Abstract
OBJECTIVE
We have provided evidence that saturated fatty acids, which are released from adipocytes via macrophage-induced adipocyte lipolysis, serve as a naturally occurring ligand for the Toll-like receptor (TLR) 4 complex in macrophages, thereby aggravating obesity-induced adipose tissue inflammation. The aim of this study was to identify the molecule(s) activated in adipose tissue macrophages in obesity.
RESEARCH DESIGN AND METHODS
We performed a cDNA microarray analysis of coculture of 3T3-L1 adipocytes and RAW264 macrophages. Cultured adipocytes and macrophages and the adipose tissue of obese mice and humans were used to examine mRNA and protein expression.
RESULTS
We found that macrophage-inducible C-type lectin (Mincle; also called Clec4e and Clecsf9), a type II transmembrane C-type lectin, is induced selectively in macrophages during the interaction between adipocytes and macrophages. Treatment with palmitate, a major saturated fatty acid released from 3T3-L1 adipocytes, induced Mincle mRNA expression in macrophages at least partly through the TLR4/nuclear factor (NF)-κB pathway. Mincle mRNA expression was increased in parallel with macrophage markers in the adipose tissue of obese mice and humans. The obesity-induced increase in Mincle mRNA expression was markedly attenuated in C3H/HeJ mice with defective TLR4 signaling relative to control C3H/HeN mice. Notably, Mincle mRNA was expressed in bone-marrow cell (BMC)-derived proinflammatory M1 macrophages rather than in BMC-derived anti-inflammatory M2 macrophages in vitro.
CONCLUSIONS
Our data suggest that Mincle is induced in adipose tissue macrophages in obesity at least partly through the saturated fatty acid/TLR4/NF-κB pathway, thereby suggesting its pathophysiologic role in obesity-induced adipose tissue inflammation.
Adipose tissue of obese animals and humans is characterized by adipocyte hypertrophy, followed by increases in angiogenesis, macrophage infiltration, and extracellular matrix and unbalanced production of pro- and anti-inflammatory adipocytokines (1–3). The dynamic change seen in adipose tissue during the course of obesity has been referred to as adipose tissue remodeling (4). Given their multifunctional roles in a variety of biological contexts, macrophages should play a central role in adipose tissue remodeling, thereby regulating adipocytokine production (2,4). Recent studies have pointed to at least two different polarization states of adipose tissue macrophages: M1 or “classically activated” (or proinflammatory) macrophages (5), which are induced by proinflammatory mediators such as lipopolysaccharide (LPS) and Th1 cytokine interferon (IFN)-γ, and M2 or “alternatively activated” (or anti-inflammatory) macrophages, which are generated in vitro by exposure to Th2 cytokines such as interleukin (IL)-4 and IL-13. It is noteworthy that macrophages, which are infiltrated into the adipose tissue during the course of obesity, exhibit the phenotypic switch from M2 to M1 polarization (6).
To explore the molecular mechanism underlying the crosstalk between adipocytes and macrophages during the course of adipose tissue remodeling, we have developed an in vitro coculture system composed of 3T3-L1 adipocytes and RAW264 macrophages and provided evidence that a paracrine loop involving saturated fatty acids and tumor necrosis factor (TNF)-α derived from adipocytes and macrophages, respectively, establishes a vicious cycle, thereby accelerating the inflammatory change in the adipose tissue in obesity (7). Interestingly, saturated fatty acids, which are released via macrophage-induced adipocyte lipolysis, may act as naturally occurring ligands for the Toll-like receptor (TLR) 4 complex, which is essential for the recognition of LPS, to induce nuclear factor (NF)-κB activation in macrophages (8). With the aid of the coculture system, we recently have identified activating transcription factor 3, a member of basic leucine zipper-type transcription factors, which is induced in adipose tissue macrophages through the saturated fatty acid/TLR4 pathway, thereby regulating transcriptionally the obesity-induced macrophage activation (9). We, therefore, think the coculture system would provide a unique in vitro experimental system with which to investigate the molecular basis underlying obesity-induced adipose tissue inflammation.
Through a combination of cDNA microarray analyses of the coculture of 3T3-L1 adipocytes and RAW264 macrophages (7), we found that macrophage-inducible C-type lectin (Mincle; also called Clec4e and Clecsf9), a type II transmembrane C-type lectin, is induced selectively in macrophages during the interaction between adipocytes and macrophages. Mincle originally was identified as a transcriptional target of CCAAT/enhancer binding protein β in macrophages in response to proinflammatory stimuli such as LPS, TNF-α, IL-6, and IFN-γ (10). Our data also suggest that Mincle is induced in M1 macrophages in the adipose tissue in obesity through the saturated fatty acid/TLR4/NF-κB pathway. This study is the first detailed analysis of a C-type lectin in adipose tissue macrophages, thereby providing a novel insight into the molecular mechanism underlying adipose tissue inflammation.
RESEARCH DESIGN AND METHODS
Reagents.
LPS (from Escherichia coli O111: B4) and BAY11-7085, an NF-κB inhibitor, were purchased from Sigma (San Diego, CA) and Merck (Whitehouse Station, NJ), respectively. Palmitate and oleate were purchased from Sigma, solubilized in ethanol, and conjugated with fatty acid- and immunoglobulin-free BSA (Sigma) at a molar ratio of 10 to 1 (fatty acids to BSA) in a low-serum medium as described (7). The concentrations of palmitate and oleate used in this study (<200 μmol/L) were within the physiologic range. All other reagents were purchased from Sigma or Nacalai Tesque (Kyoto, Japan), unless otherwise described.
Animals.
Male C3H/HeJ (HeJ) mice, which have defective LPS signaling attributed to a missense mutation in the TLR4 gene (11), and control C3H/HeN (HeN) mice were purchased from CLEA Japan (Tokyo, Japan). Male C57BL/6 J leptin-deficient ob/ob mice and their wild-type littermates were purchased from Charles River Japan (Tsukuba, Japan). The animals were housed in individual cages in a temperature-, humidity-, and light-controlled room (12-h light and 12-h dark cycle) and were allowed free access to water and standard diet (Oriental MF; 362 kcal/100 g, 5.4% energy as fat; Oriental Yeast, Tokyo, Japan), unless otherwise noted. In some experiments, mice were given free access to water and either the standard diet (SD) or the high-fat diet (HFD) (D12492; 556 kcal/100 g, 60% energy as fat; Research Diets, New Brunswick, NJ) for 16 weeks. All animal experiments were conducted according to the guidelines of the Tokyo Medical and Dental University Committee on Animal Research (no. 100098).
Cell culture.
The RAW264 macrophage cell line (Riken BioResource Center, Tsukuba, Japan) and 3T3-L1 preadipocytes (American Type Culture Collection, Manassas, VA) were maintained in Dulbecco’s modified Eagle’s medium (Nacalai Tesque) containing 10% FBS (7,8). Generation of RAW264 macrophages overexpressing a superrepressor form of the inhibitor of κB (IκB)-α (SR-IκBα; a degradation-resistant mutant of IκBα) were reported previously (9,12,13). Peritoneal and bone-marrow–derived macrophages were prepared as described (8,14,15). For macrophage polarization experiments, bone-marrow cell (BMC)-derived macrophages were cultured for 24 h in Iscove’s modified Dulbecco’s medium (Invitrogen, Carlsbad, CA) containing 5% FBS supplemented with 10 ng/mL LPS and 20 ng/mL IFN-γ (for M1 polarization) or 10 ng/mL IL-4 (for M2 polarization) (5). Control macrophages were prepared in Iscove’s modified Dulbecco’s medium containing 5% FBS alone.
Coculture of adipocytes and macrophages.
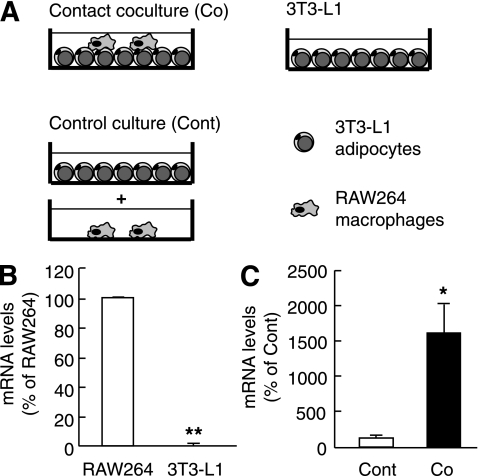
Coculture of 3T3-L1 adipocytes and macrophages (RAW264 or peritoneal macrophages) in the contact system was performed as described (7,8). In brief, serum-starved differentiated 3T3-L1 adipocytes (~0.5 × 106 cells) were cultured in a 35-mm dish, and macrophages (1.0 × 105 cells) were plated onto 3T3-L1 adipocytes (contact coculture) (Fig. 1A). The cells were cultured with direct cell-to-cell contact and harvested after a 24-h incubation, unless otherwise described. As a control (control culture), adipocytes and macrophages, the numbers of which were equal to those in the coculture, were cultured separately and mixed after harvest. Our previous data suggest that there is no apparent difference in macrophage cell number between the coculture and control culture (7).
FIG. 1.
Identification of Mincle as a target gene upregulated in the coculture of 3T3-L1 adipocytes and RAW264 macrophages. A: Illustration of the contact coculture system composed of 3T3-L1 adipocytes and RAW264 macrophages. B: Mincle mRNA expression in RAW264 and 3T3-L1. C: Effect of coculture on Mincle mRNA expression. n = 4. *P < 0.05; **P < 0.01.
In some experiments, we cocultured RAW264 macrophages with adipose tissue explants in the transwell system, in which adipose tissue explants of 8-week-old C57BL/6 J mice were plated in a transwell insert with a 0.4-μm porous membrane (Corning, Corning, NY) to separate them from RAW264 macrophages (Supplementary Fig. 1A). After incubation for 8 h, RAW264 macrophages in the lower well were harvested.
cDNA microarray analysis.
cDNA microarray analysis was performed using mouse genome 430A 2.0 (Affymetrix, Santa Clara, CA) as described (8,9). Total RNA was prepared from the contact coculture of 3T3-L1 adipocytes and RAW264 macrophages, the control culture, and 3T3-L1 adipocytes alone (Fig. 1A). In this experiment, we cocultured the cells for 8 h. We also performed a hierarchical clustering analysis using GeneSpring (Agilent Technologies, Palo Alto, CA).
Quantitative real-time PCR.
Total RNA was extracted from various tissues and cultured cells using a TRIzol reagent (Invitrogen), and quantitative real-time PCR was performed with an ABI Prism 7000 Sequence Detection System using PCR Master Mix reagent (Applied Biosystems, Foster City, CA) as described (7–9). Primers used to detect mouse and human mRNAs are described in Supplementary Tables 1 and 2, respectively. Levels of mRNA were normalized to those of 36B4 mRNA (for mouse) or β-actin (for humans).
Preparation of rabbit polyclonal anti-mouse Mincle antibody and Western blot analysis.
Details are shown in the Supplementary Research Design and Methods.
Human studies on Mincle expression in the adipose tissue and circulating monocytes.
Details are shown in Supplementary Research Design and Methods and Supplementary Table 3. The study protocol was approved by the ethical committee on human research of The University of Tokushima Graduate School, Kyoto Medical Center, and the Tokyo Medical and Dental University.
Statistical analysis.
Data were expressed as the means ± SE. Statistical analysis was performed using ANOVA. P < 0.05 was considered to be statistically significant. In the human study, linear regression analysis was used to evaluate the relationship between Mincle mRNA levels and BMI.
RESULTS
Coculture-induced Mincle mRNA expression in macrophages.
To screen the gene(s) that are upregulated selectively in macrophages during the interaction between adipocytes and macrophages, we performed cDNA microarray analysis of the coculture of 3T3-L1 adipocytes and RAW264 macrophages in the contact system (Fig. 1A). There were 316 genes upregulated (>1.5-fold) in the coculture of 3T3-L1 adipocytes and RAW264 macrophages relative to the control culture, including chemokines, proinflammatory cytokines, and acute-phase reactants (Supplementary Table 4). We also compared the control culture with 3T3-L1 adipocytes alone to examine macrophage selective expression (Supplementary Table 4). In this study, we have focused on Mincle, a type II transmembrane C-type lectin in macrophages, for the following reasons. First, Mincle is induced by LPS (10). Second, Mincle is selectively expressed in macrophages (Supplementary Table 4). We confirmed by real-time PCR that Mincle exhibits highly selective expression in RAW264 macrophages relative to 3T3-L1 adipocytes and is markedly upregulated by the coculture (P < 0.01 and P < 0.05) (Fig. 1B and C, respectively). Last, Mincle acts as a pathogen sensor to induce proinflammatory cytokine and chemokine expression such as TNF-α and macrophage inflammatory protein 2 (16–19). Indeed, cluster analysis revealed that Mincle shows a similar expression pattern with eight genes (Supplementary Fig. 2), which are all related to inflammatory responses. These observations, taken together, suggest that Mincle is selectively induced in macrophages during the interaction between adipocytes and macrophages.
Role of the saturated fatty acid/TLR4/NF-κB pathway in Mincle expression.
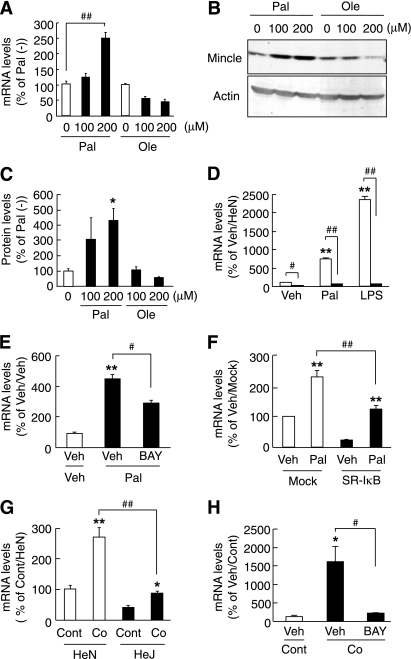
Because saturated fatty acids are a major adipocyte-derived paracrine mediator of inflammation in macrophages (8,20), we examined the effect of palmitate, a major saturated fatty acid released from 3T3-L1 adipocytes, on Mincle mRNA expression in RAW264 or peritoneal macrophages. Treatment with palmitate for 24 h induced Mincle mRNA and protein expression in RAW264 macrophages in a dose-dependent manner (Fig. 2A–C). On the other hand, there was no apparent change in Mincle mRNA and protein expression when it was treated with unsaturated fatty acid oleate (Fig. 2A–C). We also found that palmitate, as well as LPS, induces Mincle mRNA expression in peritoneal macrophages of control C3H/HeN mice. Importantly, the induction was markedly inhibited in peritoneal macrophages of TLR4 signal–deficient C3H/HeJ mice (P < 0.01) (Fig. 2D). Moreover, treatment with BAY11-7085, an NF-κB inhibitor, significantly inhibited the palmitate-induced Mincle mRNA expression in RAW264 macrophages (P < 0.05) (Fig. 2E). The palmitate-induced Mincle mRNA expression also was significantly reduced in RAW264 macrophages overexpressing SR-IκBα, a dominant-negative form of IκBα, relative to those without SR-IκBα expression (P < 0.01) (Fig. 2F). These observations suggest that the TLR4/NF-κB pathway is involved in the saturated fatty acid–induced Mincle expression in macrophages.
FIG. 2.
Effect of palmitate on Mincle expression in cultured macrophages. A–C: Effect of palmitate (Pal) and oleate (Ole) on Mincle mRNA (A) and protein (B and C) expression in RAW264 macrophages. Representative Western blots (B) and quantitative relative protein expression (C) of Mincle. D: Mincle mRNA expression stimulated by palmitate (200 μmol/L) and LPS (10 ng/mL) in peritoneal macrophages prepared from TLR4 signal–deficient C3H/HeJ (■, HeJ) and control C3H/HeN (□, HeN) mice. E: Effect of BAY11-7085 (BAY; an NF-κB inhibitor) on the palmitate-induced Mincle mRNA expression in RAW264 macrophages. F: Effect of palmitate on Mincle mRNA expression in SR-IκBα- (a dominant-negative form of IκBα) and mock-overexpressing RAW264 (SR-IκB and Mock, respectively) macrophages. G: Effect of coculture of 3T3-L1 adipocytes and peritoneal macrophages of C3H/HeJ and C3H/HeN mice on Mincle mRNA expression. H: Effect of BAY (1 μmol/L) on coculture-induced Mincle mRNA expression. Co, coculture; Cont, control culture; Veh, vehicle. n = 3–4. *P < 0.05; **P < 0.01 vs. each control; #P < 0.05; ##P < 0.01.
To elucidate the role of TLR4 in the coculture-induced Mincle mRNA expression, we performed the coculture of 3T3-L1 adipocytes and peritoneal macrophages of C3H/HeJ or C3H/HeN mice. Coculture of 3T3-L1 adipocytes with C3H/HeN peritoneal macrophages resulted in the marked upregulation of Mincle mRNA, which was significantly inhibited in the coculture with C3H/HeJ peritoneal macrophages (P < 0.05) (Fig. 2G). Moreover, treatment with BAY11-7085 effectively inhibited the coculture-induced Mincle mRNA expression (P < 0.05) (Fig. 2H). These observations, taken together, suggest the role of the TLR4/NF-κB pathway in the coculture-induced Mincle mRNA expression.
Mincle expression in adipose tissue of obese mice.
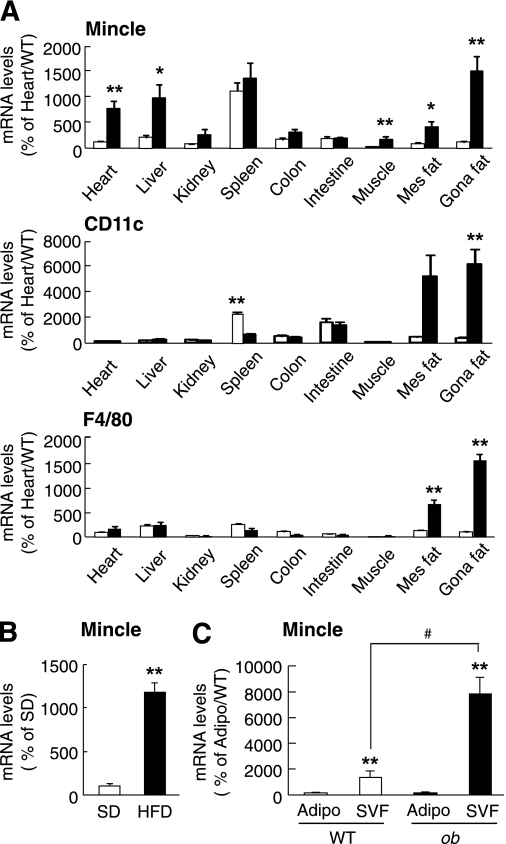
Because there is no previous report on the tissue distribution of Mincle expression in vivo, we next examined the tissue distribution of Mincle mRNA in genetically obese ob/ob mice and wild-type mice (Fig. 3A). Real-time PCR analysis revealed that Mincle mRNA is expressed most abundantly in the spleen of lean wild-type mice. Other organs such as the liver, colon, intestine, and adipose tissue also expressed Mincle mRNA, although to a lesser extent than in the spleen. Similar to macrophage marker F4/80 and M1 macrophage marker CD11c, Mincle mRNA expression was markedly increased in the adipose tissue of ob/ob mice relative to wild-type mice. We also observed a significant upregulation of Mincle mRNA in the adipose tissue of diet-induced obese mice (P < 0.01) (Fig. 3B). Collagenase digestion of adipose tissue, which is validated by F4/80 and adiponectin mRNA expression (9), revealed that Mincle mRNA expression is predominantly detected in the stromal-vascular fraction, which was markedly increased in ob/ob mice relative to wild-type mice (P < 0.05) (Fig. 3C). In this study, there was a significant increase in Mincle mRNA expression in the heart and liver (P < 0.01 and P < 0.05, respectively) (Fig. 3A). These observations, taken together, suggest that Mincle is markedly upregulated mostly in adipose tissue macrophages in obesity.
FIG. 3.
Mincle mRNA expression in mouse adipose tissue. A: Tissue distribution of Mincle (top), M1 macrophage marker CD11c (middle), and macrophage marker F4/80 (bottom) mRNAs in wild-type (WT) mice (□) and ob/ob mice (■). *P < 0.05; **P < 0.01 vs. the respective wild-type mice. B: Mincle mRNA expression in the adipose tissue of diet-induced obese mice. **P < 0.01 vs. SD. C: Mincle mRNA expression in mature adipocytes (Adipo) and stromal-vascular fraction (SVF) in the adipose tissue of wild-type mice (WT) and ob/ob mice (ob). n = 4–6. **P < 0.01 vs. each adipocyte; #P < 0.05.
Role of TLR4 in obesity-induced Mincle mRNA expression in vivo.
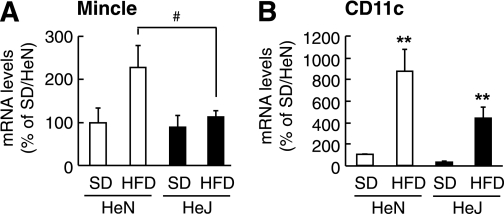
Using C3H/HeJ and C3H/HeN mice fed an HFD or an SD for 16 weeks, we examined the involvement of TLR4 signaling in obesity-induced Mincle mRNA expression in vivo. We previously demonstrated that the weight gain of C3H/HeJ mice as a result HFD feeding is roughly comparable with that of C3H/HeN mice (9,21). We also found that there is no appreciable difference in the number of F4/80-positive macrophages between the genotypes (9,21). In this study, Mincle mRNA expression in adipose tissue on an HFD was significantly attenuated in C3H/HeJ mice relative to C3H/HeN mice (P < 0.05), whereas there was no appreciable difference between the genotypes on an SD (Fig. 4A). Similarly, mRNA expression of the M1 macrophage marker, CD11c, tended to be decreased in the adipose tissue of C3H/HeJ mice relative to C3H/HeN mice, although CD11c mRNA expression was significantly increased on an HFD relative to an SD in both genotypes (Fig. 4B). These observations suggest that TLR4 signaling plays an important role in the obesity-induced Mincle mRNA expression in adipose tissue macrophages in vivo.
FIG. 4.
Role of TLR4 in obesity-induced Mincle mRNA expression in mouse adipose tissue. Mincle (A) and CD11c (B) mRNA expression in the adipose tissue of C3H/HeN and C3H/HeJ mice fed an SD or an HFD. n = 4. **P < 0.01 vs. each SD; #P < 0.05.
Mincle mRNA expression in BMC-derived M1 macrophages in vitro.
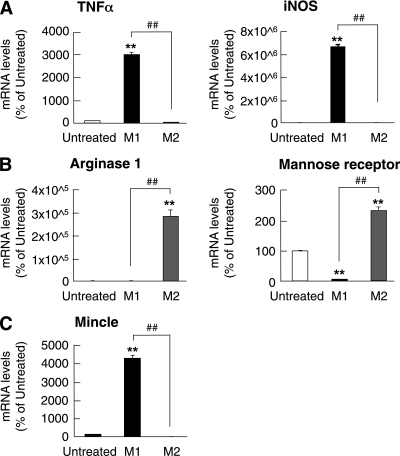
To further explore Mincle mRNA expression in M1 versus M2 macrophages, we examined Mincle mRNA expression in BMC-derived M1 and M2 macrophages in vitro. We confirmed that TNF-α and inducible nitric oxide (NO) synthase mRNAs are expressed exclusively in BMC-derived M1 macrophages (Fig. 5A), whereas BMC-derived M2 macrophages show substantial expression of arginase 1 and mannose receptor mRNAs (Fig. 5B). In this study, Mincle mRNA was predominantly expressed in BMC-derived M1 macrophages (Fig. 5C). In contrast, no appreciable amount of Mincle mRNA was detected in BMC-derived M2 macrophages (Fig. 5C). These observations suggest that Mincle is expressed in M1 macrophages rather than in M2 macrophages in vitro.
FIG. 5.
Mincle mRNA expression in M1 and M2 macrophages in vitro. mRNA expression of M1 markers (TNF-α and inducible NO synthase [iNOS]) (A), M2 markers (arginase 1 and mannose receptor) (B), and Mincle (C) in BMC-derived macrophages. M1, M1 polarization with 100 ng/mL LPS and 20 ng/mL IFN-γ; M2, M2 polarization with 10 ng/mL of IL-4. n = 4. **P < 0.01 vs. untreated; ##P < 0.01.
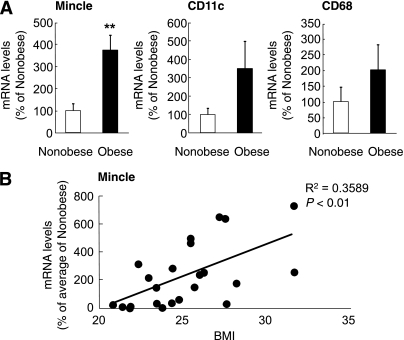
Mincle mRNA expression in the adipose tissue and circulating monocytes of obese subjects.
We also examined Mincle mRNA expression in human subcutaneous adipose tissue. We did not observe significant differences in blood pressure and serum triglycerides, HDL cholesterol, LDL cholesterol, and HbA1c levels between the groups (Table 1). On the other hand, there was a tendency of increased expression of CD11c and CD68 mRNAs in the adipose tissue of obese subjects relative to nonobese subjects (Fig. 6A). In this study, Mincle mRNA expression was markedly increased in the adipose tissue of obese subjects relative to nonobese subjects (P < 0.01) (Fig. 6A). Linear regression analysis also revealed a significantly positive correlation between Mincle mRNA levels and BMI (r2 = 0.3589, P < 0.01) (Fig. 6B).
TABLE 1.
Clinical characteristics and metabolic parameters
| BMI <25 kg/m2 group (nonobese) | BMI ≥25 kg/m2 group (obese) | |
|---|---|---|
| n | 12 | 11 |
| Age | 66.9 ± 3.5 | 62.2 ± 3.6 |
| BMI (kg/m2) | 22.9 ± 0.4 | 27.4 ± 0.7* |
| Systolic blood pressure (mmHg) | 136 ± 7 | 127 ± 4 |
| Diastolic blood pressure (mmHg) | 71.6 ± 7.1 | 74.1 ± 3.1 |
| Serum triglyceride concentration (mg/dL) | 127 ± 16 | 153 ± 35 |
| Serum HDL cholesterol (mg/dL) | 43.6 ± 2.1 | 38.9 ± 3.4 |
| Serum LDL cholesterol (mg/dL) | 105 ± 12 | 85 ± 7 |
| HbA1c (%) | 6.3 ± 0.3 | 6.4 ± 0.3 |
Data are means ± SE.
*P < 0.01.
FIG. 6.
Mincle mRNA expression in human adipose tissue. A: mRNA expression of Mincle, CD11c, and CD68 in the subcutaneous adipose tissue of nonobese (BMI <25 kg/m2, n = 12) and obese (BMI ≥25 kg/m2, n = 11) subjects. B: Linear regression analysis of correlations between adipose Mincle mRNA expression and BMI. **P < 0.01.
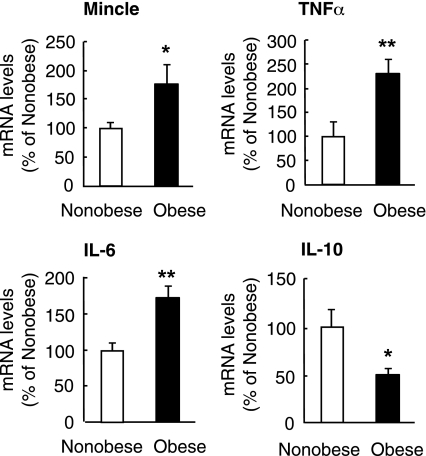
We further examined Mincle mRNA expression in circulating monocytes of nondiabetic subjects. We did not observe significant differences in diastolic blood pressure and serum lipid levels between the groups, whereas systolic blood pressure was significantly high in obese subjects relative to nonobese subjects (P < 0.05) (Supplementary Table 3). In this study, Mincle mRNA expression was significantly increased in the circulating monocytes of obese subjects relative to nonobese subjects (P < 0.05) (Fig. 7). In this setting, circulating monocytes of obese subjects exhibited higher TNF-α and IL-6 mRNA expression and lower IL-10 mRNA expression than those of nonobese subjects (Fig. 7). Collectively, these observations suggest that Mincle expression is increased in the adipose tissue and circulating monocytes in obese subjects.
FIG. 7.
Mincle mRNA expression in human circulating monocytes. mRNA expression of Mincle, TNF-α, IL-6, and IL-10 in circulating CD14-positive cells or monocytes of nonobese (BMI <25 kg/m2, n = 25) and obese (BMI ≥25 kg/m2, n = 25) subjects. *P < 0.05; **P < 0.01.
DISCUSSION
During the course of adipose tissue remodeling in obesity, infiltrating macrophages may participate in the inflammatory pathways that are activated in the adipose tissue (1,2,7). Using an in vitro coculture system composed of 3T3-L1 adipocytes and RAW264 macrophages, we have provided evidence that saturated fatty acids, which are released in large quantities from hypertrophied adipocytes via the macrophage-induced adipocyte lipolysis, serve as a naturally occurring ligand for TLR4 complex in macrophages, thereby aggravating chronic inflammatory changes in the adipose tissue in obesity (7,8). To understand the molecular mechanisms underlying the interaction between adipocytes and macrophages, it is therefore important to characterize molecules and pathways activated in macrophages in response to saturated fatty acids. Through a combination of cDNA microarray analyses of coculture of 3T3-L1 adipocytes and RAW264 macrophages, we have identified several candidate genes that are induced in macrophages during the interaction between adipocytes and macrophages. Because there are no previous reports on the role of Mincle in obesity-induced adipose tissue inflammation, it is interesting to speculate that Mincle plays a novel role in the pathophysiology of obesity and obesity-related metabolic derangements.
This study provides in vitro evidence that saturated fatty acids induce Mincle mRNA expression in macrophages at least partly through the TLR4/NF-κB pathway. This is consistent with a previous report that the 5′-flanking region of the mouse Mincle gene has a couple of putative binding motifs for NF-κB (10). We also demonstrate with a series of coculture experiments that the TLR4/NF-κB pathway is involved in the coculture-induced Mincle mRNA expression. In this study, we observed that Mincle mRNA expression is significantly increased in RAW264 macrophages in the transwell coculture system as well as the contact coculture system, supporting the role of adipocyte-derived secretory factors, such as saturated fatty acids, in the induction of Mincle expression. On the other hand, it seems that the upregulation of Mincle expression by treatment with palmitate or in the transwell coculture system is apparently modest relative to that in the contact coculture system. It is therefore conceivable that in addition to secretory factors, direct contact between adipocytes and macrophages also is involved in the induction of Mincle expression in the contact coculture system. On the other hand, there still appears to be some upregulation of Mincle expression in the absence of TLR4 signaling in coculture using HeJ peritoneal macrophages. Indeed, Matsumoto et al. (10) reported that Mincle mRNA expression is markedly induced in macrophages in response to proinflammatory cytokines such as TNF-α, IL-6, and IFN-γ. It is interesting to identify other adipocyte-derived paracrine signals than saturated fatty acids, which are involved in the coculture-induced Mincle expression in macrophages.
In this study, we demonstrate that Mincle mRNA expression is markedly induced in the stromal-vascular fraction in the adipose tissue of obese mice, suggesting the predominant expression of Mincle in adipose tissue macrophages in vivo. The obesity-induced Mincle mRNA expression is markedly attenuated in the adipose tissue of C3H/HeJ mice relative to C3H/HeN mice, suggesting the involvement of TLR4 signaling in vivo. There is considerable evidence that macrophages, which are infiltrated into the adipose tissue in obesity, exhibit the phenotypic change from anti-inflammatory M2 to proinflammatory M1 polarization (6). Given that Mincle is induced in macrophages in response to LPS (10), an important mediator of M1 macrophage polarization in vitro (5), it is conceivable that Mincle is expressed in M1 macrophages rather than in M2 macrophages. In this study, obesity-induced CD11c mRNA expression tends to be reduced in the adipose tissue of C3H/HeJ mice relative to C3H/HeN mice, suggesting the role of TLR4 signaling in M1 macrophage polarization in vivo. We also found that Mincle mRNA expression is attenuated in parallel with CD11c mRNA expression in HFD-fed C3H/HeJ mice relative to HFD-fed C3H/HeN mice. Given their critical role as a major adipocyte-derived paracrine mediator of inflammation in macrophages (7), it is likely that saturated fatty acids, when released from adipocytes, are an endogenous ligand that induces Mincle mRNA expression in macrophages at least partly through the TLR4/NF-κB pathway in vivo. These observations, taken together, suggest that Mincle is induced in M1 macrophages in adipose tissue through TLR4 signaling in obesity. This concept is supported by our in vitro observation that Mincle mRNA is expressed in BMC-derived M1 macrophages rather than in BMC-derived M2 macrophages.
In this study, we also found that Mincle mRNA expression is increased in the adipose tissue of obese subjects relative to nonobese subjects. Moreover, there is a significant correlation between Mincle mRNA expression in adipose tissue and BMI. Because there may be species differences in adipose tissue macrophages between humans and rodents, the M1/M2 paradigm of murine adipose tissue macrophages (6) may not be entirely applicable to humans (22–24). Nevertheless, our data suggest that Mincle is expressed in proinflammatory macrophages in the adipose tissue of humans and mice. Moreover, we have shown that Mincle mRNA levels in circulating monocytes are significantly increased in obese subjects relative to nonobese subjects. Although monocytes are considered not fully differentiated cell types, recent studies showed that circulating monocytes give rise to tissue macrophages and that monocyte subsets seem to reflect developmental stages of tissue macrophages (25). These observations support the notion that Mincle is overexpressed in adipose tissue macrophages in obese subjects relative to nonobese subjects. Additional studies are needed to elucidate the polarization state of adipose tissue macrophages expressing Mincle in humans. In this study, we do not exclude the possibility that differences in medication affect Mincle expression in the adipose tissue and circulating monocytes because obese subjects received more medication than nonobese subjects. On the other hand, serum free fatty acids (FFAs) are largely derived from adipose tissue, suggesting that the local FFA concentrations in adipose tissue are quite high relative to the serum FFA concentrations. It would be interesting to know the local concentrations of FFAs, especially saturated fatty acids, in the adipose tissue in obesity.
In the danger signal hypothesis (26,27), dying cells or damaged tissues are thought to release endogenous danger signals, which are recognized by innate immune receptors. We have shown that saturated fatty acids, which are released from adipocytes in response to a macrophage-derived death signal TNF-α, act as a naturally occurring ligand for the TLR4 complex to induce macrophage activation (8,28). It is therefore conceivable that saturated fatty acids also act as an endogenous danger signal that reports the dysfunctional state of hypertrophied adipocytes to adipose tissue macrophages in obesity. Sustained stimulation of other pathogen sensors expressed in macrophages by their endogenous ligands released from adipocytes should lead to chronic/homeostatic inflammatory responses, ranging from the basal homeostatic state to diseased tissue remodeling, which has been referred to as “homeostatic inflammation” (4). Recent studies (16–19) with Mincle-deficient mice demonstrated that Mincle serves as a pathogen sensor against certain types of fungi and bacteria and induces chemokine and proinflammatory cytokine production, suggesting that Mincle plays a critical role in immune responses to pathogens. On the other hand, Yamasaki et al. (29) reported that Mincle serves as a receptor for SAP130, a component of small, nuclear ribonucloprotein released from damaged cells, to sense cell death and induce proinflammatory cytokine production. Recent evidence (30,31) suggests that dead adipocytes are surrounded by macrophages (or crown-like structure) in the adipose tissue of obese humans and mice. Collectively, Mincle may play a role in sensing adipocyte death to induce proinflammatory cytokine production in the adipose tissue in obesity. Moreover, there are two previous reports (32,33) that showed that some members of the immunoglobulin-like lectin family can modulate the inflammatory response through TLRs on immune cells. It is, therefore, tempting to speculate that endogenous ligand(s) for Mincle, in concert with saturated fatty acids, report the adipose tissue state to macrophages during the course of adipose tissue remodeling and induce inflammatory cytokine production. Additional studies are required to elucidate the pathophysiologic role of Mincle in obesity-induced adipose tissue inflammation.
In conclusion, we demonstrate that Mincle is induced selectively in macrophages during the interaction between adipocytes and macrophages in vitro and markedly increased in the adipose tissue of obese mice and humans in vivo. Our data also suggest that Mincle is expressed in M1 macrophages in the adipose tissue in obesity through the TLR4/NF-κB pathway. This study is the first detailed analysis of Mincle in adipose tissue macrophages, thereby providing a novel insight into the molecular mechanism underlying adipose tissue remodeling.
ACKNOWLEDGMENTS
This work was supported in part by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and the Ministry of Health, Labour, and Welfare of Japan; and research grants from Takeda Science Foundation, Ono Medical Research Foundation, Japan Vascular Disease Research Foundation, Yamaguchi Endocrine Research Foundation, and The Naito Foundation. No other potential conflicts of interest relevant to this article were reported.
M.I. researched data, contributed to discussion, and wrote the manuscript. T.S. researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript. N.T. researched data and contributed to discussion. I.S., Y.H., N.S.-A., and Y.S. researched data. M.T. researched data and contributed to discussion. M.K.-S. and Y.M. researched data. Y.K. and M.S. contributed to discussion and reviewed and edited the manuscript. Y.O. contributed to discussion and wrote, reviewed, and edited the manuscript.
The authors thank Ai Togo (Tokyo Medical and Dental University) for secretarial assistance and Yousuke Sasaki (Kyoto Medical Center) for technical assistance.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-0864/-/DC1.
REFERENCES
- 1.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–867 10.1038/nature05485 [DOI] [PubMed] [Google Scholar]
- 3.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res 2005;96:939–949 10.1161/01.RES.0000163635.62927.34 [DOI] [PubMed] [Google Scholar]
- 4.Suganami T, Ogawa Y. Adipose tissue macrophages: their role in adipose tissue remodeling. J Leukoc Biol 2010;88:33–39 10.1189/jlb.0210072 [DOI] [PubMed] [Google Scholar]
- 5.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 2006;177:7303–7311 [DOI] [PubMed] [Google Scholar]
- 6.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007;117:175–184 10.1172/JCI29881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor α. Arterioscler Thromb Vasc Biol 2005;25:2062–2068 10.1161/01.ATV.0000183883.72263.13 [DOI] [PubMed] [Google Scholar]
- 8.Suganami T, Tanimoto-Koyama K, Nishida J, et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol 2007;27:84–91 10.1161/01.ATV.0000251608.09329.9a [DOI] [PubMed] [Google Scholar]
- 9.Suganami T, Yuan X, Shimoda Y, et al. Activating transcription factor 3 constitutes a negative feedback mechanism that attenuates saturated Fatty acid/toll-like receptor 4 signaling and macrophage activation in obese adipose tissue. Circ Res 2009;105:25–32 10.1161/CIRCRESAHA.109.196261 [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto M, Tanaka T, Kaisho T, et al. A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. J Immunol 1999;163:5039–5048 [PubMed] [Google Scholar]
- 11.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 1998;282:2085–2088 10.1126/science.282.5396.2085 [DOI] [PubMed] [Google Scholar]
- 12.Hironaka N, Mochida K, Mori N, Maeda M, Yamamoto N, Yamaoka S. Tax-independent constitutive IkappaB kinase activation in adult T-cell leukemia cells. Neoplasia 2004;6:266–278 10.1593/neo.03388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomita S, Fujita T, Kirino Y, Suzuki T. PDZ domain-dependent suppression of NF-kappaB/p65-induced Abeta42 production by a neuron-specific X11-like protein. J Biol Chem 2000;275:13056–13060 10.1074/jbc.C000019200 [DOI] [PubMed] [Google Scholar]
- 14.Yamauchi A, Kim C, Li S, et al. Rac2-deficient murine macrophages have selective defects in superoxide production and phagocytosis of opsonized particles. J Immunol 2004;173:5971–5979 [DOI] [PubMed] [Google Scholar]
- 15.Ito A, Suganami T, Yamauchi A, et al. Role of CC chemokine receptor 2 in bone marrow cells in the recruitment of macrophages into obese adipose tissue. J Biol Chem 2008;283:35715–35723 10.1074/jbc.M804220200 [DOI] [PubMed] [Google Scholar]
- 16.Wells CA, Salvage-Jones JA, Li X, et al. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol 2008;180:7404–7413 [DOI] [PubMed] [Google Scholar]
- 17.Schoenen H, Bodendorfer B, Hitchens K, et al. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J Immunol 2010;184:2756–2760 10.4049/jimmunol.0904013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamasaki S, Matsumoto M, Takeuchi O, et al. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc Natl Acad Sci USA 2009;106:1897–1902 10.1073/pnas.0805177106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishikawa E, Ishikawa T, Morita YS, et al. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med 2009;206:2879–2888 10.1084/jem.20091750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem 2001;276:16683–16689 10.1074/jbc.M011695200 [DOI] [PubMed] [Google Scholar]
- 21.Suganami T, Mieda T, Itoh M, Shimoda Y, Kamei Y, Ogawa Y. Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochem Biophys Res Commun 2007;354:45–49 10.1016/j.bbrc.2006.12.190 [DOI] [PubMed] [Google Scholar]
- 22.Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des 2008;14:1225–1230 10.2174/138161208784246153 [DOI] [PubMed] [Google Scholar]
- 23.Zeyda M, Farmer D, Todoric J, et al. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 2007;31:1420–1428 10.1038/sj.ijo.0803632 [DOI] [PubMed] [Google Scholar]
- 24.Zeyda M, Stulnig TM. Adipose tissue macrophages. Immunol Lett 2007;112:61–67 10.1016/j.imlet.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 25.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005;5:953–964 10.1038/nri1733 [DOI] [PubMed] [Google Scholar]
- 26.Medzhitov R. Origin and physiological roles of inflammation. Nature 2008;454:428–435 10.1038/nature07201 [DOI] [PubMed] [Google Scholar]
- 27.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 2007;56:1986–1998 10.2337/db06-1595 [DOI] [PubMed] [Google Scholar]
- 28.Itoh M, Suganami T, Satoh N, et al. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler Thromb Vasc Biol 2007;27:1918–1925 10.1161/ATVBAHA.106.136853 [DOI] [PubMed] [Google Scholar]
- 29.Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol 2008;9:1179–1188 10.1038/ni.1651 [DOI] [PubMed] [Google Scholar]
- 30.Strissel KJ, Stancheva Z, Miyoshi H, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 2007;56:2910–2918 10.2337/db07-0767 [DOI] [PubMed] [Google Scholar]
- 31.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 2005;46:2347–2355 10.1194/jlr.M500294-JLR200 [DOI] [PubMed] [Google Scholar]
- 32.Gringhuis SI, den Dunnen J, Litjens M, van Het Hof B, van Kooyk Y, Geijtenbeek TB. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity 2007;26:605–616 10.1016/j.immuni.2007.03.012 [DOI] [PubMed] [Google Scholar]
- 33.Nakayama M, Underhill DM, Petersen TW, et al. Paired Ig-like receptors bind to bacteria and shape TLR-mediated cytokine production. J Immunol 2007;178:4250–4259 [DOI] [PubMed] [Google Scholar]