Abstract
OBJECTIVE
During energy stress, AMP-activated protein kinase (AMPK) promotes glucose transport and glycolysis for ATP production, while it is thought to inhibit anabolic glycogen synthesis by suppressing the activity of glycogen synthase (GS) to maintain the energy balance in muscle. Paradoxically, chronic activation of AMPK causes an increase in glycogen accumulation in skeletal and cardiac muscles, which in some cases is associated with cardiac dysfunction. The aim of this study was to elucidate the molecular mechanism by which AMPK activation promotes muscle glycogen accumulation.
RESEARCH DESIGN AND METHODS
We recently generated knock-in mice in which wild-type muscle GS was replaced by a mutant (Arg582Ala) that could not be activated by glucose-6-phosphate (G6P), but possessed full catalytic activity and could still be activated normally by dephosphorylation. Muscles from GS knock-in or transgenic mice overexpressing a kinase dead (KD) AMPK were incubated with glucose tracers and the AMPK-activating compound 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) ex vivo. GS activity and glucose uptake and utilization (glycolysis and glycogen synthesis) were assessed.
RESULTS
Even though AICAR caused a modest inactivation of GS, it stimulated muscle glycogen synthesis that was accompanied by increases in glucose transport and intracellular [G6P]. These effects of AICAR required the catalytic activity of AMPK. Strikingly, AICAR-induced glycogen synthesis was completely abolished in G6P-insensitive GS knock-in mice, although AICAR-stimulated AMPK activation, glucose transport, and total glucose utilization were normal.
CONCLUSIONS
We provide genetic evidence that AMPK activation promotes muscle glycogen accumulation by allosteric activation of GS through an increase in glucose uptake and subsequent rise in cellular [G6P].
AMPK is a major regulator of cellular and whole-body energy homeostasis that coordinates metabolic pathways to balance nutrient supply with energy demand (1–4). In response to cellular stress, AMPK inhibits anabolic pathways and stimulates catabolic pathways to restore cellular energy charge. In skeletal muscle, AMPK is activated under energy-consuming conditions such as during contraction and also energy-depleting processes such as hypoxia, which leads to an increase in fatty acid oxidation, glucose uptake, and inhibition of protein synthesis (1,5). The most well established function of AMPK activation in muscle is to stimulate glucose transport by promoting the redistribution of GLUT4 from intracellular compartments to the cell surface (5–7).
The resulting increase in glucose transport and phosphorylation of glucose by hexokinase II leads to an increase in the intracellular level of glucose-6-phosphate (G6P) (8,9). G6P can be used for the synthesis of glycogen or metabolized in the glycolytic pathway to generate ATP. During glycogen synthesis, G6P is converted to uridine diphosphate (UDP) glucose, and the glucosyl moiety from UDP glucose is used to elongate a growing glycogen chain through α-1,4-glycosidic bonds by the action of glycogen synthase (GS) (10,11). There are two GS isoforms in mammals encoded by separate genes. GYS1, encoding the muscle isoform, is expressed in muscle and many other organs, including kidney, heart, and brain, whereas GYS2, encoding the liver GS isoform, is expressed exclusively in the liver (11). GS activity of both isoforms is regulated by G6P, an allosteric activator, and by covalent phosphorylation, which inhibits enzyme activity (10).
Carling and Hardie (12) reported that AMPK phosphorylates muscle GS at site 2 (Ser8 [amino acid numbering starts from the initiator methionine residue] in human, mouse, and rat), a known inhibitory site of the enzyme, in cell-free assays. Recent work has shown in intact skeletal muscle tissue that acute stimulation of AMPK by a pharmacologic activator, 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR), promotes phosphorylation of GS at site 2 (13), resulting in a decrease in enzymatic activity (13–15). From these findings, it was anticipated that activation of AMPK would reduce muscle glycogen levels. However, in apparent conflict with this anticipation, long-term/chronic activation of AMPK increases glycogen storage in skeletal (16,17) and cardiac (18) muscles. Some have speculated that AMPK-mediated increases in glucose transport and the subsequent elevation of intracellular [G6P] are able to allosterically stimulate GS and thus glycogen synthesis by overriding the inhibitory phosphorylation of GS in muscles (8,9).
This hypothesis, however, has not been directly tested, mainly because there are currently no experimental or assay systems to assess G6P-mediated regulation of GS in vivo. GS activity is routinely assayed in vitro using cell/tissue extracts in which the rate of incorporation of UDP-[14C]glucose into glycogen is measured in the absence or presence of G6P (19). GS activity in the presence of saturating concentrations of G6P is independent of the state of phosphorylation, and the activity ratio in the absence of G6P relative to that in the presence of G6P is used as an index of GS activity. However, it has been virtually impossible to prove that G6P activates GS in vivo or to assess its physiologic significance because G6P binds noncovalently to GS and therefore dissociates from it when muscle tissue is homogenized in a protein extraction buffer.
We have recently identified a key residue, Arg582, which is located in a highly basic segment comprising a putative G6P-binding pocket at the C-terminus of GS (20,21). Substitution of Arg582 to Ala (R582A) caused a complete loss of allosteric activation of GS by G6P without affecting phosphorylation-dependent enzymatic activity and robustly reduced insulin-mediated glycogen synthesis in skeletal muscle (22). To investigate the physiologic involvement of allosteric activation of GS in regulating muscle glycogen metabolism in vivo, a knock-in mouse expressing a G6P-insensitive GS mutant (GSR582A/R582A mouse) has recently been generated (22). Using this mouse model, we demonstrate here that acute activation of AMPK promotes muscle glycogen synthesis through allosteric activation of GS through increasing glucose uptake and the subsequent rise in intracellular [G6P].
RESEARCH DESIGN AND METHODS
Materials.
d-[U-14C]glucose-1-phosphate was purchased from Hartmann Analytic GmbH (Brunswick, Germany). All other radiochemicals were from Perkin Elmer (Buckinghamshire, U.K.). Human insulin (Actrapid) was from Novo-Nordisk (Bagsværd, Denmark). AICAR was from Toronto Research Chemicals (Ontario, Canada). Wortmannin was from Tocris (Avonmouth, U.K.). Horseradish peroxidase-conjugated secondary antibodies were from Thermo Fisher (Northumberland, U.K.). All other chemicals, unless specified otherwise, were obtained from Sigma (Poole, U.K.).
Animals.
Animal studies were approved by the University of Dundee Ethics Committee and performed under a U.K. Home Office project license. C57BL/6 J mice were obtained from Harlan (Leicestershire, U.K.). Generation of the muscle GSR582A/R582A knock-in (22) and transgenic AMPK kinase dead (K45R) (23) mice has been previously described.
Antibodies.
Total acetyl CoA carboxylase (ACC), phospho ACC1 (Ser79, also detects equivalent site on mouse ACC2 at Ser212), total raptor, phospho raptor (Ser792), total glycogen synthase (GS), phospho GS (Ser641), total AMPKα, phospho AMPKα (Thr172), and phospho protein kinase B (PKB; Thr308) antibodies were from Cell Signaling Technology (Beverly, MA). Hexokinase II antibody (C-14) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The phospho GS antibodies (Ser8 and Ser8/11) (24) and the total AMPKα1 and AMPKα2 antibodies used for immunoprecipitation were generated and donated by Professor D. Grahame Hardie (University of Dundee). GLUT4 antibody was generated and donated by Professor Geoffrey Holman (University of Bath). Generation of the TBC1D1 (total [S279C, 1st bleed] and antiphospho Ser231 [S131C, 2nd bleed]) (25), phosphorylase (total [S956A, 2nd bleed] and antiphospho Ser15 [S960A, 1st bleed]), and total PKBα (S742B, 2nd bleed) antibodies (26) has been previously described.
Incubation of isolated muscle.
Mice were killed by cervical dislocation, and extensor digitorum longus (EDL) muscles were rapidly removed and mounted on an incubation apparatus as described (26). Muscles were incubated in 2 mL Krebs-Ringer bicarbonate (KRB) buffer (in mmol/L: 117 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 KH2PO4, 1.2 MgSO4, and 24.6 NaHCO3, pH 7.4) containing 5.5 mmol/L d-glucose for 40 min at 37°C gassed continuously with 95% O2 and 5% CO2. At the end of the incubation period muscles were frozen in liquid nitrogen and stored at −80°C.
Measurement of glucose transport.
EDL muscles were isolated and 2-deoxy-[3H]glucose uptake was measured as described (27). Briefly, muscles were incubated in 2 mL KRB containing 2 mmol/L pyruvate for 40 min at 37°C. Muscles were transferred to 2 mL KRB containing 1 mmol/L 2-deoxyglucose (1.5 mCi/mmol 2-deoxy-d-[1,2-3H(N)]glucose) and 7 mmol/L d-mannitol (0.064 mCi/mmol d-[1-14C]mannitol) and incubated for an additional 10 min at 30°C. Muscles were frozen in liquid nitrogen and acid hydrolysates subjected to scintillation counting (27).
Measurement of glycogen synthesis.
d-[U-14C]glucose incorporation into muscle glycogen was determined as previously described (26). Briefly, EDL muscles were incubated in 2 mL KRB containing 5.5 mmol/L d-glucose and 0.1 mCi/mmol d-[U-14C]glucose for 40 min at 37°C. Muscles were frozen in liquid nitrogen and [14C]glycogen extracted by ethanol precipitation from KOH digests as described (26).
Measurement of glycolysis.
The rate of glycolysis was determined by the detritiation of [5-3H]glucose as described (22). Briefly, muscles were incubated in 2 mL KRB containing 5.5 mmol/L glucose and 0.5 mCi/mmol [5-3H]glucose for 40 min at 37°C. Muscles were frozen in liquid nitrogen, and the rate of [5-3H]glucose incorporation into glycogen was determined as described for d-[U-14C]glucose. [3H]H2O was isolated from conditioned KRB by borate complex ion exchange chromatography and measured by scintillation counting.
Preparation of muscle lysates.
Muscle lysates were prepared as described (26). Homogenates were clarified at 3,000g for 10 min at 4°C, and protein concentration was estimated using Bradford reagent and bovine serum albumin (BSA) as standard. Lysates were frozen in liquid nitrogen and stored at −80°C.
Immunoblotting.
Muscle extracts (20–30 μg) were denatured in SDS sample buffer, separated by SDS-PAGE, and transferred to polyvinylidene fluoride membrane. Membranes were blocked for 1 h in 20 mmol/L Tris-HCl (pH 7.5), 137 mmol/L NaCl, and 0.1% (v/v) Tween-20 (TBST) containing 5% (w/v) skimmed milk. Membranes were incubated in primary antibody prepared in TBST containing 5% (w/v) BSA overnight at 4°C. Detection was performed using horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence reagent.
Assay of glycogen synthase and phosphorylase.
Muscle homogenates (25 μg) were assayed for glycogen synthase and phosphorylase activity (reverse direction) by measuring the incorporation of UDP-[U-14C]glucose and [U-14C]glucose-1-phosphate respectively into glycogen, as described (22). Results are expressed as the activity ratio in the absence and presence of 10 mmol/L G6P (glycogen synthase) or 2 mmol/L AMP (phosphorylase).
AMPK activity assay.
AMPK was immunoprecipitated from 30 μg lysate with antibodies against the α1 and α2 subunits and assayed for phosphotransferase activity toward AMARA peptide (AMARAASAAALARRR) using [γ-32P]ATP, as previously described (28).
Assay of muscle glycogen.
Frozen muscles were digested in 100 μL of 1 mol/L KOH for 20 min at 80°C. The pH was adjusted to 4.8 with 50 μL of 4 mol/L acetic acid and 250 μL of 4 units/mL amyloglucosidase (Aspergillus niger) in 0.2 mol/L sodium acetate (pH 4.8 added). Samples were incubated for 2 h at 40°C, clarified at 16,000g for 10 min, and neutralized with NaOH. Glucose released from glycogen was determined using a commercial hexokinase/G6P dehydrogenase (G6PDH) coupled assay (Amresco, Solon, OH) using d-glucose as a standard.
Assay of muscle G6P.
G6P was assayed fluorometrically in HClO4 extracts of EDL muscle, as previously described (22).
Statistical analyses.
Data are expressed as means ± SEM. Statistical analysis was performed by unpaired, two-tailed Student t test or one-way or two-way ANOVA with Dunnett post hoc test. Differences between groups were considered statistically significant when P < 0.05.
RESULTS
Pharmacologic activation of AMPK leads to inactivation of muscle glycogen synthase.
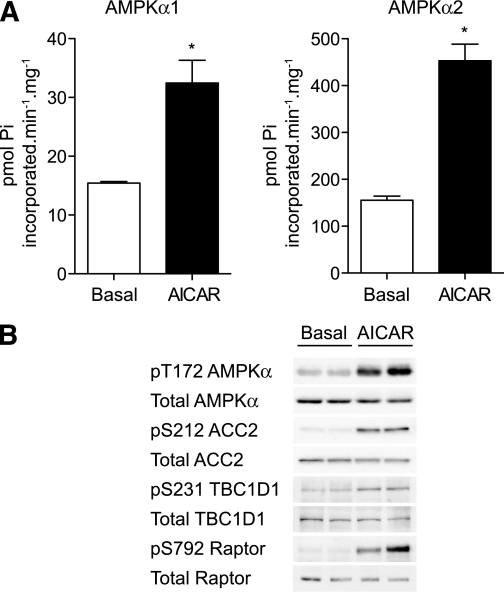
We first measured the effect of the pharmacologic AMPK activator, AICAR, on AMPK activity in EDL muscle (composed mainly of fast-twitch, glycolytic fibers) isolated from male C57BL/6 mice. EDL was used because the effects of AICAR on AMPK activity and glucose uptake are reported to be robust in this muscle (29). As previously reported (27), incubation of EDL ex vivo with AICAR promoted robust phosphorylation of the AMPKα catalytic subunit at Thr172 in the T-loop and activation of both AMPKα1 (∼twofold) and AMPKα2 (∼fourfold) compared with unstimulated muscle (Fig. 1A and B). Consistent with these observations, AICAR treatment increased phosphorylation of known AMPK substrates such as ACC2 at Ser212, raptor at Ser792 (30), and TBC1D1 at Ser231 (25,31) (Fig. 1B).
FIG. 1.
AICAR robustly stimulates activation of AMPK in EDL muscle. EDL muscles from male C57BL/6J mice (7–10 weeks old) were incubated with vehicle or 2 mmol/L AICAR for 40 min in KRB containing 5.5 mmol/L glucose. A: AMPK was immunoprecipitated from muscle lysates using anti-AMPKα1 or anti-AMPKα2 antibodies and assayed for activity using AMARA peptide. Assays were performed in duplicate from muscles derived from four mice and are expressed as mean ± SEM. B: Lysates were immunoblotted to assess phosphorylation of components of the AMPK pathway. Results are representative of experiments performed on tissues from at least three mice. *P < 0.05.
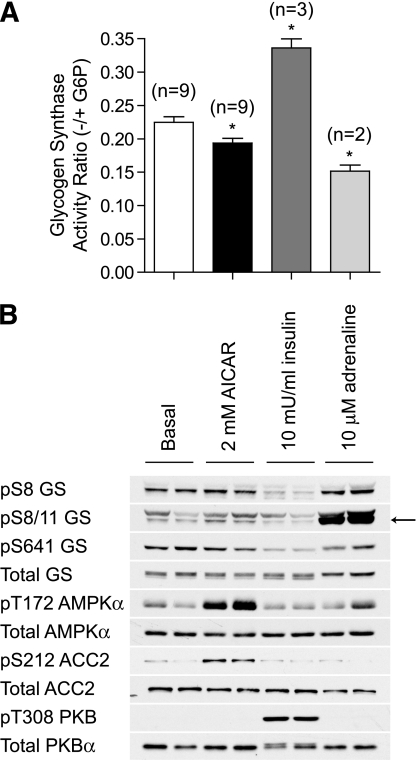
We next assessed the effect of AICAR on phosphorylation and activity of GS in isolated EDL muscle. Muscles were incubated in parallel with GS-activating (insulin) and GS-inhibiting (adrenaline) hormones as controls. AICAR treatment resulted in a modest but significant decrease in GS activity (∼10%) (Fig. 2A). However, in contrast to previous studies (13,14), no detectable change in the phosphorylation of GS at Ser8, an inhibitory site that is targeted by AMPK (12), was observed (Fig. 2B). Because phosphorylation of Ser8 is known to promote subsequent phosphorylation of Ser11 by casein kinase 1, which cannot be detected by Ser8 phospho-specific antibody, we also monitored dual phosphorylation of Ser8 and Ser11 by a phospho-specific antibody that detects only when both sites are phosphorylated. AICAR failed to induce a detectable change in the phosphorylation of Ser8 and Ser11, whereas adrenaline robustly increased phosphorylation of these sites, correlating with a marked decrease in GS activity (Fig. 2A and B). AICAR did not modify phosphorylation of other key regulatory sites such as Ser641, whereas insulin promoted dephosphorylation of this site likely via the PKB/GS kinase 3 (GSK3) pathway (Fig. 2B), resulting in a ∼1.7-fold increase in GS activity (Fig. 2A and B), as previously reported (32).
FIG. 2.
AICAR modestly inhibits GS activity in vitro. EDL muscles from C57BL/6J mice were incubated with vehicle, 2 mmol/L AICAR, 10 mU/mL insulin, or 10 μmol/L adrenaline for 40 min in KRB containing 5.5 mmol/L glucose. A: GS activity was measured in muscle homogenates as described in research design and methods. Results are presented as mean ± SEM from the indicated number (n) of mice. B: Alternatively, lysates were immunoblotted for GS phosphorylation with the indicated antibodies. *P < 0.05 compared with basal.
AICAR stimulates muscle glycogen synthesis independent of the phosphoinositide-3 kinase pathway.
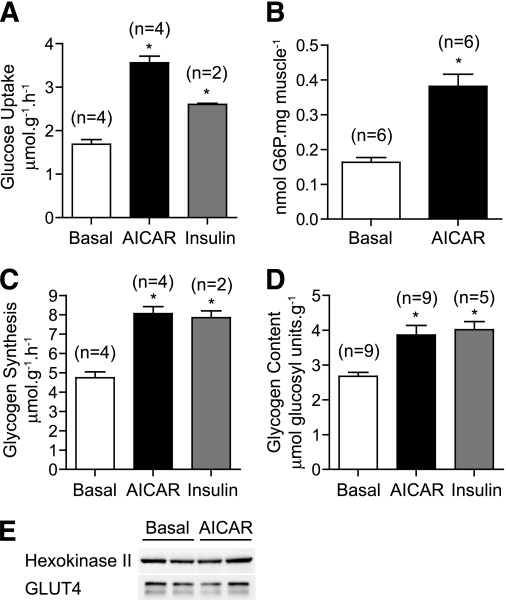
A well-established physiologic role of AMPK in muscle is to stimulate glucose transport (5). Incubation of EDL with AICAR or insulin significantly increased 2-deoxy-[3H]glucose uptake by ∼twofold and ∼1.5-fold, respectively (Fig. 3A). AICAR also caused an increase (2.5-fold) in the levels of G6P compared with resting EDL (Fig. 3B). Although chronic/continuous AICAR treatment is known to promote glucose transport and phosphorylation at least partially through an increase in the levels of GLUT4 and hexokinase II- (16,17) short-term incubation (40 min) did not cause detectable changes in the amount of these proteins (Fig. 3E). We next measured glycogen synthesis by monitoring incorporation of d-[14C-U]glucose into glycogen in response to AICAR or insulin. Both AICAR and insulin stimulated glycogen synthesis by ∼1.6-fold (Fig. 3C), which was associated with a significant increase (1.4-fold) in muscle glycogen concentration (Fig. 3D).
FIG. 3.
AICAR stimulates glucose uptake and storage into glycogen. EDL muscles from C57BL/6J mice were incubated with vehicle, 2 mmol/L AICAR, or 10 mU/mL insulin for 40 min in KRB containing 2 mmol/L pyruvate. A: Muscles were transferred to vials containing 2-deoxy-[3H]glucose and glucose transport assayed as described in research design and methods. B: Alternatively, EDL muscles incubated with vehicle or 2 mmol/L AICAR for 40 min were snap frozen in liquid nitrogen and glucose-6-phosphate (G6P) levels assayed as described in research design and methods. C: Isolated EDL muscles were incubated with the indicated stimuli for 40 min in KRB containing d-[U-14C]glucose (5.5 mmol/L), and the rate of glucose incorporation into glycogen was determined as described in research design and methods. D: Alternatively, EDL muscles were incubated in KRB containing unlabeled glucose (5.5 mmol/L) for 1 h, and glycogen content was determined by digestion of KOH extracts with amyloglucosidase as described in research design and methods. E: EDL muscles were incubated with vehicle or 2 mmol/L AICAR for 40 min and lysates immunoblotted with the indicated antibodies. Results are presented as mean ± SEM from the indicated number (n) of mice. *P < 0.05 compared with basal.
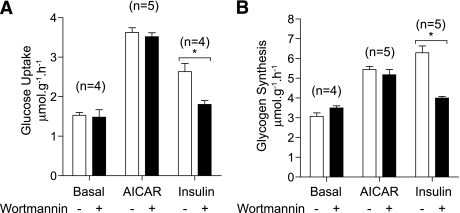
The effect of AICAR on both glucose uptake and glycogen synthesis was distinct from those by insulin, and the phosphoinositide-3 kinase pathway as wortmannin, a selective phosphoinositide-3 kinase inhibitor, abolished insulin-stimulated glucose transport and glycogen synthesis, but not in response to AICAR (Fig. 4A and B).
FIG. 4.
AICAR-stimulated glucose disposal is independent of phosphoinositide-3 (PI-3) kinase. EDL muscles from C57BL/6J mice were incubated with vehicle (0.2% DMSO) or 100 nmol/L wortmannin for 20 min in KRB/pyruvate. A: Muscles were transferred to fresh KRB/glucose containing matched vehicle/compound and the indicated stimuli for 40 min. Finally, glucose transport was assayed using 2-deoxy-[3H]glucose as described in research design and methods. B: Alternatively, muscles preincubated with vehicle or wortmannin as described in (A) were transferred to KRB containing d-[U-14C]glucose (5.5 mmol/L) and the indicated stimuli and incubated for 40 min. The rate of glucose incorporation into glycogen was determined as described in research design and methods. Results are expressed as mean ± SEM from the indicated number (n) of mice. *P < 0.05 compared with basal.
AICAR has no effect on muscle glycogen phosphorylase activity.
Net glycogen content and incorporation of [14C]glucose are dependent on both glycogen synthesis and degradation. Consequently, the increased glycogen content observed in AICAR-treated muscles could be partly due to a decrease in glycogen degradation. To rule out this possibility, we assessed the activity of muscle glycogen phosphorylase, a rate-limiting enzyme in glycogen degradation. Phosphorylase activity and phosphorylation at Ser15, an activating residue that is catalyzed by phosphorylase kinase, were both unchanged in response to AICAR or insulin (Fig. 5A and B). To confirm that our assay was sensitive enough to detect changes in phosphorylase activity, isolated EDL muscles were also incubated in the presence of adrenaline, an activator of phosphorylase through a phosphorylase kinase-dependent pathway. As expected, adrenaline caused a significant activation of phosphorylase (twofold) that was accompanied by an increase (∼50%) in phosphorylation of Ser15 (Fig. 5B).
FIG. 5.
AICAR has no effect on glycogen phosphorylase activity in vitro. EDL muscles were treated as described in Fig. 2 and lysates were assayed for phosphorylase activity (A) or immunoblotted with the indicated antibodies (B). Results are presented as mean ± SEM from the indicated number (n) of mice. *P < 0.05 compared with basal.
Catalytic activity of AMPK is required for AICAR-stimulated muscle glycogen synthesis.
To establish that AICAR-stimulated glycogen synthesis is mediated through AMPK and not by off-target action(s) of the compound, we assessed the effect of AICAR on glycogen synthesis in EDL from mice expressing catalytically inactive/kinase dead (KD) AMPKα2 (23). Immunoblot analysis using anti-pan-AMPKα antibody confirmed that KD AMPK, epitope-tagged with Myc, displayed a slightly slower mobility and endogenous AMPKα was absent (Fig. 6D). As previously reported, AMPKα2 activity was largely eliminated, and AMPKα1 was also substantially reduced at rest in EDL from AMPK KD animals (Fig. 6A), an effect likely due to the displacement of endogenous α2 and also α1 from βγ heterotrimers by over-expressed KD α2, as has previously been suggested (23). Consistent with previous studies (33), AICAR significantly stimulated both AMPKα1 and AMPKα2 activity in wild-type mice, whereas neither AMPKα1 nor α2 activity was significantly increased in muscles from AMPK KD mice (Fig. 6A). AICAR caused a robust increase in ACC2 phosphorylation, which was partially suppressed (∼30–40%) in AMPK KD mice (Fig. 6D), as previously reported (33).
FIG. 6.
AICAR-stimulated glycogen synthesis is AMPK-dependent. A: Isolated EDL muscles from 14- to 18-week-old wild-type (WT, □) or AMPKα2 KD mice (▨) were incubated with the indicated stimuli for 40 min, and AMPK activity was determined as described in Fig. 1. B: Alternatively, muscles were incubated with the indicated stimuli for 40 min in KRB containing 5.5 mmol/L glucose and GS activity measured in tissue homogenates, as described in research design and methods. C: Muscles were incubated with the indicated stimuli for 40 min in KRB containing d-[U-14C]glucose (5.5 mmol/L), and the rate of glucose incorporation into glycogen was determined as described in research design and methods. D: Muscles from WT and AMPKα2 KD mice were incubated with the indicated stimuli for 40 min and tissue lysates immunoblotted with the indicated antibodies. Results are presented as mean ± SEM from the indicated number (n) of mice. *P < 0.05.
We next measured the effect of AICAR on GS activity in EDL isolated from AMPK KD or wild-type littermate control animals. In unstimulated muscles, GS activity was significantly higher (about twofold) in AMPK KD compared with wild-type mice (Fig. 6B), which was associated with a modest decrease in GS phosphorylation at both Ser8 and Ser641 (Fig. 6D). AICAR caused a modest (∼10%) decrease in GS activity in wild-type mice but not in AMPK KD animals, demonstrating that AMPK catalytic activity, most likely α2 activity (13), is required for GS inactivation by AICAR (Fig. 6B). AICAR did not significantly promote single Ser8 and dual Ser8/11 phosphorylation in EDL from wild-type or AMPK KD mice. Insulin promoted GS dephosphorylation on Ser641 and activation in muscles of wild-type and AMPK KD mice to a relatively similar degree (Fig. 6B and D). Insulin did not cause a significant change in Ser8 phosphorylation, although some muscle showed a slightly reduced phospho signal on this site (Fig. 6D). We next measured glycogen synthesis and observed that AICAR-stimulated increases were abolished in AMPK KD animals, whereas insulin stimulated glycogen synthesis in both genotypes to the same extent (Fig. 6C). Total protein expression and phosphorylation of phosphorylase was similar between wild-type and AMPK KD muscles in both resting and AICAR-treated mice (Fig. 6D).
Allosteric activation of GS is required for AICAR-stimulated muscle glycogen synthesis.
To establish that AICAR-stimulated glycogen synthesis is mediated through allosteric activation of GS by G6P, we have used G6P-insensitive GS knock-in mice in which a critical G6P permissive residue (Arg582) is changed to Ala (GSR582A/R582A). We have recently reported that mutant GS derived from GSR582A/R582A mouse skeletal muscle is completely resistant to G6P but retains its capacity to be activated through dephosphorylation by GSK3 inhibition in response to insulin (22).
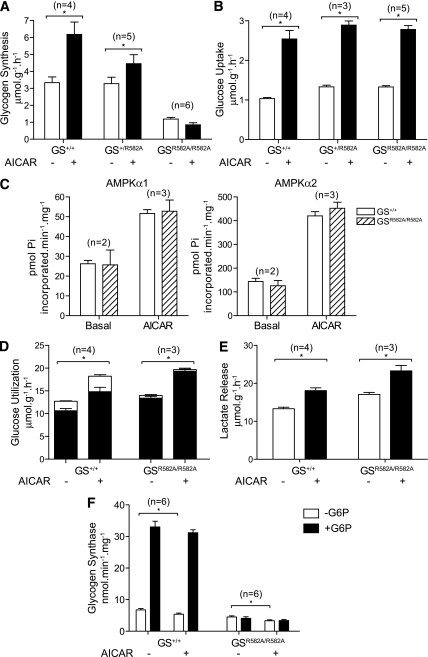
As reported previously, glycogen synthesis in resting EDL was comparable between wild-type and GSR582A/+ mice, and there was a substantial decrease (∼70–80%) in glycogen synthesis in GSR582A/R582A animals compared with wild-type or GSR582A/+. AICAR stimulated glycogen synthesis in wild-type and GSR582A/+ mice by ∼twofold and ∼1.5-fold, respectively, whereas AICAR had no effect on glycogen synthesis in GSR582A/R582A animals (Fig. 7A). There was a trend that AICAR modestly inhibited glycogen synthesis in GSR582A/R582A mice, possibly through deactivation of GS or stimulation of phosphorylase. To rule out the possibility that the reduced glycogen synthesis observed in GS+/R582A and GSR582A/R582A mice was caused by reduced glucose transport or altered AMPK activity, or both, these parameters were measured in isolated EDL muscle in the presence or absence of AICAR. We observed no difference in basal or in AICAR-stimulated glucose uptake across all genotypes (Fig. 7B). We also confirmed that resting and AICAR-stimulated AMPK activity (α1 and α2) were similar between wild-type and GSR582A/R582A mice (Fig. 7C).
FIG. 7.
AICAR-stimulated glycogen synthesis is dependent on allosteric activation of GS. GS R582A is unresponsive to allosteric activation by G6P (22). EDL muscles from the indicated genotypes were incubated with vehicle or 2 mmol/L AICAR in KRB containing d-[U-14C]glucose (5.5 mmol/L) for 40 min. A: Glycogen synthesis was assayed as described in research design and methods. B: Alternatively muscles were incubated with vehicle or 2 mmol/L AICAR and glucose transport assayed using 2-deoxy-[3H]glucose as described in research design and methods. C: Basal or AICAR-stimulated muscles from GS+/+ (□) and GSR582A/R582A (▨) mice were homogenized and assayed for AMPK activity as described in Fig. 1. D: Muscles were incubated with vehicle or 2 mmol/L AICAR in KRB containing [5-3H]glucose for 40 min and the rates of glycolysis (■) and glycogenesis (□) determined as described in research design and methods. E: Alternatively, muscles were incubated with vehicle or 2 mmol/L AICAR in KRB/glucose for 40 min, and the rate of lactate release into the superfusate was determined enzymatically. F: Muscles were incubated with vehicle or 2 mmol/L AICAR in KRB/glucose for 40 min and GS activity was measured in muscle homogenates as described in research design and methods. Results are expressed as mean ± SEM for the indicated number (n) of animals. *P < 0.05 compared with basal.
Interestingly, despite the significant impairment in glycogen synthesis, overall glucose utilization, as determined by metabolism of [5-3H]glucose was also similar between wild-type and GSR582A/R582A mice (Fig. 7D). The decrease in glycogen synthesis in GSR582A/R582A mice was compensated by a significant increase in the rate of glycolysis in both resting and AICAR-stimulated muscles. Consistent with this finding, the rate of lactate release was also elevated under resting and AICAR-stimulated conditions in GSR582A/R582A mice compared with wild-type (Fig. 7E). We confirmed that GS activity in resting EDL muscles was comparable between wild-type and GSR582A/R582A mice when assayed in the absence of G6P, whereas the robust G6P-mediated activation observed in wild-type mice was completely abolished in the muscle of GSR582A/R582A mice (Fig. 7F). AICAR modestly deactivated muscle GS in both wild-type and GSR582A/R582A animals (Fig. 7F). Potentially, hypophosphorylation of GS by inhibition of GSK3 may compensate for the lack of allosteric activation by G6P in GSR582A/R582A mice. Accordingly, we co-incubated EDL muscle with AICAR and the GSK3 selective inhibitor, CT99021, and observed a significant, albeit modest increase in glycogen synthesis compared with muscle treated with AICAR alone (Supplementary Fig. 1).
DISCUSSION
We (8) and others (16,17) previously reported that AICAR treatment caused an increase in muscle glycogen levels; however, whether this was mediated through AMPK has not been clearly demonstrated. AICAR (Z-riboside) is a widely used AMPK activator that is taken up into cells by adenosine transporters and converted by adenosine kinase to the monophosphorylated derivative ZMP (34). ZMP binds to the γ subunit of AMPK and mimics the effect of AMP on the allosteric activation of the kinase and also on inhibition of dephosphorylation (35). However, because AICAR has been found to produce AMPK-independent effects due to binding of ZMP to other AMP/ZMP-regulated enzymes and also to unknown off-target effects (34), we wished to establish that AICAR-stimulated glycogen synthesis occurs in an AMPK-dependent manner.
Abbott Laboratories has recently identified a direct and specific activator of AMPK, the thienopyridine A-769662, which is a useful tool to understand the physiologic consequences of AMPK activation in animals (36–38). However, Scott et al. (39) have demonstrated that this compound selectively activates β1-containing AMPK trimeric (αβγ) complexes, but not β2-complexes in cell-free assays. They further investigated the selectivity of A-769662 in vivo and showed that A-769662 failed to stimulate AMPK in AMPKβ1-deficient mouse tissues (39). The two β1-containing AMPK heterotrimers (α1β1γ1 and α2β1γ1), as well as activity associated with these complexes, appear to constitute only a small fraction of the total pool of AMPK trimeric complex/activity (i.e., <5%) in mouse skeletal muscle (40). A-769662 treatment resulted in only a modest activation of AMPK (37,39) and failed to promote AMPK-dependent increases in glucose uptake in isolated mouse skeletal muscles (40). Therefore, A-769662 would not be a suitable tool to study the effect of AMPK on glucose/glycogen metabolism in mouse skeletal muscle. As an alternative approach, we used a genetically engineered mouse model, AMPK KD, in which AMPK is inactivated by transgenic over-expression of a dominant inhibitory AMPKα2 mutant in muscle cells (6), and established that catalytic activity of AMPK is necessary to elicit AICAR-stimulated glycogen synthesis most likely by increased glucose transport and subsequent accumulation of intracellular G6P.
Consistent with previous studies, AICAR modestly decreased the muscle GS activity ratio (13,15), a measure of phospho-dependent activity, in cell-free assays in wild-type animals, and this effect was lost in AMPK KD mice. However, we failed to detect a consistent elevation in GS phosphorylation at site 2 (Ser8) or site 2 + 2a (Ser8 and Ser11) in EDL incubated with AICAR ex vivo. These antibodies have been stringently validated (24), so it would appear that the effect of AICAR on site 2 phosphorylation is modest (as evidenced by only ∼10% decrease in GS activity), and in the current study, it failed to reach statistical significance.
This modest effect of AICAR on GS phosphorylation probably explains why a previous study failed to observe significant GS inhibition in skeletal muscle (8). A more robust effect of AICAR on GS activity and phosphorylation has been observed in rat skeletal muscle, although the reason for this is unclear (13). We tested if G6P directly affected GS phosphorylation by AMPK in response to AICAR. AMPK-mediated Ser8 phosphorylation of GS was not significantly altered in the presence of G6P in cell-free assays, although supraphysiologic concentrations of G6P (2 mmol/L) modestly reduced GS Ser8 phosphorylation by AMPK (Supplementary Fig. 2). Although we cannot completely rule out the possibility that AMPK inactivates GS through phosphorylation at other site(s), apart from Ser8, a previous in vitro phospho-mapping study did not identify additional phosphorylation sites (12). In support of this, there were no other obvious AMPK target site(s) (the typical consensus motif is φX[B, X)XX(Ser/Thr]XXXφ, where φ is a hydrophobic residue, B is basic, and X is any residue) (1) on GS when motif scan analysis (Scansite, http://scansite.mit.edu/) was performed. Regardless of the mechanism, the modest inactivation of GS by AMPK was overridden by the allosteric stimulator, G6P, resulting in elevated GS activity in vivo as evidenced by an increase in [14C]glucose incorporation into glycogen.
Gain-of-function mutation in AMPKγ1 (R70Q) (41) and naturally occurring mutations in γ3 (R200Q) originally identified in the Hampshire pig (Sus scrofa domesticus; RN-) (42), are known to promote marked glycogen accumulation in skeletal muscle. In addition, mutations in the AMPKγ2 subunit (encoded by the PRKAG2 gene) have been implicated in the human cardiomyopathy, Wolff-Parkinson-White syndrome, which is characterized by ventricular pre-excitation, and in certain cases, cardiac hypertrophy (18). Notably, several genetic studies have revealed that γ2 mutations cause excess myocardial glycogen accumulation (43–45), which is hypothesized to cause conduction system abnormalities by unknown mechanism(s) (18,46). Interestingly, Luptak et al. (9) demonstrated that transgenic mice over-expressing one of the γ2 mutations (N488I) in cardiomyocytes displayed aberrant high activity of AMPK resulting in elevated intracellular [G6P] due to increased glucose uptake, which serves as both the carbon skeleton for glycogen synthesis and the allosteric stimulator of GS. It would be of major interest to cross AMPKγ2 mutant transgenic animals with G6P-resistant GSR582A/R582A knock-in mice and investigate if abnormal cardiac glycogen accumulation and the associated pathologies can be rescued.
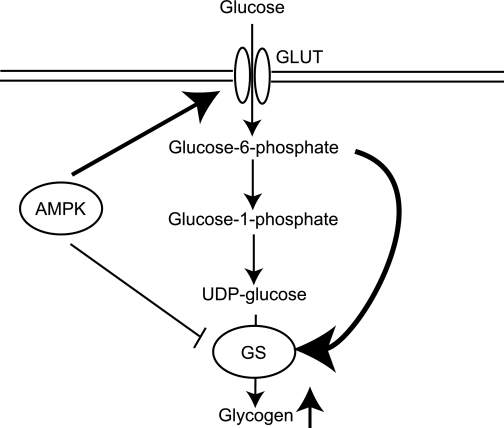
In summary, we provide genetic evidence that AMPK-mediated glycogen synthesis occurs by increased activity of GS through its allosteric stimulator, G6P, and we propose the following model (Fig. 8): elevated glucose transport promoted by increased AMPK activity causes an accumulation of intracellular G6P. This leads to allosteric activation of GS, which overrides the inhibitory effect of AMPK on GS resulting in a net increase in GS activity and excess glycogen storage in muscle cells. Our work is of particular importance when considering AMPK as a target for treating metabolic disorders such as type 2 diabetes (47) because chronic/persistent activation of AMPK may have adverse consequences on cardiac function.
FIG. 8.
Schematic summary shows the AMPK-mediated increase in muscle glycogen storage. Elevated glucose transport promoted by increased AMPK activity over an extended period, in the absence of a proportional increase in utilization of glucose, causes an accumulation of intracellular G6P. This leads to persistent allosteric activation of GS, which overrides the direct inhibitory effect of AMPK on GS and results in a net increase in GS activity and excess glycogen storage in muscle.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by Diabetes UK (07/0003529 to K.S.), Dundee and district of Diabetes U.K. volunteer group, British Heart Foundation (PG/09/059 to K.S.), the U.K. Medical Research Council, Danish Medical Research Councils, the Novo Nordisk Research Foundation, and the Lundbeck Foundation. This work was done as a part of the research program of the UNIK: Food, Fitness & Pharma for Health and Disease (see www.foodfitnesspharma.ku.dk). The UNIK project is supported by the Danish Ministry of Science, Technology and Innovation.
This study also received support from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck–Serono, and Pfizer to K.S. No other potential conflicts of interest relevant to this study were reported.
R.W.H. designed and conducted most of the experiments, analyzed data, and wrote the manuscript. J.T.T. and J.F.P.W. conducted experiments, analyzed data, reviewed and edited the manuscript, and contributed to discussion. K.S. designed and conducted experiments, analyzed data, wrote the manuscript, and also supervised the project.
The authors thank Morris Birnbaum (University of Pennsylvania) for the donation of AMPK kinase dead animals, Gail Fraser and members of the resource unit for genotyping and technical assistance, and the antibody purification teams (Division of Signal Transduction Therapy, University of Dundee, Dundee, Scotland, U.K.), coordinated by Hilary McLauchlan and James Hastie.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-1148/-/DC1.
REFERENCES
- 1.Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev 2009;89:1025–1078 10.1152/physrev.00011.2008 [DOI] [PubMed] [Google Scholar]
- 2.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 2005;1:15–25 10.1016/j.cmet.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 3.Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes (Lond) 2008;32(Suppl. 4):S7–S12 10.1038/ijo.2008.116 [DOI] [PubMed] [Google Scholar]
- 4.Carling D. The AMP-activated protein kinase cascade—a unifying system for energy control. Trends Biochem Sci 2004;29:18–24 10.1016/j.tibs.2003.11.005 [DOI] [PubMed] [Google Scholar]
- 5.Hardie DG, Sakamoto K. AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology (Bethesda) 2006;21:48–60 [DOI] [PubMed] [Google Scholar]
- 6.Mu J, Barton ER, Birnbaum MJ. Selective suppression of AMP-activated protein kinase in skeletal muscle: update on ‘lazy mice’. Biochem Soc Trans 2003;31:236–241 10.1042/BST0310236 [DOI] [PubMed] [Google Scholar]
- 7.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab 2007;5:237–252 10.1016/j.cmet.2007.03.006 [DOI] [PubMed] [Google Scholar]
- 8.Aschenbach WG, Hirshman MF, Fujii N, Sakamoto K, Howlett KF, Goodyear LJ. Effect of AICAR treatment on glycogen metabolism in skeletal muscle. Diabetes 2002;51:567–573 10.2337/diabetes.51.3.567 [DOI] [PubMed] [Google Scholar]
- 9.Luptak I, Shen M, He H, et al. Aberrant activation of AMP-activated protein kinase remodels metabolic network in favor of cardiac glycogen storage. J Clin Invest 2007;117:1432–1439 10.1172/JCI30658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrence JC, Jr, Roach PJ. New insights into the role and mechanism of glycogen synthase activation by insulin. Diabetes 1997;46:541–547 10.2337/diabetes.46.4.541 [DOI] [PubMed] [Google Scholar]
- 11.Roach PJ. Glycogen and its metabolism. Curr Mol Med 2002;2:101–120 10.2174/1566524024605761 [DOI] [PubMed] [Google Scholar]
- 12.Carling D, Hardie DG. The substrate and sequence specificity of the AMP-activated protein kinase. Phosphorylation of glycogen synthase and phosphorylase kinase. Biochim Biophys Acta 1989;1012:81–86 10.1016/0167-4889(89)90014-1 [DOI] [PubMed] [Google Scholar]
- 13.Jørgensen SB, Nielsen JN, Birk JB, et al. The alpha2-5’AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes 2004;53:3074–3081 10.2337/diabetes.53.12.3074 [DOI] [PubMed] [Google Scholar]
- 14.Wojtaszewski JF, Jørgensen SB, Hellsten Y, Hardie DG, Richter EA. Glycogen-dependent effects of 5-aminoimidazole-4-carboxamide (AICA)-riboside on AMP-activated protein kinase and glycogen synthase activities in rat skeletal muscle. Diabetes 2002;51:284–292 10.2337/diabetes.51.2.284 [DOI] [PubMed] [Google Scholar]
- 15.Miyamoto L, Toyoda T, Hayashi T, et al. Effect of acute activation of 5′-AMP-activated protein kinase on glycogen regulation in isolated rat skeletal muscle. J Appl Physiol 2007;102:1007–1013 10.1152/japplphysiol.01034.2006 [DOI] [PubMed] [Google Scholar]
- 16.Holmes BF, Kurth-Kraczek EJ, Winder WW. Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol 1999;87:1990–1995 [DOI] [PubMed] [Google Scholar]
- 17.Ojuka EO, Nolte LA, Holloszy JO. Increased expression of GLUT-4 and hexokinase in rat epitrochlearis muscles exposed to AICAR in vitro. J Appl Physiol 2000;88:1072–1075 [DOI] [PubMed] [Google Scholar]
- 18.Arad M, Seidman CE, Seidman JG. AMP-activated protein kinase in the heart: role during health and disease. Circ Res 2007;100:474–488 10.1161/01.RES.0000258446.23525.37 [DOI] [PubMed] [Google Scholar]
- 19.Thomas JA, Schlender KK, Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem 1968;25:486–499 10.1016/0003-2697(68)90127-9 [DOI] [PubMed] [Google Scholar]
- 20.Pederson BA, Cheng C, Wilson WA, Roach PJ. Regulation of glycogen synthase. Identification of residues involved in regulation by the allosteric ligand glucose-6-P and by phosphorylation. J Biol Chem 2000;275:27753–27761 [DOI] [PubMed] [Google Scholar]
- 21.Hanashiro I, Roach PJ. Mutations of muscle glycogen synthase that disable activation by glucose 6-phosphate. Arch Biochem Biophys 2002;397:286–292 10.1006/abbi.2001.2623 [DOI] [PubMed] [Google Scholar]
- 22.Bouskila M, Hunter RW, Ibrahim AF, et al. Allosteric regulation of glycogen synthase controls glycogen synthesis in muscle. Cell Metab 2010;12:456–466 10.1016/j.cmet.2010.10.006 [DOI] [PubMed] [Google Scholar]
- 23.Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 2001;7:1085–1094 10.1016/S1097-2765(01)00251-9 [DOI] [PubMed] [Google Scholar]
- 24.Højlund K, Staehr P, Hansen BF, et al. Increased phosphorylation of skeletal muscle glycogen synthase at NH2-terminal sites during physiological hyperinsulinemia in type 2 diabetes. Diabetes 2003;52:1393–1402 10.2337/diabetes.52.6.1393 [DOI] [PubMed] [Google Scholar]
- 25.Chen S, Murphy J, Toth R, Campbell DG, Morrice NA, Mackintosh C. Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem J 2008;409:449–459 10.1042/BJ20071114 [DOI] [PubMed] [Google Scholar]
- 26.Bouskila M, Hirshman MF, Jensen J, Goodyear LJ, Sakamoto K. Insulin promotes glycogen synthesis in the absence of GSK3 phosphorylation in skeletal muscle. Am J Physiol Endocrinol Metab 2008;294:E28–E35 10.1152/ajpendo.00481.2007 [DOI] [PubMed] [Google Scholar]
- 27.Sakamoto K, McCarthy A, Smith D, et al. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J 2005;24:1810–1820 10.1038/sj.emboj.7600667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakamoto K, Zarrinpashneh E, Budas GR, et al. Deficiency of LKB1 in heart prevents ischemia-mediated activation of AMPKalpha2 but not AMPKalpha1. Am J Physiol Endocrinol Metab 2006;290:E780–E788 10.1152/ajpendo.00443.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jørgensen SB, Viollet B, Andreelli F, et al. Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside but not contraction-induced glucose uptake in skeletal muscle. J Biol Chem 2004;279:1070–1079 10.1074/jbc.M306205200 [DOI] [PubMed] [Google Scholar]
- 30.Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 2008;30:214–226 10.1016/j.molcel.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chavez JA, Roach WG, Keller SR, Lane WS, Lienhard GE. Inhibition of GLUT4 translocation by Tbc1d1, a Rab GTPase-activating protein abundant in skeletal muscle, is partially relieved by AMP-activated protein kinase activation. J Biol Chem 2008;283:9187–9195 10.1074/jbc.M708934200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McManus EJ, Sakamoto K, Armit LJ, et al. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J 2005;24:1571–1583 10.1038/sj.emboj.7600633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dzamko N, Schertzer JD, Ryall JG, et al. AMPK-independent pathways regulate skeletal muscle fatty acid oxidation. J Physiol 2008;586:5819–5831 10.1113/jphysiol.2008.159814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guigas B, Sakamoto K, Taleux N, et al. Beyond AICA riboside: in search of new specific AMP-activated protein kinase activators. IUBMB Life 2009;61:18–26 10.1002/iub.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fogarty S, Hardie DG. Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer. Biochim Biophys Acta 2010;1804:581–591 [DOI] [PubMed] [Google Scholar]
- 36.Cool B, Zinker B, Chiou W, et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab 2006;3:403–416 10.1016/j.cmet.2006.05.005 [DOI] [PubMed] [Google Scholar]
- 37.Göransson O, McBride A, Hawley SA, et al. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J Biol Chem 2007;282:32549–32560 10.1074/jbc.M706536200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanders MJ, Ali ZS, Hegarty BD, Heath R, Snowden MA, Carling D. Defining the mechanism of activation of AMP-activated protein kinase by the small molecule A-769662, a member of the thienopyridone family. J Biol Chem 2007;282:32539–32548 10.1074/jbc.M706543200 [DOI] [PubMed] [Google Scholar]
- 39.Scott JW, van Denderen BJ, Jorgensen SB, et al. Thienopyridone drugs are selective activators of AMP-activated protein kinase beta1-containing complexes. Chem Biol 2008;15:1220–1230 10.1016/j.chembiol.2008.10.005 [DOI] [PubMed] [Google Scholar]
- 40.Treebak JT, Birk JB, Hansen BF, Olsen GS, Wojtaszewski JF. A-769662 activates AMPK beta1-containing complexes but induces glucose uptake through a PI3-kinase-dependent pathway in mouse skeletal muscle. Am J Physiol Cell Physiol 2009;297:C1041–C1052 10.1152/ajpcell.00051.2009 [DOI] [PubMed] [Google Scholar]
- 41.Barré L, Richardson C, Hirshman MF, et al. Genetic model for the chronic activation of skeletal muscle AMP-activated protein kinase leads to glycogen accumulation. Am J Physiol Endocrinol Metab 2007;292:E802–E811 10.1152/ajpendo.00369.2006 [DOI] [PubMed] [Google Scholar]
- 42.Milan D, Jeon JT, Looft C, et al. A mutation in PRKAG3 associated with excess glycogen content in pig skeletal muscle. Science 2000;288:1248–1251 10.1126/science.288.5469.1248 [DOI] [PubMed] [Google Scholar]
- 43.Akman HO, Sampayo JN, Ross FA, et al. Fatal infantile cardiac glycogenosis with phosphorylase kinase deficiency and a mutation in the gamma2-subunit of AMP-activated protein kinase. Pediatr Res 2007;62:499–504 [DOI] [PubMed] [Google Scholar]
- 44.Folmes KD, Chan AY, Koonen DP, et al. Distinct early signaling events resulting from the expression of the PRKAG2 R302Q mutant of AMPK contribute to increased myocardial glycogen. Circ Cardiovasc Genet 2009;2:457–466 10.1161/CIRCGENETICS.108.834564 [DOI] [PubMed] [Google Scholar]
- 45.Arad M, Benson DW, Perez-Atayde AR, et al. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J Clin Invest 2002;109:357–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gollob MH. Glycogen storage disease as a unifying mechanism of disease in the PRKAG2 cardiac syndrome. Biochem Soc Trans 2003;31:228–231 10.1042/BST0310228 [DOI] [PubMed] [Google Scholar]
- 47.Hardie DG. AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol 2007;47:185–210 10.1146/annurev.pharmtox.47.120505.105304 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.