Abstract
OBJECTIVE
Insulin resistance (IR) and cardiovascular disease may share a common genetic background. We investigated the role of IR-associated ENPP1 K121Q polymorphism (rs1044498) on cardiovascular disease in high-risk individuals.
RESEARCH DESIGN AND METHODS
A prospective study (average follow-up, 37 months) was conducted for major cardiovascular events (myocardial infarction [MI], stroke, cardiovascular death) from the Gargano Heart Study (GHS; n = 330 with type 2 diabetes and coronary artery disease), the Tor Vergata Atherosclerosis Study (TVAS; n = 141 who had MI), and the Cardiovascular Risk Extended Evaluation in Dialysis (CREED) database (n = 266 with end-stage renal disease). Age at MI was investigated in cross-sectional studies of 339 type 2 diabetic patients (n = 169 from Italy, n = 170 from the U.S.).
RESULTS
Incidence of cardiovascular events per 100 person--years was 4.2 in GHS, 10.8 in TVAS, and 11.7 in CREED. Hazard ratios (HRs) for KQ+QQ versus individuals carrying the K121/K121 genotype (KK) individuals were 1.47 (95% CI 0.80–2.70) in GHS, 2.31 (95% CI 1.22–4.34) in TVAS, and 1.36 (95% CI 0.88–2.10) in CREED, and 1.56 (95% CI 1.15–2.12) in the three cohorts combined. In the 395 diabetic patients, the Q121 variant predicted cardiovascular events among obese but not among nonobese individuals (HR 5.94 vs. 0.62, P = 0.003 for interaction). A similar synergism was observed in cross-sectional studies, with age at MI being 3 years younger in Q121 carriers than in KK homozygotes among obese but not among nonobese patients (P = 0.035 for interaction).
CONCLUSIONS
The ENPP1 K121Q polymorphism is an independent predictor of major cardiovascular events in high-risk individuals. In type 2 diabetes, this effect is exacerbated by obesity. Future larger studies are needed to confirm our finding.
Morbidity and mortality due to cardiovascular disease (CVD) are highly prevalent (1), mostly because of the epidemics of obesity and type 2 diabetes (2–4). Environmental and genetic factors both contribute to CVD. The genes that are involved are mostly unknown (5), and only few clues have been provided about their identities by recent genome-wide association studies (6–10). Insulin resistance and related abnormalities are among the factors that have been implicated in the etiology of CVD (11–17). Because insulin resistance is also under genetic control, the two conditions may share a common genetic background (18–20), with genes that contribute to impaired insulin sensitivity being prime candidates for a predisposing effect on CVD.
The ectoenzyme nucleotide pyrophosphate phosphodiesterase (ENPP1) inhibits insulin receptor signaling (21). A nonsynonymous polymorphism (K121Q, rs1044498) of the ENPP1 gene has been described (22), with the Q121 variant determining a gain of function resulting in an increased ability to inhibit insulin receptor signaling (22–24). This variant has been associated with insulin resistance in most (22,25–27) but not all (28) large studies. In agreement with the hypothesis of a common genetic “soil” between insulin resistance and CVD, the Q121 variant has been also associated with atherosclerosis-related phenotypes in European (24,29,30) but not in Brazilian (31) or in Chinese (32) samples. Most of these associations have been mainly observed among heavier individuals (25–27,30,33), thereby suggesting a gene-by-adiposity interaction.
The aim of our study was to investigate the role of the ENPP1 Q121 variant and its interaction with obesity (i.e., BMI ≥30 kg/m2) in accelerating major cardiovascular events in very high-risk individuals.
RESEARCH DESIGN AND METHODS
Study participants
Prospective studies.
Sample 1—the Gargano Heart Study, prospective analysis. The study included 340 whites from Italy with type 2 diabetes (according to American Diabetes Association 2003 criteria) and coronary artery disease (CAD), as indicated by previous myocardial infarction (MI) or >50% stenosis of at least one major vessel at coronary angiography, or both. These individuals were cases of the cross-sectional case-control Gargano Heart Study (GHS) (30) and were consecutively recruited at the Scientific Institute “Casa Sollievo della Sofferenza” from 2001 to 2008 and monitored until the end of 2009. The only exclusion criterion was the presence of poor life expectancy due to malignancies. Ten patients became untraceable before the first follow-up visit; therefore, data were available for 330 patients.
Sample 2—the Tor Vergata Atherosclerosis Study. A total of 143 whites from Italy were consecutively recruited from 2005 at ‘‘Tor Vergata’’ University Hospital (Rome). They all had been diagnosed with an acute MI according to the European Society of Cardiology and American Heart Consensus Guidelines. Exclusion criteria were the presence of malignancies and a medical record of diabetes, although 22 study participants (15.6%) were found to have subclinical diabetes after an oral glucose tolerance test. Because recruitment is still in progress, only patients who were recruited up to 2007, and as such underwent at least one follow-up visit, were included in this study. Two patients became untraceable before the first follow-up; therefore, data were available for 141 patients.
Sample 3—the Cardiovascular Risk Extended Evaluation in Dialysis database. This study comprises 283 whites with end-stage renal disease (ESRD), with 231 requiring hemodialysis and 52 receiving long-term ambulatory peritoneal dialysis (34). Exclusion criteria were dialysis for less than 6 months, left ventricular ejection fraction <35%, history of circulatory congestion, and hospitalization for intercurrent illness, including major infections. No dropouts were observed. Blood samples for DNA extraction were unavailable for 17 subjects; thus, 266 patients were included. Of these, 43 (16.2%) had diabetes, a proportion similar to that reported by a nationwide epidemiologic study of kidney disease in Italy (35).
Study end point. The end point considered in all three samples was a major cardiovascular event, defined as nonfatal stroke, nonfatal MI, or cardiovascular death. Information on the occurrence of nonfatal events was sought yearly from study participants and confirmed by a review of hospital records if cardiovascular events were reported. If patients did not report to a scheduled visit, information on the occurrence of cardiovascular events was obtained by telephone interview, from their primary care physicians, or from death certificates. Deaths were ascribed to CVD according to the International Classification of Diseases codes: 410.0–410.9, 415.1, 427.4–427.5, 428.0–428.9, 433.1, 434.1, 444.2, 444.8 (9th edition) or I21.0–I21.9, I46.1, I49.0, I50.1, I62.9, I63.0–I63.9, I71.3 (10th edition).
Cross-sectional study of age at MI.
A total of 339 white patients with type 2 diabetes who had survived an MI were studied. They are part of two ongoing investigations on the genetics of CAD in type 2 diabetes (30,36,37). Of these, 169 were recruited at the Scientific Institute “Casa Sollievo della Sofferenza” in San Giovanni Rotondo (Gargano, center east coast of Italy), as cases of the cross-sectional case-control GHS (29). Most of these patients (n = 160) were further studied for incident major cardiovascular events in the prospective GHS (see above). Another 170 were recruited in Boston from the Beth Israel Deaconess Medical Center (BIDMC) and the Joslin Clinic (which serves as the BIDMC Diabetes Clinic) as part of an ongoing investigation on the genetics of CAD in type 2 diabetes that has been described previously (37).
Data collection.
All subjects underwent a clinical examination and a standardized interview (at the time of recruitment and at each subsequent time point, if applicable), which was identical in all three prospective samples. A fasting blood sample (collected between 8:00 a.m. and 9:00 a.m.) was obtained from the prospective study participants and the cross-sectional study participants recruited in Italy. A random blood sample was obtained from the cross-sectional study participants recruited in Boston. BMI was calculated by dividing the weight (in kilograms) by the square of height (in meters). Presence of hypertension was defined as a systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg, or both, or the presence of antihypertensive treatment. Current and former smokers were considered as one group and compared with those who never smoked. All study protocols were approved by the local institutional review boards and performed according to the Helsinki Declaration. Written informed consent was obtained from each study participant.
Genotyping.
DNA was extracted from whole blood by standard methods. Genotyping of the ENPP1 K121Q polymorphism (rs1044498) was performed by TaqMan allele discrimination (assay C_16190162_10; Applied Biosystems, Foster City, CA) on the HT7900 platform (Applied Biosystems). The failure rate was <1%. Genotyping quality was assessed by including positive controls with known genotypes. The agreement rate was >99%. Genotype distribution was in Hardy-Weinberg equilibrium in all study samples.
Statistical analysis.
Patients’ characteristics are reported as mean and SD for continuous variables and as frequencies and percentages for categoric variables. Comparisons between genotype groups were performed by Pearson χ2 or Mann-Whitney U tests for continuous or categoric variables, respectively.
Deviations from Hardy-Weinberg equilibrium were investigated by exact χ2 test. Because of the low number of QQ individuals, only the dominant genetic model was tested by comparing individuals carrying the K121/Q121 or the Q121/Q121 genotype (Q121) (i.e., KQ heterozygotes + QQ homozygotes) to K121 homozygotes (KK). In prospective studies, a time-to-event analysis was conducted by means of Cox proportional hazards regression models using the Breslow approach in the case of ties and reported as hazard ratios (HRs) along with their 95% CI. The time to event was defined as the time between enrollment date and the date of the first cardiovascular event. For censored subjects, the time variable was defined as the time between the enrollment date and the date of the last available clinical data. The assumption of proportionality of the hazards was tested by using scaled Schoenfeld residuals. In cross-sectional studies, the association between ENPP1 Q121 variant and age at MI was analyzed by multiple linear regression analysis, and results are given as β regression coefficients.
Pooled data analyses were performed in an individual patient data meta-analysis fashion (38) (i.e., adjusting for “study sample”) after excluding genotype-by-sample interactions. Genotype-by-obesity interaction was tested by adding a cross-product term to the regression model.
The discriminatory power of prediction models was assessed by estimating the survival c-index (39) and by measuring the integrated discrimination improvement (IDI) (40).
A value of P < 0.05 was considered significant. All analyses were performed using SAS 9.1 software (SAS Institute, Cary, NC).
RESULTS
Characteristics of cohort members at baseline.
We studied three cohorts of subjects who were at very high risk of major cardiovascular events: the GHS—individuals with type 2 diabetes and previously diagnosed CAD; the Tor Vergata Atherosclerosis Study (TVAS)—individuals from the general population who had experienced an MI; and the Cardiovascular Risk Extended Evaluation in Dialysis (CREED)—individuals with ESRD who required dialysis. Clinical characteristics of study subjects at study entry are summarized in Table 1. No significant differences in baseline characteristics across genotype groups were observed in any of the three studies.
TABLE 1.
Clinical features of very high-risk individuals in the three prospective studies
| Variables | Whole sample | KK | Q121 |
|---|---|---|---|
| GHS | n = 330 | n = 222 | n = 108 |
| Male | 68.8 | 68.0 | 70.4 |
| Age (years) | 64 (8) | 64 (8) | 65 (7) |
| Smokers | 47.2 | 49.8 | 41.8 |
| BMI (kg/m2) | 30.2 (4.8) | 30.1 (4.6) | 30.5 (5.2) |
| Hypertension | 77.8 | 78.0 | 77.4 |
| Diabetes | 100 | 100 | 100 |
| TVAS | n = 141 | n = 102 | n = 39 |
| Male | 83.7 | 84.2 | 82.1 |
| Age (years) | 62 (10) | 62 (10) | 63 (9) |
| Smokers | 75.2 | 73.3 | 82.0 |
| BMI (kg/m2) | 28.0 (3.7) | 28.4 (3.8) | 27.0 (3.4) |
| Hypertension | 98.6 | 98.0 | 100.0 |
| Diabetes | 15.6 | 16.8 | 12.8 |
| CREED | n = 266 | n = 188 | n = 78 |
| Male | 56.0 | 52.1 | 65.4 |
| Age (years) | 61 (15) | 60 (16) | 64 (14) |
| Smokers | 40.0 | 38.3 | 43.6 |
| BMI (kg/m2) | 24.9 (4.4) | 24.9 (4.6) | 24.8 (4.0) |
| Hypertension | 72.9 | 73.9 | 70.5 |
| Diabetes | 16.2 | 16.5 | 15.4 |
Data are expressed as absolute numbers, percentage, or mean (SD).
ENPP1 K121Q polymorphism as a predictor of major cardiovascular events.
The average mean (SD) follow-up was 37.1 (19.4) months (range 1–91) in the GHS, 30.6 (11.3) months (range 1–37) in the TVAS, and 36.3 (22.0) months (range, 1–69) in the CREED. During follow-up, 43 major cardiovascular events occurred in the GHS, 39 in the TVAS, and 94 in the CREED, resulting in respective incidence rates of 4.2, 10.8, and 11.7 per 100 person-years (Table 2). In all studies, incidence rates per 100 person-years were numerically higher in Q121 carriers than in KK homozygotes: 5.4 vs. 3.6 in the GHS, 19.2 vs. 8.1 in the TVAS, and 14.1 vs. 10.8 in the CREED (Table 2). The difference was significant in the TVAS (P = 0.025) and in a pooled analysis of the three studies (P = 0.005). No difference in the magnitude of the genetic effect was observed among studies (P = 0.32 for interaction).
TABLE 2.
Incidence of major cardiovascular events in GHS, TVAS, and CREEDS
| N | Person-years | Nonfatal MI | Nonfatal stroke | CV death | Total events | Incidence rate* | P | |
|---|---|---|---|---|---|---|---|---|
| GHS | ||||||||
| All | 330 | 1,027 | 3 | 8 | 32 | 43 | 4.2 | |
| KK | 222 | 694 | 1 | 3 | 21 | 25 | 3.6 | |
| Q121 | 108 | 333 | 2 | 5 | 11 | 18 | 5.4 | 0.22 |
| TVAS | ||||||||
| All | 141 | 361 | 20 | 13 | 6 | 39 | 10.8 | |
| KK | 102 | 272 | 11 | 9 | 2 | 22 | 8.1 | |
| Q121 | 39 | 89 | 9 | 4 | 4 | 17 | 19.2 | 0.025 |
| CREED | ||||||||
| All | 266 | 804 | 1 | 10 | 83 | 94 | 11.7 | |
| KK | 188 | 592 | 1 | 8 | 55 | 64 | 10.8 | |
| Q121 | 78 | 212 | 0 | 2 | 28 | 30 | 14.1 | 0.225 |
| GHS+TVAS+CREED | ||||||||
| All | 737 | 2,192 | 24 | 31 | 121 | 176 | 8.1 | |
| KK | 512 | 1,558 | 13 | 20 | 78 | 111 | 7.0 | |
| Q121 | 225 | 634 | 11 | 11 | 43 | 65 | 10.9 | 0.005 |
*Per 100 person-years. CV, cardiovascular.
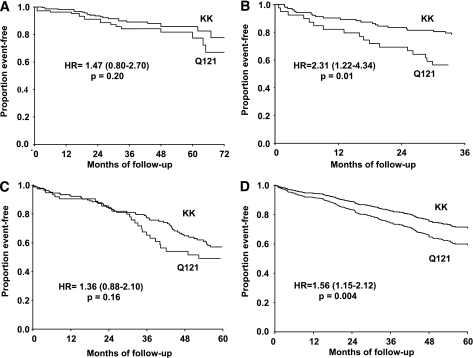
In a time-to-event analysis, the HR of cardiovascular events for Q121 carriers versus KK homozygotes was 1.47 (95% CI 0.80–2.70, P = 0.21) in the GHS, 2.31 (95% CI 1.22–4.34, P = 0.01) in the TVAS, and 1.36 (95% CI 0.88–2.10, P = 0.16) in the CREED (Fig. 1A, B, and C, respectively; P = 0.32 for gene-by-sample interaction). In a pooled analysis (i.e., individual patient data meta-analysis) of the three studies, which included 737 subjects with 176 events, the HR for Q121 carriers versus KK homozygotes was 1.56 (95% CI 1.15–2.12, P = 0.004; Fig. 1D). Age at study entry (HR 1.04 [95% CI 1.03–1.06], P < 0.0001), diabetes (2.23 [95% CI 1.50–3.31], P < 0.0001), and smoking status (1.71 [95% CI 1.24–2.36], P = 0.001) were additional predictors of incident events. BMI (HR 1.03 [95% CI 0.99–1.06], P = 0.08), hypertension (1.50 [95% CI 0.99–2.27], P = 0.054), and sex (1.30 [95% CI 0.95–1.79], P = 0.10) also tended to be associated with increased rate of events, although these did not reach statistical significance. The increased risk of events associated with the Q121 variant remained significant (HR 1.55 [95% CI 1.14–2.12], P = 0.005) after adjusting for BMI and diabetes, both of which had been reported to be associated with the Q121 variant (38–40), as well as after further adjustment for age, sex, hypertension, and smoking status (HR 1.45 [95% CI 1.05–2.00], P = 0.022).
FIG. 1.
Kaplan-Meier survival curves are shown for major cardiovascular events in GHS (A), TVAS (B), and CREED (C). D: Estimates generated by Cox regression in the pooled analysis are shown.
The addition of the K121Q genotype did not improve the risk discrimination provided by the predictive model that included age, sex, BMI, smoking status, hypertension and diabetes, as indicated by the survival c-index, which went from 0.704 to 0.713 (P = 0.94), or by the IDI, with 0.42% improvement (P = 0.16).
Interaction between Q121 variant and obesity in predicting major cardiovascular events.
Given the previous evidence for an ENPP1-by-obesity interaction in the modulation of traits related to insulin resistance (25–27,29,30,39–41), we investigated this hypothesis in our study. In the GHS, which entirely consisted of patients with type 2 diabetes, we indeed observed a significant interaction between the Q121 variant and obesity. An association between the variant and risk of events was present among the 159 subjects who had a BMI ≥30 kg/m2 (HR 3.56 [95% CI 1.21–10.5], P = 0.02), but not among the 171 individuals who had a BMI <30 kg/m2 (0.91 [95% CI 0.40–2.06], P = 0.82; P = 0.039 for interaction).
No evidence of gene-by-obesity interaction was instead observed in the TVAS (P = 0.53) and the CREED (P = 0.41). Because approximately 85% of these two cohorts consisted of nondiabetic individuals, we hypothesized that the genotype-by-obesity interaction might be specific to diabetes. Indeed, a pattern consistent with such an effect was also observed in these two studies when the analysis was restricted to individuals with diabetes, even though the small sample size prevented statistical significance (data not shown).
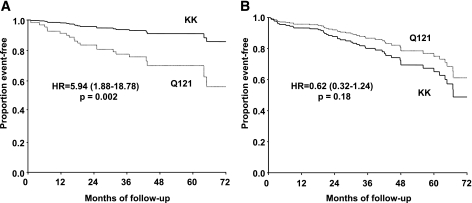
Thus, we further investigated the Q121-by-obesity interaction in a pooled analysis of the three studies after stratification by diabetes status. In the diabetic stratum (n = 395), the Q121 variant was associated with an increased risk of incident events among the 177 obese (Fig. 2A) but not among the 218 nonobese (Fig. 2B) individuals, with adjusted HRs of 5.94 (95% CI 1.88–18.78, P = 0.002) vs. 0.62 (95% CI 0.32–1.24, P = 0.18). The interaction between the Q121 variant and obesity was significant (P = 0.003). By contrast, no evidence of interaction was observed in the nondiabetic stratum (n = 344, P = 0.26), with adjusted HRs of 0.82 (95% CI 0.22–3.11, P = 0.77) in the 39 obese individuals and 1.85 (95% CI 1.18–2.90, P = 0.008) in the 305 nonobese subjects.
FIG. 2.
Survival curves for major cardiovascular events in obese (A) and nonobese (B) patients with type 2 diabetes. Curves are estimates generated by Cox regression in the pooled analysis of the three prospective studies.
Among obese diabetic individuals, the addition of the K121Q genotype to the multivariable model produced a slight improvement from 0.802 to 0.831 in risk discrimination when this was assessed by the survival c-index (P = 0.81). A much larger effect, approaching statistical significance, was observed when the improvement was assessed by IDI with a 4.56% improvement (95% CI −0.27 to 9.42, P = 0.09).
Interaction between Q121 variant and obesity on age at MI in cross-sectional studies.
To seek replication of the gene-by-obesity interaction observed in patients with diabetes, we analyzed the association between the Q121 variant and age at MI in two cross-sectional samples of individuals with type 2 diabetes who had had a previous MI. One sample was from the Gargano area in Italy, the other was from Boston. Salient clinical features of the study subjects are summarized in Table 3. Because no significant genotype-by-sample interaction was observed in the association with age at MI (P = 0.11), pooled analyses were performed by adjusting for “study sample.” To make the analysis comparable to that of prospective studies, sex, smoking status, hypertension, and BMI, but not age (due to its collinearity with age at MI—and diabetes—because all study participants were diabetic) were included as covariates. Among obese subjects, 64 Q121 carriers had had the MI almost 3 years earlier than the 124 KK homozygotes, at 54.5 (9.6) vs. 57.2 (8.9) years of age (P = 0.035). In contrast, no significant difference in age at MI was observed among nonobese subjects: 59.2 (10.5) in 41 Q121 carriers versus 57.2 (10.4) in 110 KK homozygotes (P = 0.16; P = 0.025 value for Q121-by-obesity interaction).
TABLE 3.
Clinical features of patients with type 2 diabetes who survived an MI in the two cross-sectional studies
| Whole sample | KK | Q121 | |
|---|---|---|---|
| Gargano | n = 169 | n = 112 | n = 57 |
| Male (%) | 70.4 | 72.3 | 66.7 |
| Age (years) | 64 (9) | 64 (9) | 64 (8) |
| Smokers (%) | 44.0 | 47.6 | 37.0 |
| BMI (kg/m2) | 30.7 (4.8) | 30.6 (4.6) | 30.9 (5.3) |
| Hypertension (%) | 86.9 | 89.3 | 82.1 |
| Duration of diabetes (years) | 14.2 (9.5) | 14.7 (9.9) | 13.3 (8.5) |
| HbA1c (%) | 8.6 (2.0) | 8.6 (1.8) | 8.5 (2.2) |
| Treatment of hyperglycemia | |||
| Diet alone (%) | 7.2 | 5.4 | 10.9 |
| Oral hypoglycemic agents (%) | 40.4 | 38.7 | 43.6 |
| Insulin ± oral hypoglycemic agents (%) | 52.4 | 55.9 | 45.5 |
| Boston | n = 170 | n = 122 | n = 48 |
| Male (%) | 72.4 | 73.3 | 70.8 |
| Age (years) | 64.6 (7.9) | 64.7 (7.8) | 64.3 (8.1) |
| Smokers (%) | 69.3 | 66.9 | 75.6 |
| BMI (kg/m2) | 31.8 (5.9) | 31.3 (5.6) | 33.1 (6.4) |
| Hypertension (%) | 78.2 | 77.0 | 81.3 |
| Duration of diabetes (years) | 12.5 (9.3) | 11.8 (9.4) | 14.1 (9.1) |
| HbA1c (%) | 7.4 (1.4) | 7.5 (1.5) | 7.3 (1.3) |
| Treatment of hyperglycemia | |||
| Diet alone (%) | 5.3 | 4.1 | 8.3 |
| Oral hypoglycemic agents (%) | 42.9 | 47.5 | 31.3 |
| Insulin ± oral hypoglycemic agents (%) | 51.8 | 48.4 | 60.4 |
Data are expressed as number, percentage, or mean (SD).
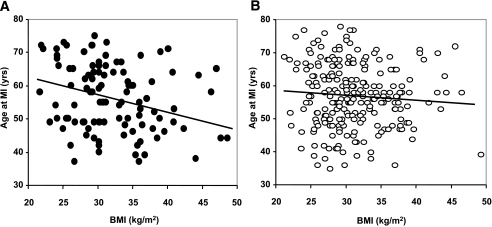
Virtually identical results were obtained when duration of diabetes was also added to the multivariate model, with Q121 carriers having had an MI at a significantly younger age than KK individuals among obese (P = 0.039) but not among nonobese (P = 0.20) individuals (P = 0.032 for interaction). In addition, when BMI was considered as a continuous trait, it was inversely related to the age at MI among Q121 carriers (β = −0.44 [95% CI −0.75 to −0.12], P = 0.008; Fig. 3A), but not among KK individuals (β = −0.17 [95% CI −0.41 to 0.07], P = 0.16; P = 0.069; for Q121-by-BMI interaction; Fig. 3B).
FIG. 3.
Linear regression between BMI and age at MI in diabetic patients with Q121 (A) and KK (B) genotypes. Data are obtained by individual data meta-analysis of the two cross-sectional studies from Gargano and Boston.
DISCUSSION
Our results indicate that the ENPP1 K121Q polymorphism predicts acceleration of major cardiovascular events in very high-risk patients. The increased risk conferred by the Q121 variant is independent of that of age, sex, BMI, diabetes, and cigarette smoking. Our findings are in agreement with a previous cross-sectional study of 445 MI survivors from Central Europe (29). By contrast, case-control genome-wide association studies reported that a single nucleotide polymorphism (SNP, rs7767502), which is in perfect linkage disequilibrium with the ENPP1 K121Q polymorphism, was not associated with CAD (6–10). Several differences between our study and the genome-wide association studies, such as the prospective versus cross-sectional designs, the different end points under investigation, and the different baseline cardiovascular risk, with only the patients enrolled in our study being very high-risk as per selection criteria, might be responsible for this apparent discordance.
An additional important result of our study is that the effect of the Q121 variant was modulated by obesity in diabetic patients among whom the risk of incident events was five times higher in Q121 than in KK genotype carriers. Although not the aim of our study, one can infer that obese individuals (Fig. 2A) as a whole tend to have a lower risk of future cardiovascular events than nonobese patients (Fig. 2B; adjusted HR 0.68 [95% CI 0.411–1.124], P = 0.13). This paradoxic protective effect of obesity resembles that observed in patients with CAD (41), ESRD (42), heart failure (43), and older age (44), all conditions heavily over-represented in our samples. In this context, the Q121 variant seems to eliminate the paradoxic protective effect of obesity.
An important finding was that the Q121 variant-by-obesity interaction observed in the prospective study was replicated in a cross-sectional study on age at MI in diabetic patients. Information on the K121Q genotypes tended to improve risk prediction in these patients when the improvement was measured by the IDI, the approach that is currently favored to evaluate predictive ability increase conferred by a new marker when added to a well-performing model (39). Thus, pending further validation in larger studies, one can hypothesize clinical implementation of the Q121 variant as a marker of early cardiovascular events among obese diabetic patients. Given the increasing incidence worldwide of both obesity and diabetes (2–4) and the poor ability to stratify cardiovascular risk among diabetic patients, a large sector of society would be likely to benefit in the future from the availability of such a test.
The synergistic effect of the genetic marker and obesity in the modulation of cardiovascular risk resembles results repeatedly reported in the risk modulation of insulin resistance and related traits (25–27,30,33,45–49). Placed in a broader perspective, this is an excellent example of genetic heterogeneity (i.e., different genetic effects being at play in different population subgroups) and clearly illustrates how accounting for such heterogeneity may be critical to dissect the genetic architecture of multifactorial diseases.
Understanding the mechanisms through which the Q121 variant is associated with CVD is beyond the scope of this study. However, one can speculate that the Q121 variant exacerbates cardiovascular risk by inducing systemic insulin resistance (22,25–27) and proatherogenic phenotypes (24,29,30,33). It may also act by way of a direct detrimental effect on insulin-dependent endothelial function, as suggested by the observation that human endothelial cells carrying the Q121 variant show impaired insulin receptor signaling and, most importantly, reduced release of nitric oxide (24), a potent vasodilator whose deficiency is an established early step in the pathway development of atherosclerosis (50).
One can hypothesize that the interaction between the Q121 variant and obesity is sustained by the different sites of action on the insulin-signaling pathway. Although ENPP1 acts at the insulin receptor level (21,23), obesity acts by different mechanisms, mostly at a postreceptor level (51). It is, therefore, possible that postreceptor insulin-signaling abnormalities are necessary for the Q121 variant to be fully effective in inducing insulin resistance and, eventually, related clinical outcomes.
The three cohorts of very high-risk individuals that we studied were quite different from each other: one comprised only patients with type 2 diabetes and CAD, another included patients with a previous MI who did not have frank type 2 diabetes, and the third included only patients with ESRDs. Despite such apparent phenotypic heterogeneity, the effect of the Q121 variant was not heterogeneous across the three studies. Not only did this allow us to analyze the three cohorts together, increasing statistical power, but it also suggests that our findings may be generalizable to all high-risk patients, irrespective of their background clinical characteristics. Whether the predictive role of the Q121 variant extends to situations characterized by a more moderate cardiovascular risk remains to be determined.
We acknowledge that, mainly because of the relatively small size of our samples, the significance level of our findings is still compatible with a false-positive result. However, this seems unlikely given that the association between Q121 was not heterogeneous across the three cohorts and, importantly, was further confirmed in cross-sectional studies as far as the interaction with obesity in diabetic patients is concerned. We also acknowledge that due to the relatively small sample size of the studies that we analyzed, we cannot exclude that the gene-by-obesity interaction that we observed among diabetic patients also occurs among nondiabetic individuals, as is the case for the modulation of insulin resistance (25–27). Therefore, our findings need further replication in larger samples before they can be considered as established. Finally, because this study was entirely performed in individuals of European ancestry, we do not know whether our findings can be extended to populations of different race.
In conclusion, pending confirmation in further larger studies, the Q121 variant has the potential to become a clinical tool for identifying those very high-risk patients who are especially prone to major cardiovascular events and need, therefore, to be targeted with specific and even more aggressive preventive strategies.
ACKNOWLEDGMENTS
This study was partly supported by the Italian Ministry of Health (“Ricerca Corrente 2009 and 2010”) (to S.P. and V.T.), by Fondazione Roma (“Sostegno alla ricerca scientifica biomedica 2008”) (to V.T.), and by the National Institutes of Health Grants HL73168 (to A.D.) and DK36836 to the Genetics Core of the Diabetes & Endocrinology Research Center at the Joslin Diabetes Center.
No potential conflicts of interest relevant to this article were reported.
S.B. designed the study; acquired, analyzed, and interpreted the data; and wrote the manuscript. S.R. and S.P. acquired, analyzed, and interpreted the data and reviewed and edited the manuscript. B.S., C.P., A.F., A.P., D.L., A.T., Y.-Y.Z., and G.D.S. acquired data and reviewed the manuscript. F.M. reviewed the manuscript. G.T., R.X., D.M., F.A., R.L., E.V.G., and T.H.H. acquired data and reviewed the manuscript. M.C. acquired and analyzed the data and reviewed and edited the manuscript. S.D.C. acquired and interpreted the data and reviewed the manuscript. F.P. acquired and analyzed the data and reviewed and edited the manuscript. C.Z. and M.F. acquired data and reviewed and edited the manuscript. A.D. and V.T. designed the study, analyzed and interpreted the data, and wrote the manuscript.
The authors are grateful to Celeste Amundsen, Erin Murphy, and Elisabeth Goheen (Joslin Diabetes Center, Boston, MA) for their technical help and acknowledge the invaluable contribution by the individuals who participated in this study.
REFERENCES
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Writing Group Members. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation 2010;121:e46–e215 [DOI] [PubMed] [Google Scholar]
- 2.Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med 2006;12:75–80 [DOI] [PubMed] [Google Scholar]
- 3.Goya Wannamethee S, Gerald Shaper A, Whincup PH, Walker M. Overweight and obesity and the burden of disease and disability in elderly men. Int J Obes Relat Metab Disord 2004;28:1374–1382 [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008;16:2323–2330 [DOI] [PubMed] [Google Scholar]
- 5.Hamsten A, Eriksson P. Identifying the susceptibility genes for coronary artery disease: from hyperbole through doubt to cautious optimism. J Intern Med 2008;263:538–552 [DOI] [PubMed] [Google Scholar]
- 6.McPherson R, Pertsemlidis A, Kavaslar N, et al. A common allele on chromosome 9 associated with coronary heart disease. Science 2007;316:1488–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helgadottir A, Thorleifsson G, Manolescu A, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science 2007;316:1491–1493 [DOI] [PubMed] [Google Scholar]
- 8.Samani NJ, Erdmann J, Hall AS, et al. WTCCC and the Cardiogenics Consortium Genomewide association analysis of coronary artery disease. N Engl J Med 2007;357:443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erdmann J, Grosshennig A, Braund PS, et al. Italian Atherosclerosis, Thrombosis, and Vascular Biology Working Group. Myocardial Infarction Genetics Consortium. Wellcome Trust Case Control Consortium. Cardiogenics Consortium New susceptibility locus for coronary artery disease on chromosome 3q22.3. Nat Genet 2009;41:280–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myocardial Infarction Genetics Consortium, Kathiresan S, Voight BF, Purcell S, et al. Genome-wide association of early-onset myocardial infarction with common single polymorphisms, common copy number variants, and rare copy number variants. Nat Genet 2009;41:334–341 [DOI] [PMC free article] [PubMed]
- 11.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988;37:1595–1607 [DOI] [PubMed] [Google Scholar]
- 12.Howard G, O’Leary DH, Zaccaro D, et al. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators Insulin sensitivity and atherosclerosis. Circulation 1996;93:1809–1817 [DOI] [PubMed] [Google Scholar]
- 13.Hedblad B, Nilsson P, Engström G, Berglund G, Janzon L. Insulin resistance in non-diabetic subjects is associated with increased incidence of myocardial infarction and death. Diabet Med 2002;19:470–475 [DOI] [PubMed] [Google Scholar]
- 14.Rutter MK, Meigs JB, Sullivan LM, D’Agostino RB, Sr, Wilson PW. Insulin resistance, the metabolic syndrome, and incident cardiovascular events in the Framingham Offspring Study. Diabetes 2005;54:3252–3257 [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Muntner P, Hamm LL, et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med 2004;140:167–174 [DOI] [PubMed] [Google Scholar]
- 16.De Cosmo S, Trevisan R, Minenna A, et al. Insulin resistance and the cluster of abnormalities related to the metabolic syndrome are associated with reduced glomerular filtration rate in patients with type 2 diabetes. Diabetes Care 2006;29:432–434 [DOI] [PubMed] [Google Scholar]
- 17.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev 2007;28:463–491 [DOI] [PubMed] [Google Scholar]
- 18.Pedersen O. Genetics of insulin resistance. Exp Clin Endocrinol Diabetes 1999;107:113–118 [DOI] [PubMed] [Google Scholar]
- 19.Rich SS, Bowden DW, Haffner SM, et al. Insulin Resistance Atherosclerosis Study Family Study Identification of quantitative trait loci for glucose homeostasis: the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes 2004;53:1866–1875 [DOI] [PubMed] [Google Scholar]
- 20.Poulsen P, Levin K, Petersen I, Christensen K, Beck-Nielsen H, Vaag A. Heritability of insulin secretion, peripheral and hepatic insulin action, and intracellular glucose partitioning in young and old Danish twins. Diabetes 2005;54:275–283 [DOI] [PubMed] [Google Scholar]
- 21.Goldfine ID, Maddux BA, Youngren JF, et al. The role of membrane glycoprotein plasma cell antigen 1/ectonucleotide pyrophosphatase phosphodiesterase 1 in the pathogenesis of insulin resistance and related abnormalities. Endocr Rev 2008;29:62–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pizzuti A, Frittitta L, Argiolas A, et al. A polymorphism (K121Q) of the human glycoprotein PC-1 gene coding region is strongly associated with insulin resistance. Diabetes 1999;48:1881–1884 [DOI] [PubMed] [Google Scholar]
- 23.Costanzo BV, Trischitta V, Di Paola R, et al. The Q allele variant (GLN121) of membrane glycoprotein PC-1 interacts with the insulin receptor and inhibits insulin signaling more effectively than the common K allele variant (LYS121). Diabetes 2001;50:831–836 [DOI] [PubMed] [Google Scholar]
- 24.Bacci S, Di Paola R, Menzaghi C, et al. ENPP1 Q121 variant, increased pulse pressure and reduced insulin signaling, and nitric oxide synthase activity in endothelial cells. Arterioscler Thromb Vasc Biol 2009;29:1678–1683 [DOI] [PubMed] [Google Scholar]
- 25.Abate N, Chandalia M, Satija P, et al. ENPP1/PC-1 K121Q polymorphism and genetic susceptibility to type 2 diabetes. Diabetes 2005;54:1207–1213 [DOI] [PubMed] [Google Scholar]
- 26.Stolerman ES, Manning AK, McAteer JB, et al. Haplotype structure of the ENPP1 Gene and Nominal Association of the K121Q missense single nucleotide polymorphism with glycemic traits in the Framingham Heart Study. Diabetes 2008;57:1971–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baratta R, Rossetti P, Prudente S, et al. Role of the ENPP1 K121Q polymorphism in glucose homeostasis. Diabetes 2008;57:3360–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grarup N, Urhammer SA, Ek J, et al. Studies of the relationship between the ENPP1 K121Q polymorphism and type 2 diabetes, insulin resistance and obesity in 7,333 Danish white subjects. Diabetologia 2006;49:2097–2104 [DOI] [PubMed] [Google Scholar]
- 29.Endler G, Mannhalter C, Sunder-Plassmann H, et al. The K121Q polymorphism in the plasma cell membrane glycoprotein 1 gene predisposes to early myocardial infarction. J Mol Med 2002;80:791–795 [DOI] [PubMed] [Google Scholar]
- 30.Bacci S, Ludovico O, Prudente S, et al. The K121Q polymorphism of the ENPP1/PC-1 gene is associated with insulin resistance/atherogenic phenotypes, including earlier onset of type 2 diabetes and myocardial infarction. Diabetes 2005;54:3021–3025 [DOI] [PubMed] [Google Scholar]
- 31.Moehlecke M, Kramer CK, Leitão CB, et al. [ENPP1 K121Q polymorphism and ischemic heart disease in diabetic patients]. Arq Bras Cardiol 2010;94:157–161, 168–173, 159–163 [DOI] [PubMed] [Google Scholar]
- 32.Chen MP, Chung FM, Chang DM, et al. ENPP1 K121Q polymorphism is not related to type 2 diabetes mellitus, features of metabolic syndrome, and diabetic cardiovascular complications in a Chinese population. Rev Diabet Stud 2006;3:21–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Cosmo S, Minenna A, Zhang YY, et al. Association of the Q121 variant of ENPP1 gene with decreased kidney function among patients with type 2 diabetes. Am J Kidney Dis 2009;53:273–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Testa A, Spoto B, Tripepi G, et al. The GLU298ASP variant of nitric oxide synthase interacts with asymmetric dimethyl arginine in determining cardiovascular mortality in patients with end-stage renal disease. J Hypertens 2005;23:1825–1830 [DOI] [PubMed] [Google Scholar]
- 35.Panzetta G, Basile C, Santoro A, et al. Diabetics on dialysis in Italy: a nationwide epidemiological study. Nephrol Dial Transplant 2008;23:3988–3995 [DOI] [PubMed] [Google Scholar]
- 36.Prudente S, Hribal ML, Flex E, et al. The functional Q84R polymorphism of mammalian Tribbles homolog TRB3 is associated with insulin resistance and related cardiovascular risk in Caucasians from Italy. Diabetes 2005;54:2807–2811 [DOI] [PubMed] [Google Scholar]
- 37.Doria A, Wojcik J, Xu R, et al. Interaction between poor glycemic control and 9p21 locus on risk of coronary artery disease in type 2 diabetes. JAMA 2008;300:2389–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olkin I, Sampson A. Comparison of meta-analysis versus analysis of variance of individual patient data. Biometrics 1998;54:317–322 [PubMed] [Google Scholar]
- 39.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med 2004;23:2109–2123 [DOI] [PubMed] [Google Scholar]
- 40.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–172; discussion 207–212 [DOI] [PubMed] [Google Scholar]
- 41.Kang X, Shaw LJ, Hayes SW, et al. Impact of body mass index on cardiac mortality in patients with known or suspected coronary artery disease undergoing myocardial perfusion single-photon emission computed tomography. J Am Coll Cardiol 2006;47:1418–1426 [DOI] [PubMed] [Google Scholar]
- 42.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int 2003;63:793–808 [DOI] [PubMed] [Google Scholar]
- 43.Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol 2004;43:1439–1444 [DOI] [PubMed] [Google Scholar]
- 44.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med 1998;338:1–7 [DOI] [PubMed] [Google Scholar]
- 45.Meyre D, Bouatia-Naji N, Tounian A, et al. Variants of ENPP1 are associated with childhood and adult obesity and increase the risk of glucose intolerance and type 2 diabetes. Nat Genet 2005;37:863–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McAteer JB, Prudente S, Bacci S, et al. ENPP1 Consortium The ENPP1 K121Q polymorphism is associated with type 2 diabetes in European populations: evidence from an updated meta-analysis in 42,042 subjects. Diabetes 2008;57:1125–1130 [DOI] [PubMed] [Google Scholar]
- 47.Prudente S, Morini E, Trischitta V. Insulin signaling regulating genes: effect on T2DM and cardiovascular risk. Nat Rev Endocrinol 2009;5:682–693 [DOI] [PubMed] [Google Scholar]
- 48.Bochenski J, Placha G, Wanic K, et al. New polymorphism of ENPP1 (PC-1) is associated with increased risk of type 2 diabetes among obese individuals. Diabetes 2006;55:2626–2630 [DOI] [PubMed] [Google Scholar]
- 49.Cauchi S, Nead KT, Choquet H, et al. The genetic susceptibility to type 2 diabetes may be modulated by obesity status: implications for association studies. BMC Med Genet 2008;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004;109(Suppl. 1):III27–III32 [DOI] [PubMed] [Google Scholar]
- 51.Abate N, Chandalia M, Di Paola R, Foster DW, Grundy SM, Trischitta V. Mechanisms of disease: Ectonucleotide pyrophosphatase phosphodiesterase 1 as a ‘gatekeeper’ of insulin receptors. Nat Clin Pract Endocrinol Metab 2006;2:694–701 [DOI] [PubMed] [Google Scholar]