Abstract
Objective
Identification of predictors of time to sputum smear conversion in patients with pulmonary tuberculosis (TB) could be used for programmatic planning and the counseling of TB patients. Time estimates of smear conversion based on the presence of risk factors may assist. We identify significant factors associated with time to sputum smear conversion using time-to-event analysis.
Methods
We performed a cohort study using proportional hazards models to identify factors associated with time to smear conversion. All cases of sputum smear positive pulmonary TB managed by Public Health - Seattle & King County TB Control Program in 2003 and 2004 were reviewed. We defined the time to sputum smear conversion as the time elapsed from the start of treatment to the first date of sustained conversion.
Results
There were 98 patients whose sputum was AFB smear positive. Lower initial smear grade (on 1+ to 4+ scale), and absence of cavitation on chest radiograph were associated with earlier sputum smear conversion on bivariate analysis. In multiple regression analysis, initial smear grade (hazard ratio [HR] 0.45, 95% confidence interval [CI] 0.35–0.57) and drug resistance (HR 2.30, 95% CI 1.08–4.89) remained significant. Culture conversion preceding smear conversion was uncommon except for patients with initial 4+ smears (occurred in 38%).
Conclusions
Initial smear grade was the strongest predictor of time to sputum smear and culture conversion in pulmonary TB patients and may be a useful predictor for programmatic planning and patient counseling.
Introduction
Patients with tuberculosis (TB) of the lungs or larynx have the potential to transmit Mycobacterium tuberculosis (MTb) to exposed individuals. The American Thoracic Society, Centers for Disease Control and Prevention (CDC), and the Infectious Diseases Society of America issued joint guidelines on controlling tuberculosis in 2005.1 According to these guidelines, patients with pulmonary TB may be considered to be noninfectious when: 1) they have received multi-drug anti-tuberculosis therapy for 2–3 weeks, 2) they have demonstrated clinical improvement, and 3) there is negligible chance of multi-drug resistant TB (MDR-TB). Criteria for infectiousness are more stringent for hospitalized patients and those residing in congregate settings due to the increased risk to contacts. The guidelines recommend that these patients remain in airborne infection isolation until they have had three consecutive sputum smears that are negative for acid-fast bacilli (AFB).1 This recommendation may also apply to patients returning to households that have infants or immunocompromised individuals.
Prior studies have identified factors associated with delayed sputum smear and culture conversion.2–7 However, time-to-event analysis has not been used to model the duration of sputum smear positivity. The ability to estimate time until the occurrence of smear conversion would be useful for patient counseling regarding anticipated length of isolation (e.g. “Patients with TB similar to your presentation require, on average, × weeks to convert their sputum”). Administrators of hospitals and congregate settings could use reliable estimates of smear positivity for programmatic planning, including efficient use of airborne infection isolation rooms.
We designed the present study to address the duration of sputum smear positivity in TB patients while they are on TB treatment. We hypothesized that initial smear grade and radiographic features would be predictive of time to sputum smear conversion since these factors are markers of disease severity. As we were interested in the durations of increased and absolute patient infectiousness, we identified predictors associated with sputum culture conversion and factors associated with culture conversion that precedes smear conversion, as this latter state represents the excretion of non-viable mycobacteria.8 Results from this study have been previously reported as an abstract.9
Methods and Materials
Patient Population
We reviewed all cases of pulmonary TB managed by Public Health - Seattle & King County TB Control Program (“TB Clinic”) from January 1, 2003, through December 31, 2004; during this time period all cases of pulmonary TB in King County were managed by the TB Clinic. We included patients with at least one spontaneously expectorated or induced sputum specimen that was AFB-smear positive and culture positive for M. tuberculosis complex. The following two groups of TB patients were excluded from this study: (1) patients who had pulmonary TB diagnosed solely on the basis of more invasive tests (e.g. bronchoscopy), and (2) patients diagnosed with pulmonary TB solely on clinical grounds without culture confirmation. All patients received directly observed therapy (DOT) throughout the course of treatment and were treated according to U.S. TB treatment guidelines.10
Study Design
We abstracted data from the TB Information Management System and medical records. The study was approved by the Human Subjects Review Committee of the University of Washington.
Outcome and Predictor Variables
Study end points were time to sputum smear and culture conversion for all subjects. The TB Clinic protocol includes the collection of sputum every two weeks while patients are smear positive or weekly if there is a need to detect smear conversion earlier. All cultures were performed with conventional Lowenstein-Jensen solid media and in BACTEC broth media. We calculated time to sputum smear and culture conversion by determining the time elapsed from the date of TB treatment initiation to the date of sustained conversion, which was defined as the first of at least three consecutive negative specimens.
The predictor variables for evaluation were selected based on known and potential risk factors for delayed time to sputum smear or culture conversion. Race and ethnicity were based on self-report. Chest radiographs were read by one of two TB Clinic providers (CS, MN) and cavitary disease was defined as cavitation present on plain film. Smear grade was defined as the highest smear grade from initially collected sputum samples within 7 days of initiation of TB treatment and based on the standard fluorochrome quantitation scale (results are graded based on the number of AFB observed: 1+, 1–9 AFB/10 fields; 2+, 1–9 AFB/field; 3+, 10–90 AFB/field; 4+, >90 AFB/field).11 Drug resistance was defined as resistance to any of the “first-line” anti-TB medications (isoniazid, rifampin, pyrazinamide, ethambutol, and streptomycin). Other variable definitions were according to Report of Verified Case of TB guidelines.12
Statistical Analyses
Baseline characteristics were compared by smear grade. We assessed times to smear and culture conversion using the log-rank statistic. We performed analyses using the Cox proportional hazards model. In building our multivariable models, we considered variables that were significant in the univariate analysis at the 0.20 level; variables were manually deleted and effects on the model compared using partial likelihood ratio tests. We used logistic regression analysis to evaluate predictors of culture conversion preceding smear conversion in a similar manner.
Modeling of initial smear grade as a continuous or categorical variable had similar results. Patients who had an unknown HIV status were categorized as HIV negative in our analyses. Proportional hazards assumptions were confirmed using Schoenfeld residuals.13 Potential interactions were assessed between smear grade and gender or age. The level for determining statistical significance was set at p<0.05. We performed all analyses with Stata 10 (StataCorp, College Station, TX). Subjects with data missing on time to smear or sputum conversion were included in the time-to-event analysis.
Results
During the study period, 196 patients with pulmonary TB were managed at the TB Clinic and this formed the cohort that was initially reviewed. A total of 31 patients were excluded for the following reasons: 5 patients were diagnosed with bronchoscopy or gastric lavage, 3 patients died on treatment for TB prior to sputum smear conversion, 3 patients had missing charts and 20 patients were culture negative cases. An additional 67 patients had sputum smear negative pulmonary TB. Ninety-eight patients were included in the study.
Characteristics of the study subjects by smear grade are presented in Table 1. Mean age of the patients was 44.3 years and 79% were men. No patients had been previously treated for active TB. HIV coinfection was present in 4% and 64% of the patients were born outside of the United States. In patients with documented sputum smear conversion, 78% had their conversion smear collected within 14 days of their last positive smear; 88% had this specimen collected within 21 days. Nine percent of isolates were resistant to one or more “first-line” medications (Table 1). One patient had MDR-TB.
Table 1.
Subject Characteristics According to Sputum Smear Status
| Total | Sputum Smear Grade | ||||
|---|---|---|---|---|---|
| Population | 1+ | 2+ | 3+ | 4+ | |
| [N=98] | [n=13] | [n=24] | [n=22] | [n=39] | |
| 41.5 | 51.5 | 43.4 | 41.5 | ||
| Age* (years) | 44.3 (20.1) | (17.4) | (18.9) | (18.8) | (21.9) |
| Male sex | 74 (76) | 12 (92) | 14 (58) | 18 (82) | 29 (74) |
| Cavitation** | 44 (45) | 3 (23) | 4 (17) | 12 (55) | 24 (62) |
| Bilateral Lung | |||||
| Involvement** | 42 (43) | 5 (38) | 13 (54) | 6 (27) | 17 (44) |
| Concurrent EPTB† | 8 (8) | 0 | 5 (21) | 0 | 3 (8) |
| Drug Resistance | |||||
| Any | 8 (8) | 0 | 2 (8) | 2 (9) | 4 (10) |
| Isoniazid | 2 (2) | 0 | 0 | 1 (5) | 1 (3) |
| Rifampin | 2 (2) | 0 | 0 | 1 (5) | 1 (3) |
| Streptomycin | 7 (7) | 0 | 2 (8) | 2 (9) | 3 (8) |
| Pyrazinamide | 1 (1) | 0 | 1 (4) | 0 | 0 |
| Ethambutol | 2 (2) | 0 | 0 | 1 (5) | 1 (3) |
| Tobacco use | 38 (39) | 7 (54) | 7 (29) | 13 (59) | 11 (28) |
| Diabetes Mellitus | 7 (7) | 0 | 1 (4) | 2 (9) | 4 (10) |
| HIV‡ Status: | |||||
| Positive | 4 (4) | 0 | 3 (13) | 1 (5) | 0 |
| Refused/Not Done | 17 (17) | 1 (8) | 6 (25) | 2 (9) | 8 (21) |
| Intravenous Drug Use | 5 (5) | 1 (8) | 1 (4) | 1 (5) | 2 (5) |
| Alcohol abuse | 43 (44) | 7 (54) | 6 (25) | 12 (56) | 18 (46) |
| Treatment non-adherent | 15 (15) | 4 (31) | 3 (13) | 4 (18) | 4 (10) |
| Race/ethnicity | |||||
| Asian | 33 (34) | 6 (46) | 11 (46) | 3 (14) | 13 (33) |
| Black | 29 (30) | 2 (15) | 7 (29) | 10 (45) | 10 (26) |
| Latino | 12 (12) | 2 (15) | 1 (4) | 3 (14) | 6 (15) |
| Native American | 10 (10) | 3 (23) | 1 (4) | 4 (18) | 2 (5) |
| White | 14 (14) | 0 | 4 (17) | 2 (9) | 8 (21) |
| Foreign born | 64 (65) | 6 (46) | 18 (75) | 13 (59) | 27 (69) |
Mean (SD), else N (%);
According to chest x-ray;
Extra-pulmonary TB;
Human immunodeficiency virus
Sputum Smear Status and Conversion Time
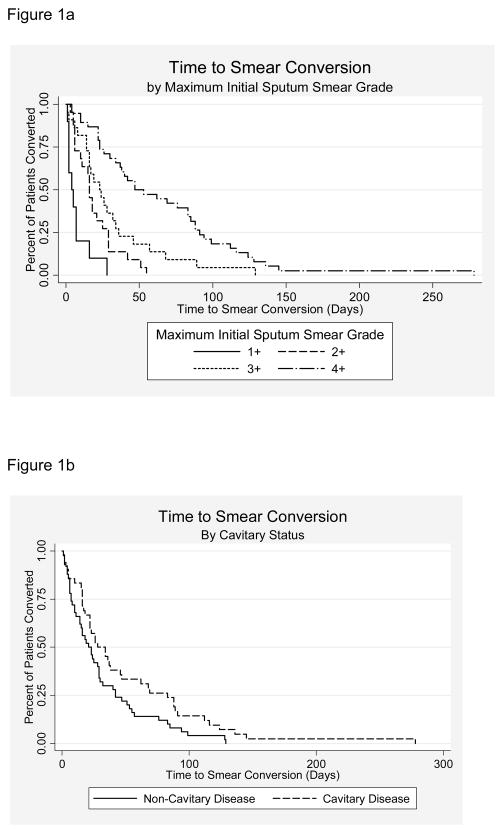
Median time to sputum smear conversion of all study subjects was 24 days (IQR 10–53). Four patients had experienced sputum smear conversion at the time of treatment initiation. The Kaplan-Meier curves for variables with a significant log-rank test (smear grade and radiographic cavitations) are presented in Figure 1. Median times to smear conversion by smear grade are presented in Table 2.
Figure 1.
Figure 1a and 1b. Kaplan-Meier Curves of Time to Sputum Smear Conversion by Initial Sputum Smear Grad (1a) and Cavitary Status on Chest Radiograph (1b).
Table 2.
Time to Sputum Conversion by Maximum Sputum Smear Grade
| Maximum | Days to Smear Conversion | Days to Culture Conversion | ||
|---|---|---|---|---|
| Smear Grade | N | Median (IQR*) | N | Median (IQR1) |
| 1+ | 10 | 4 (2,7) | 12 | 16 (6, 28) |
| 2+ | 22 | 16 (6, 29) | 24 | 28 (16, 51) |
| 3+ | 22 | 23 (14, 36) | 22 | 42 (23, 64) |
| 4+ | 38 | 47 (23, 91) | 38 | 62 (48, 84) |
IQR = interquartile range
We performed Cox proportional hazards regression analysis to evaluate predictors of time to smear conversion. In univariate analyses, higher smear grade and cavitation on chest radiographs were associated with increased time to sputum smear conversion (Table 3). In our multivariable model, significant associations with time to sputum smear conversion were found for initial smear grade (hazard ratio [HR] 0.45, 95% confidence interval [CI] 0.35–0.57) and drug resistance (HR 2.30, 95% CI 1.08–4.89). For each 1+ increase in smear grade, the risk of sputum smear conversion decreased by 55%. Disease caused by drug resistant MTb isolates increased the rate of sputum smear conversion by 130% compared to patients with pan-susceptible isolates. No interactions were detected.
Table 3.
Univariate and Multivariable Models of Time to Sputum Smear Conversion
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Age (year) | 1.00 (0.99, 1.00) | 0.95 | - | |
| Male sex | 0.84 (0.52, 1.35) | 0.46 | - | |
| Sputum smear grade* | 0.47 (0.37, 0.59) | 0.00 | 0.45 (0.35, 0.57) | 0.000 |
| Cavitation | 0.65 (0.42, 0.99) | 0.05 | - | |
| Bilateral lung involvement | 1.08 (0.71, 1.64) | 0.73 | - | |
| Drug resistance | 1.47 (0.71, 3.05) | 0.30 | 2.30 (1.08, 4.89) | 0.03 |
| Tobacco use | 1.40 (0.91, 2.17) | 0.13 | - | |
| Alcohol abuse | 0.96 (0.63, 1.47) | 0.85 | - | |
| Diabetes mellitus | 0.79 (0.36, 1.73) | 0.56 | - | |
| HIV** infection | 1.43 (0.45, 4.54) | 0.55 | - | |
1+ to 4+ scale;
Human immunodeficiency virus
Sputum Culture Status and Conversion Time
We performed a similar analysis of time to sputum culture conversion. Time to sputum culture conversion by smear grade is presented in Table 2. In univariate analyses, higher smear grade and cavitation on chest radiographs were associated with increased time to sputum culture conversion; in multivariable analysis, smear grade and any drug resistance were associated with time to culture conversion (Table 4).
Table 4.
Univariate and Multivariable Models of Time to Sputum Culture Conversion
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Age (year) | 1.00 (0.99, 1.02) | 0.40 | - | |
| Male sex | 0.68 (0.42, 1.09) | 0.12 | - | |
| Sputum smear grade* | 0.53 (0.44, 0.67) | 0.00 | 0.52 (0.40, 0.67) | 0.00 |
| Cavitation | 0.57 (0.38, 0.87) | 0.01 | - | |
| Bilateral lung involvement | 1.29 (0.85, 1.95) | 0.23 | - | |
| Drug resistance** | 1.16 (0.56, 2.41) | 0.69 | 2.30 (1.02, 5.21) | 0.05 |
| Tobacco use | 1.11 (0.73, 1.68) | 0.64 | - | |
| Alcohol abuse | 0.91 (0.60, 1.38) | 0.65 | - | |
| Diabetes mellitus | 1.00 (0.46, 2.17) | 1.0 | - | |
| HIV† infection | 2.77 (0.99, 7.74) | 0.09 | - | |
1+ to 4+ scale;
To any 1st-line drug;
Human immunodeficiency virus
Relationship Between Smear and Culture Conversion
Ninety-five smear positive patients had complete sputum smear and culture conversion information. Table 5 details the timing of smear conversion relative to culture conversion in these patients. While less than 10% of patients with smear grades 1–3+ experienced sputum smear conversion after culture conversion, 38% of smear grade 4+ patients experienced this event. On multivariable logistic regression analysis, using the same candidate variables as in the time-to-event analyses, only higher initial smear grade (OR 3.7, 95% CI 1.6–8.7, p=0.002) was associated with sputum culture conversion before smear conversion.
Table 5.
Timing of Sputum Conversion by Initial Smear Grade
| Smear Conversion after | Initial Sputum Smear Grade N(%) | |||
|---|---|---|---|---|
| Culture Conversion | 1+ | 2+ | 3+ | 4+ |
| No [n=78] | 13 (16.7) | 21 (26.9) | 21 (26.9) | 23 (29.5) |
| Yes [n=17] | 0 (0) | 2 (11.8) | 1 (5.9) | 14 (82.4) |
Additional Analyses
Sensitivity analyses were performed in both sputum smear and culture conversion models to assess the affect of patient adherence with anti-tuberculosis therapy (defined as completion of the intensive phase of therapy (8 week-equivalents) in less than 9 weeks). Fifteen percent of the patients met our definition of non-adherence. Modeling medication adherence did not significantly affect the results of our final models.
As we were interested in improving patient counseling and programmatic planning, we compared the final model for time to sputum smear conversion (containing sputum smear grade and drug resistance) with one that contained only sputum smear grade (information which would be available at the time of diagnosis with TB or shortly thereafter). Using the likelihood-ratio test, compared with the final model (chi-square = 41.58), the nested model (chi-square = 37.71) did not fit as well. (chi-square 3.87, df = 1, p = 0.049).13
Discussion
This cohort study of sputum smear positive TB patients was designed to improve the ability of clinicians to counsel TB patients on the duration of increased infectiousness and help administrators of congregate settings in programmatic planning. We identified predictors for the duration of AFB sputum smear positivity while on TB treatment. In our final model, we found that initial sputum smear grade and the presence of drug resistance were associated with time to sputum smear conversion.
The novelty of our study is in the use of Cox proportional survival analysis to model our hypothesis and provide time estimates. In survival analysis, the usual hazard that is being assessed is an undesirable state. However, in our study the “hazard” is the conversion of sputum smear or culture and a hazard ratio below one indicates delayed conversion. We found that lower initial smear grade and any drug resistance are associated with earlier smear conversion. We compared this final model to one that contained only initial smear grade, as this variable would be available at the time of patient counseling. The difference between the models was of borderline significance (p=0.049); initial smear grade contributes to more than 90% of the variability in time to smear conversion in the final model. This supports the clinical use of initial sputum smear grade alone in predicting time to smear conversion.
Previous studies have found that patient age, initial sputum AFB smear grade, and chest radiographic findings of cavitation and bilateral lung involvement were associated with time to smear conversion.3–6 Our finding of a strong association between initial smear grade and time to sputum smear conversion is consistent with our hypothesis. While the presence of cavitation was significant in univariate analysis, this was not confirmed in the multivariate analysis. This may be due to collinearity of the variables. An association between the presence of drug resistance and decreased time to sputum smear conversion has not been previously reported and appears counterintuitive. In our study, there were 8 patients with any drug resistance: 7 patients had streptomycin-resistant isolates, one patient had rifampin monoresistance and one patient had MDR-TB with streptomycin resistance. While the noted association may be spurious, it may also represent decreased fitness in MTb isolates that have developed drug resistance. Studies have suggested that specific mutations for isoniazid and rifampin resistance may affect the competitive fitness of MTb; no study has evaluated the impact of streptomycin resistance on virulence. 14–16
Our study found that patients with initial sputum smear grade of 4+ commonly experienced sputum culture conversion before smear conversion. In this group of patients, 38% had culture conversion precede sputum smear conversion (36% of patients with non-cavitary disease and 39% of patients with cavitary disease). In a logistic regression analysis, culture conversion occurring before smear conversion was associated with higher smear grade and concurrent extra-pulmonary TB. Culture conversion before smear conversion is a well-reported phenomenon and is thought to represent the continued excretion of non-viable or “dead” bacilli. 8 These patients might have been maintained in isolation, despite not presenting an infectious risk. Previous studies showed that culture conversion before smear conversion was associated with disease extent, drug resistance, poor compliance and the use of rifampin containing regimens.8,17 The frequency of this phenomenon in our cohort is higher than that previously described by Kim et al, where approximately 12% of patients with far-advanced TB without cavities and 28% of patients with cavitary far-advanced TB were reported to have this event.8
There are several limitations to our current study. First, our study findings may not be generalizable to other unique TB populations. In our cohort, the prevalence of HIV coinfection (5%), diabetes mellitus (7%) and drug resistant isolates (8%) were relatively low. Second, our primary endpoint was time to TB smear conversion. Estimates of time to sputum smear and culture conversion will be influenced by the frequency with which sputum specimens are collected. While the TB Clinic protocol was to collect sputum specimen at least every two weeks while patients were smear positive, more frequent sputum collection may have improved precision in our analysis.
The CDC promulgated infection control guidelines for TB patients in institutionalized settings in 1994 in the wake a number of institutional outbreaks of MDR-TB. Although these recommendations have been criticized as too stringent, they were maintained in the most recent joint statement on controlling TB. 10,18 In determining when isolation may be discontinued in TB patients, it has been noted that “Where is the patient being discharged to?” may be a more pertinent question than “When can the patient be discharged?”19 In order to minimize the isolation period of TB patients while preventing TB transmission in congregate settings, further studies are needed to determine the infectiousness of patients who remain persistently smear positive beyond 2–3 weeks of treatment despite adherence to DOT and clinical improvement.
Until more is known about the infectiousness of TB patients on treatment, our study may be useful in counseling patients and in anticipating discharges of TB patients to congregate settings. Further, our results may be used in determining the efficient frequency of sputum collections to document smear conversion. In conclusion, we have identified several predictors of time to sputum conversion. Of these, initial sputum smear grade status is the most important factor in predicting a patient’s time to conversion.
Acknowledgments
Financial support. D.J.H. received grant support from the Firland Foundation and NIH/NHLBI Grant F32HL094031.
Footnotes
Abstract previously presented at the American Thoracic Society International Conference, May 2008.
Conflict of interest. D.J.H., C.O.J., E.O., C.S., and M.N. have no conflicts of interest to disclose.
Contributor Information
David J Horne, Division of Pulmonary and Critical Care Medicine, Department of Medicine, University of Washington School of Medicine, Seattle WA.
Catherine O. Johnson, Department of Epidemiology, School of Public Health, University of Washington, Seattle WA.
Eyal Oren, Public Health - Seattle & King County, Tuberculosis Control Program.
Christopher Spitters, Public Health - Seattle & King County, Tuberculosis Control Program; Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington School of Medicine, Seattle WA.
Masahiro Narita, Division of Pulmonary and Critical Care Medicine, Department of Medicine, University of Washington School of Medicine, Seattle WA; Department of Epidemiology, School of Public Health, University of Washington, Seattle WA; Public Health - Seattle & King County, Tuberculosis Control Program.
References
- 1.American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: controlling tuberculosis in the United States. Am J Respir Crit Care Med. 2005;172:1169–1227. doi: 10.1164/rccm.2508001. [DOI] [PubMed] [Google Scholar]
- 2.Lienhardt C, Manneh K, Bouchier V, et al. Factors determining the outcome of treatment of adult smear-positive tuberculosis cases in The Gambia. Int J Tuberc Lung Dis. 1998;2:712–718. [PubMed] [Google Scholar]
- 3.Singla R, Osman MM, Khan N, et al. Factors predicting persistent sputum smear positivity among pulmonary tuberculosis patients 2 months after treatment. Int J Tuberc Lung Dis. 2003;7:58–64. [PubMed] [Google Scholar]
- 4.Dominguez-Castellano A, Muniain MA, Rodriguez-Bano J, et al. Factors associated with time to sputum smear conversion in active pulmonary tuberculosis. Int J Tuberc Lung Dis. 2003;7:432–438. [PubMed] [Google Scholar]
- 5.Telzak EE, Fazal BA, Pollard CL, et al. Factors influencing time to sputum conversion among patients with smear-positive pulmonary tuberculosis. Clin Infect Dis. 1997;25:666–670. doi: 10.1086/513772. [DOI] [PubMed] [Google Scholar]
- 6.Guler M, Unsal E, Dursun B, et al. Factors influencing sputum smear and culture conversion time among patients with new case pulmonary tuberculosis. Int J Clin Pract. 2007;61:231–235. doi: 10.1111/j.1742-1241.2006.01131.x. [DOI] [PubMed] [Google Scholar]
- 7.Rieder HL. Sputum smear conversion during directly observed treatment for tuberculosis. Tuber Lung Dis. 1996;77:124–129. doi: 10.1016/s0962-8479(96)90026-x. [DOI] [PubMed] [Google Scholar]
- 8.Kim TC, Blackman RS, Heatwole KM, et al. Acid-fast bacilli in sputum smears of patients with pulmonary tuberculosis. Prevalence and significance of negative smears pretreatment and positive smears post-treatment. Am Rev Respir Dis. 1984;129:264–268. [PubMed] [Google Scholar]
- 9.Horne DJ, Johnson CO, Oren E, et al. Factors Affecting Time to Sputum Smear and Culture Conversion in Patients with Pulmonary Tuberculosis [abstract] Am J Respir Crit Care Med. 2008;177:A790. [Google Scholar]
- 10.Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 11.Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000;161:1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 12.Report of Verified Case of Tuberculosis Manual. http://www.doh.wa.gov/cfh/TB/RVCTmanual.pdf.
- 13.Hosmer DW, Lemeshow S. Applied survival analysis: regression modeling of time to event data. New York: Wiley; 1999. [Google Scholar]
- 14.van Soolingen D, Borgdorff MW, de Haas PE, et al. Molecular epidemiology of tuberculosis in the Netherlands: a nationwide study from 1993 through 1997. J Infect Dis. 1999;180:726–736. doi: 10.1086/314930. [DOI] [PubMed] [Google Scholar]
- 15.Mariam DH, Mengistu Y, Hoffner SE, et al. Effect of rpoB mutations conferring rifampin resistance on fitness of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2004;48:1289–1294. doi: 10.1128/AAC.48.4.1289-1294.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Garcia ML, Ponce de Leon A, Jimenez-Corona ME, et al. Clinical consequences and transmissibility of drug-resistant tuberculosis in southern Mexico. Arch Intern Med. 2000;160:630–636. doi: 10.1001/archinte.160.5.630. [DOI] [PubMed] [Google Scholar]
- 17.Al-Moamary MS, Black W, Bessuille E, et al. The significance of the persistent presence of acid-fast bacilli in sputum smears in pulmonary tuberculosis. Chest. 1999;116:726–731. doi: 10.1378/chest.116.3.726. [DOI] [PubMed] [Google Scholar]
- 18.Iseman MD. An unholy trinity--three negative sputum smears and release from tuberculosis isolation. Clin Infect Dis. 1997;25:671–672. doi: 10.1086/513774. [DOI] [PubMed] [Google Scholar]
- 19.Sepkowitz KA. Tuberculosis control in the 21st century. Emerg Infect Dis. 2001;7:259–262. doi: 10.3201/eid0702.010222. [DOI] [PMC free article] [PubMed] [Google Scholar]