Abstract
Although a fraction of human blood memory CD4+ T cells expresses chemokine (C-X-C motif) receptor 5 (CXCR5), their relationship to T follicular helper (Tfh) cells is not well-established. Here we show that human blood CXCR5+ CD4+ T cells share functional properties with Tfh cells, and appear to represent their circulating memory compartment. Blood CXCR5+ CD4+ T cells comprised three subsets; T helper 1 (Th1), Th2 and Th17 cells. Th2 and Th17 cells within CXCR5+, but not within CXCR5−, compartment efficiently induced naïve B cells to produce immunoglobulins via interleukin-21 (IL-21). In contrast, Th1 cells from both CXCR5+ and CXCR5− compartments lacked the capacity to help B cells. Patients with juvenile dermatomyositis, a systemic autoimmune disease, displayed a profound skewing of blood CXCR5+ Th subsets towards Th2 and Th17 cells. Importantly, the skewing of subsets correlated with disease activity and frequency of blood plasmablasts. Collectively, our study suggests that an altered balance of Tfh subsets contributes to human autoimmunity.
Introduction
Antibody responses are largely dependent on the help provided by CD4+ T cells CD4+ T cells are fundamental for the generation of germinal centers (GCs), a discrete structure in secondary lymphoid organs where selection of high-affinity B cells and development of B cell memory occur (Allen et al., 2007; MacLennan, 1994). Recently, CD4+ T cells present in B cell follicles, named T follicular helper cells (Tfh), have been established as a T helper (Th) cell subset specialized for providing help to B cells in GCs (Fazilleau et al., 2009; King et al., 2008). Tfh cells express the chemokine (C-X-C motif) receptor 5 (CXCR5) (Breitfeld et al., 2000; Kim et al., 2001; Schaerli et al., 2000), which allows their migration into B cell follicles in response to the specific ligand CXCL13. Tfh cells secrete IL-4, IL-10, and IL-21, cytokines that promote growth, differentiation, and class-switching of B cells (Ettinger et al., 2005; Good et al., 2006; Pene et al., 2004). Tfh cells also express surface molecules essential for helper functions, including CD40-ligand (CD40L), and inducible co-stimulator (ICOS) (King et al., 2008). Tfh cells express large amounts of B cell lymphoma 6 (Bcl-6) (Chtanova et al., 2004; Rasheed et al., 2006), which is necessary and sufficient for the development of Tfh cells in vivo (Johnston et al., 2009; Nurieva et al., 2009; Yu et al., 2009). In contrast, B lymphocyte-induced maturation protein 1 (Blimp-1), a transcription repressor that regulates the function of Bcl-6, inhibits the generation of Tfh cells (Johnston et al., 2009). Thus, Tfh generation is controlled by the balance of these two transcription repressors. This supports the hypothesis that the developmental pathway of Tfh cells is distinct from that of other canonical Th subsets (Nurieva et al., 2008). Alternatively, there is evidence that mouse Tfh cells are heterogeneous, and encompass distinct subsets secreting cytokines characteristic of Th1, Th2, and Th17 cells (Bauquet et al., 2009; Fazilleau et al., 2009; King and Mohrs, 2009; Reinhardt et al., 2009; Zaretsky et al., 2009). Furthermore, mouse Th2 (Zaretsky et al., 2009) and T reg cells (Tsuji et al., 2009) were shown to be convertible into Tfh cells in vivo. Therefore, the relationship between Tfh cells and other Th subsets still remains unclear. Notably, whereas all these studies were performed with inbred mouse strains, whether Tfh cells in humans comprise of different subsets is largely unknown. Previous studies have shown that tonsillar Tfh cells display distinct phenotype and genetic profiles from other canonical Th subsets (Chtanova et al., 2004; Kim et al., 2004; Rasheed et al., 2006). However, as suggested in mouse studies, the precursors of Tfh cells might be composed of heterogeneous cell populations also in humans, and they might differentiate into distinct types of Tfh cells.
Furthermore, although several mouse studies show that over-representation of Tfh cells is associated with the development of systemic autoimmunity (Linterman et al., 2009; Subramanian et al., 2006; Vinuesa et al., 2005), their association with human autoimmune diseases remains largely unknown. Patients with autoimmune diseases such as lupus or rheumatoid arthritis display high-affinity somatically mutated autoantibodies in sera (Mietzner et al., 2008; Shlomchik et al., 1987), suggesting the involvement of Tfh cells (or Tfh-committed extrafollicular cells (Poholek et al., 2010)) in the pathogenesis. Although a systematic approach would be required to define the role of Tfh cells in human autoimmune diseases, obtaining lymph node samples from patients routinely and/or longitudinally is extremely challenging. Therefore, there is a strong need to establish surrogate strategies to assess the quality of Tfh responses in humans. In this regard, analysis of blood CD4+ T cells expressing CXCR5 (Forster et al., 1994) might facilitate such studies. Several observations suggest a relationship between CXCR5+ CD4+ T cells and Tfh cells. For example, humans who show severely impaired GC formation through deficiency of CD40-ligand or ICOS display substantially fewer circulating CXCR5+ CD4+ T cells (Bossaller et al., 2006). On the contrary, CXCR5+ CD4+ T cells expressing ICOS are present at a higher frequency in blood of lupus patients (Simpson et al., 2010). However, whether circulating CXCR5+ CD4+ T cells indeed share the phenotypic and functional properties of Tfh cells remains to be established.
Here we show that human blood CXCR5+ CD4+ T cells appear to represent a circulating pool of memory Tfh cells, and can be distinguished into Th1, Th2, and Th17 subsets with different capacities to regulate B cell responses. We further show an alteration of blood CXCR5+ CD4+ T cell subsets in an autoimmune disease, juvenile dermatomyositis (JDM).
Results
Human blood CXCR5+ CD4+ T cells induce the differentiation of naïve B cells towards plasmablasts
In healthy adult blood, CXCR5 was expressed by 8.3 ± 1.8 % of CD4+ T cells, and 18.9 ± 3.6 % of memory (CD45RA−) CD4+ T cells (mean ± s.d., n=10)(Figure 1A). Consistent with previous observations (Breitfeld et al., 2000; Kim et al., 2001; Schaerli et al., 2000), blood CXCR5+ CD4+ T cells expressed CCR7 and CD62L, but few expressed activation molecules expressed by Tfh cells, such as ICOS and CD69 (Figure S1A), suggesting a resting state. Both CXCR5 and CCR7 were functional, as blood CXCR5+ CD4+ T cells migrated in response to the ligands, CXCL13 and CCL19, respectively (Figure S1B).
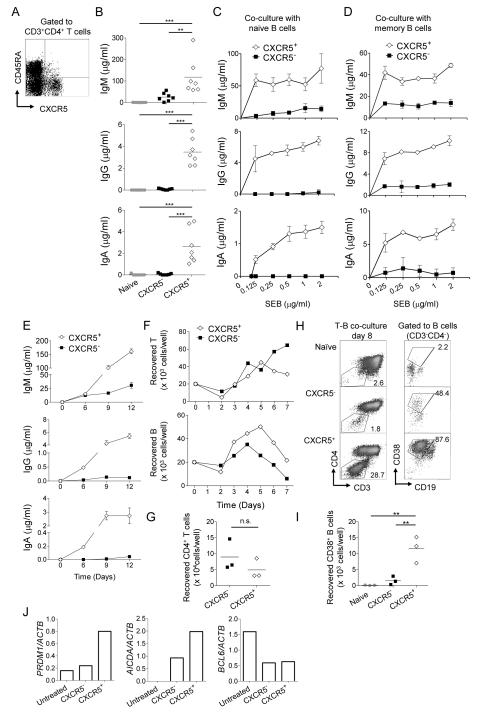
Figure 1. Blood CXCR5+ CD4+ T cells induce naïve B cells to differentiate into Ig-producing plasmablasts.
A. CXCR5 expression by blood CD4+ T cells. PBMCs were stained with CD3, CD4, CD45RA, and CXCR5 mAbs. Gated to CD3+CD4+ T cells.
B. Blood naive, CXCR5−, and CXCR5+ CD4+ T cells were cultured with autologous naïve B cells in the presence of SEB. Ig concentrations were measured at day 12. One way ANOVA test. Data from seven independent experiments. ** p<0.01, *** p<0.001.
C-D. CXCR5− or CXCR5+ CD4+ T cells were cultured with naïve (C) or memory (D) B cells in the presence of titrated doses of SEB. Ig concentrations at day 12 in (C) and day 6 in (D). n=3, Mean ± s.d. Representative data from three independent experiments.
E. Ig concentrations at different time points in the cultures of CXCR5− or CXCR5+ CD4+ T cells with naïve B cells. n=3, Mean ± s.d. Representative data from four independent experiments.
F. Number of viable CD4+ T and B cells at different time points. Representative data from two independent experiments.
G. Number of viable CD4+ T cells at day 8. Paired t-test. Data from three independent experiments.
H. CD38+ plasmablast population in the co-culture of blood Th subsets and naive B cells at day 8. Representative data from three independent experiments.
I. Number of plasmablasts in the co-cultures. One way ANOVA test. Data from three independent experiments.
J. Measurement of BCL6, PRDM1, and AICDA mRNA expression by real-time RT-PCR in naïve B cells cultured with blood Th subsets. Expression of each mRNA was normalized to that of ACTB mRNA. Before and after 7 d culture with CXCR5− or CXCR5+ CD4+ T cell subsets (B cells were purified after culture). Representative data from two independent experiments.
To determine their capacity to help B cells, memory (CD45RA−) CXCR5+ cells were sorted, and cultured with autologous naïve B (IgD+CD27−CD19+) cells. Naïve (CD45RA+) and memory CXCR5− CD4+ T cells were also sorted for comparison. To mimic the antigen-specific interaction between T and B cells, staphylococcal enterotoxin B (SEB), a superantigen, was added to the cultures. Naïve CD4+ T cells did not induce naïve B cells to produce immunoglobulins (Igs) (Figure 1B). Memory CXCR5− CD4+ T cells induced naïve B cells to produce only low amounts of IgM, but no IgG or IgA. In contrast, CXCR5+ CD4+ T cells were potent at inducing naive B cells to produce IgM, IgG, and IgA (Figure 1B). The Ig production was totally dependent on cognate interactions between T and B cells, as naïve B cells co-cultured with CXCR5+ CD4+ T cells did not produce Igs in the absence of SEB (Figure 1C). CXCR5+ CD4+ T cells were also more efficient than CXCR5− CD4+ T cells in inducing memory B cells to produce Igs (Figure 1D).
Kinetics studies revealed that CXCR5+ CD4+ T cells induced naïve B cells to produce IgG and IgA as early as day 6 of culture, whereas CXCR5− CD4+ T cells did not induce either IgG or IgA secretion even at day 12 (Figure 1E). The number of viable T cells was similar between the cultures of CXCR5− and CXCR5+ CD4+ T cells with naïve B cells (Figures 1F top and 1G), indicating that the inability of CXCR5− CD4+ T cells to induce naïve B cells to produce IgG or IgA was not due to their poor survival. In contrast, the number of viable B cells was constantly higher when naïve B cells were cultured with CXCR5+ cells than with CXCR5− CD4+ T cells (Figure 1F bottom). Of note, the number of viable B cells decreased after day 5 even when cultured with CXCR5+ CD4+ T cells, suggesting that the T cells could not fully support the survival of B cells in vitro. Nonetheless, culturing naïve B cells with CXCR5+ CD4+ T cells yielded higher numbers of CD38+CD19lo cells than with CXCR5−CD4+ T cells (Figure 1H, I), indicating that a fraction of surviving B cells differentiated into plasmablasts. Consistently, CXCR5+ CD4+ T cells efficiently induced naïve B cells to express activation-induced cytidine deaminase (AID, encoded by AICDA gene), a factor required for class-switching, and Blimp-1 (encoded by PRDM1 gene), a transcription factor critical for the differentiation of plasma cells, but not Bcl6 (encoded by BCL6 gene) (Figure 1J).
Collectively, these observations show that blood CXCR5+ CD4+ T cells were more efficient than CXCR5− CD4+ T cells at inducing naive B cells to differentiate into plasmablasts and to promote class switching.
Blood CXCR5+ CD4+ T cells help naïve B cells through IL-21
Similar to tonsillar Tfh cells, blood CXCR5+ CD4+ T cells secreted IL-21 upon contact with naïve B cells, whereas CXCR5− CD4+ T cells barely secreted IL-21 (Figure 2A). Although CXCR5+ CD4+ T cells secreted IL-21 within 24 h after interaction with naïve B cells, very low amounts, if any, of IL-21 was secreted by CXCR5− CD4+ T cells up to 96 h (Figure S2A). Notably, CXCR5+ CD4+ T cells also secreted larger amounts of CXCL13 than CXCR5− CD4+ T cells (Figure 2B), a chemokine produced by tonsillar Tfh cells (Kim et al., 2004; Rasheed et al., 2006).
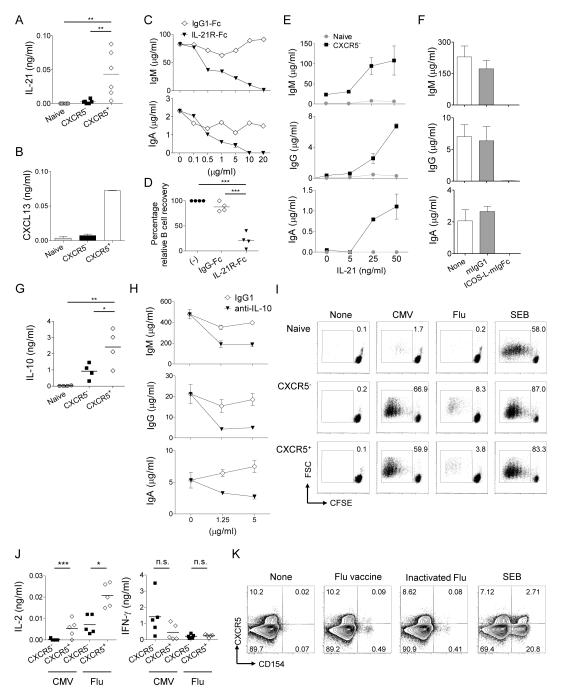
Figure 2. Blood CXCR5+ CD4+ T cells depends on IL-21, IL-10 and ICOS for B cell help.
A. IL-21 secretion by blood Th subsets cultured with naïve B cells. Data from six independent experiments. One way ANOVA test. ** p<0.01
B. CXCL13 secretion by blood Th subsets cultured with naïve B cells. Mean ± s.d, n=3. Representative data from three independent experiments.
C. Titrated amounts of IL-21R-Fc were added to the co-culture of CXCR5+ CD4+ T cells and naïve B cells. Ig concentrations at day 12. IgG is not shown due to the cross-reactivity to the Fc portion of IL-21R-Fc. Representative data from two independent experiments.
D. Recovery of viable B cells at day 12. Normalized to the culture of CXCR5+ CD4+ T cells and naïve B cells. One way ANOVA test, n=4. *** p<0.001.
E. Titrated amounts of IL-21 were added to the co-culture of naïve or CXCR5− CD4+ T cells with naïve B cells. Ig concentrations at day 12. n=3, Mean ± s.d. Representative data from three independent experiments.
F. Ig concentrations at day 12 in the co-culture of CXCR5+ CD4+ T cells and naïve B cells with an ICOS blocking reagent. Mean ± s.d, n=3. Representative data from two independent experiments.
G. IL-10 secretion in supernatants of blood Th subsets cultured with naïve B cells. Data from four independent experiments. One way ANOVA test. * p<0.05, ** p<0.01.
H. Ig concentrations at day 12 in the co-culture of CXCR5+ CD4+ T cells and naïve B cells with indicated amounts of anti-IL-10. Mean ± s.d., n=3. Representative data from three independent experiments.
I. CFSE-labeled blood Th subsets were cultured with autologous monocytes incubated with inactivated Flu virus or CMV. Cell proliferation was analyzed at day 5. Representative data from two independent experiments.
J. IL-2 and IFN-γ secretion in supernatants at day 2. Data from five independent experiments. Paired t-test. *** p<0.001, * p<0.05.
K. PBMCs were stimulated with none, Flu vaccine, inactivated Flu virus, or SEB for 6 h in the presence of Brefeldin A and monensin, and the intracytoplasmic expression of CD154 in CXCR5+ or CXCR5− CD4+ T cells was analyzed. Representative data from four independent experiments.
Previous studies demonstrated that IL-21 secreted by tonsillar Tfh cells plays a central role in the expansion and plasma cell differentiation of co-cultured B cells (Bryant et al., 2007). Similarly, blocking IL-21 during the co-culture of naïve B cells with blood CXCR5+ CD4+ T cells resulted in a dose-dependent inhibition of Ig secretion (Figure 2C), and B cell recovery (Figure 2D). Conversely, addition of IL-21 resulted in the enhancement of IgM secretion as well as the induction of IgG and IgA secretion by naïve B cells cultured with CXCR5−, but not naïve, CD4+ T cells (Figure 2E). Consistent with previous studies using Tfh cells (Bauquet et al., 2009; Odegard et al., 2008; Vogelzang et al., 2008), B cell help by blood CXCR5+ CD4+ T cells was also dependent on ICOS, as blocking of ICOS-ICOS ligand interaction inhibited both IL-21 secretion (Figure S2B) and Ig secretion (Figure 2F). Large amounts of IL-10 was also detected in the co-cultures of CXCR5+ CD4+ T cells and naïve B cells (Figure 2G), and blocking IL-10 resulted in a partial inhibition of Ig secretion (Figure 2H).
Whether blood CXCR5+ CD4+ T cells contain antigen-specific memory cells has been controversial (Breitfeld et al., 2000; Rivino et al., 2004; Schaerli et al., 2001). To address this issue, isolated CXCR5− and CXCR5+ CD4+ T cells were co-cultured with autologous monocytes that had been pulsed with inactivated influenza (Flu) virus, or cytomegalovirus, and the proliferation of T cells was analyzed at day 5. Whereas CXCR5− CD4+ T cells proliferated robustly in response to the stimulation with both viruses, these stimulations also induced CXCR5+ CD4+ T cells to proliferate (Figure 2I) and to secrete cytokines, including IL-2 and IFN-γ (Figure 2J). Notably, CXCR5+ CD4+ T cells secreted more IL-2 than CXCR5− CD4+ T cells in response to the stimulation with virus antigens (Figure 2J). To directly illustrate the Flu-specific CXCR5+ CD4+ T cells, PBMCs obtained from healthy donors (who did not receive influenza vaccines more than 1 year) were incubated for 6 h with either a seasonal Flu vaccine (Fluzone) or a heat-inactivated Flu virus (PR8). Then cells were analyzed for the expression of CD4 and CXCR5 together with the expression of intracytoplasmic CD154, which permits the sensitive identification of antigen-specific CD4+ T cells (Chattopadhyay et al., 2006). Whereas CXCR5 is expressed by activated CD4+ T cells (Schaerli et al., 2001), CXCR5− CD4+ T cells stimulated for 6 h with SEB remained negative for CXCR5 expression (Figure S2C). As shown in Figure 2K, Flu-specific CD4+ T cells were detected as CD154+ cells in both stimulations, which contained CXCR5+ cells. Furthermore, a fraction of Flu antigen-specific (CD154+) CXCR5+ CD4+ T cells also expressed intracytoplasmic IL-2 and/or IFN-γ upon stimulation (Figure S2D).
Collectively, these observations show that CXCR5+ CD4+ T cells shared functional properties of Tfh cells. Inasmuch as CXCR5+ CD4+ T cells contained antigen-specific memory cells, blood CXCR5+ CD4+ T cells appear to represent circulating memory Tfh cells.
Three distinct Tfh cell subsets in human blood
The expression of chemokine receptors has been instrumental for defining human CD4+ T cell subsets. The expression of CXCR3 is preferentially maintained by cells committed to the Th1 pathway (Bonecchi et al., 1998; Rabin et al., 2003; Sallusto et al., 1998), whereas CCR6 is expressed by Th17 cells (Acosta-Rodriguez et al., 2007; Annunziato et al., 2007; Singh et al., 2008). Though blood CXCR5+ CD4+ T cells were previously shown to co-express other chemokine receptors (Lim et al., 2008), the relationship between chemokine receptor expression and their function has not been established. As illustrated in Figure 3A, differential expression of CXCR3 and CCR6 defined three major subsets within blood CXCR5+ CD4+ T cells: CXCR3+CCR6−, CXCR3−CCR6−, and CXCR3−CCR6+ cells. CXCR5− CD4+ T cells contained four subpopulations including CXCR3+CCR6+ cells. When compared to CXCR5+ CD4+ T cells, CXCR5− CD4+ T cells contained less CXCR3−CCR6+ T cells (p<0.01, paired t-test) and more CXCR3+CCR6+ T cells (p<0.001) (Figure 3B).
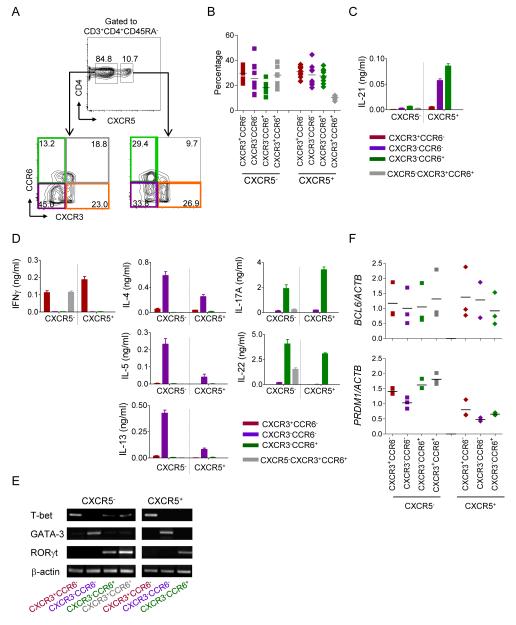
Figure 3. Blood CXCR5+ CD4+ T cells are composed of Th1, Th2, and Th17 cells.
A. CXCR3 and CCR6 expression on blood CXCR5− or CXCR5+ CD4+ T cell population. Gated to CD3+CD4+CD45RA− cells.
B. Frequency of populations within blood CXCR5− and CXCR5+ CD4+ T cells of ten healthy adults.
C. The seven blood Th populations were co-cultured with naïve B cells and the secreted IL-21 was measured at 48 h. n=3, Mean ± s.d. Representative data from four independent experiments.
D. Other cytokine secretion in the co-cultures of the seven blood Th populations and naïve B cells. n=3, Mean ± s.d. Representative data from four independent experiments.
E. Expression of each transcriptional factor in the seven blood Th populations was assessed by RT-PCR. Representative data from two independent experiments.
F. The seven Th populations were sorted from PBMCs of three donors, and expression of BCL6 and PRDM1 mRNA was analyzed by real-time RT-PCR. Normalized to ACTB mRNA expression in each Th subset.
To analyze the functional differences of blood memory CD4+ T cell populations, seven major subpopulations (four CXCR5− and three CXCR5+) were isolated according to the expression of CXCR5, CXCR3 and CCR6. To analyze cytokine secretion patterns, each subpopulation was co-cultured for 2 days with SEB-pulsed naïve B cells. As expected (Figure 2A), all four subpopulations within the CXCR5− compartment secreted very little, if any, IL-21 upon interaction with naïve B cells (Figure 3C). Within the CXCR5+ CD4+ T cell compartment, only CXCR3−CCR6− and CXCR3−CCR6+ cells produced IL-21. Measurement of other cytokines revealed that each cell population secreted different sets of cytokines (Figure 3D). CXCR3+CCR6− cells in both CXCR5+ and in CXCR5− compartments secreted IFN-γ, but not Th2 or Th17 cytokines. Th2 cytokines, i.e., IL-4, IL-5, and IL-13, were exclusively secreted by CXCR3−CCR6− cells, whereas Th17 cytokines, IL-17A and IL-22, were produced by CXCR3−CCR6+ cells, in both CXCR5+ and CXCR5− cells. Expression profiling of transcription factors demonstrated that within the CXCR5+ CD4+ T cell compartment, CXCR3+CCR6− cells expressed T-bet, a transcription factor of Th1 cells, CXCR3−CCR6− cells expressed GATA3, a transcription factor of Th2 cells, whereas CXCR3−CCR6+ cells expressed RORγT, a transcription factor of Th17 cells (Figure 3E). The similar pattern of transcription factor expression was also observed within CXCR5− CD4+ T cell compartment. Thus, both blood CXCR5+ and CXCR5− CD4+ T cells included Th1, Th2, and Th17 cells.
Next we analyzed the expression of BCL6 and PRDM1 transcripts in blood memory Th subsets with real-time RT-PCR. Several reports demonstrated that blood CXCR5+ CD4+ T cells express much lower amounts of BCL6 transcript than tonsillar Tfh cells (Chtanova et al., 2004; Rasheed et al., 2006; Simpson et al., 2010). Consistently, the expression of BCL6 transcript was similar among CXCR5+ and CXCR5− Th subsets (Figure 3F). However, PRDM1 transcript expression was lower in all the CXCR5+ Th subsets than in their counterparts in CXCR5− cells. Thus, the balance between Bcl-6 and Blimp-1 appears to be differentially regulated between CXCR5+ and CXCR5− Th subsets.
CXCR5+ Th2 and CXCR5+ Th17 cells help B cell differentiation
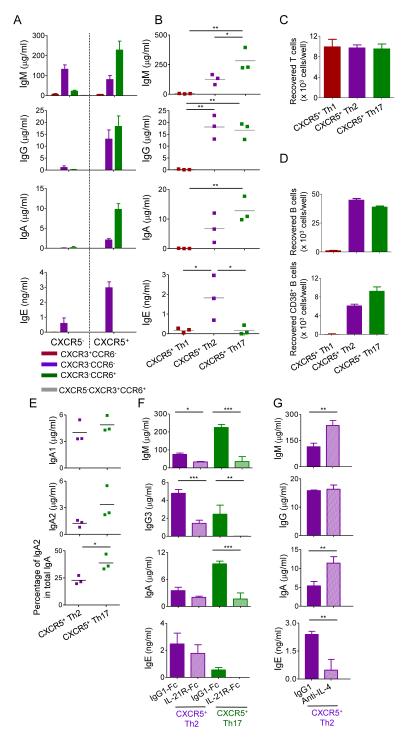
We next examined the ability of the seven blood CD4+ T cell populations to help naïve B cells. The sorted CD4+ T cells were cultured with SEB-pulsed naïve B cells for 12 days, and secreted Ig concentrations were measured. Both CXCR5+ and CXCR5− Th1 (CXCR3+CCR6−) cells failed to induce naïve B cells to produce Igs (Figure 4A, B). CXCR5+ Th1 cells were also incapable of inducing memory B cells to produce Igs (Figure S3A). Among Th2 (CXCR3−CCR6−) cells, CXCR5+ cells induced naïve B cells to produce IgM, IgG, IgA, and IgE. In contrast, CXCR5− Th2 cells induced B cells to secrete only IgM and small amounts of IgE, but virtually no IgG and IgA (Figure 4A, B). Among Th17 (CXCR3−CCR6+) cells, CXCR5+ cells potently induced naïve B cells to produce IgM, IgG and IgA, but not IgE. However, CXCR5− Th17 cells completely lacked the capacity to help naïve B cells (Figure 4A, B). The inability of CXCR5+ Th1 cells to help naïve B cells was not due to the poor survival of T cells in the cultures (Figure 4C), but due to the lack of capacity to maintain the survival of B cells (Figure 4D). In contrast, CXCR5+ Th2 and Th17 cells efficiently induced naïve B cells to proliferate and to differentiate into CD19loCD38+ plasmablasts (Figure 4D).
Figure 4. CXCR5+ Th2 and Th17 cells efficiently help naïve B cells.
A. Ig secretion by naïve B cells co-cultured with the seven blood Th populations for 12 d. n=3-4, Mean ± s.d. Representative data from three independent experiments.
B. Ig secretion from naïve B cells co-cultured with blood CXCR5+ Th subsets. Data of three independent experiments. One way ANOVA test. * p<0.05, ** p<0.01.
C. Number of viable CD4+ T cells at day 8. n=3-4, Mean ± s.d. Representative data from two independent experiments.
D. Number of viable B cells and plasmablasts at day 8. n=3-4, Mean ± s.d. Representative data from two independent experiments.
E. IgA1 and IgA2 production. Data from three independent experiments. Student’s t-test. * p<0.05.
F. Addition of IL-21R-Fc chimera protein to the co-cultures of naïve B cells and CXCR5+ Th2 or Th17 cells. n=3, Mean ± s.d. Student’s t-test. * p<0.05, ** p<0.01, *** p<0.001. Representative data from three independent experiments.
G. Addition of IL-4 blocking antibody to the culture of CXCR5+ Th2 cells and naïve B cells. n=3, Mean ± s.d. Student’s t-test. ** p<0.01. Representative data from two independent experiments.
Whereas CXCR5+ Th2 cells and Th17 cells induced naïve B cells to produce comparable amounts of IgG (Figure 4B) and its subclasses (Figure S3B), CXCR5+ Th17 cells induced naïve B cells to produce higher amounts of IgA (Figure 4B), in particular IgA2 (Figure 4E), than did CXCR5+ Th2 cells. Blocking IL-21 in the culture of naïve B cells with CXCR5+ Th2 cells resulted in a substantial decrease in IgM and IgG3 (the IgG isotype which does not crossreact to IL-21R-Fc) production (Figure 4F), whereas blocking IL-4 resulted in a substantial inhibition of IgE production (Figure 4G). The help of CXCR5+ Th17 cells to naïve B cells was largely dependent on IL-21, as blocking IL-21 strongly inhibited the production of IgM, IgG3, and IgA (Figure 4F).
Thus, the capacity to induce naïve B cells to differentiate into Ig-producing cells was different among blood CD4+ T cell populations.
CXCR5+ Th subsets are altered in juvenile dermatomyositis
The identification of functionally distinct Th subsets within blood CXCR5+ compartment led us consider that their analysis might reveal dysregulation of Tfh responses in autoimmune diseases. Juvenile dermatomyositis (JDM) is a chronic, multisystem autoimmune disease involving muscle, skin, gastrointestinal tract, and other organs. JDM patients with active disease typically show proximal muscle weakness and skin rash (Feldman et al., 2008; Suber et al., 2008). Studies on JDM have revealed several mediators common to systemic lupus erythematosus, including type I IFN (Walsh et al., 2007). Autoantibodies can be found in JDM patients serum, though relatively little is known regarding their specificities (Suber et al., 2008). The pathogenesis of JDM remains largely unknown.
We analyzed the blood CXCR5+ T cell subsets in samples from JDM patients (total 52 samples from 45 patients) and age-matched healthy pediatric controls (43 donors) (Summarized in Table 1). Blood samples were also obtained from age-matched pediatric patients with psoriatic arthritis (PSOA, 31 patients), a systemic inflammatory disease mediated by inflammatory T cells (Lewkowicz and Gottlieb, 2004). Thirty-five JDM patients were under standard treatment including corticosteroids, methotrexate, Etanercept (TNF antagonist), and/or high-dose immunoglobulin (Table S1). Twenty-six samples were obtained from symptomatic JDM patients who displayed skin rash and/or muscular weakness (measured by the Childhood Myositis Assessment Scale (CMAS)) at the time of sampling. These patients included four untreated active patients (Table S1).
Table 1.
Summary of patients and donors in the study
| p-value (t-test) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JDM (52 samples) | PSOA (31 samples) | Health (43 samples) | JDM Symptomatic vs Asynptomatic |
JDM Symptomatic vs PSOA |
JDM Asymptomatic vs PSOA |
JDM Symptomatic vs Health |
JDM Asymptomatic vs Health |
||||||
| Symptomatic | Asymptomatic | ||||||||||||
| Mean | Range | Mean | Range | Mean | Range | Mean | Range | ||||||
| Age | 10.1 ± 4.7 | 3-18 | 10.8 ± 4.0 | 5-18 | 8.7 ± 4.0 | 3-17 | 10.6 ± 4.5 | 2-18 | 0.54 | 0.23 | 0.21 | 0.82 | 0.64 |
| Gender | M10 / F17 | M4 / F21 | M6 / F25 | M29 / F14 | |||||||||
| WBC | 7.8 ± 3.5 | 3.7-15.2 | 7.0 ± 2.1 | 4.5-11 | 6.6 ± 1.9 | 3.6-13.1 | 0.36 | 0.09 | 0.40 | ||||
| Hgb | 12.7 ± 0.9 | 10.9-14.4 | 13.5 ± 1.0 | 11.7-15.9 | 12.8 ± 1.1 | 9.9-14.8 | 0.007 | 0.550 | 0.03 | ||||
| Plat | 300 ± 66 | 173-424 | 306 ± 84 | 151-422 | 326 ± 88 | 194-501 | 0.74 | 0.21 | 0.43 | ||||
| Neu | 4.6 ± 2.7 | 1.44-10.39 | 3.7 ± 1.8 | 1.71-9.72 | 3.4 ± 1.5 | 0.9-8.7 | 0.19 | 0.03 | 0.44 | ||||
| Lym | 2.0 ± 0.8 | 0.4-3.47 | 2.4 ± 1.0 | 0.85-5.06 | 2.4 ± 0.7 | 1.10-4.12 | 0.08 | 0.11 | 0.58 | ||||
| Mon | 0.9 ± 0.5 | 0.22-2.31 | 0.6 ± 0.2 | 0.25-1.3 | 0.6 ± 0.2 | 0.17-0.91 | 0.03 | 0.002 | 0.34 | ||||
| ESR | 17.0 ± 11.7 | 3-53 | 10.7 ± 10.1 | 1-41 | 8.8 ± 6.0 | 2-25 | 0.06 | 0.001 | 0.38 | ||||
| CPK | 1381 ± 3207 | 25-11332 | 87 ± 47 | 19-207 | 91 ± 30 | 56-162 | 0.07 | 0.12 | 0.80 | ||||
| ALD | 6.9 ± 5.8 | 2.6->25 | 4.5 ± 1.7 | 2.5-8.5 | 4.8 ± 1.3 | 2.3-8.0 | 0.07 | 0.17 | 0.51 | ||||
| LDH | 327 ± 249 | 126-1236 | 199 ± 45 | 127-285 | 218 ± 46 | 123-319 | 0.02 | 0.02 | 0.15 | ||||
| AST | 68 ± 100 | 16-389 | 22.6 ± 7.2 | 12-35 | 26 ± 9 | 11-46 | 0.04 | 0.02 | 0.13 | ||||
| ALT | 43 ± 59 | 7-267 | 15.2 ± 6.1 | 9-35 | 19 ± 10 | 7-49 | 0.03 | 0.03 | 0.20 | ||||
| CMAS | 40.0 ± 13.6 | 0-52 | 51.6 ± 1.0 | 48-52 | N/A | 0.0002 | |||||||
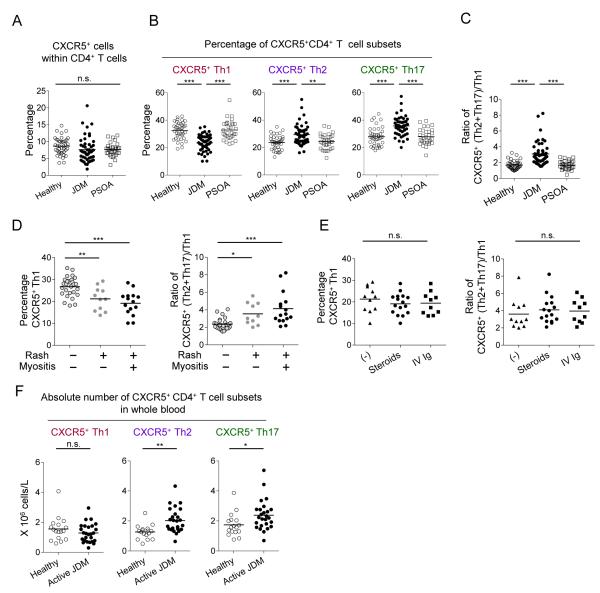
The frequency of CXCR5+ cells within CD4+ T cells was not substantially different among the three groups (Figure 5A). However, the frequency of Th1 cells within the CXCR5+ CD4+ T cell compartment was significantly lower in JDM patients when compared to PSOA patients and healthy controls (Figure 5B. JDM 23.5 ± 0.8%, PSOA 32.8 ± 1.3%, and control 32.4 ± 1.0%. Mean ± s.e.m. both p<0.0001, One way ANOVA test). In contrast, the frequencies of Th2 and Th17 cells within CXCR5+ CD4+ T cells were significantly higher in JDM compared to PSOA patients and healthy controls (Th2: JDM 29.4 ± 1.0%, PSOA 24.4 ± 1.0%, and control 23.7 ± 0.8%. both p<0.0001. Th17: JDM 35.8 ± 1.0%, PSOA 27.9 ± 1.0%, and control 28.1 ± 1.0%. both p<0.0001). The skewing of CXCR5+ Th subsets resulted in a significant increase in B helpers over non-B helpers in JDM, as determined by the ratio of Th2+Th17 (B helpers) over Th1 (non B-helpers) (Figure 5C. JDM 3.1 ± 0.2, PSOA 1.7 ± 0.1, and control 1.7 ± 0.1, Mean ± s.e.m., both p<0.0001). The Th subsets within the CXCR5− compartment were also skewed towards Th2 and Th17 in JDM patients (Figure S4A). Of note, in the PSOA group, patients receiving methotrexate or Etanercept showed comparable frequencies of the CXCR5+ Th subsets, indicating that these treatments did not alter the composition of CXCR5+ Th subsets (Figure S4B).
Figure 5. Blood CXCR5+ Th subsets are altered in JDM.
A. Percentage of CXCR5+ cells within CD4+ T cells in samples from JDM patients (n=52), PSOA patients (n=31), and age-matched healthy controls (n=43).
B. Percentage of each Th subset within blood CXCR5+ CD4+ T cells. One way ANOVA test, ** p<0.01, *** p<0.001.
C. Ratio of CXCR5+ (Th2 + Th17) / Th1 cells. One way ANOVA test.
D. Frequency of CXCR5+ Th1 cells and ratio of (Th2+Th17) / Th1 in JDM patients with different disease activities. One way ANOVA test.
E. Frequency of CXCR5+ Th1 cells and ratio of (Th2+Th17) / Th1 in active JDM patients receiving none, intravenous corticosteroids or high-dose Ig treatments.
F. The absolute cell numbers in blood were calculated based on the complete blood cell count, lymphocyte frequency within white blood cells, and the frequency of CXCR5+ Th subsets within the lymphocyte population. Student’s t-test.
Thus, blood CXCR5+ Th subsets were skewed towards Th2 and Th17 cells in JDM patients.
Tfh subsets skewing is associated with disease activity
To determine whether the skewing in Th subsets is associated with disease activity in JDM, patients were subgrouped according to the severity of clinical manifestations. Active patients with skin rash and muscular weakness showed a lower frequency of Th1 cells within CXCR5+ cells than asymptomatic patients (Figure 5D). Accordingly, patients with skin rash and muscular weakness displayed a higher ratio of Th2+Th17/Th1 in CXCR5+ cells (Figure 5D). The skewing of Th subsets is not due to the treatment, as neither the frequency of Th1 cells nor the ratio of Th2+Th17/Th1 in CXCR5+ cells were different among active patients receiving intravenous corticosteroids, high-dose intravenous immunoglobulins, or no treatment (Figure 5E). Furthermore, patients with skin rash and muscular weakness displayed a significant increase in the absolute number of CXCR5+ Th2 and Th17 cells in blood, when compared to healthy controls (Figure 5F. Th2: active JDM (n=25) 2.0 ± 0.2 vs. Healthy (n=17) 1.3 ± 0.1 × 106 cells/L. Mean ± s.e.m. p=0.001, t-test; Th17: active JDM 2.4 ± 0.2 vs. Healthy 1.7 ± 0.2 × 106 cells/L. p=0.03). Lastly, JDM patients displayed higher serum IgG concentrations than PSOA and control groups (Figure S4C).
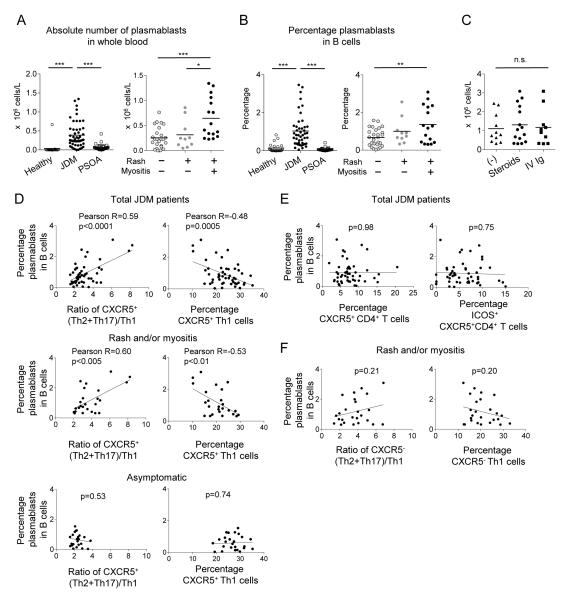
Analysis of blood B cell subsets revealed that JDM patients, particularly in those displaying both skin rash and muscular weakness, displayed higher numbers of circulating plasmablasts (CD19+CD20−CD27+CD38++ cells (Arce et al., 2001)) than PSOA patients and controls (Figure 6A, B). The number of circulating plasmablasts was similar among symptomatic patients regardless of treatment modality (Figure 6C). The frequency of plasmablasts within CD19+ B cells correlated positively with the extent of skewing of CXCR5+ Th subsets towards Th2+Th17 (Figure 6D, top left), and negatively with the frequency of CXCR5+ Th1 cells (Figure 6D, top right). The correlation between the frequency of plasmablasts and the skewing of CXCR5+ Th subsets was limited to symptomatic patients (rash and/or muscular weakness) (Figure 6D, middle and bottom). In contrast, the frequency of plasmablasts did not correlate with the frequency of either total CXCR5+ CD4+ T cells (Figure 6E, left), or ICOS+CXCR5+CD4+ T cells (Figure 6E, right). Furthermore, neither the skewing of Th subsets within CXCR5− CD4+ T cells nor the frequency of CXCR5− Th1 cells correlated with the frequency of plasmablasts (Figure 6E).
Figure 6. Skewing in blood CXCR5+ Th subsets correlates with B cell alteration.
A. The absolute number of plasmablasts in blood of JDM patients, PSOA patients, and healthy controls (left), and in JDM patients with different disease activities (right). One way ANOVA test.
B. Percentage of plasmablast within total CD19+ B cells.
C. The absolute number of plasmablasts in blood of active JDM patients receiving none, intravenous corticosteroids or high-dose Ig treatments.
D. Correlation between the percentage of plasmablasts within CD19+ B cells and the ratio of CXCR5+ (Th2+Th17) / Th1 cells (left) or the frequency of CXCR5+ Th1 cells (right) in JDM. Pearson correlation coefficient and two-tailed p-value are shown.
E. Correlation between the percentage of plasmablasts within CD19+ B cells and the frequency of total CXCR5+ CD4+ T cells (left) or of ICOS+ CXCR5+ CD4+ T cells (right).
F. Correlation between the percentage of plasmablasts and the ratio of CXCR5− (Th2+Th17) / Th1 (left) or the frequency of CXCR5− Th1 cells (right) in active JDM patients.
Collectively, in JDM patients, the alteration of blood CXCR5+ Th subsets correlated with disease activity and with an increase in circulating plasmablasts.
Discussion
Our study shows that blood CXCR5+ CD4+ T cells share functional properties with Tfh cells from secondary lymphoid organs. In concordance with Tfh cells, blood CXCR5+ CD4+ T cells induced naïve and memory B cells to become Ig-producing cells via IL-21, IL-10, and ICOS, and secreted CXCL13. At variance with Tfh cells, blood CXCR5+ CD4+ T cells barely expressed CD69 and ICOS, and PD-1 only at low intensity (Kim et al., 2001; Ma et al., 2009; Simpson et al., 2010), suggesting that they are in a resting state. Consistently, blood CXCR5+ CD4+ T cells required cell activation to provide help to B cells through cognate interaction. Whereas CXCR5 can be expressed by any activated CD4+ T cells (Schaerli et al., 2001), dissociation in the expression of CXCR5 and activation molecules such as CD69 and ICOS suggests that blood CXCR5+ CD4+ T cells do not represent recently activated cells. In contrast to Tfh cells, blood CXCR5+ CD4+ T cells express CCR7 and CD62L, suggesting their capacity to migrate into secondary lymphoid organs. Thus, it is plausible that upon microbial invasion, CXCR5+ CD4+ T cells draining into lymphoid organs interact with B cells presenting microbial antigens, and induce their differentiation into Ig-producing cells or germinal center B cells through secretion of IL-21 (Linterman et al., 2010; MacLennan et al., 2003; Zotos et al., 2010).
Our study shows that human blood CXCR5+ CD4+ T cells are composed of three subsets: Th1, Th2, and Th17 cells. CXCR5+ Th2 and CXCR5+ Th17 cells induced naïve B cells to secrete Igs through IL-21, but differentially modulated isotype switch. Whereas CXCR5+ Th2 cells promoted IgG and IgE secretion, CXCR5+ Th17 cells promoted IgG and, in particular, IgA secretion. These findings suggest that Tfh cells associated with different Th subsets differentially shape the quality of human humoral immunity. In support of this hypothesis, only a fraction of tonsillar Tfh cells produces IL-4 together with IL-21 (Lane et al., 2005; Ma et al., 2009; Yu et al., 2009). Th2-type Tfh cells were also demonstrated in mice (King and Mohrs, 2009; Reinhardt et al., 2009; Zaretsky et al., 2009). The difference between mouse and human Tfh cells might lie on the role of Th1-type cells. Mouse studies identified IFN-γ-secreting Th1-type Tfh cells in GCs, which promote the class-switching of GC B cells towards IgG2a (Reinhardt et al., 2009). This is in contrast to human CXCR5+ Th1 cells, which were incapable of helping B cells. Indeed, IFN-γ does not have any impact on isotype switching of human B cells (Banchereau et al., 1994).
Among blood CXCR5− CD4+ T cells, only CXCR5− Th2 cells induced naïve B cells to become plasmablasts producing IgM and IgE. CXCR5− Th17 cells were unable to induce naïve B cells to secrete Igs. Neither CXCR5− Th2 nor CXCR5− Th17 cells secreted IL-21 upon interaction with naïve B cells. The molecular mechanisms whereby two different types of effectors, i.e., B-helpers and non-B-helpers, emerge from Th2 and Th17 subsets remain to be established. Multiple factors are likely involved in this process, including DC subsets that prime naive CD4+ T cells (Klechevsky et al., 2008), cytokines secreted by DCs (Deenick et al., 2010; Dienz et al., 2009; Schmitt et al., 2009) or other cell types including neighboring Th cells (Nurieva et al., 2008; Vogelzang et al., 2008), T cell receptor affinity against peptide-MHC complex (Fazilleau et al., 2009), and the interaction with B cells (Nurieva et al., 2008; Zaretsky et al., 2009).
Whether human blood CXCR5+ CD4+ T cells originate from cells that migrated out of GCs or Tfh-committed extrafollicular helper cells (MacLennan et al., 2003; Poholek et al., 2010) will be challenging to address in humans. Notably, consistent with a previous report (Simpson et al., 2010), the expression of BCL6 transcript was similar between blood CXCR5+ and CXCR5− Th subsets. However, the expression of PRDM1 transcript was lower in all the Th subsets in CXCR5+ cells than their counterparts in CXCR5− cells. Given the reciprocal regulation between Bcl-6 and Blimp-1 in the generation of Tfh cells (Johnston et al., 2009), maintaining the expression of Blimp-1 at low concentrations might be a feature of memory Tfh cells. Alternatively, in concordance with B cells (Kuo et al., 2007), down regulation of Bcl-6 might be necessary for germinal center Tfh cells to become memory cells.
Our study on JDM suggests that the alteration in the balance of Tfh subsets can be associated with autoimmunity in humans. Higher numbers of circulating plasmablasts in active JDM patients and a high relevance of antinuclear antibodies prompted us to analyze blood CXCR5+ subsets in these patients. Over-representation of Th2 and Th17 and under-representation of Th1 cells in both CXCR5+ and CXCR5− cells suggest that the overall regulation of Th differentiation is altered in JDM patients. The alteration in CXCR5+ Th subsets, however, correlated better with the frequency of circulating plasmablasts than that in CXCR5− Th subsets, and thus represents a better biomarker to assess the dysregulation of B cell responses in this disease.
Our findings support the idea that humoral responses are differentially regulated by different subsets of Tfh cells in humans. This might have important implications for the design of novel vaccines. For example, induction of Th2- and Th17-, but not Th1-type, Tfh cells would be desired for efficient antibody responses to vaccination. In particular, the discovery of the Th17-type CXCR5+ CD4+ T cell subset as a potent IgA inducer could guide the design of vaccines for protective mucosal immunity. Further characterization of blood CXCR5+ CD4+ T cells as well as secondary lymphoid organ Tfh cells will provide insights into the pathogenesis and perhaps identify novel therapeutic targets for human autoimmune diseases.
Experimental Procedures
Blood samples
PBMCs purified from apheresis blood samples obtained from adult healthy volunteers were used in the experiments. Fresh blood samples were also collected from JDM (n=52), PSOA patients (n=31) and age-matched pediatric controls (n=43). Detailed clinical characteristics, clinical lab data, and treatment at the time of analysis are shown in Table S1. The study was approved by the Institutional Review Boards (IRBs) of UT Southwestern Medical Center, Texas Scottish Rite Hospital, and Baylor Health Care System. Informed consent was obtained from parents or legal guardians.
Cell Isolation
CD4+ T cells enriched by negative selection were stained with the anti-CD4 FITC (RPA-T4), anti-CXCR5 PE (51505.111), anti-CD45RA TC (MEM-56), anti-CD14 APC (61D3), and anti-CD123 APC (AC145). Then, naïve, CXCR5− memory, and CXCR5+ memory CD4+ T cells were sorted from APC-negative cell fractions. For CXCR5+ Th subsets sorting, enriched CD4+CD45RA− T cells were stained with anti-CCR6 biotin (11A9) + SA-TC, anti-CXCR3 FITC (49801), anti-CXCR5 PE, and anti-CD4 APC. Positively selected CD19+ B cells were stained with anti-IgD FITC (IA6-2), anti-CD27 PE (L128), and anti-CD3 APC. Naïve and memory B cells were sorted as IgD+CD27−CD3− and CD27+CD3−CD19+ cells, respectively.
CD4+ T cell and B cell Co-culture
Naïve B cells were co-cultured with sorted CD4+ T cells (2 × 104 cells each/well. 5 × 104 cells each/well for cytokine measurement) in the presence of endotoxin-reduced SEB (1 μg/ml) in RPMI 1640 complete medium supplemented with 10% heat-inactivated FBS. Cytokine concentrations were measured in the culture supernatants at day 2 by Luminex, and the Ig concentrations were measured at day 12 by ELISA.
Real time RT-PCR
Total RNA was extracted from blood Th subpopulations or cultured B cells. Real-time PCR was set up with Roche Probes Master reagents and Universal Probe Library hydrolysis probes. PCR reaction was performed on the LightCycler 480 (Roche Applied Science) followed these conditions: step 1 (denaturation) at 95°C for 5 min, step 2 (amplification) at 60°C for 30 min, step 3 (cooling) at 40°C for 30 sec. The expression of each gene was normalized to housekeeping gene ACTB.
Phenotypical analysis of blood samples
Whole blood cells were stained with these mAbs: CXCR5-Alexa488 (RF8B2), CCR6-PE (11A9), CD45RA-ECD (2H4LDH11LDB9), CXCR3-PECy5 (1C6), CD3-AF700 (UCHT1), CD4-Pacific Blue (RPA-T4), CD45-Pacific Orange (HI30), CD19-ECD (J3.119), CD20-PECy5 (2H7), CD38-PECy7 (HB7), CD27-APCH7 (O323). The stained cells were analyzed with BD LSRII.
Statistics
The significance of the difference between groups was analyzed with One way ANOVA test with Bonferroni correction. The significance of the difference between two groups was evaluated by F-test followed by the two-tailed Student’s, or paired t-test. A Wilcoxon signed-ranks test was applied when data did not show Gaussian distribution. Pearson correlation coefficient and two-tailed p-value were determined in the analysis of correlations.
Supplementary Material
Acknowledgments
We thank E. Kowalski, S. Coquery for cell sorting; L. Walters for cell processing from apheresis blood; S. Zurawski for IL-21 Luminex analysis; and I. Munagala for real time RT-PCR. We thank A. Karolina Palucka for critical reading and discussions. This study was supported by U19-AI057234, R01-CA84512, R01-CA078846, AR054083-01 (J.B.), U19-AI082715-01 (H.U., Program PI: VP), and Baylor Health Care System (H.U. and J.B.). J.B. holds the W. W. Caruth, Jr. Chair for Transplantation Immunology Research.
References
- Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nature immunology. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, et al. Phenotypic and functional features of human Th17 cells. The Journal of experimental medicine. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce E, Jackson DG, Gill MA, Bennett LB, Banchereau J, Pascual V. Increased frequency of pre-germinal center b cells and plasma cell precursors in the blood of children with systemic lupus erythematosus. J Immunol. 2001;167:2361–2369. doi: 10.4049/jimmunol.167.4.2361. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, van Kooten C, Liu YJ, Rousset F, Saeland S. The CD40 antigen and its ligand. Annual review of immunology. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nature immunology. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. The Journal of experimental medicine. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossaller L, Burger J, Draeger R, Grimbacher B, Knoth R, Plebani A, Durandy A, Baumann U, Schlesier M, Welcher AA, et al. ICOS Deficiency Is Associated with a Severe Reduction of CXCR5+CD4 Germinal Center Th Cells. J Immunol. 2006;177:4927–4932. doi: 10.4049/jimmunol.177.7.4927. [DOI] [PubMed] [Google Scholar]
- Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. The Journal of experimental medicine. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, de Waal Malefyt R, Tangye SG. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay PK, Yu J, Roederer M. Live-cell assay to detect antigen-specific CD4+ T-cell responses by CD154 expression. Nature protocols. 2006;1:1–6. doi: 10.1038/nprot.2006.1. [DOI] [PubMed] [Google Scholar]
- Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, Tangye SG. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010;33:241–253. doi: 10.1016/j.immuni.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienz O, Eaton SM, Bond JP, Neveu W, Moquin D, Noubade R, Briso EM, Charland C, Leonard WJ, Ciliberto G, et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. The Journal of experimental medicine. 2009;206:69–78. doi: 10.1084/jem.20081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, Leonard WJ, Lipsky PE. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman BM, Rider LG, Reed AM, Pachman LM. Juvenile dermatomyositis and other idiopathic inflammatory myopathies of childhood. Lancet. 2008;371:2201–2212. doi: 10.1016/S0140-6736(08)60955-1. [DOI] [PubMed] [Google Scholar]
- Forster R, Emrich T, Kremmer E, Lipp M. Expression of the G-protein--coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood. 1994;84:830–840. [PubMed] [Google Scholar]
- Good KL, Bryant VL, Tangye SG. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J Immunol. 2006;177:5236–5247. doi: 10.4049/jimmunol.177.8.5236. [DOI] [PubMed] [Google Scholar]
- Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science (New York, N.Y. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Lim HW, Kim JR, Rott L, Hillsamer P, Butcher EC. Unique gene expression program of human germinal center T helper cells. Blood. 2004;104:1952–1960. doi: 10.1182/blood-2004-03-1206. [DOI] [PubMed] [Google Scholar]
- Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. The Journal of experimental medicine. 2001;193:1373–1381. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annual review of immunology. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. The Journal of experimental medicine. 2009;206:1001–1007. doi: 10.1084/jem.20090313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo TC, Shaffer AL, Haddad J, Jr., Choi YS, Staudt LM, Calame K. Repression of BCL-6 is required for the formation of human memory B cells in vitro. The Journal of experimental medicine. 2007;204:819–830. doi: 10.1084/jem.20062104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane PJ, Gaspal FM, Kim MY. Two sides of a cellular coin: CD4(+)CD3- cells regulate memory responses and lymph-node organization. Nat Rev Immunol. 2005;5:655–660. doi: 10.1038/nri1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowicz D, Gottlieb AB. Pediatric psoriasis and psoriatic arthritis. Dermatol Ther. 2004;17:364–375. doi: 10.1111/j.1396-0296.2004.04039.x. [DOI] [PubMed] [Google Scholar]
- Lim HW, Lee J, Hillsamer P, Kim CH. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J Immunol. 2008;180:122–129. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. The Journal of experimental medicine. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, Schwartzberg PL, Cook MC, Walters GD, Vinuesa CG. Follicular helper T cells are required for systemic autoimmunity. The Journal of experimental medicine. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, Deenick EK, Tangye SG. Early commitment of naive human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunology and cell biology. 2009;87:590–600. doi: 10.1038/icb.2009.64. [DOI] [PubMed] [Google Scholar]
- MacLennan IC. Germinal centers. Annual review of immunology. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunological reviews. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- Mietzner B, Tsuiji M, Scheid J, Velinzon K, Tiller T, Abraham K, Gonzalez JB, Pascual V, Stichweh D, Wardemann H, Nussenzweig MC. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9727–9732. doi: 10.1073/pnas.0803644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 mediates the development of T follicular helper cells. Science (New York, N.Y. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, Flavell RA, Craft J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. The Journal of experimental medicine. 2008;205:2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pene J, Gauchat JF, Lecart S, Drouet E, Guglielmi P, Boulay V, Delwail A, Foster D, Lecron JC, Yssel H. Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J Immunol. 2004;172:5154–5157. doi: 10.4049/jimmunol.172.9.5154. [DOI] [PubMed] [Google Scholar]
- Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, Dong X, Odegard JM, Kaech SM, Dent AL, et al. In Vivo Regulation of Bcl6 and T Follicular Helper Cell Development. J Immunol. 2010 doi: 10.4049/jimmunol.0904023. doi:10.4049/jimmunol.0904023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin RL, Alston MA, Sircus JC, Knollmann-Ritschel B, Moratz C, Ngo D, Farber JM. CXCR3 is induced early on the pathway of CD4+ T cell differentiation and bridges central and peripheral functions. J Immunol. 2003;171:2812–2824. doi: 10.4049/jimmunol.171.6.2812. [DOI] [PubMed] [Google Scholar]
- Rasheed AU, Rahn HP, Sallusto F, Lipp M, Muller G. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. European journal of immunology. 2006;36:1892–1903. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nature immunology. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivino L, Messi M, Jarrossay D, Lanzavecchia A, Sallusto F, Geginat J. Chemokine receptor expression identifies Pre-T helper (Th)1, Pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. The Journal of experimental medicine. 2004;200:725–735. doi: 10.1084/jem.20040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. The Journal of experimental medicine. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaerli P, Loetscher P, Moser B. Cutting edge: induction of follicular homing precedes effector Th cell development. J Immunol. 2001;167:6082–6086. doi: 10.4049/jimmunol.167.11.6082. [DOI] [PubMed] [Google Scholar]
- Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. The Journal of experimental medicine. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, Ueno H. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31:158–169. doi: 10.1016/j.immuni.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik MJ, Marshak-Rothstein A, Wolfowicz CB, Rothstein TL, Weigert MG. The role of clonal selection and somatic mutation in autoimmunity. Nature. 1987;328:805–811. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, Manku H, Vyse TJ, Roncador G, Huttley GA, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis and rheumatism. 2010;62:234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- Singh SP, Zhang HH, Foley JF, Hedrick MN, Farber JM. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol. 2008;180:214–221. doi: 10.4049/jimmunol.180.1.214. [DOI] [PubMed] [Google Scholar]
- Suber TL, Casciola-Rosen L, Rosen A. Mechanisms of disease: autoantigens as clues to the pathogenesis of myositis. Nat Clin Pract Rheumatol. 2008;4:201–209. doi: 10.1038/ncprheum0760. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, Liang C, Bartov G, McDaniel LD, Zhou XJ, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science (New York, N.Y. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, Yu D, Domaschenz H, Whittle B, Lambe T, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Walsh RJ, Kong SW, Yao Y, Jallal B, Kiener PA, Pinkus JL, Beggs AH, Amato AA, Greenberg SA. Type I interferon-inducible gene expression in blood is present and reflects disease activity in dermatomyositis and polymyositis. Arthritis and rheumatism. 2007;56:3784–3792. doi: 10.1002/art.22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Zaretsky AG, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. The Journal of experimental medicine. 2009;206:991–999. doi: 10.1084/jem.20090303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotos D, Coquet JM, Zhang Y, Light A, D’Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. The Journal of experimental medicine. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.