Abstract
OBJECTIVE
Bariatric surgery causes durable weight loss. Gut hormones are implicated in obesity pathogenesis, dietary failure, and mediating gastrointestinal bypass (GIBP) surgery weight loss. In mice, we determined the effects of diet-induced obesity (DIO), subsequent dieting, and GIBP surgery on ghrelin, peptide YY (PYY), and glucagon-like peptide-1 (GLP-1). To evaluate PYY’s role in mediating weight loss post-GIBP, we undertook GIBP surgery in PyyKO mice.
RESEARCH DESIGN AND METHODS
Male C57BL/6 mice randomized to a high-fat diet or control diet were killed at 4-week intervals. DIO mice underwent switch to ad libitum low-fat diet (DIO-switch) or caloric restriction (CR) for 4 weeks before being killed. PyyKO mice and their DIO wild-type (WT) littermates underwent GIBP or sham surgery and were culled 10 days postoperatively. Fasting acyl-ghrelin, total PYY, active GLP-1 concentrations, stomach ghrelin expression, and colonic Pyy and glucagon expression were determined. Fasting and postprandial PYY and GLP-1 concentrations were assessed 30 days postsurgery in GIBP and sham pair-fed (sham.PF) groups.
RESULTS
DIO progressively reduced circulating fasting acyl-ghrelin, PYY, and GLP-1 levels. CR and DIO-switch caused weight loss but failed to restore circulating PYY to weight-appropriate levels. After GIBP, WT mice lost weight and exhibited increased circulating fasting PYY and colonic Pyy and glucagon expression. In contrast, the acute effects of GIBP on body weight were lost in PyyKO mice. Fasting PYY and postprandial PYY and GLP-1 levels were increased in GIBP mice compared with sham.PF mice.
CONCLUSIONS
PYY plays a key role in mediating the early weight loss observed post-GIBP, whereas relative PYY deficiency during dieting may compromise weight-loss attempts.
Obesity is a global health concern, yet nonsurgical therapies remain limited. Body weight is controlled by complex physiologic systems in which hormones signal body energy stores and nutrient intake to central nervous system pathways controlling energy homeostasis. Gut hormones play an important role in regulating body weight (1) and may be involved in the pathogenesis of obesity (2–4). However, the temporal relationship between obesity development and altered gut hormones is unknown.
Dietary modifications, such as altering macronutrient composition or restricting caloric intake, are the first-line obesity treatments. However, weight-loss maintenance is often difficult (5). Dieting-induced compensatory gut hormone changes may contribute to the failure of weight loss through dietary means (6). Gastrointestinal bypass (GIBP) surgery is an effective obesity treatment, significantly reducing morbidity and mortality (7). Although mechanisms underlying the weight-loss effects of GIBP remain largely unknown, alterations in gut hormones engendered by these procedures may play a causal role (8,9). Two hormones implicated in the pathogenesis of obesity, dietary failure, and GIBP weight-loss benefits are the orexigenic hormone acyl-ghrelin and the anorectic hormone peptide YY (PYY) (8,9). Ghrelin is produced predominantly by the stomach; circulating levels increase during fasting and decrease postprandially (6). Acylation by the enzyme ghrelin O-acyl transferase (GOAT; mBOAT4) is essential for ghrelin’s biological activity (10,11). Diet-induced weight loss increases circulating ghrelin, potentially compromising weight-loss maintenance (12). The effects of GIBP on circulating ghrelin concentrations is controversial, with some studies reporting low levels despite marked weight loss (9,13,14).
The truncated form of PYY, PYY3–36, is an anorectic hormone (1). PYY is produced by enteroendocrine l-cells found mainly in the distal gastrointestinal tract. Circulating PYY3–36 concentrations increase postprandially and remain elevated during the intermeal period (15). Plasma PYY levels are reduced in obesity (3). However, unlike leptin, obese subjects remain responsive to the anorectic actions of exogenous PYY3–36 (2). Mouse genetic studies also implicate PYY in regulating body weight: PyyKO mice are hyperphagic and obese (3), whereas Pyy overexpressing mice are protected against the development of diet-induced and genetic obesity (16). Although the development of obesity results in reduced circulating PYY (3,4), the effects of subsequent diet-induced weight loss are unclear (17–19). GIBP surgery has been reported to increase fasting and nutrient-stimulated circulating PYY concentrations (20,21). The gut l-cells also synthesize the incretin hormone glucagon-like peptide-1 (GLP-1) (1). The effects of obesity and weight loss through dieting on circulating GLP-1 levels are unclear with variable findings reported. In contrast, the majority of studies report that circulating nutrient-stimulated GLP-1 levels are increased after GIBP (8).
Rodents fed a high-fat diet (HFD), analogous in fat content and calorie density to Western diets, represent an animal model of common human obesity (22). Thus, we investigated the temporal changes in ghrelin, PYY, and GLP-1 and the changes that occur with the development of diet-induced obesity (DIO) in mice in response to an HFD. Next, we evaluated the effects of weight loss induced by dietary modification and GIBP surgery on ghrelin, PYY, and GLP-1. To determine PYY’s role in mediating the early weight loss seen after GIBP, we undertook GIBP in PyyKO mice.
RESEARCH DESIGN AND METHODS
Mice.
Mice were maintained on a 12-h light/dark cycle (0700–1900 h) under constant temperature and housed in specific pathogen-free facilities. All studies were performed in accordance to the Home Office Animal Procedures Act (1986) U.K. and the principles and guidelines established by the European Convention for the Protection of Laboratory Animals. Six-week-old male C57BL/6 mice were purchased from Charles River U.K. Ltd. (Margate, U.K.). PyyKO mice and littermate control mice on a C57BL/6 background were generated and genotyped as described previously (3). HFD (D12451) and control low-fat diet (LFD) (control diet) (D12450B) were obtained from Research Diets (New Brunswick, NJ). The dietary composition is presented in Supplementary Table 1.
Study 1: investigation of the temporal changes in ghrelin, PYY, and GLP-1 with HFD-induced obesity.
Male C57BL/6 mice, aged 88 weeks, were randomized to ad libitum HFD or control diet (n = 40 per group). Body weight was monitored weekly. Ten mice from each diet group were killed after a 16-h overnight fast after 4, 8, 12, and 16 weeks of dietary exposure. Fasted bloods and tissues were harvested as described next.
Study 2: investigation of the effects of dietary modification on ghrelin, PYY, and GLP-1 in DIO mice.
Male C57BL/6 mice, aged 48 weeks, were randomized to ad libitum HFD (n = 30) or LFD (n = 10). After 16 weeks, the HFD group was randomized to one of two different dietary groups for 4 weeks: continued ad libitum HFD (DIO) (n = 10) or switched to ad libitum control diet (n = 20). After switching to the control diet for 1 week, mice were then further randomized to either dieting/caloric restriction (CR) or to continue ad libitum control diet (DIO-switch) (n = 10). CR was carried out by a step-down regimen (23). Mice were killed after an overnight 16-h fast, and blood and tissue were collected.
Study 3: evaluation of the effects of GIBP surgery on ghrelin, PYY, and GLP-1 and the role of endogenous PYY in mediating weight loss.
Male PyyKO mice (n = 30) and their wild-type (WT) littermates (n = 30) were weaned at 3 weeks of age. PyyKO mice gain more weight on control diet and HFD than their WT littermates (3). Thus, to enable weight-matching, 6-week-old WT mice were commenced on HFD and the PyyKO mice continued on control diet until age 14 weeks when they were switched to HFD. At 24 weeks of age, 16 weight-matched mice from each group were transported to facilities where the surgery was to be performed. Mice were acclimatized for 1 week before randomization to bypass or sham surgery (n = 8).
Mice were fasted overnight for 16 h preoperatively. The GIBP group underwent entero-gastro anastomosis (EGA) as previously described under isoflurane anesthesia (24). Briefly, a midline laparotomy was performed and the pyloric sphincter was ligatured, followed by an EGA between the mid-jejunum and stomach fundus, excluding the duodenum and the proximal jejunum from nutrient flow (Supplementary Fig. 1). Sham-operated counterparts were identically anesthetized, a midline incision was made, and the stomach and intestines were exposed and manipulated. The incision was kept open for an amount of time corresponding to that for the EGA surgery. Two mice died postoperatively, one WT sham and one PyyKO bypass, leaving seven WT sham-operated (WT.S), eight WT bypass (WT.BP), eight PyyKO sham-operated (PyyKO.S), and seven PyyKO bypass (PyyKO.BP). Mice were monitored daily for general well-being and killed 10 days postoperatively after an overnight 16-h fast. Blood and tissue were collected. The pyloric ligation was checked and was intact in all EGA animals.
Study 4: evaluation of the effects of weight loss induced by GIBP and pair-feeding on fasting and postprandial GLP-1 and PYY.
Male C57Bl6 mice, aged 8 weeks, were ad libitum fed an HFD for 3 months. Weight-matched groups were then randomized to either EGA or sham.pair-fed (sham.PF) (n = 5). Postsurgery the food intake/day per mouse was monitored in the EGA group, and the pair-fed group received the same amount of HFD food (sham.PF). Thirty days postsurgery, mice were fasted overnight and a tail bleed was taken. The EGA group was re-fed for 90 min, and the amount of food consumed was calculated (0.3 ± 0.1 g). A postprandial tail bleed was taken. The sham.PF group was re-fed the same amount of food in 90 min, and a postprandial tail bleed was taken. Fasted and fed plasma active GLP-1 and total PYY concentrations were assayed.
Terminal procedures and tissue harvesting.
Mice in studies 1–3 were killed by terminal anesthesia. Blood was collected by cardiac puncture and processed as previously described (15). The gastrointestinal tract was dissected; the whole stomach and a 3-cm section of descending colon were rapidly removed, cleared of peritoneum, fat, and digestive contents, snap-frozen in liquid nitrogen, and stored at −80°C until analysis.
Quantitative PCR analyses of gene expression.
Total RNA was extracted using TRIzol reagent, and 2 μg of RNA was reverse transcribed to cDNA. Real-time quantitative PCR was performed as previously described using proprietary sequence Taqman Gene Expression assay FAM/TAMRA probes (Applied Biosystems, Warrington, U.K.) (3).
Hormone assays.
All samples were run in duplicate. To reduce interassay variation, all samples from studies 1 and 2 were run in one assay, as were all samples from studies 3 and 4. Plasma leptin was measured in studies 1–3 as a marker of adiposity (25). Plasma leptin and active GLP-1 concentrations were measured using commercially available ELISA kits (Millipore, Watford, U.K.). There are no specific PYY3–36 assays for rodents; thus, we measured total PYY. Plasma acyl-ghrelin and total PYY concentrations were measured by commercially available radioimmunoassay (Millipore, Watford, U.K.) (Supplementary Table 2 for further assay details).
Statistical analysis.
Data are presented as means ± SEM. Comparisons between groups were made using one-way ANOVA with Dunnett post hoc tests or Student t test where appropriate. For all statistical analyses, P < 0.05 was considered significant.
RESULTS
Study 1: temporal changes in ghrelin, PYY, and GLP-1 with the development of HFD-induced obesity.
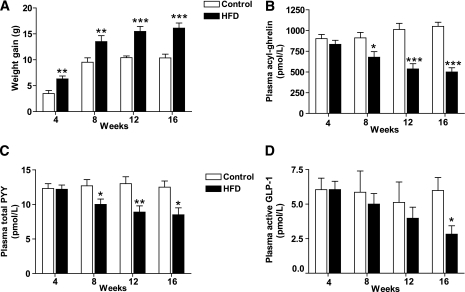
Ad libitum HFD access caused greater weight gain than control diet (Fig. 1A, Table 1). HFD mice displayed the expected fasting hyperleptinemia (Table 1). Fasting circulating acyl-ghrelin levels remained stable over time in the control-diet mice, whereas fasting circulating acyl-ghrelin progressively decreased in HFD mice (Fig. 1B). A negative correlation between body weight and acyl-ghrelin was observed at all time points studied (Supplementary Fig. 2A–D). In contrast with reports of reduced stomach ghrelin mRNA expression in DIO mice (26–28), no differences were observed in fasted stomach ghrelin expression between control and HFD groups (Table 1). At the 4-week time point, there was a trend toward an inverse relationship between stomach ghrelin and body weight (r = −0.43, P = 0.06). No difference was observed in stomach Mboat4 expression between HFD and control groups (Table 1). Correlation analysis between body weight and stomach Mboat4 expression revealed an inverse relationship at the 4-week (r = −0.48, P = 0.03; Supplementary Fig. 2E) and 8-week time points (r = −0.49, P = 0.03; Supplementary Fig. 2F). Similarly, after 12 weeks there was a trend toward a negative correlation (r = −0.39, P = 0.09), but after 16 weeks there was no relationship (r = 0.02, P = 0.93). We found no relationship between plasma acyl-ghrelin and stomach Mboat4 expression or between acyl-ghrelin and stomach ghrelin expression.
FIG. 1.
Effect of HFD on body weight, fasting plasma concentrations of leptin, acyl-ghrelin, total PYY, and active GLP-1. Cohorts of mice were exposed to control diet or HFD for 4, 8, 12, and 16 weeks and then killed in the fasted state. Body weight gain (A), plasma acyl-ghrelin (B), plasma total PYY (C), and plasma active GLP-1 (D) were assessed. Data are expressed as means ± SE. n = 10. *P < 0.05; **P < 0.01; ***P < 0.001 for HFD vs. control diet.
TABLE 1.
Effect of HFD on fasting body weight, plasma leptin, stomach expression of ghrelin and Mboat4, and colonic expression of Pyy and glucagon
| Weeks of diet |
||||||||
|---|---|---|---|---|---|---|---|---|
| 4 |
8 |
12 |
16 |
|||||
| Control | HFD | Control | HFD | Control | HFD | Control | HFD | |
| Body weight (g) | 24.5 ± 1.6 | 27.9 ± 0.7 | 28.0 ± 1.2 | 32.7 ± 1.2 | 28.4 ± 0.4 | 34.3 ± 1.1 | 29.9 ± 1.0 | 35.5 ± 1.1 |
| P < 0.05 | P < 0.01 | P < 0.001 | ||||||
| Leptin (ng/mL) | 0.6 ± 0.1 | 3.2 ± 1.0 | 0.8 ± 0.2 | 3.1 ± 1.0 | 1.2 ± 0.5 | 8.3 ± 2.7 | 1.0 ± 0.1 | 8.5 ± 2.1 |
| P < 0.05 | P < 0.05 | P < 0.01 | ||||||
| Stomach | ||||||||
| ghrelin (AU) | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.1 | 1.3 ± 0.1 |
| Stomach | ||||||||
| Mboat4 (AU) | 0.7 ± 0.5 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.1 |
| Colonic Pyy (AU) | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.1 |
| Colonic | ||||||||
| glucagon (AU) | 1.0 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.1 |
Data are means ± SE. Body weights and fasting expression of stomach ghrelin and Mboat4 and colonic Pyy and glucagon after 4, 8, 12, and 16 weeks' exposure to control diet or HFD.
Next, we evaluated the effects of HFD on fasting circulating total PYY levels. After 4 weeks, total PYY levels were similar in both groups. However, continued HFD exposure and the development of obesity resulted in reduced fasting total PYY levels in the HFD group (Fig. 1C). Consistent with previous findings in humans (2,29), a negative correlation was observed between body weight and circulating PYY concentrations at 8, 12, and 16 weeks (Supplementary Fig. 3). In contrast, fasted colonic Pyy expression was unaffected (Table 1). After 4 weeks of HFD, fasted active GLP-1 concentrations were similar in both groups (Fig. 1D). Active GLP-1 levels tended to be lower in the HFD group after 8 and 12 weeks of different diets and by 16 weeks were significantly reduced (Fig. 1D). Colonic glucagon expression was unaltered (Table 1).
Study 2: investigation of the effects of dietary modification on ghrelin, PYY, and GLP-1 in DIO mice.
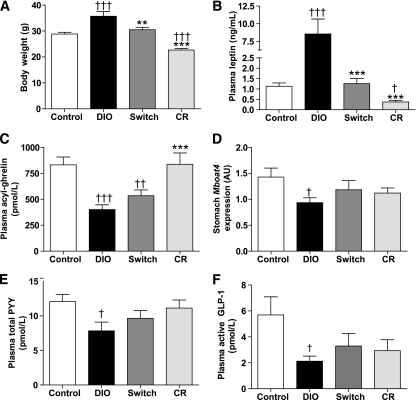
Dietary modification, DIO-switch or CR, reduced body weight, whereas weight remained stable in DIO and control mice (Supplementary Fig. 4A). After 4 weeks of dietary intervention, control and DIO-switch mice had similar body weights and CR mice weighed significantly less (Fig. 2A). Fasting leptin levels reflected body weight: highest in the DIO group, lowest in the CR group, and similar in control and DIO-switch mice (Fig. 2B).
FIG. 2.
Effect of dietary modification on body weight, fasting concentrations of leptin, acyl-ghrelin, total PYY, active GLP-1, and stomach expression of Mboat4. DIO mice were randomized to three groups, continued HFD (DIO), switch to control diet (Switch), or calorically restricted (CR), for 4 weeks and then killed after an overnight fast. Body weight (A), plasma leptin (B), plasma acyl-ghrelin (C), stomach Mboat4 expression (D), plasma total PYY (E), and plasma active GLP-1 (F) were assessed. Data are expressed as means ± SE. n = 10. **P < 0.01; ***P < 0.001 vs. DIO; †P < 0.05; ††P < 0.01; †††P < 0.001 vs. control diet.
As in study 1, fasting acyl-ghrelin levels were reduced in the DIO mice compared with control and CR mice (Fig. 2C). DIO-switch mice had fasting acyl-ghrelin concentrations intermediate between those observed in the control and DIO groups. Fasting acyl-ghrelin concentrations in the CR group were comparable to those in the control group despite CR mice weighing 21.3 ± 2.0% less than control mice. We evaluated the relationship between body weight and plasma acyl-ghrelin and found a negative correlation (r = −0.46, P = 0.003; Supplementary Fig. 4B). No effect of HFD or dietary modification on stomach ghrelin expression was apparent (ghrelin expression: control = 1.00 ± 0.08 arbitrary units [AU], DIO = 0.94 ± 0.06 AU, DIO-switch = 0.94 ± 0.06 AU, and CR = 0.91 ± 0.08 AU). However, stomach Mboat4 expression was reduced in the DIO compared with the control-diet mice (P = 0.02, Fig. 2D). Correlation analyses revealed no relationship among stomach ghrelin, Mboat4, and circulating acyl-ghrelin (data not shown).
DIO mice had reduced circulating total PYY and active GLP-1 concentrations compared with control mice (Fig. 2E and F). Fasting total PYY and active GLP-1 levels tended to increase in DIO mice subjected to dietary modification (DIO-switch and CR) but did not reach significance compared with DIO mice (Fig. 2E and F). CR mice had fasting circulating total PYY and active GLP-1 levels similar to those observed in control mice despite weighing significantly less. Colonic Pyy and glucagon expression were similar in all groups (Supplementary Table 3).
Study 3: effect of EGA surgery on ghrelin, PYY, and GLP-1 and the role of endogenous PYY in mediating weight loss.
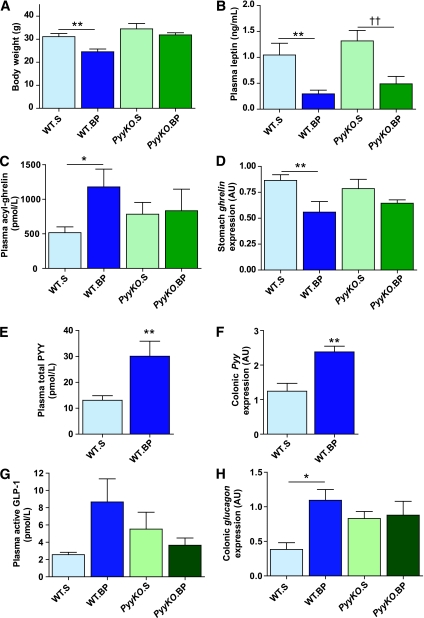
The preoperative body weights of the four different groups—WT.S, WT.BP, PyyKO.S, and PyyKO.BP—were similar (body weight: WT.S = 35.9 ± 1.2 g, WT.BP = 36.0 ± 1.1 g, PyyKO.S = 37.7 ± 2.3 g, and PyyKO.BP = 37.0 ± 1.5 g). Ten days postoperatively, WT.BP mice weighed less (Fig. 3A, Supplementary Fig. 5A) and had lost more body weight than WT.S mice (weight loss: WT.S = 4.7 ± 0.6 g, WT.BP = 11.4 ± 1.3 g, WT.S vs. WT.BP g; P < 0.001). However, body weight and weight loss in PyyKO.S and PyyKO.BP groups were similar (weight loss: PyyKO.S = 4.3 ± 0.6 g, PyyKO.BP = 5.1 ± 0.8 g; P = 0.43). WT.BP mice lost significantly more weight than PyyKO.BP mice (P = 0.002). Circulating leptin levels were reduced in WT.BP and PyyKO.BP mice compared with sham-operated mice (Fig. 3B). Fasting plasma acyl-ghrelin concentrations were elevated in WT.BP mice compared with WT.S mice (Fig. 3C). In contrast, no difference in circulating fasting acyl-ghrelin levels was observed between PyyKO.S and PyyKO.BP groups (Fig. 3C). In WT mice, fasting acyl-ghrelin concentrations correlated negatively with body weight (r = −0.58, P = 0.03). However, in the KO group no correlation between body weight and circulating acyl-ghrelin concentrations was observed. Stomach ghrelin expression was reduced in WT.BP mice (Fig. 3D). In the PyyKO.BP group, there was a trend for reduced stomach ghrelin expression (P = 0.18, Fig. 3D). Stomach Mboat4 expression was similar in all four groups (stomach Mboat4: WT.S = 1.00 ± 0.13 AU, WT.BP = 0.85 ± 0.10 AU, PyyKO.S = 0.86 ± 0.10 AU, and PyyKO.BP = 0.94 ± 0.09 AU). No relationship was found between stomach expression of ghrelin or Mboat4 and circulating acyl-ghrelin levels. Fasting circulating total PYY concentrations were markedly elevated in the WT.BP mice compared with WT.S mice (Fig. 3E). Similarly, colonic Pyy expression was increased in the WT.BP group (Fig. 3F). In the WT group, there was a negative correlation between body weight and circulating total PYY (r = −0.74, P = 0.002, Supplementary Fig. 5B) and between body weight and colonic Pyy expression (r = −0.6, P = 0.02, Supplementary Fig. 5C). Fasting active GLP-1 concentrations were higher in the WT.BP group compared with the WT.S group, but this failed to reach significance (Fig. 3E). Similar fasting active GLP-1 concentrations were observed in the PyyKO.S and PyyKO.BP groups (Fig. 3E). Fasting colonic glucagon expression was increased in WT.BP compared with WT.S mice (Fig. 3F). PyyKO.S mice had higher fasting colonic glucagon expression than WT.S mice (P = 0.01), but no difference was observed between PyyKO.S and PyyKO.BP groups.
FIG. 3.
Effect of GIBP surgery in WT and PyyKO mice. Male weight-matched DIO WT mice and PyyKO littermates underwent GIBP surgery or sham procedure. Ten days postoperatively mice were killed in the fasted state. Body weight (A), plasma leptin (B), plasma acyl-ghrelin (C), stomach ghrelin expression (D), plasma total PYY (E), colonic Pyy expression (F), plasma active GLP-1 (G), and colonic glucagon expression (H) were evaluated. Data are expressed as means ± SE. *P < 0.05; **P < 0.01; ††P < 0.01. WT.S, n = 7; WT.BP, n = 8; PyyKO.S, n = 8; PyyKO.BP, n = 7.
Study 4: evaluation of the effects of EGA and pair-feeding on fasting and postprandial GLP-1 and PYY.
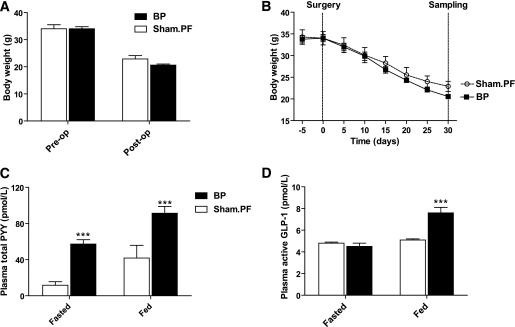
Preoperatively, the weights of EGA and sham.pair-fed groups were matched (body weight: EGA group = 34.0 ± 0.8 g, sham.pair-fed = 34.0 ± 1.5 g). Daily food intake of all mice preoperatively was 3.7 ± 1.2 g per mouse. Post-EGA food intake was reduced (food intake/mouse/day = 0.8 ± 0.2 g). Postsurgery, body weight decreased in both groups (Fig. 4A and B). There was a tendency for the EGA group to weigh less, but this did not reach significance (body weight: EGA = 20.6 ± 0.4 g, sham.pairfed = 22.9 ± 1.2 g, P = 0.10). Both fasting and postprandial levels of PYY were significantly higher in the EGA group compared with the sham.pair-fed group (Fig. 4C). Although fasting active GLP-1 concentrations were similar in EGA, postprandially active GLP-1 levels were significantly higher in the EGA group (Fig. 4D).
FIG. 4.
Effect of GIBP and sham.PF on body weight and fasting and postprandial concentrations of total PYY and active GLP-1. Male weight-matched DIO mice underwent GIBP surgery or sham procedure. The sham group was then pair-fed (sham.PF) to the GIBP group. Thirty days postsurgery, a tail bleed was taken after an overnight fast and 90 min postprandially. A: Body weight presurgery and 30 days postsurgery. B: Body weight curves in GIBP and sham.PF groups. C: Fasting and postprandial plasma total PYY. D: Plasma fasted and postprandial active GLP-1. Data are expressed as means ± SE. n = 5. ***P < 0.001 vs. sham.PF. BP, bypass.
DISCUSSION
We show that short-term HFD exposure per se does not alter circulating fasted acyl-ghrelin, total PYY, or active GLP-1 concentrations, because levels were unaltered after 4 weeks of HFD. However, with continued HFD and the development of obesity, circulating acyl-ghrelin, total PYY, and active GLP-1 become progressively reduced. In agreement with our previous studies (3), no effect of HFD or DIO on expression of stomach ghrelin was observed. HFD mice tended to have lower stomach Mboat4 expression, but this failed to reach significance until 20 weeks of HFD (Table 2). In study 1, we observed an inverse relationship between stomach Mboat4 expression and body weight at 4, 8, and 12 weeks, but this disappeared by 16 weeks of HFD. These Mboat4 changes may reflect alterations in leptin sensitivity because leptin has been shown to increase stomach Mboat4 expression in the fasted state (30).
TABLE 2.
Summary of changes in circulating hormone, stomach ghrelin and Mboat4 expression, and colonic Pyy and glucagon expression
| Study 1: 16 weeks of HFD vs. control diet | Study 2: Dietary modification |
Study 3: GIBP in WT and PyyKO mice | Study 4: EGA and sham.PF | |||
|---|---|---|---|---|---|---|
| HFD vs. control diet | Compared with control diet | Compared with DIO group | WT.BP vs. WT.S | PyyKO.BP vs. PyyKO.S | EGA vs. sham.PF | |
| Fasting acyl-ghrelin (pmol/L) | ↓*** | DIO ↓*** Switch ↓** CR ↔ | Switch ↔ CR↑*** | ↑* | ↔ | NA |
| Fasting total PYY (pmol/L) | ↓* | DIO ↓* Switch ↔ CR ↔ | Switch ↔ CR ↔ | ↑** | NA | ↑*** |
| Fed total PYY (pmol/L) | NA | NA | NA | NA | NA | ↑*** |
| Fasting active GLP-1 (pmol/L) | ↓* | DIO ↓* Switch ↔ CR ↔ | Switch ↔ CR ↔ | ↔ | ↔ | ↔ |
| Fed active GLP-1 (pmol/L) | NA | NA | NA | NA | NA | ↑*** |
| Stomach ghrelin (AU) | ↔ | ↔ | ↔ | ↓** | ↔ | NA |
| Stomach Mboat4 (AU) | ↔ | DIO ↓* Switch ↔ CR ↔ | Switch ↔ CR ↔ | ↔ | ↔ | NA |
| Colonic Pyy (AU) | ↔ | ↔ | ↔ | ↑** | NA | NA |
| Colonic glucagon (AU) | ↔ | ↔ | ↔ | ↑* | ↔ | NA |
Arrows indicate statistically significant differences between groups.
NA, not applicable.
*P < 0.05;
**P < 0.01;
***P < 0.001.
DIO-switch mice lost weight and exhibited body weights and leptin concentrations similar to those of the control mice, yet their acyl-ghrelin levels remained suppressed. These findings agree with those of Weigle et al. (31), who found that switching obese subjects from an HFD to ad libitum LFD resulted in a reduced caloric intake and weight loss without a concomitant increment in circulating ghrelin. One explanation for this is that reduced dietary fat intake improves leptin sensitivity, which in turn prevents an increase in ghrelin levels. Our study is the first to report the effect of dietary modification on stomach ghrelin and Mboat4 expression in DIO mice subjected to dietary modification as opposed to dietary restriction undertaken in normal-weight animals (32). We observed no effect of diet-induced weight loss on either stomach ghrelin or Mboat4 expression, suggesting that altered acyl-ghrelin levels relate to factors independent of ghrelin and Mboat4 expression, such as acylated-ghrelin degradation, GOAT enzymatic activity, or substrate availability (28). Further studies are now warranted to investigate the temporal effect of high-fat feeding and subsequent dietary modification on stomach expression of ghrelin, Mboat4, and circulating concentrations of acyl-ghrelin, des-acylghrelin, and GOAT.
Consistent with human studies (2,29), a negative correlation between body weight and fasting total PYY concentrations was observed in study 1. However, when previously DIO mice lost weight through dietary modification, this relationship between body weight and plasma PYY concentrations was lost, with lower circulating PYY levels observed than would have been predicted from body weight alone. In obese human subjects, reduced circulating PYY concentrations result in diminished satiety (4), whereas PYY administration to obese subjects decreases caloric intake (2). PYY administration to obese rats during dieting reduces maladaptive food-taking behavior (33), and chronic administration reduces adiposity (34). Moreover, in humans, PYY administration modulates neuronal activity within homeostatic and reward brain regions; the latter play a key role in determining feeding behavior in our “obesogenic” environment (35). Taken together, these findings suggest that PYY deficiency in obesity is likely to reinforce obesity and that the failure of circulating PYY concentrations to return to “weight-appropriate levels” during dieting may hamper both weight loss and weight-loss maintenance.
We show that colonic Pyy and glucagon expression remain unaltered during the development of obesity and during subsequent weight loss through dietary means. These findings suggest that obesity-induced modulation of circulating total PYY and active GLP-1 levels may occur posttranslationally because of defective secretion or altered breakdown.
Reports of circulating ghrelin post-GIBP in humans are highly variable (9,12–14). These inconsistencies likely reflect whether patients are studied while actively losing weight or when weight-stable. In our studies, fasting acyl-ghrelin levels were elevated in WT.BP mice compared with WT.S mice and were inversely related to body weight. However, we observed no difference in fasting acyl-ghrelin between PyyKO.BP and PyyKO.S groups, suggesting increased acyl-ghrelin was not due to the EGA surgical procedure itself. Exclusion of ghrelin-producing X/A-cells from nutrient contact has been proposed as a potential mechanism leading to increased circulating ghrelin levels in the early postoperative period (36). However, in our studies the stomach was not excluded from nutrient contact. Our findings suggest that increased fasting acyl-ghrelin concentration post-GIBP is due to weight loss (36). We found reduced stomach ghrelin expression in WT.BP mice compared with WT.S and a similar trend was seen in PyyKO.BP mice. This observation of diminished stomach ghrelin expression in both WT and PyyKO groups suggests that this is not due to weight loss, is independent of PYY, and does not depend on diversion of nutrients from the stomach. Reduced stomach ghrelin expression could antedate the development of subsequent suppressed circulating acyl-ghrelin levels that have been reported (12). Thus, further studies are needed to examine the temporal effects of GIBP on stomach ghrelin expression and circulating ghrelin concentrations.
To directly evaluate PYY’s role in mediating the early weight loss post-GIBP, we undertook EGA surgery in DIO WT mice and PyyKO mice. We found that GIBP increased fasting circulating PYY concentrations in WT mice and caused a twofold increment in Pyy colonic expression. Moreover, although WT.BP mice lost significantly more weight than WT.S mice, there was no difference in weight loss between PyyKO.BP and PyyKO.S groups, suggesting that PYY plays a key role in mediating early postoperative weight loss.
Our studies in sham-operated mice pair-fed to the GIBP group enabled us to investigate whether the PYY changes post-GIBP were due to CR or weight loss. Circulating concentrations of PYY and active GLP-1 are highest in the postprandial state; thus, in this experiment we also evaluated postprandial circulating PYY and active GLP-1. Weight loss in the EGA and sham-PF groups were similar, but fasting and postprandial total PYY and postprandial active GLP-1 concentrations were markedly elevated in the EGA group. These findings suggest that EGA alters total PYY and active GLP-1 independently of CR or weight loss.
Several different surgical procedures, sleeve gastrectomy (37), jejuno-ileal bypass (38,39), Roux-en-Y gastric bypass (RYGB) (37), and now EGA, result in increased circulating PYY concentrations, suggesting that neither stomach nor foregut exclusion is a prerequisite. Increased nutrient delivery to hindgut l-cells has been suggested to lead to increased postprandial PYY release. However, increased nutrient delivery cannot explain increased fasted PYY levels. This finding suggests that l-cell function is altered directly or indirectly by GIBP. Moreover, the finding that fasting PYY levels remain altered 20 years postsurgery suggests that this change is long-lasting (38).
Our studies have several limitations. RYGB involves bypassing the majority of the stomach with direct nutrient emptying from the stomach pouch into the mid-jejunum. Thus, nutrients are excluded from the majority of the stomach, duodenum, and proximal jejunum. EGA surgery undertaken in our studies (Supplementary Fig. 1) differs from RYGB in that the stomach was not excluded but nutrient passage into the duodenum was prevented by pyloric ligation. The stomach was then anastomosed to the mid-jejunum leading to bypass of the duodenum and proximal jejunum. Thus, our findings cannot be directly translated to RYGB surgery because the hormonal changes may be different. To standardize feeding status, our mice in studies 1–3 were culled in the fasted state. Circulating acyl-ghrelin levels are highest in the fasted state and then decrease postprandially, whereas PYY and active GLP-1 levels increase postprandially. Thus, additional insights would be gained from undertaking nutrient-stimulated studies. The numbers used in our studies were relatively low, and this may have contributed to the lack of significant differences between groups in some of our studies. Our sham EGA operation did not control for any inflammatory processes or possible postoperative ileus, which may have resulted in greater weight loss in the bypass mice. However, these factors would have affected WT and PyyKO mice alike, enabling us to conclude that PYY plays a key role in early postoperative weight reduction.
In summary, our studies provide important new information in three areas. First, our HFD temporal studies reveal that circulating acyl-ghrelin, total PYY, and active GLP-1 concentrations are progressively reduced with the development of DIO. Second, dietary modification induces weight loss but fails to restore circulating total PYY to weight-appropriate levels, potentially compromising ongoing dieting efforts. Third, the results from our EGA and sham-PF groups confirm that neither CR nor weight loss per se mediate the observed increase in fasting total PYY or increased postprandial total PYY and active GLP-1 concentration. Finally, we provide the first direct evidence that PYY plays a key role in mediating weight loss after GIBP. These results suggest that an increased understanding of the mechanisms regulating gut hormone expression and secretion and their roles in GIBP may result in new treatment strategies for obesity.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by the Rosetrees Trust, the Medical Research Council, and the Benjamin Delessert Institute.
No potential conflicts of interest relevant to this article were reported.
K.C. researched data and wrote the article. C.G. and E.K. researched data and reviewed the article. A.I.G., M.E.D., V.F., B.V., and F.A. researched data. D.J.W. researched data, contributed to discussion, and reviewed the article. R.L.B. researched data and wrote the article.
The authors thank Elaine Robins (University College London, London, U.K.) and Jenny Jones (University College London, London, U.K.) for technical help, and Dr. Colin Selman (Aberdeen University, Aberdeen, U.K.) for advice regarding the design of the CR experiments.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-0566/-/DC1.
REFERENCES
- 1.Karra E, Batterham RL. The role of gut hormones in the regulation of body weight and energy homeostasis. Mol Cell Endocrinol 2010;316:120–128 [DOI] [PubMed] [Google Scholar]
- 2.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med 2003;349:941–948 [DOI] [PubMed] [Google Scholar]
- 3.Batterham RL, Heffron H, Kapoor S, et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab 2006;4:223–233 [DOI] [PubMed] [Google Scholar]
- 4.le Roux CW, Batterham RL, Aylwin SJB, et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 2006;147:3–8 [DOI] [PubMed] [Google Scholar]
- 5.Tsai AG, Wadden TA. Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Intern Med 2005;142:56–66 [DOI] [PubMed] [Google Scholar]
- 6.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001;50:1714–1719 [DOI] [PubMed] [Google Scholar]
- 7.Sjöström L, Narbro K, Sjöström CD, et al. Swedish Obese Subjects Study Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–752 [DOI] [PubMed] [Google Scholar]
- 8.Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. JCEM 2004;89:2608–2615 [DOI] [PubMed] [Google Scholar]
- 9.Karra E, Yousseif A, Batterham RL. Mechanisms facilitating weight loss and resolution of type 2 diabetes following bariatric surgery. Trends Endocrinol Metab 2010;21:337–344 [DOI] [PubMed]
- 10.Hosoda H, Kojima M, Kangawa K. Ghrelin and the regulation of food intake and energy balance. Mol Interv 2002;2:494–503 [DOI] [PubMed]
- 11.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 2008;132:387–396 [DOI] [PubMed] [Google Scholar]
- 12.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 2002;346:1623–1630 [DOI] [PubMed] [Google Scholar]
- 13.Faraj M, Havel PJ, Phélis S, Blank D, Sniderman AD, Cianflone K. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab 2003;88:1594–1602 [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Fuentes E, Garrido-Sanchez L, Garcia-Almeida JM, et al. Different effect of laparoscopic Roux-en-Y gastric bypass and open biliopancreatic diversion of Scopinaro on serum PYY and ghrelin levels. Obes Surg 2008;18:1424–1429 [DOI] [PubMed] [Google Scholar]
- 15.Chandarana K, Drew ME, Emmanuel J, et al. Subject standardization, acclimatization, and sample processing affect gut hormone levels and appetite in humans. Gastroenterology 2009;136:2115–2126 [DOI] [PubMed] [Google Scholar]
- 16.Boey D, Lin S, Enriquez RF, et al. PYY transgenic mice are protected against diet-induced and genetic obesity. Neuropeptides 2008;42:19–30 [DOI] [PubMed] [Google Scholar]
- 17.Moran LJ, Noakes M, Clifton PM, et al. Postprandial ghrelin, cholecystokinin, peptide YY, and appetite before and after weight loss in overweight women with and without polycystic ovary syndrome. Am J Clin Nutr 2007;86:1603–1610 [DOI] [PubMed] [Google Scholar]
- 18.Roth CL, Enriori PJ, Harz K, Woelfle J, Cowley MA, Reinehr T. Peptide YY is a regulator of energy homeostasis in obese children before and after weight loss. J Clin Endocrinol Metab 2005;90:6386–6391 [DOI] [PubMed] [Google Scholar]
- 19.Valderas JP, Irribarra V, Boza C, et al. Medical and surgical treatments for obesity have opposite effects on peptide YY and appetite: a prospective study controlled for weight loss. J Clin Endocrinol Metab 2010;95:1069–1075 [DOI] [PubMed]
- 20.Korner J, Inabnet W, Conwell IM, et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring) 2006;14:1553–1561 [DOI] [PubMed] [Google Scholar]
- 21.Morínigo R, Vidal J, Lacy AM, Delgado S, Casamitjana R, Gomis R. Circulating peptide YY, weight loss, and glucose homeostasis after gastric bypass surgery in morbidly obese subjects. Ann Surg 2008;247:270–275 [DOI] [PubMed] [Google Scholar]
- 22.Lin S, Thomas TC, Storlien LH, Huang XF. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord 2000;24:639–646 [DOI] [PubMed] [Google Scholar]
- 23.Miller RA, Chang Y, Galecki AT, Al-Regaiey K, Kopchick JJ, Bartke A. Gene expression patterns in calorically restricted mice: partial overlap with long-lived mutant mice. Mol Endocrinol 2002;16:2657–2666 [DOI] [PubMed] [Google Scholar]
- 24.Troy S, Soty M, Ribeiro L, et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab 2008;8:201–211 [DOI] [PubMed] [Google Scholar]
- 25.Van Heek M, Compton DS, France CF, et al. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest 1997;99:385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HM, Wang G, Englander EW, Kojima M, Greeley GH., Jr Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology 2002;143:185–190 [DOI] [PubMed] [Google Scholar]
- 27.Moesgaard SG, Ahrén B, Carr RD, Gram DX, Brand CL, Sundler F. Effects of high-fat feeding and fasting on ghrelin expression in the mouse stomach. Regul Pept 2004;120:261–267 [DOI] [PubMed] [Google Scholar]
- 28.Gahete MD, Córdoba-Chacón J, Salvatori R, Castaño JP, Kineman RD, Luque RM. Metabolic regulation of ghrelin O-acyl transferase (GOAT) expression in the mouse hypothalamus, pituitary, and stomach. Mol Cell Endocrinol 2010;317:154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo Y, Ma L, Enriori PJ, et al. Physiological evidence for the involvement of peptide YY in the regulation of energy homeostasis in humans. Obesity (Silver Spring) 2006;14:1562–1570 [DOI] [PubMed] [Google Scholar]
- 30.González CR, Vázquez MJ, López M, Diéguez C. Influence of chronic undernutrition and leptin on GOAT mRNA levels in rat stomach mucosa. J Mol Endocrinol 2008;41:415–421 [DOI] [PubMed] [Google Scholar]
- 31.Weigle DS, Cummings DE, Newby PD, et al. Roles of leptin and ghrelin in the loss of body weight caused by a low fat, high carbohydrate diet. J Clin Endocrinol Metab 2003;88:1577–1586 [DOI] [PubMed] [Google Scholar]
- 32.Reimer RA, Maurer AD, Lau DC, Auer RN. Long-term dietary restriction influences plasma ghrelin and GOAT mRNA level in rats. Physiol Behav 2010;99:605–610 [DOI] [PMC free article] [PubMed]
- 33.Ghitza UE, Nair SG, Golden SA, et al. Peptide YY3-36 decreases reinstatement of high-fat food seeking during dieting in a rat relapse model. J Neurosci 2007;27:11522–11532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vrang N, Madsen AN, Tang-Christensen M, Hansen G, Larsen PJ. PYY(3-36) reduces food intake and body weight and improves insulin sensitivity in rodent models of diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 2006;291:R367–R375 [DOI] [PubMed] [Google Scholar]
- 35.Batterham RL, Ffytche DH, Rosenthal JM, et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature 2007;450:106–109 [DOI] [PubMed] [Google Scholar]
- 36.Aprahamian CJ, Tekant G, Chen M, et al. A rat model of childhood diet-induced obesity: Roux-en-Y gastric bypass induced changes in metabolic parameters and gastric peptide ghrelin. Pediatr Surg Int 2007;23:653–657 [DOI] [PubMed] [Google Scholar]
- 37.Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg 2008;247:401–407 [DOI] [PubMed] [Google Scholar]
- 38.Näslund E, Grybäck P, Hellström PM, et al. Gastrointestinal hormones and gastric emptying 20 years after jejunoileal bypass for massive obesity. Int J Obes Relat Metab Disord 1997;21:387–392 [DOI] [PubMed] [Google Scholar]
- 39.le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 2006;243:108–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.