Abstract
OBJECTIVE
We have previously shown that serum insulin levels decrease threefold and blood glucose levels remain normal in mice fed a leucine-deficient diet, suggesting increased insulin sensitivity. The goal of the current study is to investigate this possibility and elucidate the underlying cellular mechanisms.
RESEARCH DESIGN AND METHODS
Changes in metabolic parameters and expression of genes and proteins involved in regulation of insulin sensitivity were analyzed in mice, human HepG2 cells, and mouse primary hepatocytes under leucine deprivation.
RESULTS
We show that leucine deprivation improves hepatic insulin sensitivity by sequentially activating general control nonderepressible (GCN)2 and decreasing mammalian target of rapamycin/S6K1 signaling. In addition, we show that activation of AMP-activated protein kinase also contributes to leucine deprivation–increased hepatic insulin sensitivity. Finally, we show that leucine deprivation improves insulin sensitivity under insulin-resistant conditions.
CONCLUSIONS
This study describes mechanisms underlying increased hepatic insulin sensitivity under leucine deprivation. Furthermore, we demonstrate a novel function for GCN2 in the regulation of insulin sensitivity. These observations provide a rationale for short-term dietary restriction of leucine for the treatment of insulin resistance and associated metabolic diseases.
Insulin resistance is a common feature of many metabolic diseases, including type 2 diabetes and nonalcoholic fatty liver diseases. The hallmark of insulin resistance is reduced glucose uptake in muscle and adipose tissue, and increased glucose production in liver (1,2). Various strategies have been proposed to treat insulin resistance, including lifestyle modifications and pharmacologic interventions (3,4).
Recently, there has been a growing interest in treating insulin resistance with dietary manipulation of micronutrients, including branched-chain amino acids (BCAAs). The BCAAs comprise the three essential amino acids having nonlinear aliphatic side chains: leucine, isoleucine, and valine. These amino acids not only serve as precursors in protein synthesis but also play regulatory roles in intracellular signaling (5).
Several studies have shown that dietary supplementation of leucine influences insulin sensitivity. For example, Zhang and colleagues (6) recently demonstrated that increased oral intake of leucine improves whole-body glucose metabolism in mice maintained on a high-fat diet (HFD). The effect of increasing dietary leucine, however, is controversial. For example, other studies reported that increased serum levels of leucine have no effect (7) or increase insulin resistance in humans and in animal models of obesity (8,9).
By contrast, our research has focused on the effect of eliminating leucine from the diet. As shown in our previous study (10), serum insulin levels decrease threefold in mice fed a leucine-deficient [(−) leu] diet. Blood glucose levels remain normal in these mice, however, suggesting increased insulin sensitivity. The goal of our current study is to investigate this possibility and elucidate the underlying molecular and cellular mechanisms.
In our current study, we observed that leucine deprivation increases whole-body insulin sensitivity and insulin signaling in liver. Furthermore, we show that leucine deprivation improves hepatic insulin sensitivity by sequentially activating general control nonderepressible (GCN)2 and decreasing mammalian target of rapamycin (mTOR) signaling. In addition, we show that activation of AMP-activated protein kinase (AMPK) also contributes to increased hepatic insulin sensitivity under leucine deprivation. Finally, we show that leucine deprivation improves insulin sensitivity under insulin-resistant conditions.
RESEARCH DESIGN AND METHODS
Chemicals and plasmids.
Insulin and rapamycin were from Sigma and Tauto Biotech (Shanghai, China), respectively. The vector p-CAGGS-DN-AMPK-IRES-EGFP encoding dominant-negative AMPK protein was a gift from Dr. Jia Li, Shanghai Institute of Materia Medica, China. The HA-tagged constitutively active S6K1 expression plasmid was the Addgene plasmid 8991, originally from John Blenis’s laboratory. Effectene Transfection Reagent was from Qiagen (Hilden, Germany).
Animals and treatment.
Male C57BL/6J mice were obtained from Shanghai Laboratory Animal Co., Ltd. (Shanghai, China). GCN2 knockout (Gcn2−/−) and leptin receptor–deficient (db/db) mice were provided by Dr. Douglas Cavener, Penn State University, and Dr. Xiang Gao, Nanjing University, China, respectively. Eight- to ten-week-old mice were maintained on a 12-h light/dark cycle at 25°C. Control (nutritionally complete amino acid) and (−) leu diets were obtained from Research Diets, Inc. (New Brunswick, NJ). All diets were isocaloric and compositionally the same in terms of carbohydrate and lipid component. At the start of the feeding experiments, mice were acclimated to a control diet for 7 days and then randomly divided into control and (−) leu diet groups, with free access to control and (−) leu diets, respectively, for 7 days. In addition, a pair-fed group was included to distinguish the possible influence of the minor reduction in food intake, as previously observed in the (−) leu group (11). A subset of mice underwent glucose tolerance tests (GTTs) and insulin tolerance tests (ITTs) before they were killed by CO2 inhalation. These experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Institute for Nutritional Sciences, Shanghai Institute for Biological Sciences, Chinese Academy of Sciences.
Cell culture and treatments.
HepG2 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 25 mmol/L glucose (Gibco, Invitrogen, Carlsbad, CA), 10% FBS, 50 μg/mL penicillin and streptomycin at 37°C, and 5% CO2–95% air. Control and (−) leu medium were prepared from amino acid-free DMEM (Invitrogen) by adding back all the amino acids contained in regular DMEM and without leucine only, respectively. For the rapamycin treatment, cells were incubated with 50 nmol/L rapamycin for 1 h. To induce insulin resistance, HepG2 cells were incubated with 18 mmol/L glucosamine for 18 h in serum-free medium as previously described (12), followed by incubation in serum-free (−) leu or control medium for 24 h in the presence of glucosamine. The GCN2-knockdown (KO) stable cell lines were obtained by transfecting pSilencer4.1-CMV-puro-GCN2 small interfering (si)RNA vector into HepG2 cells and selecting for stable clones in the presence of 0.5 μg/mL puromycin.
Isolation of primary hepatocytes.
Male C57BL/6J mice were anesthetized by intraperitoneal injection with chloral hydrate. Hepatocytes were prepared by collagenase perfusion as described previously (13). Isolated hepatocytes were resuspended in 10% FBS DMEM and incubated for 1 day before switching to (−) leu medium.
Generation and administration of recombinant adenoviruses.
The recombinant adenoviruses used for the S6K1 expression were generated using AdEasy Vector System (Qbiogene, Montreal, Canada). Briefly, S6K1 gene was cloned into a transfer vector. The resulting plasmid was then linearized with PmeI and cotransformed into Escherichia coli strain BJ5183 together with pAdEasy-1. After transformation in DH5α for greater yields of DNA production, the recombinant adenoviral construct was cleaved with PacI and transfected into QBI-293A cells to produce viral particles. Adenoviruses were purified by ultracentrifugation in cesium chloride gradient and quantified. Viruses were diluted in PBS and administered via tail vein injection, using 5 × 108 pfu/mice.
Blood glucose, serum insulin, GTT, ITT, and homeostasis model assessment–insulin resistance index.
Blood glucose levels were measured using a Glucometer Elite monitor. Serum insulin levels were measured using the Mercodia Ultrasensitive Rat Insulin ELISA kit (ALPCO Diagnostic, Salem, NH). GTTs and ITTs were performed by i.p. injection of 2 g/kg glucose after overnight fasting and 0.75 units/kg insulin after 4-h fasting, respectively. Homeostasis model assessment–insulin resistance(HOMA-IR) index was calculated according to the formula: [fasting glucose levels (mmol/L)] × [fasting serum insulin (μU/mL)]/22.5.
Glycogen synthesis assay.
HepG2 cells were incubated in (−) leu medium for the indicated times before adding 100 nmol/L insulin and 1 μCi/mL [3H]glucose. Cells were incubated with control or (−) leu medium for 3 h before harvesting for glycogen synthesis assays, as previously described (12).
In vivo insulin signaling assay.
Mice maintained on different diets were fasted for 6 h before insulin injection as previously described (13). Sections of liver, soleus muscle, and abdominal fat were excised from anesthetized mice and snap-frozen, as untreated controls. Three to five minutes after injection with 2 units/kg of insulin via the portal vein, pieces of tissue section were excised and snap-frozen for Western blot analysis.
Western blot analysis.
Whole-cell lysates were isolated using RIPA lysis buffer (150 mmol/L Tris-HCl, 50 mmol/L NaCl, 1% NP-40, 0.1% Tween 20). Protease and phosphatase inhibitors were added to all buffers before experiments. Western blot analysis was performed as previously described (11). Protein concentrations were assayed using the BCA Kit (Pierce, Rockford, IL). Primary antibodies (anti–p-insulin receptor, anti-insulin receptor, anti–p-AKT, anti-AKT, anti–p-IRS1, anti-IRS1, anti–p-mTOR, anti-mTOR, anti–p-p70 S6K1, anti-p70 S6K1, anti–p-S6, anti-S6, anti–p-GCN2, anti-GCN2, anti–p-ACC, anti-ACC, anti–p-AMPK, anti–t-AMPK [all from Cell Signaling Technology, Danvers, MA]), anti–p-IRS1, and HA probe (Y-11) were incubated overnight at 4°C, and specific proteins were visualized by ECL Plus (Amersham Biosciences, Uppsala, Sweden). Band intensities were measured using Quantity One (Bio-Rad Laboratories, Hercules, CA) and normalized to total protein or actin.
Statistics.
All data are expressed as means ± SEM. Significant differences were assessed by two-tailed Student t test or one-way ANOVA followed by the Student-Newman-Keuls test. P < 0.05 was considered statistically significant.
RESULTS
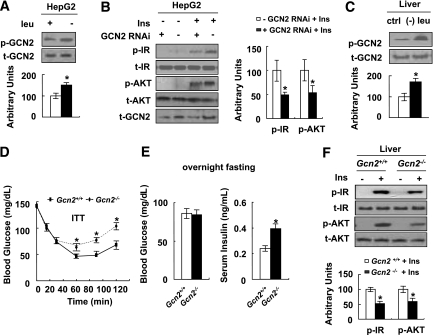
Leucine deprivation increases whole-body insulin sensitivity.
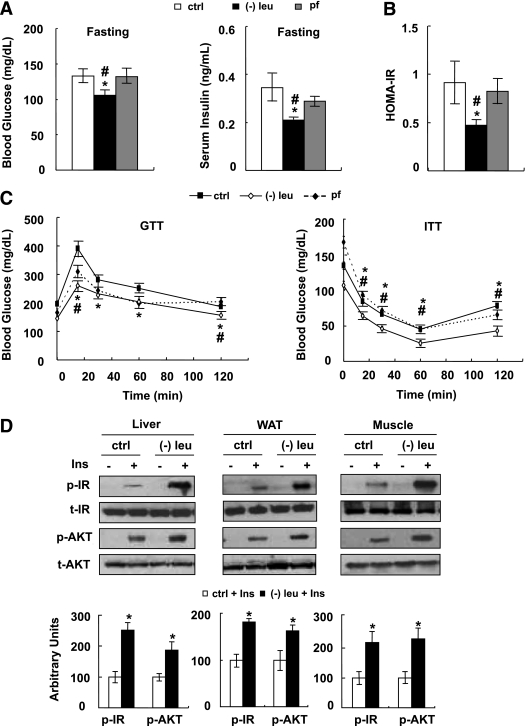
Mice were fed a control, (−) leu, or pair-fed diet for 7 days. As shown previously (14), leucine deprivation results in a decrease in leucine and increases in isoleucine, valine, and several other amino acids in the serum (Supplementary Fig. 1). Consistent with previous observations (10), levels of serum insulin decreased, but blood glucose levels remained unchanged, in leucine-deprived mice in the fed state (data not shown). By contrast, fasting blood glucose levels were lower in leucine-deprived mice compared with control or pair-fed mice. Levels of serum insulin were also decreased 50% in these mice (Fig. 1A). Consistent with these changes, the HOMA-IR index was decreased in leucine-deprived mice (Fig. 1B). Glucose tolerance and clearance were further examined by GTTs and ITTs, respectively. Fifteen minutes after injection of glucose, blood glucose levels were significantly lower in pair-fed and leucine-deprived mice compared with controls, with lowest levels in leucine-deprived mice. In addition, blood glucose levels were decreased more quickly after administration of insulin in leucine-deprived mice compared with control or pair-fed mice (Fig. 1C).
FIG. 1.
Leucine deprivation improves insulin sensitivity in vivo. A–D: Mice were fed a control (ctrl), (−) leu, or pair-fed (pf) diet for 7 days, followed by fasting overnight or indicated times before measuring blood glucose and serum insulin levels (A), calculating HOMA-IR index (B), and carrying out GTT and ITT (C). Insulin signaling in liver, WAT, and muscle was examined before (− Ins) and after (+ Ins) 2 units/kg insulin stimulation for 3 to 5 min (D). Data are means ± SEM of at least two independent experiments with mice of each group for each experiment (n = 5–6 each group). Statistical significance is calculated by one-way ANOVA followed by the Student-Newman-Keuls test for the effects of either group vs. control diet (*P < 0.01) and (−) leu vs. pair-fed diet (#P < 0.01) (A–C), or by two-tailed Student t test for the effects of (−) leu vs. control diet after insulin stimulation (*P < 0.05) (D).Blood glucose and serum insulin levels (A), HOMA-IR index (B), GTT and ITT (C), and p-IR and p-AKT protein (D) (top, Western blot; bottom, quantitative measurements of p-IR and p-AKT protein relative to their total protein).
Increased insulin sensitivity in mice under leucine deprivation suggests an increase in insulin sensitivity in one or more peripheral tissues, including liver, white adipose tissue (WAT), and muscle. To test this possibility, we examined the levels of phosphorylation of two key components in the insulin signaling pathways (15), insulin receptor (IR) and protein kinase B (AKT), in these tissues after infusion of insulin (2 units/kg) into the hepatic portal vein, as described previously (13). As expected, insulin-stimulated phosphorylation of IR and AKT was increased in these tissues in leucine-deprived mice compared with mice maintained on a control diet (Fig. 1D).
Leucine deprivation increases insulin sensitivity in vitro.
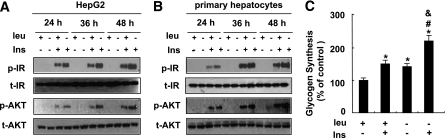
To determine whether leucine deprivation has a direct effect on the insulin signaling in liver, we examined the effect of leucine deprivation on insulin signaling in both the human hepatoma–derived cell line HepG2 and primary cultured mice hepatocytes. Consistent with our in vivo observations (Fig. 1D), insulin-stimulated phosphorylation of IR and AKT was significantly elevated under leucine deprivation in HepG2 cells (Fig. 2A) and primary cultured hepatocytes (Fig. 2B). Furthermore, insulin-stimulated incorporation of glucose into glycogen was significantly increased in cells incubated in (−) leu medium for 36 h. Leucine deprivation, however, also increased baseline levels of glucose incorporation into glycogen (Fig. 2C).
FIG. 2.
Leucine deprivation improves insulin sensitivity in vitro. A and B: Cells were incubated in control (+ leu) or leucine-deficient (− leu) medium for indicated times, followed with (+ Ins) or without (− Ins) 100 nmol/L insulin stimulation for 20 min. Data represent results from at least three independent experiments. C: HepG2 cells were incubated in control or leucine-deficient medium for 36 h, followed by a glycogen synthesis assay. Data are means ± SEM of at least three independent experiments. Statistical significance is calculated by one-way ANOVA followed by Student-Newman-Keuls test for the effects of any group vs. control medium without insulin stimulation (*P < 0.01), with vs. without insulin stimulation in (−) leu medium (#P < 0.01), or (−) leu vs. control medium after insulin stimulation (&P < 0.01). A and B: p-IR and p-AKT protein. C: Glycogen synthesis assay.
Leucine deprivation increases insulin sensitivity by decreasing mTOR/S6K1 signaling in vitro.
Decreased mTOR/S6K1 signaling has been shown to increase insulin sensitivity (16,17). Because mTOR activity is decreased in the livers of mice maintained on a (−) leu diet (14), we speculated that mTOR/S6K1 signaling may regulate insulin sensitivity under leucine deprivation.
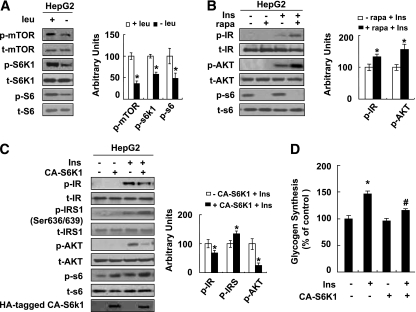
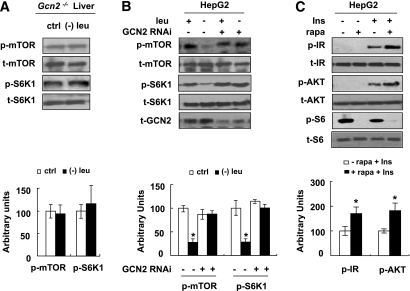
To investigate this possibility, we examined levels of phosphorylation of mTOR and its downstream targets, including S6K1 and ribosomal protein S6, in HepG2 cells incubated in (−) leu medium for 24 h. Levels of phosphorylation of these proteins were also used as indicators for the activation status of mTOR (18). Consistent with previous results (19), phosphorylation of these proteins was decreased by leucine deprivation in HepG2 cells (Fig. 3A). Similar to the effect of leucine deprivation, pretreatment with the mTOR inhibitor 50 nmol/L rapamycin for 1 h significantly decreased phosphorylation of S6 in HepG2 cells compared with pretreatment with vehicle. By contrast, insulin-stimulated phosphorylation of IR and AKT was increased significantly in these cells (Fig. 3B).
FIG. 3.
Leucine deprivation increases insulin sensitivity by decreasing mTOR/S6K1 signaling in vitro. A: HepG2 cells were incubated in control (+ leu) or leucine-deficient (− leu) medium for 24 h. B: HepG2 cells were treated with (+ rapa) or without (− rapa) 50 nmol/L rapamycin for 1 h, followed with (+ Ins) or without (− Ins) 100 nM insulin stimulation for 20 min. C and D: HepG2 cells were transfected with HA-tagged constitutively active S6K1 (+ CA-S6K1) or control pRK7 vectors (− CA-S6K1). Cells were incubated with (−) leu medium for 24 h followed by 100 nmol/L insulin stimulation for 20 min (C), or for 36 h followed by glycogen synthesis assay (D). Data are means ± SEM of at least three independent experiments. Statistical significance is calculated by two-tailed Student t test for the effects of (−) leu vs. control medium (*P < 0.01) (A), with vs. without rapamycin after insulin stimulation (*P < 0.01) (B), with vs. without CA-S6K1 after insulin stimulation (*P < 0.01) (C), or by one-way ANOVA followed by Student-Newman-Keuls test for the effects of any group vs. control medium without insulin stimulation (*P < 0.01), and with vs. without insulin stimulation in (−) leu medium (#P < 0.01) (D). A: p-mTOR, p-S6K1, and p-S6 protein (left, Western blot; right, quantitative measurements of p-mTOR, p-S6K1, and p-S6 protein relative to their total protein). B and C: p-IR, p-IRS1, p-AKT, p-S6 protein (left, Western blot; right, quantitative measurements of p-IR, p-IRS1, p-AKT protein relative to their total protein). D: Glycogen synthesis assay.
If decreased mTOR/S6K1 signaling regulates hepatic insulin sensitivity under leucine deprivation, upregulation of S6K1 activity should block the effect of leucine deprivation on enhancing insulin sensitivity. As predicted, overexpression of CA-S6K1 (as confirmed by Western blotting using anti–HA-tagged antibody) increased S6 phosphorylation, but impaired phosphorylation of IR and AKT, in HepG2 cells compared with control cells (Fig. 3C). Consistent with these changes, phosphorylation of IRS1 Ser636/639, a residue that has been shown to be regulated by S6K1 (16,20), was increased in cells overexpressing CA-S6K1 (Fig. 3C). Furthermore, insulin-stimulated incorporation of glucose into glycogen was also significantly decreased in these cells (Fig. 3D).
Leucine deprivation increases insulin sensitivity by decreasing mTOR/S6K1 signaling in vivo.
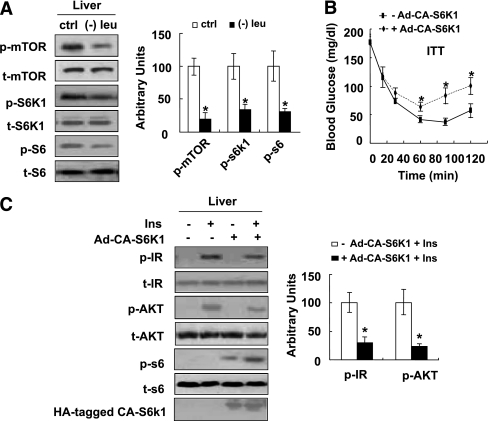
Consistent with changes in vitro, phosphorylation of mTOR, S6K1, and S6 was also decreased significantly in the livers of leucine-deprived mice compared with mice fed a control diet (Fig. 4A). By contrast, no differences in the levels of phosphorylation of these proteins were observed in the livers of pair-fed mice compared with control diet–fed mice (data not shown).
FIG. 4.
Leucine deprivation increases insulin sensitivity by decreasing mTOR/S6K1 signaling in vivo. A: Mice were fed a control (ctrl) or (−) leu diet for 7 days. B and C: Mice were infected with adenovirus HA-tagged constitutively active S6K1 (+ Ad-CA-S6K1) or control green fluorescent protein adenovirus (− Ad-CA-S6K1) via tail-vein injection. ITT was examined after mice were fed a (−) leu diet at day 6 (B), and insulin signaling was examined before (− Ins) and after (+ Ins) 2 units/kg insulin stimulation for 3 min at day 7 (C). Data are means ± SEM of at least two independent experiments with mice of each group for each experiment (n = 5–7 each group). Statistical significance is calculated by two-tailed Student t test for the effects of (−) leu vs. control diet (*P < 0.05) (A) and with vs. without CA-S6K1 after insulin stimulation (*P < 0.01) (B and C). p-mTOR, p-S6K1, and p-S6 protein (A) (left, Western blot; right, quantitative measurements of p-mTOR, p-S6K1, and p-S6 protein relative to their total protein); ITT (B); p-IR, p-AKT, p-S6 protein (C) (left, Western blot; right, quantitative measurements of p-IR and p-AKT, protein relative to their total protein).
To demonstrate the importance of S6K1 in increased hepatic insulin sensitivity under leucine deprivation in vivo, we investigated whether increased insulin sensitivity can be blocked in leucine-deprived mice by infecting mice with adenoviruses expressing CA-S6K1 (as confirmed by Western blotting using anti–HA-tagged antibody). As predicted, these mice exhibited decreased glucose clearance, compared with mice injected with adenovirus that expresses green fluorescent protein (Fig. 4B). Consistent with decreased whole-body insulin sensitivity, insulin-stimulated phosphorylation of IR and AKT was also greatly impaired in the livers of these mice (Fig. 4C).
Leucine deprivation increases insulin sensitivity by activation of GCN2.
GCN2 is a serine protein kinase that is well known to function as a sensor for amino acid deprivation (21–23). To examine the possible involvement of GCN2 in the regulation of insulin sensitivity under leucine deprivation, levels of GCN2 phosphorylation in HepG2 cells incubated in (−) leu medium were examined. Consistent with previous results in mouse embryonic fibroblasts (24), GCN2 phosphorylation was increased in these cells (Fig. 5A). Furthermore, knocking down expression of GCN2 in HepG2 cells (a stable cell line transfected with siRNA GCN2 as examined by decreased levels of GCN2 protein) significantly decreased insulin-stimulated phosphorylation of IR and AKT under leucine deprivation (Fig. 5B).
FIG. 5.
Leucine deprivation increases insulin sensitivity by activation of GCN2. A: HepG2 cells were incubated with control (+ leu) or (−) leu medium for 24 h. B: The GCN2-KO stable HepG2 (+ GCN2 RNAi) and negative control cells (− GCN2 RNAi) were incubated with (−) leu medium for 24 h and followed with (+ Ins) or without (− Ins) 100 nmol/L insulin stimulation for 20 min. Data are means ± SEM of at least three independent experiments (A and B). C: Gcn2+/+ mice were fed a control (ctrl) or (−) leu diet for 7 days. D–F: Gcn2+/+ and Gcn2−/− mice were fed a (−) leu diet for 7 days, followed by ITT (D), overnight fasting blood glucose and serum insulin measurement (E), and insulin signaling examination before (− Ins) and after (+ Ins) 2 units/kg insulin stimulation for 3 min (F). Data are means ± SEM of at least two independent experiments with mice of each group for each experiment (n = 5–6 each group). Statistical significance is calculated by two-tailed Student t test for the effects of (−) leu vs. control medium (*P < 0.01) (A), GCN2-KO vs. control cells after insulin stimulation (B), (−) leu vs. control diet (C), and Gcn2−/−vs. Gcn2+/+ mice (D and E). p-GCN2 protein (A) (top, Western blot; bottom, p-GCN2 protein relative to total protein); p-IR and p-AKT protein (B) (left, Western blot; right, quantitative measurements of p-IR and p-AKT protein relative to their total protein); p-GCN2 protein (C) (top, Western blot; bottom, p-GCN2 protein relative to total protein); ITT (D); fasting blood glucose and serum insulin (E); p-IR and p-AKT protein (F) (top, Western blot; bottom, quantitative measurements of p-IR and p-AKT protein relative to their total protein).
Consistent with our in vitro results, levels of GCN2 phosphorylation were also increased in the livers of mice maintained on a (−) leu diet for 7 days (Fig. 5C). The role of GCN2 in the regulation of increased insulin sensitivity under leucine deprivation was further examined in ITT experiments using Gcn2+/+ and Gcn2−/− mice. We found that glucose clearance was decreased in Gcn2−/− mice, compared with Gcn2+/+ mice, fed a (−) leu diet (Fig. 5D). Although fasting glucose levels in both strains of mice were not different, fasting serum insulin levels were higher in leucine-deprived Gcn2−/− mice (Fig. 5E). Consistent with these changes, insulin-stimulated phosphorylation of IR and AKT was decreased in the livers of Gcn2−/− mice (Fig. 5F).
GCN2 functions as an upstream inhibitor of mTOR under leucine deprivation.
As shown previously (14), we found that mTOR activity was not reduced by leucine deprivation in the livers of Gcn2−/− mice (Fig. 6A), suggesting that GCN2 may function as an upstream regulator of mTOR. Consistent with this possibility, decreased phosphorylation of mTOR and S6K1 by leucine deprivation was not observed in GCN2-KO HepG2 cells (Fig. 6B). If unrepressed mTOR/S6K1 activity was the cause of decreased insulin signaling in GCN2-KO HepG2 cells in (−) leu medium (Fig. 5B), treatment with the mTOR inhibitor rapamycin should reverse these effects. As predicted, treatment with 50 nmol/L rapamycin increased insulin-stimulated phosphorylation of IR and AKT in these cells (Fig. 6C).
FIG. 6.
GCN2 functions as an upstream inhibitor of mTOR under leucine deprivation. A: Gcn2−/− mice were fed a control (ctrl) or (−) leu diet for 7 days. B: GCN2-KO stable HepG2 (+ GCN2 RNAi) and negative control cells (− GCN2 RNAi) were incubated with control (+ leu) or leucine-deficient (− leu) medium for 24 h. C: GCN2-KO stable HepG2 cells were incubated in (−) leu medium for 24 h, then treated with (+ rapa) or without (− rapa) 50 nmol/L rapamycin for 1 h, followed with (+ Ins) or without (− Ins) 100 nM insulin stimulation for 20 min. Data are means ± SEM of at least two independent experiments with mice of each group for each experiment (n = 5–7 each group) (A) and at least three independent experiments (B and C). Statistical significance is calculated by two-tailed Student t test for the effects of (−) leu vs. control diet (A), one-way ANOVA followed by Student-Newman-Keuls test for the effects of any group vs. control medium in control cells (*P < 0.01) (B), and with vs. without rapamycin after insulin stimulation (*P < 0.01) (C). p-mTOR and p-S6K1 protein (A and B) (top, Western blot; bottom, quantitative measurements of p-mTOR and p-S6K1 protein relative to their total protein); p-IR, p-AKT, and p-S6 protein (C) (top, Western blot; bottom, quantitative measurements of p-IR and p-AKT protein relative to their total protein).
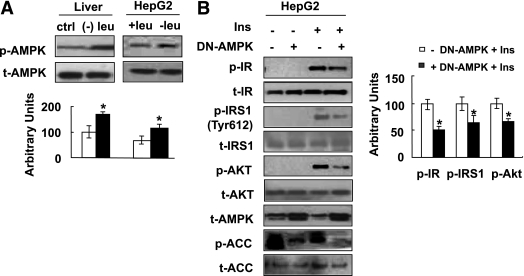
Leucine deprivation increases insulin sensitivity via activation of AMPK.
It has been shown previously that AMPK regulates insulin sensitivity and that activation of AMPK is indirectly controlled by nutrient availability (25,26). To determine a role of AMPK in regulating insulin sensitivity under leucine deprivation, we first examined the levels of AMPK phosphorylation in the livers of mice and in HepG2 cells under leucine deprivation. As predicted, AMPK phosphorylation was increased in the livers of mice fed a (−) leu diet for 7 days, compared with mice fed a control diet, and in HepG2 cells incubated in (−) leu medium for 24 h (Fig. 7A).
FIG. 7.
Leucine deprivation improves insulin sensitivity via activation of AMPK. A: Mice were fed a control (ctrl) or (−) leu diet for 7 days, and HepG2 cells were incubated with control (+ leu) or leucine-deficient (− leu) medium for 24 h. B: HepG2 cells were transfected with dominant-negative form of AMPKα1 (+ DN-AMPK) or empty vectors (− DN-AMPK). After incubation with (−) leu medium for 24 h, cells were stimulated with (+ Ins) or without (− Ins) 100 nmol/L insulin for 20 min. Data are means ± SEM of at least three independent experiments. Statistical significance is calculated by two-tailed Student t test for the effects of (−) leu vs. control treatment (*P < 0.01) (A) and with vs. without DN-AMPK after insulin stimulation (B). p-AMPK protein (A) (top, Western blot; bottom, quantitative measurements of p-AMPK protein relative to their total protein); p-IR, p-IRS1, p-AKT, p-ACC protein (B) (left, Western blot; right, quantitative measurements of p-IR, p-IRS1, and p-AKT proteins relative to their total protein).
To gain further insight into the significance of AMPK activation in regulating insulin sensitivity under leucine deprivation, we examined whether downregulation of AMPK reduces the effects of leucine deprivation on insulin sensitivity. HepG2 cells were transfected with a plasmid encoding a dominant-negative form of AMPKα1 (DN-AMPK), the effects of which were confirmed by decreased phosphorylation of acetyl-CoA carboxylase, before incubation with (−) leu medium for 24 h. As predicted, overexpression of DN-AMPK decreased insulin-stimulated phosphorylation of IR and AKT compared with control cells (Fig. 7B). Levels of phosphorylation of IRS1 at Tyr612, a residue that has been shown to be regulated by AMPK (27), were also decreased in these cells.
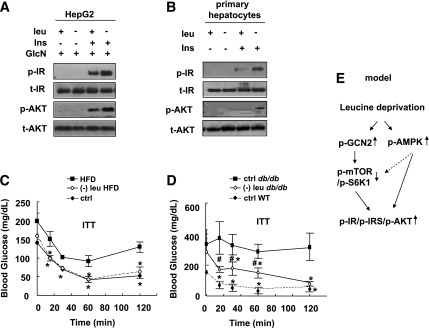
Leucine deprivation increases insulin sensitivity under insulin-resistant conditions in vivo and in vitro.
To investigate whether leucine deprivation could also increase insulin sensitivity in insulin-resistant conditions, we examined the effect of leucine deprivation on insulin sensitivity in HepG2 cells, in which insulin resistance was induced by pretreatment with 18 mmol/L glucosamine for 18 h (28). The impaired insulin signaling was partially reversed by incubating cells in (−) leu medium for 24 h (Fig. 8A). Similar results were obtained in primary cultured hepatocytes isolated from insulin-resistant mice maintained on an HFD for 16 weeks (Fig. 8B).
FIG. 8.
Leucine deprivation improves insulin sensitivity under insulin-resistant conditions. A: HepG2 cells were incubated with 18 mmol/L glucosamine for 18 h before being incubated with control (+ leu) or leucine-deficient (− leu) medium for 24 h in the presence of glucosamine, followed with (+ Ins) or without (− Ins) 100 nM insulin stimulation for 20 min. B: Primary hepatocytes isolated from insulin-resistant mice, induced by HFD feeding for 16 weeks, were incubated in control or leucine-deficient medium for 24 h, followed by stimulation with 100 nmol/L insulin for 20 min. Data represent results from at least three independent experiments (A and B). C: Mice were fed an HFD for 16 weeks and then an HFD or (−) leu HFD for 7 days, followed by ITT. Age-matched, chow-diet fed (ctrl) mice were also included. D: Leptin receptor–deficient (db/db) were fed control or (−) leu diet for 7 days, followed by ITT. Their control littermates (wild type) fed a control diet were also included. Data are means ± SE of at least two independent experiments with mice of each group for each experiment (n = 5–7 each group) (C and D). Statistical significance is calculated by one-way ANOVA followed by Student-Neuman-Keuls test for the effects of either group vs. HFD (*P < 0.01) (C), either group vs. control diet in db/db mice (*P < 0.01) (D), and (−) leu diet in db/db mice vs. control diet in wild-type mice (#P < 0.01) (D). p-IR and p-AKT protein (A and B); ITT (C and D); working model (E). GlcN, glucosamine; WT, wild type.
We next investigated whether leucine deprivation can increase insulin sensitivity under insulin-resistant conditions in vivo. Animal models of insulin resistance are divided into two categories: nutritionally induced models, such as that induced by HFD feeding (29), and genetically induced models, such as leptin receptor–deficient (db/db) mice (30). After maintenance on an HFD for 16 weeks, mice exhibited impaired glucose clearance, as demonstrated in ITT assays (Fig. 8C). Decreased glucose clearance in these mice, however, improved significantly after maintenance on a (−) leu HFD for 7 days (Fig. 8C). Similar results were obtained in db/db mice (Fig. 8D).
DISCUSSION
We have previously shown that leucine deprivation significantly decreases serum insulin levels without affecting blood glucose levels in mice in the fed state (10), suggesting increased insulin sensitivity. This possibility was confirmed in our current study by the decreased HOMA-IR index and increased glucose clearance and tolerance in leucine-deprived mice. Lower baseline levels of blood glucose, however, could also account for the lower peak as shown in the GTT and ITT results in these mice. As previously reported, mice maintained on a (−) leu diet reduced their food intake by 15% (11). By using pair-fed mice as a control, we demonstrated that this increased insulin sensitivity is caused primarily by deficiency of leucine, rather than the small reduction in food intake. Consistent with previous reports (14), our study found that leucine deprivation results in a decrease in leucine and increases in isoleucine and valine in the serum. The increased levels of serum isoleucine and valine, however, are unlikely to account for the increased insulin sensitivity under leucine deprivation because the levels of all three BCAAs and other amino acids are elevated in insulin-resistant conditions in vivo (8,9) and in vitro (31). Taken together, these results strongly suggest that low levels of leucine are the critical factor correlating with the increased insulin sensitivity in leucine-deprived mice.
Insulin is secreted by pancreatic β-cells in response to feeding and other stimuli and functions to regulate blood glucose levels. Binding of insulin to its receptor leads to tyrosine phosphorylation of IRS (15), which results in the recruitment of the regulatory subunit of phosphoinositide 3-kinase (PI3K) to IRS. Activated PI3K phosphorylates and activates 3-phosphoinositide–dependent protein kinase 1, which stimulates AKT activation. Activated AKT in turn activates glycogen synthase, stimulating the synthesis of glycogen in the liver (15) or glucose uptake in the adipose tissue and muscle (32). Our current results show that leucine deprivation increases insulin signaling in all the tissues examined, including liver, WAT, and muscle, consistent with increased insulin sensitivity.
mTOR is a serine-threonine protein kinase that has been shown to be essential for protein synthesis, growth, development, and proliferation (18,33,34). Studies have shown that mTOR activity is regulated by BCAA availability, especially leucine (35). For example, mTOR activity is increased by elevated extracellular levels of leucine (33). By contrast, its activity is decreased in the livers of mice under leucine deprivation (14). Activation of mTOR/S6K1 signaling has been shown to contribute to the development of insulin resistance. Recent studies demonstrate that the mTOR downstream target S6K1 directly phosphorylates IRS1 serine residues, including Ser-302/307 (36), Ser-307/312 (37), Ser-632/636 (20), and Ser-1097/1101 (38). Increased IRS1 serine phosphorylation reduces the activity of IRS1, thereby impairing PI3K/AKT signaling and increasing insulin resistance (39,40). This effect is reversed in vitro by rapamycin (37). Consistent with these results, mice deleted for S6K1 have reduced phosphorylation of IRS1 Ser-636 (16) and do not develop diet-induced insulin resistance.
On the basis of these studies, we hypothesized that leucine deprivation increases insulin sensitivity by decreasing mTOR/S6K1 activity. Consistent with our hypothesis, decreased mTOR/S6K1 signaling in the livers of mice and HepG2 cells under leucine deprivation was observed. Furthermore, overexpression of CA-S6K1 inhibited insulin signaling in HepG2 cells incubated in (−) leu medium. A key role for S6K1 in the regulation of insulin sensitivity was confirmed in mice injected with adenoviruses expressing CA-S6K1. These mice exhibited decreased glucose clearance and insulin signaling in liver, compared with control mice, when fed a (−) leu diet. Taken together, our findings demonstrate an important role for mTOR/S6K1 in the regulation of insulin sensitivity under leucine deprivation.
To look for upstream regulators for mTOR, our attention was drawn to the amino acid sensor GCN2, a kinase that is activated by uncharged tRNAs in response to deprivation of essential amino acids, including leucine, in yeast and mammals (21–23). Activated GCN2 phosphorylates eukaryotic initiation factor 2-α, thereby repressing general protein synthesis, but increasing translation of proteins related to amino acid biosynthesis and transport (41–44). We recently demonstrated that GCN2 also regulates lipid metabolism during leucine deprivation (10). Because deficiency in amino acid is sensed by GCN2, we speculated that GCN2 may also regulate glucose utilization by modulating insulin sensitivity during leucine deprivation.
Consistent with this hypothesis, knocking down expression of GCN2 using siRNA decreased insulin signaling in HepG2 cells incubated in (−) leu medium. The key role for GCN2 in the regulation of insulin sensitivity was confirmed by the observation that Gcn2−/− mice exhibited decreased glucose clearance, compared with wild-type mice, when fed a (−) leu diet. Consistent with these results, higher serum insulin levels were required to maintain the same levels of fasting blood glucose in Gcn2−/− mice compared with Gcn2+/+ mice. In addition, we provide evidence that GCN2 regulates insulin sensitivity via inhibition of mTOR activity during leucine deprivation. Thus, in addition to regulation of amino acid and lipid metabolism (10,14), GCN2 also functions in glucose metabolism. Taken together, our results strongly suggest that GCN2 functions as a master regulator of metabolic adaptation to deprivation of essential amino acids. The mechanism by which leucine deprivation activates GCN2 requires further investigation.
The fact that overexpression of CA-S6K1 does not completely block the increased insulin signaling under leucine deprivation suggests the possible involvement of other regulatory pathways. Recent studies indicate that AMPK also plays a role in the regulation of insulin sensitivity by directly phosphorylating IRS1 (45) or by phosphorylating the mTOR upstream inhibitor TSC2 (46). AMPK activators, including metformin and rosiglitazone, increase insulin sensitivity and are used clinically in the treatment of type 2 diabetes (2). These studies raise the possibility that activation of AMPK may contribute to the enhancement of insulin sensitivity under leucine deprivation.
As predicted, leucine deprivation increased levels of activated AMPK in vivo and in vitro. A key role for AMPK in regulating insulin sensitivity under leucine deprivation was confirmed by the observed decreases in insulin signaling in HepG2 cells transfected with DN-AMPK. The mechanism by which leucine deprivation activates AMPK is unknown. Furthermore, our current results did not distinguish whether AMPK directly regulates IRS1 phosphorylation or indirectly regulates insulin sensitivity by modulating mTOR activity. These issues will be investigated in future studies.
A previous study showed that mice deleted for the BCATm gene encoding the enzyme catalyzing the first step in peripheral BCAA metabolism, which would also be expected to have low leucine, valine, or isoleucine use, exhibited increased sensitivity (47). These results suggest that deficiency of other BCAAs may also have similar effects on insulin sensitivity. Consistent with this hypothesis, our study found that valine deprivation also increases hepatic insulin sensitivity compared with those under control or glycine (a nonessential amino acid)–deficiency treatment (F.X., F.G., unpublished observations). Furthermore, the effects of valine deprivation on insulin sensitivity are mediated by GCN2 activation (F.X., F.G., unpublished observations). On the basis of these observations, we plan to carry out a systematic analysis of the effects of deficiency of isoleucine and other essential amino acids in the future.
The current study also found that leucine deprivation increases insulin sensitivity under insulin-resistant conditions, both in vitro and in vivo, suggesting potential application of this diet in treating type 2 diabetes. Clinical use of this diet, however, is still premature, because the safety of short- and long-term leucine deprivation in humans has not been examined. Determining the optimal concentration of leucine and the duration of therapeutic (−) leu diets will be important in future studies.
In summary, we have shown that leucine deprivation improves insulin sensitivity under normal and insulin-resistant conditions, both in vitro and in vivo. Furthermore, the GCN2/mTOR/S6K1 and AMPK pathways play important roles in the regulation of hepatic insulin sensitivity by leucine deprivation. The relative contribution of each of these pathways remains to be demonstrated. It also remains to be demonstrated whether these pathways function in the regulation of insulin sensitivity in other tissues, including muscle and WAT.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Ministry of Science and Technology of China (973 Program 2009CB919001 and 2010CB912502); National Natural Science Foundation (30871208 and 30890043); Chief Scientist Program of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (SIBS2008006); Science and Technology Commission of Shanghai Municipality (08DJ1400601); 2010 Key Program of Clinical Research Center, Institute for Nutritional Sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (CRC2010005); Key Program of Shanghai Scientific and Technological Innovation Action Plan (10JC1416900); and CAS-Pfizer Project (Pfizer-SIBS2 01002). F.G. was also supported by the One Hundred Talents Program of the Chinese Academy of Sciences and the Pujiang Talents Program of Shanghai Municipality (08PJ1410700).
No potential conflicts of interest relevant to this article were reported.
F.X. researched data, contributed to discussion, and wrote, reviewed, and edited the article. Z.H. researched data and contributed to discussion. H.L. researched data, contributed to discussion, and reviewed and edited the article. J.Y., C.W., S.C., Q.M., and Y.C. researched data and contributed to discussion. X.G. and J.L. provided research material and contributed to discussion. Y.L. contributed to discussion and reviewed and edited the article. F.G. contributed to discussion and wrote, reviewed, and edited the article.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-1246/-/DC1.
REFERENCES
- 1.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 2007;87:507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab 2009;9:407–416 [DOI] [PubMed] [Google Scholar]
- 3.Monzillo LU, Hamdy O, Horton ES, et al. Effect of lifestyle modification on adipokine levels in obese subjects with insulin resistance. Obes Res 2003;11:1048–1054 [DOI] [PubMed] [Google Scholar]
- 4.Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes 2002;51:2796–2803 [DOI] [PubMed] [Google Scholar]
- 5.Nair KS, Short KR. Hormonal and signaling role of branched-chain amino acids. J Nutr 2005;135(Suppl.):1547S–1552S [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 2007;56:1647–1654 [DOI] [PubMed] [Google Scholar]
- 7.Nairizi A, She P, Vary TC, Lynch CJ. Leucine supplementation of drinking water does not alter susceptibility to diet-induced obesity in mice. J Nutr 2009;139:715–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tremblay F, Krebs M, Dombrowski L, et al. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes 2005;54:2674–2684 [DOI] [PubMed] [Google Scholar]
- 10.Guo F, Cavener DR. The GCN2 eIF2alpha kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab 2007;5:103–114 [DOI] [PubMed] [Google Scholar]
- 11.Cheng Y, Meng Q, Wang C, et al. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes 2010;59:17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun C, Zhang F, Ge X, et al. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab 2007;6:307–319 [DOI] [PubMed] [Google Scholar]
- 13.Wang Q, Jiang L, Wang J, et al. Abrogation of hepatic ATP-citrate lyase protects against fatty liver and ameliorates hyperglycemia in leptin receptor-deficient mice. Hepatology 2009;49:1166–1175 [DOI] [PubMed] [Google Scholar]
- 14.Anthony TG, McDaniel BJ, Byerley RL, et al. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J Biol Chem 2004;279:36553–36561 [DOI] [PubMed] [Google Scholar]
- 15.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001;414:799–806 [DOI] [PubMed] [Google Scholar]
- 16.Um SH, Frigerio F, Watanabe M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 2004;431:200–205 [DOI] [PubMed] [Google Scholar]
- 17.Krebs M, Brunmair B, Brehm A, et al. The mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes 2007;56:1600–1607 [DOI] [PubMed] [Google Scholar]
- 18.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 2006;124:471–484 [DOI] [PubMed] [Google Scholar]
- 19.Sarbassov DD, Sabatini DM. Redox regulation of the nutrient-sensitive raptor-mTOR pathway and complex. J Biol Chem 2005;280:39505–39509 [DOI] [PubMed] [Google Scholar]
- 20.Ozes ON, Akca H, Mayo LD, et al. A phosphatidylinositol 3-kinase/Akt/mTOR pathway mediates and PTEN antagonizes tumor necrosis factor inhibition of insulin signaling through insulin receptor substrate-1. Proc Natl Acad Sci U S A 2001;98:4640–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wek RC, Jackson BM, Hinnebusch AG. Juxtaposition of domains homologous to protein kinases and histidyl-tRNA synthetases in GCN2 protein suggests a mechanism for coupling GCN4 expression to amino acid availability. Proc Natl Acad Sci U S A 1989;86:4579–4583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wek SA, Zhu S, Wek RC. The histidyl-tRNA synthetase-related sequence in the eIF-2 alpha protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol Cell Biol 1995;15:4497–4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinnebusch AG. The eIF-2 alpha kinases: regulators of protein synthesis in starvation and stress. Semin Cell Biol 1994;5:417–426 [DOI] [PubMed] [Google Scholar]
- 24.Harding HP, Zhang Y, Zeng H, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 2003;11:619–633 [DOI] [PubMed] [Google Scholar]
- 25.Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov 2004;3:340–351 [DOI] [PubMed] [Google Scholar]
- 26.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest 2006;116:1776–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C, Mao X, Wang L, et al. Adiponectin sensitizes insulin signaling by reducing p70 S6 kinase-mediated serine phosphorylation of IRS-1. J Biol Chem 2007;282:7991–7996 [DOI] [PubMed] [Google Scholar]
- 28.Sakai K, Clemmons DR. Glucosamine induces resistance to insulin-like growth factor I (IGF-I) and insulin in Hep G2 cell cultures: biological significance of IGF-I/insulin hybrid receptors. Endocrinology 2003;144:2388–2395 [DOI] [PubMed] [Google Scholar]
- 29.Winzell MS, Ahrén B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 2004;53(Suppl. 3):S215–S219 [DOI] [PubMed] [Google Scholar]
- 30.Kodama H, Fujita M, Yamaguchi I. Development of hyperglycaemia and insulin resistance in conscious genetically diabetic (C57BL/KsJ-db/db) mice. Diabetologia 1994;37:739–744 [DOI] [PubMed] [Google Scholar]
- 31.Iwanaka N, Egawa T, Satoubu N, et al. Leucine modulates contraction- and insulin-stimulated glucose transport and upstream signaling events in rat skeletal muscle. J Appl Physiol 2010;108:274–282 [DOI] [PubMed] [Google Scholar]
- 32.Whiteman EL, Cho H, Birnbaum MJ. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab 2002;13:444–451 [DOI] [PubMed] [Google Scholar]
- 33.Cota D, Proulx K, Smith KA, et al. Hypothalamic mTOR signaling regulates food intake. Science 2006;312:927–930 [DOI] [PubMed] [Google Scholar]
- 34.Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet 2005;37:19–24 [DOI] [PubMed] [Google Scholar]
- 35.Lynch CJ. Role of leucine in the regulation of mTOR by amino acids: revelations from structure-activity studies. J Nutr 2001;131:861S–865S [DOI] [PubMed] [Google Scholar]
- 36.Harrington LS, Findlay GM, Gray A, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol 2004;166:213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carlson CJ, White MF, Rondinone CM. Mammalian target of rapamycin regulates IRS-1 serine 307 phosphorylation. Biochem Biophys Res Commun 2004;316:533–539 [DOI] [PubMed] [Google Scholar]
- 38.Tremblay F, Brûlé S, Hee Um S, et al. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci U S A 2007;104:14056–14061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Draznin B. Molecular mechanisms of insulin resistance: serine phosphorylation of insulin receptor substrate-1 and increased expression of p85alpha: the two sides of a coin. Diabetes 2006;55:2392–2397 [DOI] [PubMed] [Google Scholar]
- 40.Pederson TM, Kramer DL, Rondinone CM. Serine/threonine phosphorylation of IRS-1 triggers its degradation: possible regulation by tyrosine phosphorylation. Diabetes 2001;50:24–31 [DOI] [PubMed] [Google Scholar]
- 41.Bruhat A, Jousse C, Fafournoux P. Amino acid limitation regulates gene expression. Proc Nutr Soc 1999;58:625–632 [DOI] [PubMed] [Google Scholar]
- 42.Kilberg MS, Pan YX, Chen H, Leung-Pineda V. Nutritional control of gene expression: how mammalian cells respond to amino acid limitation. Annu Rev Nutr 2005;25:59–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Averous J, Bruhat A, Mordier S, Fafournoux P. Recent advances in the understanding of amino acid regulation of gene expression. J Nutr 2003;133(Suppl. 1):2040S–2045S [DOI] [PubMed] [Google Scholar]
- 44.Anthony TG, Reiter AK, Anthony JC, Kimball SR, Jefferson LS. Deficiency of dietary EAA preferentially inhibits mRNA translation of ribosomal proteins in liver of meal-fed rats. Am J Physiol Endocrinol Metab 2001;281:E430–E439 [DOI] [PubMed] [Google Scholar]
- 45.Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE. 5′-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J Biol Chem 2001;276:46912–46916 [DOI] [PubMed] [Google Scholar]
- 46.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003;115:577–590 [DOI] [PubMed] [Google Scholar]
- 47.She P, Reid TM, Bronson SK, et al. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab 2007;6:181–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.