Abstract
OBJECTIVE
The objective of this study was to test if the proportion of new-onset diabetic subjects with the HLA-DR3/4-DQB1*0302 genotype is decreasing over time.
RESEARCH DESIGN AND METHODS
We analyzed HLA class II genotype frequencies over time in two large populations with type 1 diabetes diagnosed at ≤18 years of age. There were 4,075 subjects from the Type 1 Diabetes Genetics Consortium (T1DGC) and 1,675 subjects from the Barbara Davis Center (BDC).
RESULTS
Both T1DGC and BDC cohorts showed a decrease of the highest-risk HLA-DR3/4-DQB1*0302 genotype over time. This decrease was greatest over time in T1DGC subjects with age of onset ≤5 years (P = 0.004) and onset between ages 6 and 10 years (P = 0.002). The overall percent of HLA-DR3/4-DQB1*0302 was greater in the T1DGC population compared with the BDC population. There was an increased percent over time of other HLA genotypes without HLA-DR3 or -DR4 in T1DGC new onsets (P = 0.003), and the trend was similar in BDC subjects (P = 0.08). Analyzing time trend, there appears to be a large stepwise decrease in percent DR3/4 in the 1980s in T1DGC subjects with onset age <5 years (P = 0.0001).
CONCLUSIONS
The change in frequency of multiple different genotypes and a possible stepwise decrease in percent DR3/4 suggest a change in genetic risk factors and environmental determinants of type 1 diabetes. Larger studies are needed to confirm the changing pattern of genetic risk because a stepwise change may have direct bearing on defining critical environmental determinants of type 1 diabetes.
The incidence of type 1 diabetes has been increasing worldwide by approximately 3% per year (1,2), with the highest increase occurring in young children (3,4). The major type 1 diabetes susceptibility locus maps to the HLA class II genes and accounts for 30–50% of genetic type 1 diabetes risk (5,6). Age at onset is inversely related to the frequency of high-risk HLA genotypes, with young children having the greatest proportion of HLA-DR3/4-DQB1*0302 genotype (7,8). Although 40% of Caucasians in the U.S. have an HLA-DR3 (with DQB1*0201) or -DR4 (with DQB1*0302) allele, at least one of these alleles is present in 95% of patients with type 1 diabetes. The estimated risk of developing type 1 diabetes for children in the general population who have the HLA DR3-DQB1*0201/DR4-DQB1*0302 genotype is ∼1:15–1:25 (9). Only 2.4% of the general population carries this genotype compared with 30–40% of type 1 diabetic patients. In siblings, HLA-DR3/4 heterozygosity identifies higher risk of type 1 diabetes than HLA identity (10). A combination of HLA-DR3/4 heterozygosity and HLA identity in siblings is associated with extreme diabetes risk of 55% by age 12 years in the Diabetes Autoimmunity Study in the Young (DAISY) population (11).
However, as type 1 diabetes incidence increases, the percentage of those cases with high-risk HLA genotype is decreasing (12). A study from the U.K. (13) compared two cohorts and found a decrease in the high-risk HLA-DR3/4-DQB1*0302 genotype from 47% (Golden Years cohort diagnosed more than 50 years ago) to 35% in the more recently diagnosed cohort (between 1985–2002). Hermann et al. (8) reported a similar decrease in high-risk HLA genotypes over time (from 25 to 18%), although both of these studies (8,13) compared contemporary subjects with selective cohorts of surviving adults with childhood-onset type 1 diabetes. A study from Australia by Fourlanos et al. (14) with continuous information available reports a decrease in the high-risk HLA genotype from 79% in 1950–1969 to 28% in 2000–2005 in 462 subjects diagnosed before age 18 years; the authors conclude that the rising incidence of type 1 diabetes in childhood is accounted for by cases with lower-risk HLA genotypes.
In this study, we analyze HLA class II allele frequencies over a time period ranging from 1965 to 2008 in two large populations of type 1 diabetic subjects diagnosed at age ≤18 years and describe a possible stepwise decrease in percent DR3/4.
RESEARCH DESIGN AND METHODS
The Type 1 Diabetes Genetics Consortium (T1DGC) has created a resource base of well-characterized families from multiple ethnic groups in order to facilitate the localization and characterization of type 1 diabetes susceptibility genes (http://www.t1dgc.org). The T1DGC has recruited families consisting mainly of nuclear families with an affected sib pair. In this study, we analyzed 4,075 subjects diagnosed between 1965 and 2006 with onset age ≤18 years and complete HLA information available. The majority of the subjects were Caucasian (93.6%), and there were no sex differences (male/female = 51%). The mean age of onset was 8.4 years (range: 6 months to 18 years).
Subjects from the Barbara Davis Center (BDC) diagnosed between 1965 and 2008 with age of onset ≤18 years and positive islet autoantibodies were included (n = 1,675). For subjects diagnosed before 1995, ICA512 and GAD65 antibodies were measured and available (on samples not obtained at time of onset), and ICA512, GAD65, ZnT8 antibodies, and insulin autoantibody within 10 days of diagnosis were included for those diagnosed after 1995. The mean age of onset was 9.2 years (range: 6 months to 18 years), and there were no sex differences (male/female = 52%). The majority of the subjects were Caucasian (86.4%) with 4.8% Hispanic, 3.2% African American, and 5.6% other ethnicity. The Colorado Multiple Institutional Review Board approved the study protocol with informed consent.
Genotyping.
HLA class II genotyping at the BDC was performed using sequence specific oligonucleotide (SSO) typing for DQA1 and DQB1 as described previously (15) or real time PCR. For realtime PCR based typing, DNA was isolated from whole blood using a Quickgene 610 L instrument (Autogen, Holliston, MA). Seven Custom probes (Applied Biosystems, Foster City, CA) and 10 primers (Integrated DNA Technologies, Coralville, IA) were used in 4 PCR wells. In addition, one Taqman premixed probe/primer mix was used in one additional well (Applied Biosystems, Foster City, CA). The PCR was set up in 5 wells per sample. In well 1, probes detected the presence or absence of DR4, and in well 2, probes detected the presence or absence of DR3. Wells 3, 4, and 5 contained probes which detected the presence or absence of DQB*0302 or DQB*0602.
SSO typing for DQA1 and DQB1 was done in the same way in the last few years in both BDC and T1DGC populations as described previously (15,16).
Statistical analysis.
Comparisons of genotype frequencies between the 2 cohorts over time were assessed by Fisher exact test or χ2 statistics. A P value of 0.05 or less was considered to be statistically significant. Genotype frequencies were analyzed by decades for both T1DGC and BDC population as well as by age of onset (ages ≤5, 6–10, and 11–18 years). HLA genotypes were classified into the following groups: high-risk HLA-DR3/4-DQB1*0302; HLA-DR4/4 and 4/X; HLA-DR3/3 and 3/X; HLA-DRX/X and protective HLA-DR2-DQB1*0602 (where X is not DR3, DR4, or DQB1*0602). The protective DRB1*0403 was removed from the DR4 category in the T1DGC population. For T1DGC population, HLA-DR3/4-DQB1*0302 genotype was also analyzed in 2-year interval periods (4-year intervals before 1977 because of small numbers during that period).
RESULTS
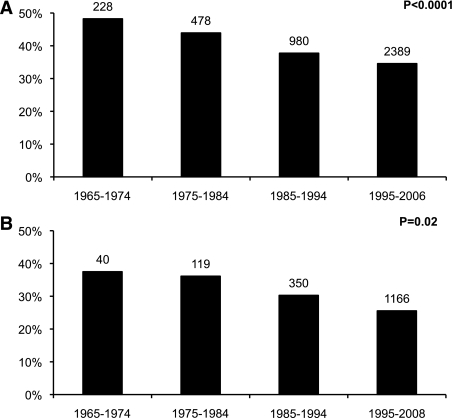
Both T1DGC and BDC cohorts showed a significant decrease of the high-risk HLA-DR3/4-DQB1*0302 genotype over time. In the T1DGC cohort, the frequency of the high-risk HLA genotype decreased from 48% in 1965–1974 to 35% in 1995–2006 (Fig. 1A). In the BDC population, the overall percent of HLA-DR3/4-DQB1*0302 was less than in the T1DGC, but the difference over time was similar, decreasing from 38% in 1965–1974 to 26% in 1995–2008 (Fig. 1B).
FIG. 1.
Percent HLA-DR3/4-DQB1*0302 in T1DGC (A) and BDC (B) new onsets at age <18 years by decades (1965–2008).
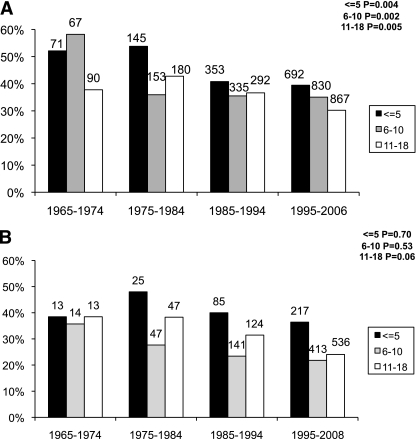
Decrease in high-risk HLA percentage was greatest over time in T1DGC subjects with age of onset ≤10 years. In the group diagnosed at age ≤5 years, the presence of HLA-DR3/4-DQB1*0302 decreased from over 50% before 1985 to 39% in 1995–2006 (P = 0.004). T1DGC subjects with onset between ages 6 and 10 years had an even larger drop over time in HLA-DR3/4-DQB1*0302 from 58 to 35% (P = 0.002), whereas subjects diagnosed after age 10 had a more moderate decrease from 38 to 30% (P = 0.005) (Fig. 2A).
FIG. 2.
Percent HLA-DR3/4-DQB1*0302 in T1DGC (A) and BDC (B) type 1 diabetic subjects by age of onset over 4 decades. Age-of-onset categories: ages <5, 6–10, and 11–18 years.
High-risk HLA-DR3/4-DQB1*0302 genotype showed a similar decrease in frequency over time in the BDC population, although not all age groups had statistically significant differences, which was likely due to smaller numbers (especially in the earlier decades). The percentage of HLA-DR3/4-DQB1*0302 decreased from 48% in 1975–1984 to 36% in 1995–2008 for those diagnosed at age ≤5 years (P = 0.70). The age 6–10 years onset group had a decrease in HLA-DR3/4-DQB1*0302 from 36 to 22% (P = 0.53) and those with onset between ages 11 and 18 years decreased from 38 to 24% (P = 0.06) (Fig. 2B).
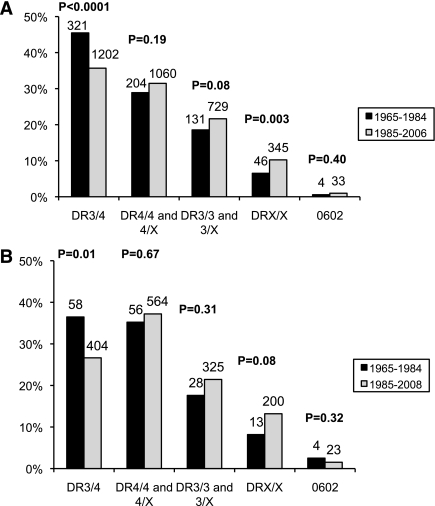
There was a significant increase over time of other HLA genotypes. Patients without HLA-DR3 or -DR4 (DRX/X) increased in the T1DGC population from 6.5 to 10% (P = 0.003). HLA DR4/4 and 4/X as well as DR3/3 and 3/X also showed an increase over time, although not significantly (Fig. 3A). In the BDC population, the trend was similar with an increase in DRX/X from 8% between 1965–84 to 13% in 1985–2008 (P = 0.08) (Fig. 3B).
FIG. 3.
Frequency of HLA genotypes over time in T1DGC (A) and BDC (B) populations. HLA genotypes were classified into the following groups: high-risk HLA-DR3/4-DQB1*0302; HLA-DR4/4 and 4/X; HLA DR3/3 and 3/X; HLA DRX/X (where X is not DR3, DR4, or DQB1*0602); and protective HLA-DR2-DQB1*0602.
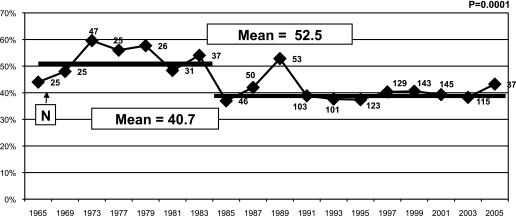
Linear regression performed in T1DGC subjects with onset age ≤5 years showed an overall linear decrease of HLA-DR3/4-DQB1*0302 genotype over time (P = 0.007, R2 = 0.33). However, when analyzing HLA-DR3/4-DQB1*0302 genotype in 2-year interval periods, we found a significant decrease in percent DR3/4 in the mid 1980s in subjects with onset age ≤5 years (P = 0.0001) (Fig. 4). We sampled the data 20,000 times with replacement using bootstrap and found a significant difference between those time points (before and after 1985), although this decrease could also have happened in the early 1990s.
FIG. 4.
Percent HLA-DR3/4-DQB1*0302 in T1DGC new onsets at age <5 years. HLA-DR3/4-DQB1*0302 genotype was analyzed in 2-year interval periods (4-year intervals before 1977 because of small numbers during that period).
We performed the same analyses in Caucasians only and found overall very similar results (Supplementary Figs. 1–4).
DISCUSSION
This study confirms that the highest risk HLA-DR3/4-DQB1*0302 genotype has been decreasing in new onset type 1 diabetic subjects in more recent decades. This finding is consistent with several important smaller studies reporting a similar decrease in the percent DR3/4 (8,12–14), although some studies have not found any temporal changes in HLA genotypes (17). This is the largest study so far with HLA information on new onset subjects over a period of 40 years; other studies have mainly compared two time periods.
Previous studies have mainly selected populations from one geographic region for HLA analysis, whereas the T1DGC includes multiple populations and large numbers of type 1 diabetic patients over many years. The T1DGC data suggests that the change in high-risk HLA frequency can be more precipitous than previously thought.
A stepwise decrease in percent DR3/4 would suggest a relatively acute environmental change with an increase of disease penetrance in individuals with lower-risk HLA genotypes and/or a lower disease penetrance in subjects with high-risk genotypes. We hypothesize that disease penetrance might be increasing for both DR3/4 and non-DR3/4 genotypes, but with the much larger population proportion of non-DR3/4 individuals, the percentage DR3/4 decreases as the incidence of type 1 diabetes is increasing. Multiple environmental changes over the past few decades have been discussed as factors that might relate to the increasing incidence of diabetes, including exposure to infections in early life (enteroviruses) or decreasing frequency of infections (hygiene hypothesis) (18,19), eating habits and lifestyle modifications leading to increased obesity and insulin resistance (accelerator hypothesis) (20,21). Most of these environmental changes are most likely to change slowly and progressively with time.
Other factors that could influence decreasing DR3/4 frequencies over time include different methods of ascertainment of type 1 diabetes or decreasing DR3/4 frequencies over time in the general population. If detection of type 1 diabetes in very young children had increased in recent years through better ascertainment, we would expect an increase overall in DR3/4 frequency because subjects with younger age of onset are those with the highest percentage of DR3/4. Percentage of DR3/4 is low in the general Colorado population and did not change significantly over time (2.6% in 1945–1975 compared with 1.9% in 2002–2004).
Although triggers of type 1 diabetes are still unknown, it is clear that there is a long prodrome of islet autoimmunity preceding the onset of type 1 diabetes, and there is growing evidence that activation of innate immunity and inflammation can be associated with induction of islet autoimmunity in animal models. In rat strains with the equivalent of high-risk class II HLA alleles, islet autoimmunity leading to diabetes can be induced by infection with Kilham rat virus or even injections of poly-IC that induces interferon-α. In the Kilham infectious model, five injections of dexamethasone, beginning on the day of infection, completely blocks diabetes. Strikingly, a single dose of dexamethasone was sufficient to prevent islet destruction (22). This raises the possibility that targeting innate immune pathways at the initiation of islet autoimmunity may be a useful strategy for disease prevention.
Because so many environmental factors have changed in the last 4 decades, it would be helpful to narrow down the time frame of change for environmental exposures. For instance in the 1980s, given association with Reyes syndrome, aspirin therapy in children was acutely stopped in many countries including the U.S. with substitution of acetaminophen. Type 1 diabetes is not the only immune-mediated disease with an increasing incidence rate, and it has been suggested that changing anti-inflammatory therapy in children may relate to increasing incidence of asthma. There is epidemiological evidence that the use of acetaminophen may increase the risk of developing asthma (23) as well as rhinoconjunctivitis and eczema (24). Larger studies are needed to confirm the apparent stepwise changing pattern of genetic risk; if confirmed, this observation may help define critical environmental determinants of type 1 diabetes and eventually have direct bearing on therapies to prevent islet autoimmunity.
ACKNOWLEDGMENTS
This research uses resources provided by the T1DGC, a collaborative clinical study sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Diseases (NIAID), National Human Genome Research Institute (NHGRI), National Institute of Child Health and Human Development (NICHD), and Juvenile Diabetes Research Foundation International (JDRF), and was supported by NIDDK Grant U01 DK062418. This research was supported by National Institutes of Health grants DK320083 and DK057516. A.K.S. was supported by the JDRF Grant 11-2010-206 Early Career Patient-Oriented Diabetes Award.
No potential conflicts of interest relevant to this article were reported.
A.K.S. wrote the manuscript and contributed to discussion. T.K.A. researched data. S.R.B. researched data. G.S.E. contributed to discussion and reviewed and edited the manuscript.
REFERENCES
- 1.Gale EA. The rise of childhood type 1 diabetes in the 20th century. Diabetes 2002;51:3353–3361 [DOI] [PubMed] [Google Scholar]
- 2.Onkamo P, Vaananen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of Type I diabetes—the analysis of the data on published incidence trends. Diabetologia 1999;42:1395–1403 [DOI] [PubMed] [Google Scholar]
- 3.Vehik K, Hamman RF, Lezotte D, et al. Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes Care 2007;30:503–509 10.2337/dc06-1837 [DOI] [PubMed] [Google Scholar]
- 4.Hathout EH, Hartwick N, Fagoaga OR, et al. Clinical, autoimmune, and HLA characteristics of children diagnosed with type 1 diabetes before 5 years of age. Pediatrics 2003;111:860–863 [DOI] [PubMed] [Google Scholar]
- 5.Sheehy MJ, Scharf SJ, Rowe JR, et al. A diabetes-susceptible HLA haplotype is best defined by a combination of HLA-DR and -DQ alleles. J Clin Invest 1989;83:830–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noble JA, Valdes AM, Cook M, Klitz W, Thomson G, Erlich HA. The role of HLA class II genes in insulin-dependent diabetes mellitus: molecular analysis of 180 Caucasian, multiplex families. Am J Hum Genet 1996;59:1134–1148 [PMC free article] [PubMed] [Google Scholar]
- 7.Komulainen J, Kulmala P, Savola K, et al. Clinical, autoimmune, and genetic characteristics of very young children with type 1 diabetes: Childhood Diabetes in Finland (DiMe) Study Group. Diabetes Care 1999;22:1950–1955 10.2337/diacare.22.12.1950 [DOI] [PubMed] [Google Scholar]
- 8.Hermann R, Knip M, Veijola R, et al. Temporal changes in the frequencies of HLA genotypes in patients with Type 1 diabetes—indication of an increased environmental pressure? Diabetologia 2003;46:420–425 [DOI] [PubMed] [Google Scholar]
- 9.Rewers M, Bugawan TL, Norris JM, et al. Newborn screening for HLA markers associated with IDDM: Diabetes Autoimmunity Study in the Young (DAISY). Diabetologia 1996;39:807–812 [DOI] [PubMed] [Google Scholar]
- 10.Deschamps I, Boitard C, Hors J, et al. Life table analysis of the risk of type I (insulin-dependent) diabetes mellitus in siblings according to islet cell antibodies and HLA markers. An 8-year prospective study. Diabetologia 1992;35:951–957 [DOI] [PubMed] [Google Scholar]
- 11.Aly TA, Ide A, Jahromi MM, et al. Extreme genetic risk for type 1A diabetes. Proc Natl Acad Sci USA 2006;103:14074–14079 10.1073/pnas.0606349103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vehik K, Hamman RF, Lezotte D, et al. Trends in high-risk HLA susceptibility genes among Colorado youth with type 1 diabetes. Diabetes Care 2008;31:1392–1396 10.2337/dc07-2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillespie KM, Bain SC, Barnett AH, et al. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet 2004;364:1699–1700 10.1016/S0140-6736(04)17357-1 [DOI] [PubMed] [Google Scholar]
- 14.Fourlanos S, Varney MD, Tait BD, et al. The rising incidence of type 1 diabetes is accounted for by cases with lower-risk human leukocyte antigen genotypes. Diabetes Care 2008;31:1546–1549 10.2337/dc08-0239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erlich H, Valdes AM, Noble J, et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the Type 1 Diabetes Genetics Consortium families. Diabetes 2008;57:1084–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mychaleckyj JC, Noble JA, Moonsamy PV, et al. HLA genotyping in the international Type 1 Diabetes Genetics Consortium. Clin Trials 2010;7(Suppl. 1):S75–S87 10.1177/1740774510373494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Awa WL, Boehm BO, Kapellen T, et al. ; DPV-Wiss Study Group and the German Competence Network Diabetes Mellitus. HLA-DR genotypes influence age at disease onset in children and juveniles with type 1 diabetes mellitus. Eur J Endocrinol 2010;163:97–104 10.1530/EJE-09-0921 [DOI] [PubMed] [Google Scholar]
- 18.Gale EA. A missing link in the hygiene hypothesis? Diabetologia 2002;45:588–594 [DOI] [PubMed] [Google Scholar]
- 19.Peng H, Hagopian W. Environmental factors in the development of Type 1 diabetes. Rev Endocr Metab Disord 2006;7:149–162 10.1007/s11154-006-9024-y [DOI] [PubMed] [Google Scholar]
- 20.Fourlanos S, Narendran P, Byrnes GB, Colman PG, Harrison LC. Insulin resistance is a risk factor for progression to type 1 diabetes. Diabetologia 2004;47:1661–1667 [DOI] [PubMed] [Google Scholar]
- 21.Wilkin TJ. The accelerator hypothesis: weight gain as the missing link between Type I and Type II diabetes. Diabetologia 2001;44:914–922 [DOI] [PubMed] [Google Scholar]
- 22.Londono P, Komura A, Hara N, Zipris D. Brief dexamethasone treatment during acute infection prevents virus-induced autoimmune diabetes. Clin Immunol 2010;135:401–411 10.1016/j.clim.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 23.Etminan M, Sadatsafavi M, Jafari S, Doyle-Waters M, Aminzadeh K, Fitzgerald JM. Acetaminophen use and the risk of asthma in children and adults: a systematic review and metaanalysis. Chest 2009;136:1316–1323 10.1378/chest.09-0865 [DOI] [PubMed] [Google Scholar]
- 24.Beasley RW, Clayton TO, Crane J, et al. Acetaminophen use and risk of asthma, rhinoconjunctivitis and eczema in adolescents: ISAAC phase three. Am J Respir Crit Care Med. 13 August 2010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]