. Notably, females with the ‘CC’ genotype were significantly less able to discriminate CS+ from CS− signals (Figure 3F, sex by genotype interaction, p<0.05, and ‘CC’ vs ‘G’ carriers in females, p<0.05).
We next examined whether a difference in dark-enhanced startle, a measure of increased anxiety in humans that is similar to light-enhanced startle in rodents26–28, was differentially associated with rs2267735. Again, we found that females, but not males, with the ‘CC’ genotype showed significantly more startle in the dark compared to the light (Fig 3G, males, N=35, p=0.71; females, N=53, p=0.02). Together, these data suggest that the ADCYAP1R1 rs2267735 SNP may be relatively specific in its association with PTSD psychological and physiological phenotypes. Further, the robust association of rs2267735 with hyperarousal symptoms suggests that the role of PACAP/PAC1 may be specifically involved in the normalization of the stress response, a process which is particularly dysregulated in PTSD.
ADCYAP1R1 methylation and mRNA expression
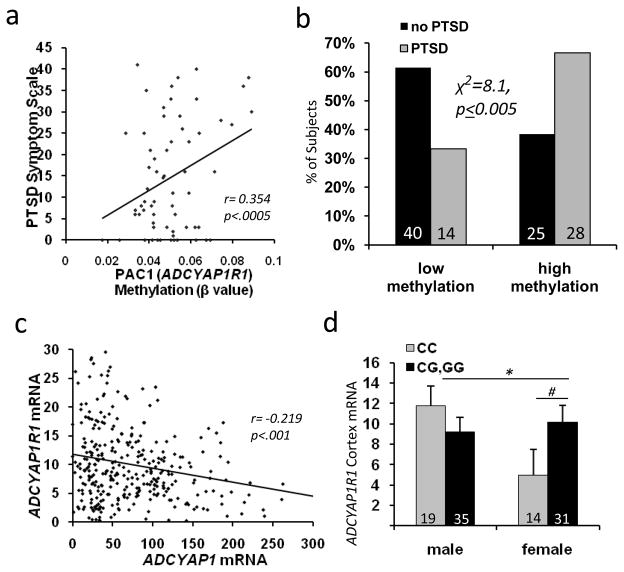
Environmental, genetic and epigenetic mechanisms likely moderate the long-term effects of trauma exposure. Using the Illumina HumanMethylation27 BeadChip, we interrogated methylation in DNA extracted from peripheral blood at the first site within the ADCYAP1R1 CpG island (Suppl. Fig. 2). Methylation at this site was significantly associated with total PTSD symptoms (Figure 4A, N=94, r = 0.354, p<0.0005) in a sex-independent manner. Further, CpG methylation level (median split) was associated with PTSD diagnosis (Figure 4B, χ2=8.1, p < 0.005), but not depression (p > 0.05, Suppl. Fig. 3E). There was no significant association between methylation of ADCYAP1 and PTSD symptoms. These data suggest that ADCYAP1R1 is regulated, in part, through epigenetic mechanisms that contribute to differential function of the PAC1 receptor in PTSD.
Figure 4. ADCYAP1R1 Methylation and mRNA expression.
A) Methylation within the first CpG island of ADCYAP1R1 (beta value, Illumina #cg27076139) is positively correlated with total PTSD symptoms (both sexes; N=107; r = 0.354, p < 0.0005). B) Subjects with PTSD have higher levels of ADCYAP1R1 methylation (median split, N = 107; chi-squared analyses, p < 0.005). C) ADCYAP1 mRNA levels are inversely correlated with ADCYAP1R1 mRNA levels in cortex (from prior dataset13)(r=−0.219; p<0.001). D) ADCYAP1R1 mRNA levels are differentially expressed in females compared to males based on imputed ADCYAP1R1 rs2267735 genotype (from prior dataset13)(*p<0.05 male vs. female CC carriers, # p<0.05, one-tailed, CC vs G-carriers). Bars represent mean ± SEM, N’s for each group at bottom of each graph.
To examine the potential relationship of genotype and brain mRNA expression as described previously29, we utilized a brain mRNA expression data set30 to test whether ADCYAP1R1 rs2267735 is associated with differential gene expression. We first examined whether cortical ADCYAP1R1 and ADCYAP1 mRNA levels were correlated. As shown in Fig 4C, these mRNA levels were significantly inversely correlated (r = −0.219, p < 0.001, including males and females), suggesting that brain levels of PACAP peptide and PAC1 mRNA are tightly regulated.
We next utilized a prior analyzed genome-wide association and brain mRNA expression data30 to examine whether ADCYAP1R1 rs2267735 imputed genotypes were associated with differential ADCYAP1R1 expression in brain. We found a sex x genotype effect (Fig 4D, F(3,99) = 4.3, p < 0.05) with females with the ‘CC’ genotype expressing significantly less ADCYAP1R1 mRNA than males(F(1,33) = 5.5, p < 0.05) or than females who are ‘G’ carriers (one-tailed, F(1, 45)=2.87, p<0.05). Thus, mRNA encoding the PACAP peptide and PAC1 receptor appeared to be tightly regulated within the human cortex, and ADCYAP1R1 mRNA levels were associated with the ADCYAP1R1 rs2267735 SNP.
Fear induces ADCYAP1R1 in mouse amygdala
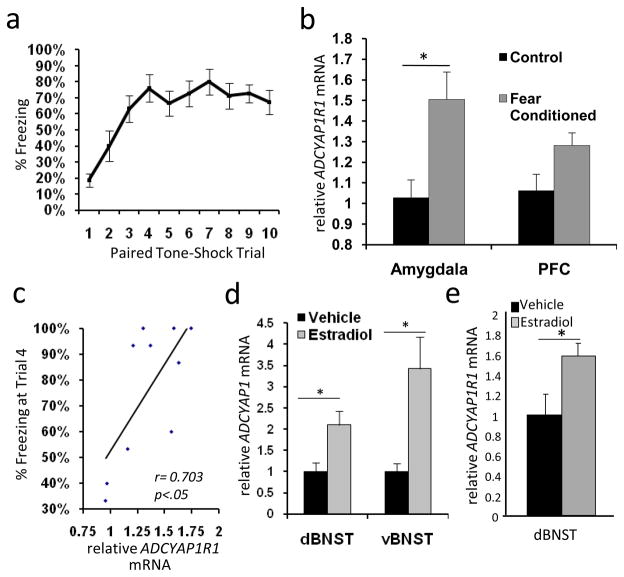
Despite prior studies examining PACAP/PAC1 receptor function in CNS/PNS development, endocrine homeostasis, metabolism, cellular protection/regeneration, and chronic stress responses2,3,6,31–34, a role for PACAP signaling in fear conditioning has not been evaluated. Given our data implicating PACAP in PTSD, we wondered if ADCYAP1R1 mRNA was differentially regulated in mice using Pavlovian fear conditioning35–40, a means of studying acute fear and trauma responses that has been proposed to model PTSD19,22. We performed classical fear conditioning experiments using male mice, in which a previously neutral tone CS (6kHz) was paired with 10 footshocks (1mA, 0.5sec; Figure 5A). This conditioning paradigm consistently provides robust fear learning in mice leading to changes in gene expression within the amygdala, a region critical for fear learning and expression. Quantitative PCR analyses shows that amygdala ADCYAP1R1 mRNA increased ~1.5-fold during the consolidation of fear (Figure 5B, p<0.05), with a similar trend within the mPFC. When peak freezing was compared with brain mRNA levels, we find a significant correlation between fear learning and ADCYAP1R1 mRNA (Fig 5C, r2=0.49, p<0.05).
Figure 5. Regulation of ADCYAP1R1 and ADCYAP1 mRNA in rodent models.
A) Percentage of time freezing, in mice, to the conditioned tone (CS+) following tone-shock pairings during the conditioned fear trials. B) rtPCR analyses of mRNA levels within mouse amygdala and mPFC 2 hrs after fear conditioning or in control handling conditions, showing a significant increase in amygdala ADCYAP1R1 mRNA (N = 15, 1.47 fold, p < 0.05) and a non-significant trend in mPFC (1.19 fold change). C) Correlation between average amygdala and PFC ACYAP1R1 mRNA and % freezing at trial 4, demonstrating an association between ADCYAP1R1 mRNA with rate of fear learning (r2=0.49, p<0.05). D) ADCYAP1 mRNA in rat BNST in female rats (N = 12/group) following ovarectomy and estradiol implant vs. vehicle replacements. ADCYAP1 mRNA is increased in both dorsal (2.1-fold) and ventral (3.4-fold) BNST after estradiol implantation. E) ADCYAP1R1 transcripts are also increased in dorsal BNST (1.6-fold, N=4 per group). Bars represent mean ± SEM, (* p < 0.05).
Estrogen induces ADCYAP1R1 in rat BNST
To further establish the relationship between PACAP/PAC1 receptors and estrogen in a validated model of sex hormone regulation, we examined estrogen-induced changes in ADCYAP1 and ADCYAP1R1 transcripts in the bed nucleus of stria terminalis (BNST) in female rats. The BNST is a component of the extended amygdala that is subject to significant gonadal hormonal control7,27,28. In rodents, it is critical for emotional behavior, mediating stress responses and the light-enhanced startle response. We examined gene expression in the BNST in ovarectomized (OVX) female rats following 21-day implantation of continuous release estrogen pellets. Compared to control implants, estradiol increased ADCYAP1 transcripts in the dorsal and ventral BNST 2.1- and 3.4-fold, respectively (p ≤ 0.01, Figure 5D). Additionally, estradiol increased ADCYAP1R1 mRNA 1.5-fold in the dorsal BNST samples (p<0.05, Figure 5E), and future studies should also examine estradiol sensitivity of these genes in amygdala. While these rodent studies are complex and have differing experimental designs, these data clearly illustrate dynamic PACAP/PAC1 receptor regulation within central areas mediating fear, stress and estrogen responsiveness.
Discussion
Since its identification more than 20 years ago, PACAP has been increasingly implicated in diverse cellular stress response pathways and neurotrophic function. However, the organizational role of the PACAP system in orchestrating behavioral stress responses has yet to be clarified. Our data suggest that PACAP/PAC1 receptor expression and signaling may be integrally involved in regulating the psychological and physiological responses to traumatic stress. Further, we report association of an ERE-embedded ADCYAP1R1 SNP with PTSD, and we demonstrate fear- and estrogen-dependent regulation of PACAP systems within stress-responsive regions of the brain. These data may begin to explain sex-specific differences in PTSD diagnosis, symptoms, and fear physiology. Future work targeting the PACAP/PAC1 receptor system may lead to novel and robust biomarkers as well as to further our understanding of the neural mechanisms underlying pathological responses to stress with potential therapeutic targets towards the prevalent and debilitating syndrome of PTSD.
Methods Summary (Please see detailed Online Supplementary Methods)
This highly traumatized, civilian, cross-sectional cohort has been previously described in candidate gene-association studies of PTSD and depression11–13. Research interviews, salivary DNA and blood samples were collected from patients receiving services in the primary care clinics at Grady Memorial Hospital (Atlanta, GA). All study procedures have been reviewed and approved by the Emory Institutional Review Board and the Grady Hospital Research Oversight Committee. PTSD measures in this manuscript are based on the PTSD symptom scale 14, which has been validated within this population using the Clinician Administered PTSD Scale. PACAP38 radioimmunoassay (1:30,000, Peninsula Laboratories, Belmont, CA) was performed at University of Vermont, using double antibody immunoprecipitation as previously described 41. For genotyping, pairwise tagging (R2 > 0.8, MAF > 0.1) was used to choose tag-SNPs for both ADCYAP1 and ADCYAP1R1. The coordinates were chr18:885000-906000 and chr7:31048667-31117836 for ADCYAP1 and ADCYAP1R1, respectively (NCBI B36) which includes approximately 10kb upstream and 5kb downstream of the coding regions for both genes. Genotypes for the tag-SNPs were generated using Sequenom iPlex with follow-up analyses using Taqman. For methylation analyses, DNA was whole-genome amplified, fragmented, and hybridized to the HumanMethylation27 BeadChip (Illumina, San Diego, CA). Individual samples were stratified to separate BeadChips according to PTSD status to limit bias. The BeadChips were scanned using a BeadStation 500GX, and the methylation level (beta value) was calculated using the Methylation Module of the BeadStudio software. The eyeblink component of the acoustic startle response was measured by EMG recordings of the right orbicularis oculi muscle with two 5-mm Ag/AgCl electrodes filled with electrolyte gel, as previously described18–19. The mouse fear conditioning and rat estrogen replacement studies are described in detail in Supplementary Methods.
Supplementary Material
Acknowledgments
This work was primarily supported by National Institutes Health Grants MH071537, DA019624 (KJR) and HD27468 (VM). Support was also received from Emory and Grady Memorial Hospital General Clinical Research Center, NIH National Centers for Research Resources (M01RR00039 and P20RR16435), the American Foundation for Suicide Prevention (BB) and the Burroughs Wellcome Fund (KJR). RNA samples were run using the Oncogenomics Core Facility at University of Miami. Dr Myers is supported by The National Institute on Aging (AG034504). We thank Drs. Joseph F. Cubells, Yilang Tang, and Karen Conneely for excellent discussions. We thank the Grady Trauma Project, including Drs. Charles F. Gillespie, Ann Schwartz, Aliza Wingo, David A. Gutman, and Tamara Weiss for medical support; Allen Graham, Angelo Brown, Justine Phifer, Daniel Crain, Asante Kamkwalala, James Poole, Dorthie Cross, Negar Fani, and Dr. Ami Smith for clinical research support; and Kristin Schutz, Emily Reiser and Caitlin Fitzgerald for excellent molecular/genetics technical support. Methylation chip assays were performed by the Emory University Biomarkers Service Center.
Footnotes
Supplementary Online Material: Detailed Materials and Methods (13 pages), with 4 Tables and 3 Figures
Reprints and permissions information is available at www.nature.com/reprints.
The authors declare no competing financial interests.
Author Contributions: K.J.R. designed the experiments and wrote the paper. K.J.R., B.B., and V.M. organized collaborations, obtained funding, supervised data collection and analyses, and revised the paper. K.B.M., K.K., and E.B.B. performed the genetics experiments and analyses on the primary, replication, and GAIN cohorts, and revised the paper. T.J. and S.D.N. performed and supervised the human physiology studies and revised the paper. V.K. and A.K.S. performed and supervised the methylation studies and revised the paper. A.M., S.E.H., D.T., K.J.R., and V.M. performed and supervised the rodent fear conditioning and estrogen replacement studies and rtPCR analyses and revised the paper. A.J.M., M.R., and A.E. performed and supervised the human mRNA expression analyses and the Alzheimer’s disease gene association studies. K.M.B. and V.M. performed and supervised the PACAP38 radioimmunoassays and related data analyses.
References
- 1.Miyata A, et al. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–74. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- 2.Ghzili H, et al. Role of PACAP in the physiology and pathology of the sympathoadrenal system. Front Neuroendocrinol. 2008;29:128–41. doi: 10.1016/j.yfrne.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto H, Shintani N, Baba A. New insights into the central PACAPergic system from the phenotypes in PACAP- and PACAP receptor-knockout mice. Ann N Y Acad Sci. 2006;1070:75–89. doi: 10.1196/annals.1317.038. [DOI] [PubMed] [Google Scholar]
- 4.Spengler D, et al. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–5. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe J, et al. Localization, characterization and function of pituitary adenylate cyclase-activating polypeptide during brain development. Peptides. 2007;28:1713–9. doi: 10.1016/j.peptides.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 6.Zhong Y. Mediation of PACAP-like neuropeptide transmission by coactivation of Ras/Raf and cAMP signal transduction pathways in Drosophila. Nature. 1995;375:588–92. doi: 10.1038/375588a0. [DOI] [PubMed] [Google Scholar]
- 7.Hammack SE, et al. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology. 2009;34:833–43. doi: 10.1016/j.psyneuen.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaudry D, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- 9.Breslau N. The epidemiology of posttraumatic stress disorder: what is the extent of the problem? J Clin Psychiatry. 2001;62 (Suppl 17):16–22. [PubMed] [Google Scholar]
- 10.Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. Jama. 2006;295:1023–32. doi: 10.1001/jama.295.9.1023. [DOI] [PubMed] [Google Scholar]
- 11.Binder EB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradley RG, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillespie CF, Phifer J, Bradley B, Ressler KJ. Risk and resilience: genetic and environmental influences on development of the stress response. Depress Anxiety. 2009;26:984–92. doi: 10.1002/da.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foa EB, Tolin DF. Comparison of the PTSD Symptom Scale-Interview Version and the Clinician-Administered PTSD scale. J Trauma Stress. 2000;13:181–91. doi: 10.1023/A:1007781909213. [DOI] [PubMed] [Google Scholar]
- 15.Bangasser DA, et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15:877, 896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McEwen BS. Steroid hormones: effect on brain development and function. Horm Res. 1992;37 (Suppl 3):1–10. doi: 10.1159/000182393. [DOI] [PubMed] [Google Scholar]
- 17.Shansky RM, et al. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex. 2010;20:2560–7. doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jovanovic T, et al. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety. 2010;27:244–51. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry. 2010;167:648–62. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–82. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–91. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Gillespie CF, et al. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry. 2009;31:505–14. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aenlle KK, Kumar A, Cui L, Jackson TC, Foster TC. Estrogen effects on cognition and hippocampal transcription in middle-aged mice. Neurobiol Aging. 2009;30:932–45. doi: 10.1016/j.neurobiolaging.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corneveaux JJ, et al. Association of CR1, CLU and PICALM with Alzheimer's disease in a cohort of clinically characterized and neuropathologically verified individuals. Hum Mol Genet. 2010;19:3295–301. doi: 10.1093/hmg/ddq221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grillon C, Morgan CA, 3rd, Davis M, Southwick SM. Effect of darkness on acoustic startle in Vietnam veterans with PTSD. Am J Psychiatry. 1998;155:812–7. doi: 10.1176/ajp.155.6.812. [DOI] [PubMed] [Google Scholar]
- 27.Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–83. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker DL, Davis M. Light-enhanced startle: further pharmacological and behavioral characterization. Psychopharmacology (Berl) 2002;159:304–10. doi: 10.1007/s002130100913. [DOI] [PubMed] [Google Scholar]
- 29.McMahon FJ, et al. Meta-analysis of genome-wide association data identifies a risk locus for major mood disorders on 3p21.1. Nat Genet. 2010;42:128–31. doi: 10.1038/ng.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myers AJ, et al. A survey of genetic human cortical gene expression. Nat Genet. 2007;39:1494–9. doi: 10.1038/ng.2007.16. [DOI] [PubMed] [Google Scholar]
- 31.Botia B, et al. Neurotrophic effects of PACAP in the cerebellar cortex. Peptides. 2007;28:1746–52. doi: 10.1016/j.peptides.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Brenneman DE. Neuroprotection: a comparative view of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Peptides. 2007;28:1720–6. doi: 10.1016/j.peptides.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Dejda A, et al. Inhibitory effect of PACAP on caspase activity in neuronal apoptosis: a better understanding towards therapeutic applications in neurodegenerative diseases. J Mol Neurosci. 2008;36:26–37. doi: 10.1007/s12031-008-9087-1. [DOI] [PubMed] [Google Scholar]
- 34.Hamelink C, et al. Pituitary adenylate cyclase-activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. Proc Natl Acad Sci U S A. 2002;99:461–6. doi: 10.1073/pnas.012608999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–75. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 36.Ehrlich I, et al. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–71. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 37.Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–32. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 38.LeDoux JE. Emotional memory: in search of systems and synapses. Ann N Y Acad Sci. 1993;702:149–57. doi: 10.1111/j.1749-6632.1993.tb17246.x. [DOI] [PubMed] [Google Scholar]
- 39.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–52. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 40.Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–63. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Girard BA, et al. Noncompensation in peptide/receptor gene expression and distinct behavioral phenotypes in VIP- and PACAP-deficient mice. J Neurochem. 2006;99(2):499–513. doi: 10.1111/j.1471-4159.2006.04112.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.