Abstract
OBJECTIVE
AMP-activated protein kinase (AMPK) signaling acts as a sensor of nutrients and hormones in the hypothalamus, thereby regulating whole-body energy homeostasis. Deletion of Ampkα2 in pro-opiomelanocortin (POMC) neurons causes obesity and defective neuronal glucose sensing. LKB1, the Peutz-Jeghers syndrome gene product, and Ca2+-calmodulin–dependent protein kinase kinase β (CaMKKβ) are key upstream activators of AMPK. This study aimed to determine their role in POMC neurons upon energy and glucose homeostasis regulation.
RESEARCH DESIGN AND METHODS
Mice lacking either Camkkβ or Lkb1 in POMC neurons were generated, and physiological, electrophysiological, and molecular biology studies were performed.
RESULTS
Deletion of Camkkβ in POMC neurons does not alter energy homeostasis or glucose metabolism. In contrast, female mice lacking Lkb1 in POMC neurons (PomcLkb1KO) display glucose intolerance, insulin resistance, impaired suppression of hepatic glucose production, and altered expression of hepatic metabolic genes. The underlying cellular defect in PomcLkb1KO mice involves a reduction in melanocortin tone caused by decreased α-melanocyte–stimulating hormone secretion. However, Lkb1-deficient POMC neurons showed normal glucose sensing, and body weight was unchanged in PomcLkb1KO mice.
CONCLUSIONS
Our findings demonstrate that LKB1 in hypothalamic POMC neurons plays a key role in the central regulation of peripheral glucose metabolism but not body-weight control. This phenotype contrasts with that seen in mice lacking AMPK in POMC neurons with defects in body-weight regulation but not glucose homeostasis, which suggests that LKB1 plays additional functions distinct from activating AMPK in POMC neurons.
AMP-activated protein kinase (AMPK) is an evolutionarily conserved guardian of both cellular and organismal energy status, regulating whole-body metabolism through multiple effects in peripheral tissues (1). Recently, AMPK has emerged as an important energy sensor and integrator of nutrient and hormonal signals in the hypothalamus, a key region for the regulation of whole-body energy homeostasis (2). We have generated mice deficient in the AMPKα2 catalytic subunit specifically in agouti-related protein (AgRP)- and pro-opiomelanocortin (POMC)-expressing neurons (POMCα2KO mice). These studies demonstrated a role for AMPK in both the acute responses of these neurons to nutrient signals and in long-term body-weight regulation (3), thereby implicating this signaling pathway in these critical neuronal components of the hypothalamic arcuate nucleus (ARC), which regulates food intake, energy expenditure, and glucose metabolism.
AMPK activity is allosterically regulated by 5′-AMP and by phosphorylation of the α-catalytic subunit. Two major upstream kinases have been identified: LKB1, the Peutz-Jeghers syndrome tumor-suppressor gene product (4,5), and Ca2+-calmodulin–dependent protein kinase kinases (CaMKKs) (6,7). Evidence is accumulating that these upstream kinases also may be involved in the regulation of energy homeostasis. For example, global deletion of Camkkβ in mice has been suggested to regulate food intake and body weight through the neuropeptide Y system (8). No insights were gained, however, into the role of CaMKKβ in POMC neurons. Global deletion of Lkb1 in mice is lethal, but tissue-specific gene targeting has implicated LKB1 in the regulation of glucose homeostasis in peripheral tissues. Deletion of Lkb1 in the adult liver results in hyperglycemia and lack of response to the antidiabetic effects of metformin (9), although recent data indicate that metformin acts independently of LKB1/AMPK (10). Disruption of Lkb1 in skeletal muscle has discordant physiological consequences: either no effect on whole-body glucose or energy balance while being key for exercise-stimulated muscle glucose uptake (11) or resulting in a paradoxical improvement in insulin sensitivity and glucose tolerance (12). In the adult pancreas, loss of LKB1 in β-cells led to increased β-cell mass, alterations in polarity, and enhanced glucose tolerance (13,14). Collectively, these studies suggest a role for LKB1 in glucose metabolism in peripheral tissues but have not investigated the role of hypothalamic LKB1 in whole-body energy homeostasis.
Here, we demonstrate that mice lacking CaMKKβ in POMC neurons have no defects in glucose or energy homeostasis. In contrast, mice with specific deletion of Lkb1 in POMC neurons display impaired hepatic glucose metabolism with an underlying reduction in hypothalamic α-−melanocyte–stimulating hormone (α-−MSH) release. Our findings indicate that LKB1 signaling plays a key role in the central melanocortinergic regulation of peripheral glucose homeostasis.
RESEARCH DESIGN AND METHODS
An expanded, more detailed section is available in the Supplemental Research Design and Methods.
Generation of null and conditional knockout mice.
The generation and genotyping of null Camkkβ, Camkkβ flox, Lkb1 flox, and POMC-Cre mice have been previously described (11,15,16). To generate mice lacking floxed alleles but expressing green fluorescent protein in cells harboring the deletion event, mice were intercrossed with Z/EG indicator mice (17). Mice were maintained on a 12-h light/dark cycle with free access to water and standard murine diet (RM1, 4% fat; Special Diet Services). Mice were handled and all in vivo studies performed in accordance to the Animal Scientific Procedures Act (1986).
Insulin sensitivity and hepatic glucose production.
Euglycemic-hyperinsulinemic clamps were performed as previously described (18).
Hypothalamic immunohistochemistry.
Hypothalamic immunohistochemistry was performed as previously described (19).
Quantitative RT-PCR analysis.
Quantitative RT-PCR was performed as previously described (19).
Hypothalamic explants.
Hypothalamic explant studies were performed as described (20). Mice were killed, and the whole brain was mounted with the ventral surface uppermost and placed in a vibrating microtome. A 2.0-mm slice was taken from the base of the brain and immediately transferred to artificial cerebrospinal fluid (aCSF) equilibrated with 95% O2/5% CO2 and maintained at 37°C. After an initial 2-h equilibration period, the hypothalami were incubated for 45 min in aCSF. The viability of the tissue was verified by a 45-min exposure to 56 mmol/L KCl. At the end of each period, the aCSF was frozen until it was assayed for α-MSH by radioimmunoassay (Phoenix Pharmaceuticals).
Experimental groups and statistical analysis.
Because of the existence of a hypomorphic phenotype (11), all relevant controls (wild-type, Cre+/−Lkb1+/+, and Cre−/−Lkb1fl/fl mice) were included in all the studies, unless otherwise stated. We did not observe differences between wild-type and Cre+/−Lkb1+/+ mice in any of the studies performed. Therefore, for clarity purposes, data from these two experimental groups were pooled and referred to as controls. Data are expressed as means ± SEM. P values were calculated using nonparametric (Mann-Whitney U test), paired, two-tailed and unpaired Student t tests and one-way ANOVA with post hoc Tukey tests, performed as appropriate. P values ≤0.05 were considered statistically significant.
RESULTS
Generation and characterization of POMC-deleted and global null Camkkβ mice.
Floxed Camkkβ mice (Supplementary Fig. 1A and B) were bred with mice expressing Cre recombinase in >90% of POMC neurons (16) to generate Cre+/−Camkkβfl/fl mice, which lack CaMKKβ in POMC neurons (hereafter referred to as PomcCamkkβKO). Deletion of Camkkβ in POMC neurons was restricted to the hypothalamus (Supplementary Fig. 2A). Hypothalamic architecture, neuron number, and distribution within the ARC were normal (Supplementary Fig. 2B–D). Male and female PomcCamkkβKO mutants exhibited normal energy homeostasis (Supplementary Figs. 3A–C and 4A–C and data not shown) and glucose handling (Supplementary Figs. 3D–F and 4D and Eand data not shown). We further explored the role of Camkkβ in energy homeostasis by studying Camkkβ global null mutants (15). Body weight, feeding behavior, and glucose metabolism were unaltered in both male and female Camkkβ global mice (Supplementary Fig. 5A–F and data not shown). Therefore, we undertook subsequent studies in male mice to compare our findings with the reported phenotypes of this strain in male animals (8). The response of these mice to the melanocortin 3/4 receptor agonist melanotan II (MT-II) also was normal (Supplementary Fig. 6A and B). Furthermore, their response to a low- or high-fat Surwit diet did not differ from wild-type littermates (Supplementary Fig. 6Cand data not shown). These results indicate that neither global nor POMC-specific deletion of Camkkβ impacts on whole-body energy balance and glucose metabolism.
Generation and validation of mice lacking LKB1 in POMC neurons.
In view of these findings, we next used floxed Lkb1 (11) and POMC Cre animals (16) to generate Cre+/−Lkb1fl/fl mice lacking LKB1 in POMC neurons (hereafter referred to as PomcLkb1KO). PCR for the recombination event demonstrated deletion of Lkb1 specifically in the hypothalamus (Fig. 1A). Immunostaining studies demonstrated the expression of LKB1 in 93 ± 3% of POMC neurons in control animals but only in 12 ± 3% of POMC neurons in PomcLkb1KO mice, indicating that the specific loss of LKB1 occurred with ~90% efficiency, as previously described for this allele (3,16) (Fig. 1B and C). Because of the nature of the targeting event, mice homozygous for the Lkb1 floxed allele (Cre−/−Lkb1fl/fl, hereafter referred to as hypomorphic) display reduced LKB1 activity in several tissues ([11] and data not shown). Together, the hypomorphic Lkb1 mutant line and mice further deleted for Lkb1 in POMC neurons provided a series of mutant animals permitting the examination of the effect of reduced LKB1 signaling and the specific role of LKB1 in POMC neurons in whole-body energy homeostasis and glucose metabolism.
FIG. 1.
Lkb1 deletion, neuron integrity, and hypothalamic anatomical studies in PomcLkb1KO mice. A: Detection of the deletion of the Lkb1 allele in PomcLkb1KO mice. DNA was extracted from different tissues (C, cerebral cortex; F, fat; H, heart; Hy, hypothalamus; K, kidney; L, liver; M, skeletal muscle) and recombination of the floxed Lkb1 allele detected by PCR. Recombination was only detected in the hypothalamus of PomcLkb1KO mice. Il-2 internal control PCR reaction also is shown. B: Immunofluorescence analysis for LKB1 (red) and POMC (green) expression in the hypothalami of control Cre+/−Lkb1+/+ZEG and PomcLkb1KO ZEG mice. LKB1 staining colocalized with POMC neurons in control sections (indicated by arrows) and reduced colocalization was seen in PomcLkb1KO sections. Confocal images of representative ARC fields are shown. C: Quantification of LKB1 loss in POMC neurons. A minimum of 225 neurons from 2–3 mice per group were analyzed. D: Representative images of hypothalamic sections. POMC staining (red) is shown. E: POMC neuron distribution throughout the ARC in control, hypomorphic, and PomcLkb1KO female mice (n = 2–3). 3V, third ventricle. Scale bars: 50 μm. Data are means ± SEM. **P < 0.01. (A high-quality digital representation of this figure is available in the online issue.)
Because LKB1 has been reported to regulate neuronal structure (21,22), we assessed the effect of deleting Lkb1 upon POMC neuronal anatomy and basic electrophysiological parameters. No obvious perturbations in the hypothalamic architecture were seen in PomcLkb1KO mice (Fig. 1D). POMC neurons from PomcLkb1KO mice exhibited normal number (control: 2,630 ± 185; hypomorphic: 2,834 ± 107; PomcLkb1KO: 2,869 ± 121), distribution, cell body structure, and POMC fiber anatomy (Fig. 1E and Supplementary Fig. 7A). In Lkb1-deficient POMC neurons, membrane potential (−54 ± 1 mV, n = 34), input resistance (2.2 ± 0.1 GΩ, n = 34), and spike firing frequency (2.4 ± 0.4 Hz, n = 34) were not different from those of control cells (3,19,23). The POMC-Cre transgene also is expressed in the pituitary, but both basal and stressed-induced plasma corticosterone levels were unaltered (Supplementary Fig. 7B). These results indicate normal POMC neuron development and anatomy and preserved hypothalamo-pituitary-adrenal axis function in PomcLkb1KO mice.
Deletion of Lkb1 in POMC neurons does not alter food intake and energy expenditure.
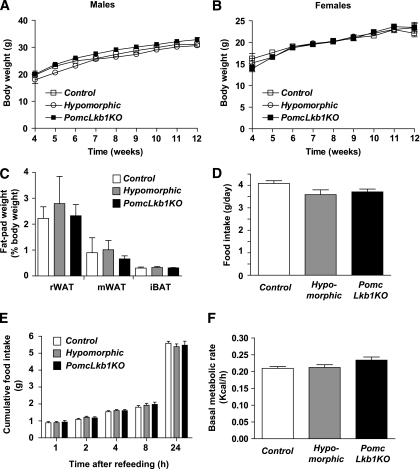
Body-weight profiles and fat-pad mass in male and female hypomorphic and PomcLkb1KO mice were normal (Fig. 2A–C and data not shown). Food intake, both under freely fed conditions and in response to an overnight fast (Fig. 2D and E), and basal metabolic rate (Fig. 2F) were not significantly different among the different experimental groups. Plasma leptin concentration, as well as content, in white adipose explants, and sensitivity to this hormone, was unaltered in PomcLkb1KO mice (data not shown).
FIG. 2.
Unaltered energy homeostasis in PomcLkb1KO mice. Weight curves of male (A) and female (B) control (n = 9–14), hypomorphic (n = 13–14), and PomcLkb1KO (n = 17–25) mice on a standard diet. C: Fat-pad weights in control, hypomorphic, and PomcLkb1KO female mice (n = 4–10). iBAT, interscapular brown adipose tissue; mWAT, mesenteric white adipose tissue; rWAT, reproductive white adipose tissue. Daily food intake under ad libitum conditions (n = 7–12) (D) and cumulative 24-h food intake after a fast-refeeding test in 10- to 11-week-old control, hypomorphic, and PomcLkb1KO female mice (n = 13–21) (E). F: Basal metabolic rate in control, hypomorphic, and PomcLkb1KO female mice (n = 7–12). Data are means ± SEM.
Deletion of Lkb1 in POMC neurons leads to sexually dimorphic alterations of glucose metabolism.
Fasting and random-fed blood glucose levels were not different in male (Supplementary Fig. 8A) or female mice from all experimental groups (Fig. 3A). Fasted plasma insulin levels also were equivalent (Fig. 3B). However, on glucose tolerance testing, female PomcLkb1KO mice exhibited impaired glucose handling (area under the curve in control mice: 992 ± 53 mmol/L per 120 min; hypomorphic mice: 1,046 ± 36 mmol/L per 120 min; PomcLkb1KO mice: 1,281 ± 40 mmol/L per 120 min; n = 9–17, P < 0.01 vs. hypomorphic, P < 0.001 vs. control) (Fig. 3C). In contrast, no alterations were found in male mice (Supplementary Fig. 8B).
FIG. 3.
Altered glucose metabolism in female PomcLkb1KO mice.Overnight-fasted and randomly fed blood glucose (n = 15–33) (A)and plasma insulin (B) levels in 12-week-old control, hypomorphic, and PomcLkb1KO female mice (n = 6–9). C: Glucose tolerance test performed in control (n = 16), hypomorphic (n = 9), and PomcLkb1KO (n = 16) female mice. At 30 min, **P < 0.01 vs. hypomorphic and ***P < 0.001 vs. control mice. At 60 minutes, **P < 0.01 vs. control and *P < 0.05 vs. hypomorphic mice. At 120 min, *P < 0.05 vs. control mice. D: Insulin tolerance test performed in control (n = 22), hypomorphic (n = 20), and PomcLkb1KO (n = 21) female mice. At 30, 60, and 120 min, **P < 0.01 vs. control and ***P < 0.001 vs. hypomorphic mice. E: Glucose turnover. F: Glycolysis rate. G: Whole-body glycogen synthesis in control, hypomorphic, and PomcLkb1KO female mice (n = 6–12) determined by euglycemic-hyperinsulinemic clamps. All data are means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Increased hepatic glucose production and expression of gluconeogenic genes in livers from PomcLkb1KO mice.
Defects in pancreatic β-cells or in peripheral metabolic tissues may cause abnormal glucose homeostasis. Pancreatic morphometric analysis revealed no alterations in islet architecture, β-cell area, or in vivo glucose-stimulated insulin secretion in PomcLkb1KO mice (Supplementary Fig. 9A–C). However, female PomcLkb1KO mice displayed a significant reduction of glucose clearance after an insulin tolerance test (area under the curve in control mice: 444 ± 21 mmol/L per 120 min; hypomorphic mice: 395 ± 19 mmol/L per 120 min; PomcLkb1KO mice: 541 ± 21 mmol/L per 120 min; n = 20–22, P < 0.01 vs. hypomorphic, P < 0.001 vs. control) (Fig. 3D), suggesting insulin resistance. We next assessed whole-body insulin sensitivity by euglycemic-hyperinsulinemic clamp studies using insulin concentrations of 18 mU ⋅ kg−1 ⋅ min−1 (18). Whole-body glucose disposal was significantly reduced in female PomcLkb1KO mice (Fig. 3E). This alteration was not associated with changes in the rate of glycolysis (Fig. 3F), but whole-body glycogen synthesis was reduced (Fig. 3G). Together, these results demonstrate that the deletion of Lkb1 in POMC neurons alters peripheral glucose metabolism. Consistent with previous reports (11), widespread partial knockdown of Lkb1 did not impact on whole-body glucose homeostasis (Fig. 3A–G).
Hepatic glucose production (HGP) by gluconeogenesis is a key metabolic pathway involved in glucose homeostasis and has been suggested to be under neuronal control by melanocortin pathways (24–26). We assessed HGP initially by administrating pyruvate, a gluconeogenic substrate, and observed that blood glucose levels were significantly higher in PomcLkb1KO mice (Fig. 4A), suggesting enhanced gluconeogenesis. We next measured HGP by euglycemic-hyperinsulinemic clamp studies using insulin concentrations of 4 mU ⋅ kg−1 ⋅ min−1 (18). PomcLkb1KO mice exhibited significantly increased HGP (Fig. 4B). These results demonstrate that the lack of LKB1 in POMC neurons impairs the ability of insulin to suppress hepatic gluconeogenesis.
FIG. 4.
Deletion of Lkb1 in POMC neurons leads to increased HGP and gluconeogenic gene expression levels. A: Pyruvate tolerance test in control (n = 13), hypomorphic (n = 6), and PomcLkb1KO (n = 6) female mice. At 60 min, *P < 0.05 vs. control and hypomorphic mice. B: Assessment of HGP by euglycemic-hyperinsulinemic clamp in control, hypomorphic, and PomcLkb1KO female mice (n = 4–7). C: Expression analysis of key hepatic genes involved in glucose metabolism and transcriptional regulation in control, hypomorphic, and PomcLkb1KO female mice (n = 6–22) assessed by quantitative RT-PCR and expressed relative to controls (dashed line). Probes for either glyceraldehyde-3-phosphate dehydrogenase (Gapdh) or hypoxanthine guanine phosphoribosyl transferase (Hprt) were used to adjust for total RNA content. D and E: Lkb1 mRNA expression in the hypothalamus from control female (D) and male (E) mice fed with a standard diet or HFD. All data are means ± SEM. *P < 0.05; ***P < 0.001.
mRNA levels of key gluconeogenic enzymes, such as glucose 6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (Pepck), were increased in the liver of PomcLkb1KO female mice (Fig. 4C). mRNA levels of suppressor of cytokine signaling-3 (Socs-3) and peroxisome proliferator–activated receptor-α (Ppar-α) also were significantly upregulated in the liver of these animals (Fig. 4C). However, liver interleukin-6 (Il-6) mRNA expression, which has been implicated in the central regulation of hepatic gluconeogenesis by insulin (27), was unaltered (Fig. 4C). Likewise, no abnormalities in the expression of glucokinase (Gck), diacylglycerol acyltransferase-2 (Dgat-2), forkhead transcription factor-1 (Foxo1), hepatic nuclear factor 4-α (Hnf4-α), and PPAR-γ coactivator-1α (Pgc-1α) were observed (Fig. 4C). Expression of a panel of metabolic genes in white adipose tissue (Glut-4, lipoprotein lipase [Lpl], Pepck, and Ppar-γ) and skeletal muscle (Foxo1, Glut-4, Il-6, and Pgc1-α) was not altered in PomcLkb1KO mice (Supplementary Fig. 10A and B). Collectively, these data indicate that Lkb1 deletion in POMC neurons impairs the regulation of HGP and alters gene expression of the key enzymes involved in gluconeogenesis.
Interestingly, Lkb1 mRNA was significantly reduced in the hypothalami from high-fat diet (HFD)-fed female mice when compared with standard diet–fed counterparts (Fig. 4D). Consistent with the sexual dimorphic phenotype, hypothalamic Lkb1 expression was equivalent in male mice fed with either the standard or HFD (Fig. 4E).
POMC neurons lacking LKB1 exhibit normal glucose sensing.
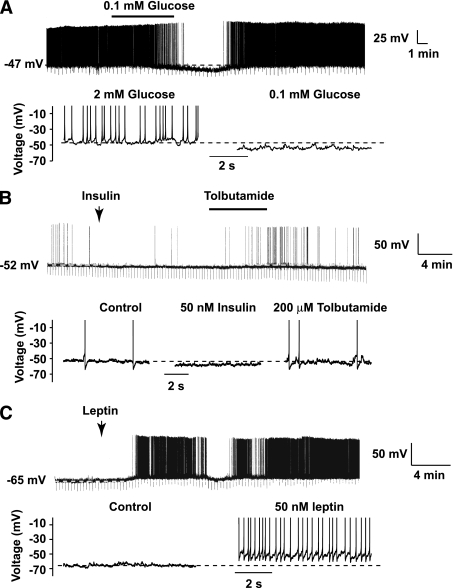
Deletion of Lkb1 in POMC neurons could impair peripheral glucose homeostasis via different mechanisms, including alteration in the responses of these neurons to nutrient and hormonal inputs, alterations in intrinsic signaling mechanisms such as AMPK, and reduced melanocortin output. Given that the majority of POMC neurons express LKB1 and that POMC neurons lacking AMPKα2 fail to respond to changes in extracellular glucose concentration (3), we next examined the electrophysiological properties of PomcLkb1KO neurons. However, the majority of PomcLkb1KO neurons responded normally to a reduction in extracellular glucose from 2 to 0.1 mmol/L by reversible hyperpolarization (−11.9 ± 3.6 mV, n = 7 of 10 neurons; P < 0.05) (Fig. 5A), which was associated with a reduction in spike firing frequency (2.3 ± 0.6 Hz vs. 0.4 ± 0.3 Hz, n = 7 of 10 neurons; P < 0.05) (Fig. 5A). Insulin and leptin action upon melanocortin circuits also has been implicated in the regulation of peripheral glucose homeostasis. However, similar to control POMC neurons, insulin hyperpolarized a subpopulation of PomcLkb1KO neurons by −5.4 ± 1.7 mV (n = 5 of 12 neurons; P < 0.05) (Fig. 5B), and this was subsequently occluded by bath-applied tolbutamide (200 μmol/L), suggesting the activation of ATP-sensitive K+ channels (Fig. 5B). Like control POMC neurons, leptin depolarized a proportion of PomcLkb1KO neurons by +7.3 ± 2.4 mV (n = 7 of 12 neurons; P < 0.05) (Fig. 5C).
FIG. 5.
POMC neurons lacking LKB1 are glucose sensitive and respond to anorexigenic hormones. Current-clamp recordings were made using the perforated-patch technique from PomcLkb1KO ARC neurons. Uninterrupted current traces are above and expanded sections shown underneath. A: Reducing glucose concentration from 2 to 0.1 mmol/L reversibly hyperpolarized and reduced the firing frequency of POMC neurons lacking LKB1. LKB1-deficient POMC neurons responded normally to insulin (B) and leptin (C) by long-lasting membrane hyperpolarization and depolarization, respectively. Note that insulin-induced hyperpolarization was occluded by bath-applied tolbutamide (200 μmol/L).
The lack of a defect in POMC neuron glucose sensing, together with the phenotypic differences between PomcLkb1KO and POMCα2KO mice (3), suggested that altered AMPK activity in LKB1-deficient POMC neurons did not underlie the phenotype. Consistent with this, we did not detect a significant reduction in AMPKα2 activity in the hypothalami of PomcLkb1KO mice (percentage of control: hypomorphic mice, 108 ± 4%; PomcLkb1KO mice, 87 ± 10%; n = 7–17; P = NS). Hypothalamic CaMKKβ activity also was unchanged (control mice: 1.49 ± 0.10 mmol32p ⋅ mg AMPK−1 ⋅ min−1; PomcLkb1KO mice: 1.31 ± 0.13 mmol32p ⋅ mg AMPK−1 ⋅ min−1; n = 3; P = NS). Together, the phenotypic differences between PomcLkb1KO and POMCα2KO mice and the normal hypothalamic AMPKα2 activity in PomcLkb1KO mice suggest that the defect in glucose homeostasis is not a result of either impaired glucose sensing in POMC neurons or reduced AMPK activity.
Enhanced melanocortin agonist sensitivity altered expression of hypothalamic melanocortin system genes but reduced α-MSH release in PomcLkb1KO mice.
PomcLkb1KO mice displayed increased sensitivity to the anorectic effects of acute MT-II administration (Fig. 6A), suggesting reduced melanocortin tone. To investigate the basis of this abnormality, which could be attributed to either reduced production or release of α-MSH, we performed a quantitative RT-PCR analysis of key components involved in hypothalamic melanocortin circuits. Although Agrp, Npy, and cocaine- and amphetamine-regulated transcript (Cart) mRNA levels were equivalent among the different experimental groups (Fig. 6B), expression of Pomc mRNA was significantly upregulated in PomcLkb1KO mice (Fig. 6B). The enhanced melanocortin agonist sensitivity and increased hypothalamic pomc mRNA levels found in PomcLkb1KO mice could reflect a compensatory response to reduced α-MSH peptide translation and/or release. To assess this hypothesis, we performed ex vivo α-MSH secretion studies in hypothalamic explants. Under basal conditions, hypothalamic slices from PomcLkb1KO mice exhibited significantly reduced α-MSH secretion (Fig. 6C). In contrast, KCl stimulation, which triggers the release of all stored α-MSH, was equivalent in all experimental groups (percentage of control: hypomorphic mice, 125 ± 19%; PomcLkb1KO mice: 103 ± 25%; n = 4–6; P = NS). To further investigate these findings, we analyzed α-MSH peptide expression in the paraventricular nucleus of the hypothalamus (PVH) by immunohistochemistry. Sections from PomcLkb1KO mice displayed reduced α-MSH staining in neuronal projections to the PVH (Fig. 6D), suggesting that, at the site of α-MSH release, there was less peptide present.
FIG. 6.
Enhanced melanocortin pathway sensitivity and reduced α-MSH release in hypothalamic explants from female PomcLkb1KO mice. A: Food intake after an acute (2-h) MT-II administration in control, hypomorphic, and PomcLkb1KO female mice (n = 14–18). The data are expressed relative to each group of mice injected with vehicle (dashed line). B: Expression analysis of melanocortin system hypothalamic genes assessed by quantitative RT-PCR in control, hypomorphic, and PomcLkb1KO female mice (n = 7–10). Data are expressed relative to controls (dashed line). C: Basal α-MSH release in hypothalamic explants from control, hypomorphic, and PomcLkb1KO female mice (n = 10–12). D: Representative α-MSH staining of PVH from control, hypomorphic, and PomcLkb1KO female mice. α-MSH–integrated density quantification also is shown. E: Expression analysis of relevant hypothalamic genes assessed by quantitative RT-PCR in control, hypomorphic, and PomcLkb1KO female mice (n = 7–10). Data are expressed relative to controls (dashed line). Probes for Hprt were used to adjust for total RNA content. All data are means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. (A high-quality digital representation of this figure is available in the online issue.)
It recently has been reported that deletion of Foxo1 in POMC neurons increases carboxypeptidase E (cpe) expression, leading to increased hypothalamic α-MSH (28). However, neither Foxo1 nor cpe hypothalamic mRNA levels were changed in PomcLkb1KO mice (data not shown), indicating that the defective α-MSH release observed is not caused by alterations in the Foxo1-cpe pathway. However, the hypothalamic expression of proprotein convertase 1/3 (Pc1/3), an enzyme involved in the posttranslational POMC processing and α-MSH production (29), was significantly increased in PomcLkb1KO mice (Fig. 6E). Expression of Mcr4, the cognate receptor for α-MSH, also was upregulated in PomcLkb1KO mice (Fig. 6E), suggesting a compensatory response (or loss of ligand-mediated receptor downregulation) and provided additional indirect evidence for reduced α-MSH release.
Collectively, these results suggest that the deletion of Lkb1 in POMC neurons results in impaired α-MSH secretion, which in turn reduces central melanocortin tone, an abnormality that has been implicated as playing an important role in the pathogenesis of insulin resistance and type 2 diabetes.
DISCUSSION
Here, we reveal a significant role for LKB1 in hypothalamic POMC neurons in the regulation of glucose homeostasis. Female, but not male, mice lacking LKB1 in POMC neurons displayed glucose intolerance, insulin resistance, impaired suppression of HGP, and altered expression of hepatic metabolic genes. Our results indicate that the underlying cellular defect in PomcLkb1KO mice involves a reduction in melanocortin tone caused by decreased α-MSH secretion. This, in turn, was reflected in increased sensitivity to an exogenous melanocortin agonist and compensatory responses in molecular components of the melanocortin system. Our findings are in agreement with the reported role of the hypothalamic melanocortinergic system in the regulation of peripheral glucose metabolism via effects on hepatic metabolism (24,26) and now demonstrate that LKB1 is a molecular component of such mechanisms.
Sexually dimorphic differences in metabolic phenotypes previously have been shown for a number of other hypothalamic mutants, including mice with deletion of specific signaling molecules in POMC neurons (30–33). Although the exact underlying causes remain unknown, these observations suggest that different genes in specific cell populations are involved in the development of metabolic disorders depending on the sex of the animal. Consistent with a sexually dimorphic role for POMC LKB1 in energy homeostasis, we also found that HFD reduces hypothalamic Lkb1 expression in female, but not male, mice. Although male hypomorphic LKB1 mice are infertile because of a defect in spermatogenesis (34), their reproductive behavior is normal and female PomcLkb1KO mice are completely fertile (M.C. and A.Wo., unpublished observations). Together, these observations suggest that defective reproductive function is unlikely to account for the sexually dimorphic metabolic phenotype. This, in turn, is more likely to reflect, as is increasingly being recognized, that both distinct and overlapping pathways in each sex are involved in the complex physiology of metabolic regulation and the pathophysiology of metabolic disorders (35).
The study of hypomorphic phenotypes can provide useful information on the role of a particular protein or highlight previously unknown functions (36,37). Widespread partial loss of function also may be useful for modeling the potential effects of pharmacological manipulation of a signaling pathway. Although widespread reduction of LKB1 expression could theoretically contribute to the observed phenotype, exhaustive phenotyping of hypomorphic mice revealed no effects on whole-body metabolism, suggesting that complete loss of LKB1 in POMC neurons is required to develop the defects in glucose handling. In contrast to the defective glucose homeostasis, we found no alterations in body-weight regulation and feeding behavior in PomcLkb1KO mice. These results are consistent with evidence indicating that central melanocortin signaling regulates systemic insulin sensitivity and energy balance via independent, but complementary, mechanisms (24,38,39). Alternatively, the enhanced melanocortin sensitivity observed in PomcLkb1KO mice could compensate for the inadequate levels of the ligand α-MSH. This also could explain the lack of an energy homeostasis phenotype in these mice despite reduced levels of α-MSH.
The phenotype observed in PomcLkb1KO mice could arise from disruption of AMPK signaling in POMC neurons. However, several features of the PomcLkb1KO mice, when compared with those seen in mice lacking Ampkα2 in POMC neurons (3), suggest that this is not the case. First, at a whole-body level the energy homeostasis phenotypes of PomcLkb1KO and POMCα2KO mice are distinct, with the former displaying defects in glucose homeostasis but not body-weight regulation, whereas the latter have essentially the opposite phenotype (3). Second, AMPKα2-deficient neurons are unable to sense a reduction in external glucose concentrations (3), whereas this response was preserved in PomcLkb1KO mice. Third, α-MSH release from LKB1-deficient POMC neurons is defective, a phenotype absent in POMCα2KO mice (3). Together, these findings demonstrate that the deletion of Lkb1 or Ampkα2 in POMC neurons has different effects on energy and glucose homeostasis, suggesting that the phenotype of PomcLkb1KO mice was not a result of the loss of AMPK signaling. Consistent with this, and in agreement with other conditional Lkb1 mutants (22), we were unable to detect reduced hypothalamic AMPK activity in PomcLkb1KO mice. It is, of course, possible that CaMKKβ compensates for the loss of LKB1, thereby preserving AMPK activity. However, the absence of metabolic phenotype in PomcCamkkβKO mice suggests that this molecule does not play a significant role in this cell type and that compensation is unlikely. Indeed, our studies with mice globally lacking CaMKKβ fail to confirm a major role for this kinase in whole-body energy homeostasis regulation. The reasons for this discrepancy with previous findings (8) are not clear but could include differences in the genetic background or the gene-targeting event.
The phenotypic differences between POMCα2KO (3) and PomcLkb1KO mice are in line with recent studies showing that pancreatic β-cell deletion of Ampk (40,41) or Lkb1 (13,14) lead to strongly divergent phenotypes. These observations suggest that LKB1 may play additional roles distinct from activating AMPK. For example, LKB1 also acts as a master upstream kinase for a number of AMPK-related kinases (42). Of these, the salt-inducible kinases (SIKs) have been implicated in the regulation of energy metabolism, and, in particular, SIK2 has been shown to regulate hepatic glucose metabolism (43). LKB1 also regulates the brain-specific kinases 1 and 2 (44), which have been implicated in the development of neuronal polarization in the cerebral cortex (22,45). However, we were unable to detect morphological or electrophysiological abnormalities in LKB1-deficient POMC neurons, suggesting that Lkb1 deletion does not have major effects on POMC neuron anatomy and function. An alternative explanation for the apparent lack of consistency between AMPK and LKB1 mutant mice phenotypes would be that either kinase has additional roles independent of their kinase activity, such as scaffolding or other structural-dependent functions.
A number of additional conclusions can be drawn from our findings in mice lacking either LKB1 or AMPKα2 in POMC neurons. First, the effects on peripheral glucose homeostasis do not correlate with defects in glucose sensing by POMC neurons. Therefore, as suggested by others (46), it is unlikely that glucose sensing, per se, in this neuronal population is required for the regulation of systemic glucose metabolism. Our findings also add further weight to the idea that there are divergent pathways in the regulation of feeding behavior and energy expenditure compared with the regulation of peripheral glucose homeostasis in POMC neurons. Such differences in melanocortin signaling have previously been demonstrated for food intake and energy expenditure (47). The cellular basis of such distinctions remains undetermined but may be attributed to the heterogeneity observed in POMC neurons, not only in their responses to hormones and nutrients but also in their outputs (3,19,23,30,48). The presence or absence of particular signaling cascades in POMC neurons may provide a molecular underpinning for such findings.
In summary, we find that LKB1, but not CaMKKβ, plays a key role in POMC neurons and that deletion of this molecule in this cell type disrupts hypothalamic melanocortin function leading to defective peripheral glucose homeostasis. Our results provide further understanding into the mechanisms involved in the pathogenesis of disordered glucose metabolism and may give new insights for the development of potential therapeutic agents for type 2 diabetes.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Biotechnology and Biological Sciences Research Council (to D.J.W.), the Medical Research Council (to D.J.W., R.L.B., K.P.G., and D.C.), the Wellcome Trust (to D.J.W., K.P.G., and M.L.A.), and the European Commission (contract no. LSHM-CT-2004-005272; to D.C.). M.C. is a recipient of a Miguel Servet contract (CP09/00233) from the Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación (Spain).
No potential conflicts of interest relevant to this article were reported.
M.C. and M.A.S. designed experiments, researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript. C.K. researched data, contributed to discussion, and reviewed and edited the manuscript. H.A.-Q. researched data and reviewed and edited the manuscript. A.Wo. researched data, contributed to discussion, and reviewed and edited the manuscript. A.H., K.P., J.J.E., A.C., P.V., P.D.C., and G.B. researched data and reviewed and edited the manuscript. A.Wh. researched data, contributed to discussion, and reviewed and edited the manuscript. P.M. researched data and reviewed and edited the manuscript. M.P. and K.M. provided reagents and reviewed and edited the manuscript. R.L.B. researched data, contributed to discussion, and reviewed and edited the manuscript. K.P.G. and A.A. provided reagents and reviewed and edited the manuscript. R.B. researched data, contributed to discussion, and reviewed and edited the manuscript. M.L.A. and D.C. contributed to discussion and reviewed and edited the manuscript. D.J.W. designed experiments, researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript.
The authors are grateful to Dr. Gregory S. Barsh (Standford University) for providing the POMC-cre mouse line. The authors also thank Dr. Mariona Graupera (Queen Mary University of London), Dr. Benoit Billotet (Queen Mary University of London), Francisco M. Vega (King’s College London), and members of Withers’ Laboratory for help and advice.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-1055/-/DC1.
REFERENCES
- 1.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest 2006;116:1776–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 2005;1:15–25 [DOI] [PubMed] [Google Scholar]
- 3.Claret M, Smith MA, Batterham RL, et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest 2007;117:2325–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawley SA, Boudeau J, Reid JL, et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol 2003;2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woods A, Johnstone SR, Dickerson K, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol 2003;13:2004–2008 [DOI] [PubMed] [Google Scholar]
- 6.Woods A, Dickerson K, Heath R, et al. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab 2005;2:21–33 [DOI] [PubMed] [Google Scholar]
- 7.Hawley SA, Pan DA, Mustard KJ, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2005;2:9–19 [DOI] [PubMed] [Google Scholar]
- 8.Anderson KA, Ribar TJ, Lin F, et al. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab 2008;7:377–388 [DOI] [PubMed] [Google Scholar]
- 9.Shaw RJ, Lamia KA, Vasquez D, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005;310:1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foretz M, Hébrard S, Leclerc J, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest 2010;120:2355–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakamoto K, McCarthy A, Smith D, et al. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J 2005;24:1810–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koh HJ, Arnolds DE, Fujii N, et al. Skeletal muscle-selective knockout of LKB1 increases insulin sensitivity, improves glucose homeostasis, and decreases TRB3. Mol Cell Biol 2006;26:8217–8227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu A, Ng AC, Depatie C, et al. Loss of Lkb1 in adult beta cells increases beta cell mass and enhances glucose tolerance in mice. Cell Metab 2009;10:285–295 [DOI] [PubMed] [Google Scholar]
- 14.Granot Z, Swisa A, Magenheim J, et al. LKB1 regulates pancreatic beta cell size, polarity, and function. Cell Metab 2009;10:296–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters M, Mizuno K, Ris L, Angelo M, Godaux E, Giese KP. Loss of Ca2+/calmodulin kinase kinase beta affects the formation of some, but not all, types of hippocampus-dependent long-term memory. J Neurosci 2003;23:9752–9760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest 2005;115:951–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis 2000;28:147–155 [PubMed] [Google Scholar]
- 18.Simmgen M, Knauf C, Lopez M, et al. Liver-specific deletion of insulin receptor substrate 2 does not impair hepatic glucose and lipid metabolism in mice. Diabetologia 2006;49:552–561 [DOI] [PubMed] [Google Scholar]
- 19.Choudhury AI, Heffron H, Smith MA, et al. The role of insulin receptor substrate 2 in hypothalamic and beta cell function. J Clin Invest 2005;115:940–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Qassab H, Smith MA, Irvine EE, et al. Dominant role of the p110beta isoform of PI3K over p110alpha in energy homeostasis regulation by POMC and AgRP neurons. Cell Metab 2009;10:343–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shelly M, Cancedda L, Heilshorn S, Sumbre G, Poo MM. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell 2007;129:565–577 [DOI] [PubMed] [Google Scholar]
- 22.Barnes AP, Lilley BN, Pan YA, et al. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell 2007;129:549–563 [DOI] [PubMed] [Google Scholar]
- 23.Smith MA, Hisadome K, Al-Qassab H, Heffron H, Withers DJ, Ashford ML. Melanocortins and agouti-related protein modulate the excitability of two arcuate nucleus neuron populations by alteration of resting potassium conductances. J Physiol 2007;578:425–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obici S, Feng Z, Tan J, Liu L, Karkanias G, Rossetti L. Central melanocortin receptors regulate insulin action. J Clin Invest 2001;108:1079–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutiérrez-Juárez R, Obici S, Rossetti L. Melanocortin-independent effects of leptin on hepatic glucose fluxes. J Biol Chem 2004;279:49704–49715 [DOI] [PubMed] [Google Scholar]
- 26.Demuro G, Obici S. Central nervous system and control of endogenous glucose production. Curr Diab Rep 2006;6:188–193 [DOI] [PubMed] [Google Scholar]
- 27.Inoue H, Ogawa W, Asakawa A, et al. Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab 2006;3:267–275 [DOI] [PubMed] [Google Scholar]
- 28.Plum L, Lin HV, Dutia R, et al. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nat Med 2009;15:1195–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pritchard LE, White A. Neuropeptide processing and its impact on melanocortin pathways. Endocrinology 2007;148:4201–4207 [DOI] [PubMed] [Google Scholar]
- 30.Plum L, Ma X, Hampel B, et al. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest 2006;116:1886–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi H, Strader AD, Sorrell JE, Chambers JB, Woods SC, Seeley RJ. Sexually different actions of leptin in proopiomelanocortin neurons to regulate glucose homeostasis. Am J Physiol Endocrinol Metab 2008;294:E630–E639 [DOI] [PubMed] [Google Scholar]
- 32.Xu AW, Ste-Marie L, Kaelin CB, Barsh GS. Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (Pomc) neurons causes decreased pomc expression, mild obesity, and defects in compensatory refeeding. Endocrinology 2007;148:72–80 [DOI] [PubMed] [Google Scholar]
- 33.Hill JW, Elias CF, Fukuda M, et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab 2010;11:286–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Towler MC, Fogarty S, Hawley SA, et al. A novel short splice variant of the tumour suppressor LKB1 is required for spermiogenesis. Biochem J 2008;416:1–14 [DOI] [PubMed] [Google Scholar]
- 35.Brown LM, Gent L, Davis K, Clegg DJ. Metabolic impact of sex hormones on obesity. Brain Res 2010;1350:77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawlor MA, Mora A, Ashby PR, et al. Essential role of PDK1 in regulating cell size and development in mice. EMBO J 2002;21:3728–3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pollizzi K, Malinowska-Kolodziej I, Doughty C, et al. A hypomorphic allele of Tsc2 highlights the role of TSC1/TSC2 in signaling to AKT and models mild human TSC2 alleles. Hum Mol Genet 2009;18:2378–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan W, Dinulescu DM, Butler AA, Zhou J, Marks DL, Cone RD. The central melanocortin system can directly regulate serum insulin levels. Endocrinology 2000;141:3072–3079 [DOI] [PubMed] [Google Scholar]
- 39.Heijboer AC, van den Hoek AM, Pijl H, et al. Intracerebroventricular administration of melanotan II increases insulin sensitivity of glucose disposal in mice. Diabetologia 2005;48:1621–1626 [DOI] [PubMed] [Google Scholar]
- 40.Sun G, Tarasov AI, McGinty J, et al. Ablation of AMP-activated protein kinase alpha1 and alpha2 from mouse pancreatic beta cells and RIP2.Cre neurons suppresses insulin release in vivo. Diabetologia 2010;53:924–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beall C, Piipari K, Al-Qassab H, et al. Loss of AMP-activated protein kinase alpha2 subunit in mouse beta-cells impairs glucose-stimulated insulin secretion and inhibits their sensitivity to hypoglycaemia. Biochem J 2010;429:323–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lizcano JM, Göransson O, Toth R, et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J 2004;23:833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dentin R, Liu Y, Koo SH, et al. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature 2007;449:366–369 [DOI] [PubMed] [Google Scholar]
- 44.Bright NJ, Carling D, Thornton C. Investigating the regulation of brain-specific kinases 1 and 2 by phosphorylation. J Biol Chem 2008;283:14946–14954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kishi M, Pan YA, Crump JG, Sanes JR. Mammalian SAD kinases are required for neuronal polarization. Science 2005;307:929–932 [DOI] [PubMed] [Google Scholar]
- 46.Levin BE. Neuronal glucose sensing: still a physiological orphan? Cell Metab 2007;6:252–254 [DOI] [PubMed] [Google Scholar]
- 47.Balthasar N, Dalgaard LT, Lee CE, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 2005;123:493–505 [DOI] [PubMed] [Google Scholar]
- 48.Hisadome K, Smith MA, Choudhury AI, Claret M, Withers DJ, Ashford ML. 5-HT inhibition of rat insulin 2 promoter Cre recombinase transgene and proopiomelanocortin neuron excitability in the mouse arcuate nucleus. Neuroscience 2009;159:83–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.