Abstract
OBJECTIVE
Type 1 diabetes is an incurable chronic autoimmune disease. Although transplantation of pancreatic islets may serve as a surrogate source of insulin, recipients are subjected to a life of immunosuppression. Interleukin (IL)-21 is necessary for type 1 diabetes in NOD mice. We examined the efficacy of an IL-21–targeted therapy on prevention of diabetes in NOD mice, in combination with syngeneic islet transplantation. In addition, we assessed the role of IL-21 responsiveness in islet allograft rejection in mouse animal models.
RESEARCH DESIGN AND METHODS
NOD mice were treated with IL-21R/Fc, an IL-21–neutralizing chimeric protein. This procedure was combined with syngeneic islet transplantation to treat diabetic NOD mice. Survival of allogeneic islet grafts in IL-21R–deficient mice was also assessed.
RESULTS
Evidence is provided that IL-21 is continually required by the autoimmune infiltrate, such that insulitis was reduced and reversed and diabetes inhibited by neutralization of IL-21 at a late preclinical stage. Recovery from autoimmune diabetes was achieved by combining neutralization of IL-21 with islet transplantation. Furthermore, IL-21–responsiveness by CD8+ T-cells was sufficient to mediate islet allograft rejection.
CONCLUSIONS
Neutralization of IL-21 in NOD mice can inhibit diabetes, and when paired with islet transplantation, this therapeutic approach restored normoglycemia. The influence of IL-21 on a graft-mounted immune response was robust, since the absence of IL-21 signaling prevented islet allograft rejection. These findings suggest that therapeutic manipulation of IL-21 may serve as a suitable treatment for patients with type 1 diabetes.
In type 1 diabetes, activated immune cells lead to the destruction of the insulin-producing β-cells in the islets of Langerhans of the pancreas (1). Clinical diabetes occurs in the nonobese diabetic (NOD) model after months of chronic pancreatic inflammation, progressing from peri-insulitis to destructive insulitis (2,3). Importantly, therapies designed to modulate lymphocyte activation are compatible with the prevention of the destructive form of insulitis, i.e., the movement of immune cells into the islet, and subsequent loss of insulin production from β-cells (4,5).
Insulin replacement is the current standard for treating type 1 diabetes, however, transplantation of pancreatic islets has the potential to serve as a viable alternative (6). Challenges of islet transplantation include finding alternatives to broad-spectrum immunosuppression (7) while preventing graft rejection and recurrence of the underlying autoimmune destruction of pancreatic islets. As T-cells constitute an integral component in both autoimmune responses of diabetes and the rejection of transplanted islet allografts (8,9), therapies addressing modulation of T-cell function may provide an appropriate strategy.
IL-21 is a member of the common γ-chain signaling family of cytokines that is necessary for the development of diabetes in the NOD mouse (10–12). The receptor for IL-21, comprising the α unit (IL-21Rα) and the common γ chain, is expressed on immune cells including T-, B-, NK, and dendritic cells, whereas IL-21 expression is largely limited to CD4+ T-cells (13). Several studies demonstrate that IL-21 acts as a lymphocyte costimulator, enhancing the proliferation and effector function of CD8+ T-cells (14,15), and transgenic over-expression of murine IL-21 revealed that IL-21 predominantly expands memory phenotype CD8+ T-cells (16). The prosurvival effect of IL-21 is important for CD8+ T-cells during chronic viral infection (17–19), with IL-21 also potently effecting the activation and differentiation of numerous CD4+ T-helper subsets, including Th17 cells (20,21).
Consistent with its actions on lymphocyte populations, IL-21 has been found to contribute to the development of autoimmune diseases in several animal models (22). Likewise, IL-21 has the potential to influence the outcome of islet graft transplantation (23–25). For instance, IL-21 has a well-documented ability to promote the production of granzyme and perforin in differentiating CD8+ cytotoxic T-cells. Direct killing of islet cells by antigen-specific cytotoxic T-cells is an important component of both allograft rejection and the autoimmune destruction of β-cells (26,27). Secondly, IL-21 costimulates the activation and differentiation of antigen specific CD4+ T-cells, and these cells can produce proinflammatory cytokines that are toxic to the islets, such as IL-1β, tumor necrosis factor-α, and γ-interferon (IFNγ) (28–31).
In this study, we demonstrate that IL-21 acts on immune cells to elicit autoimmune destruction of endogenous pancreatic islet tissue in autoimmune diabetes and islet graft rejection caused by both autoimmune and allogeneic immune responses. We provide evidence that through the modulation of IL-21, a potential therapeutic intervention for type 1 diabetes may be attainable.
RESEARCH DESIGN AND METHODS
NOD Ltj, C57BL/6, and BALB/c mice were obtained from Animal Resources Centre, Perth. NOD/Scid mice were obtained from Walter and Eliza Hall Institute of Medical Research, Melbourne. The Il21−/− mice were created through a National Institutes of Health initiative with Lexicon and Deltagen, on the 129 background and backcrossed to C57BL/6. These mice were backcrossed to NOD N10 and selected for known NOD Idd loci (vs. B6 regions) by PCR of genomic DNA (speed congenics), as previously described (32). Il21r−/− mice (33) were kindly provided by M. Smyth (Melbourne) at C57BL/6 N6 and backcrossed to N7 for experimental use; both Il21r−/− and Il21−/− NOD mice underwent an additional genome scan of +600 NOD microsatellite markers. Major histocompatibility complex (MHC) class II–deficient mice (stock 3584, Jackson Laboratories) were kindly provided by R. Brink (Sydney). Animals were housed under specific pathogen-free conditions and handled in accordance with the Garvan Institute of Medical Research and St. Vincent Hospital’s Animal Experimentation and Ethics Committee. Blood glucose levels were determined using Accu-chek Advantage blood glucose strips (Roche).
In vivo IL-21 neutralization.
IL-21R/Fc chimera was generated in-house, as previously described (34). In brief, the DNA encoding the predicted extracellular domain (aa 1–235) of mouse IL-21R with a GSGS linker were amplified by PCR, and linked to mIgG2a Fc. The Fc domain contains four amino acid mutations (L285E, E368A, K370A, and K372A) to minimize Fc binding and complement fixation (34). The resulting construct was subcloned into pEE12.4 (Lonza), a mammalian glutamine synthase expression vector, transfected into Chinese hamster ovary-K1SV cells, and grown in the presence of 25 μmol/L methionine sulphoximine. Following protein A purification, purity of IL-21R/Fc was checked by SDS/PAGE (Coomassie blue staining and silver stain, Lonza) (Supplementary Fig. 1) and tested for presence of endotoxin (Lonza). In vitro BAF-3 proliferation assay measuring biological activity of IL-21R/Fc construct was conducted as previously described (35) (Supplementary Fig. 1).
Complete treatment of NOD mice consisted of a total of 20 μg IL-21R/Fc given every other day for 11 days. Flow cytometric analyses were conducted at time points relative to this treatment regimen. Treatment of NOD transplant recipients consisted of a total of 80 μg IL-21R/Fc given on day −1, day 0, and every other day until day 12. A monoclonal Ig control was provided with a nonspecific antibody (affinity purified, produced in house). Mice received 60 μg polyclonal IL-21 antibody (goat IgG; R&D Systems) as an alternative IL-21 neutralizing reagent. The frequency of diabetes was compared between treatment groups with the Kaplan-Meier log-rank test using Prism software (GraphPad).
Immunohistochemistry.
Paraffin-embedded sections of pancreas and engrafted kidney were conventionally stained with hematoxylin and eosin or insulin by immunocytochemical techniques. For insulitis scoring, serial sections of pancreata were matched and stained for insulin (DakoCytomation) and glucagon (Chemicon). For islet mass scoring, sections of pancreata were stained for insulin. Images were processed using the Leica acquisition and analysis software ImageJ (Freeware; National Institutes of Health, Bethesda, MD).
Flow-cytometric analysis.
We stained lymphocytes extracted from the pancreas and pancreatic lymph nodes with conjugated antibodies to Annexin V, B220, CD4, CD8, CD45 (BD Biosciences), and CD11b, CD11c, CD44, CD69, Foxp3, and IL-17 (eBioscience). For flow-cytometric analysis of lymphocytes isolated from the pancreas, mice were killed by intraperitoneal injection of ketamine and perfused with ice-cold PBS for 10 min. Pancreas samples were then subjected to lymphocyte isolation as described (36). For IL-21 analysis, after ex vivo staining of surface markers, cells were fixed and permeated (BD Biosciences), followed by intracellular staining with biotin labeled polyclonal IL-21 Ab (R&D Systems). For IL-17 analysis, cells were stimulated with CD3 (2.5 μg/mL) and CD28 mAb (2.5 μg/mL) in culture medium containing monensin (GolgiStop 1 μL/mL; BD Biosciences) at 37°C for 6 h. Intracellular staining was performed according to the manufacturer’s instructions, for IL-17 (BD Biosciences) and Foxp3 (eBioscience). Data were collected on a Canto flow cytometer (BD Biosciences), and analyzed using FlowJo software (Tree Star).
RNA and quantitative RT-PCR.
Total RNA was harvested from tissues or cells using TRIzol (Invitrogen). Pancreas samples were perfused through the bile duct with RNAlater (Ambion). Tail vein bleeds were collected into RNAlater. Preparation of complementary DNA and analysis of IL-21 gene expression was performed with Taqman gene expression assays (Applied Biosystems) as described previously, with values normalized to the level of expression of the housekeeping gene GAPDH (37).
Islet transplantation.
Autoimmune diabetic NOD mice received islets prepared from NOD/Scid mice (for syngeneic transplants), and chemically induced diabetic mice (C57BL/6 and Il21r−/− mice, H-2b), injected with 180 mg/kg streptozotocin (Sigma-Aldrich), received islets prepared from BALB/c (H-2d) (for allogeneic transplants). Islets were prepared as described previously (38).
Statistical analysis.
We used an F test to compare variances, and when variances were significantly different t tests were performed with Welch correction (without assuming equal variances). P values between datasets were determined by two-tailed Student t test assuming equal variance, with log-rank test used in Figs. 1B, D, and G, 4A, 5A, and 6A. Data are reported as the mean ± SEM, along with the calculated P values.
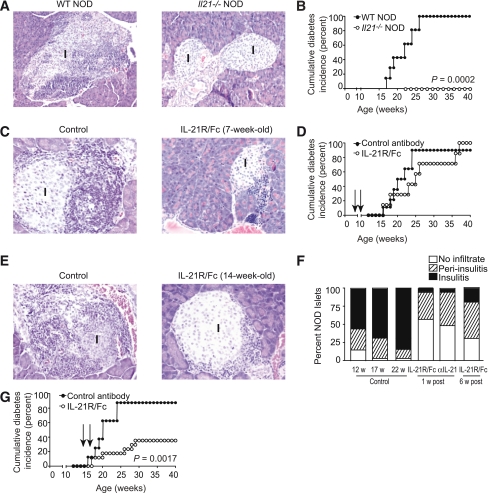
FIG. 1.
IL-21 helps to maintain pancreatic immune cell infiltrate. A: Sections from paraffin-embedded pancreata were stained with hematoxylin and eosin to reveal mononuclear infiltrate in and around islets of 15-week-old WT NOD and Il21−/− NOD mice, with cumulative incidence of diabetes in WT NOD and Il21−/− NOD mice (B), where n = 12 mice per group. C: Sections from paraffin-embedded pancreata from NOD mice treated at 7 weeks of age with a total of 20 μg control antibody or IL-21R/Fc, given every other day for 11 days are shown at 5 weeks after completion of treatment, with cumulative incidence of diabetes (D), where n = 7 mice per group. Arrows indicate beginning and end of treatment period. E: Sections from paraffin-embedded pancreata from NOD mice treated at 14 weeks of age with a total of 20 μg control antibody or IL-21R/Fc, given every other day for 11 days are shown at 1 week after completion of treatment, with insulitis indices from sections of pancreata showing the percent of islets exhibiting peri-insulitis or insulitis in 12-week-old, 17-week-old, and 22-week-old control mice, and 17-week-old mice treated with IL-21R/Fc or IL-21 polyclonal antibody (1 week after completion of treatment) and 22-week-old mice treated with IL-21R/Fc (6 weeks after completion of treatment) (F), n ≥ 220 islets from 10 mice per group. G: Cumulative incidence of diabetes in NOD mice commencing treatment at 14 weeks of age, where n = 13 mice per group. Arrows indicate beginning and end of treatment period. w, week; WT, wild type. (A high-quality digital representation of this figure is available in the online issue.)
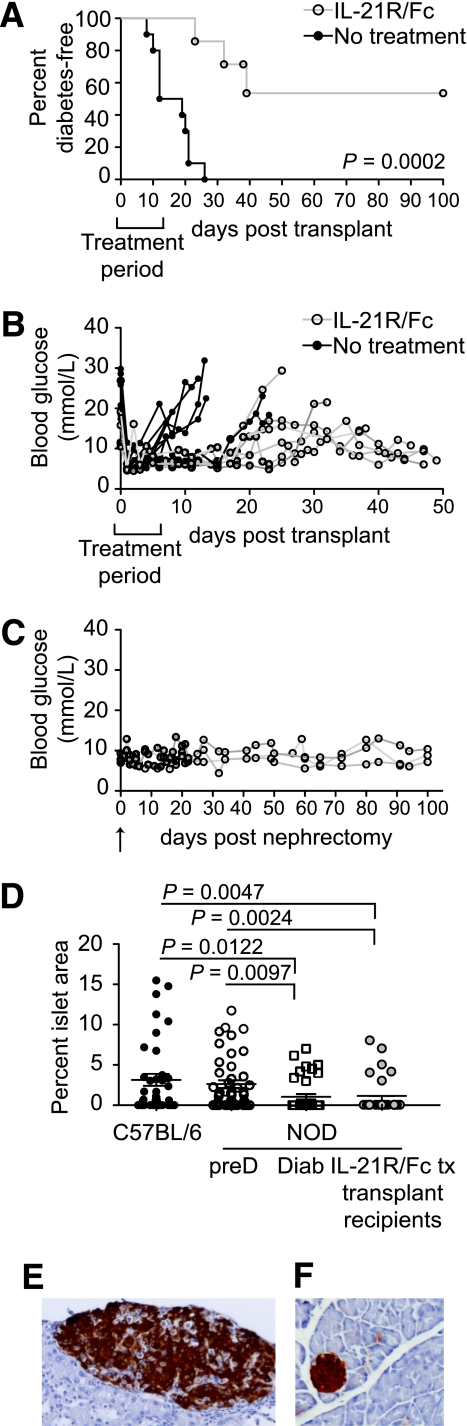
FIG. 4.
IL-21 neutralization prolongs survival of autoimmune diabetic mice. A: Percent diabetes-free NOD mice after syngeneic islet transplants with IL-21R/Fc, with treatment period indicated on the graph. n = 10 for control NOD group, no treatment, mean survival time 16.1 days. n = 7 for treated NOD group, three of seven mice returned to a diabetic state. P = 0.0002 (log-rank test). B: Individual blood glucose readings are shown for a cohort of untreated and IL-21R/Fc–treated mice. Treatment period is indicated on the graphs. C: Individual blood glucose readings are shown for long-term surviving IL-21R/Fc–treated NOD mice (n = 3) after nephrectomy (indicated by arrow). D: Frequency of islet mass area in pancreas of long-term IL-21R/Fc–treated NOD syngeneic graft recipients compared with 10-week-old C57BL/6 and prediabetic (preD) NOD mice, and newly diabetic (Diab) NOD mice (15–17 weeks of age). Enumerated from histological sections, with at least 50 fields scored, from three mice per group. Representative histological analyses of a long-term surviving islet graft (E) and pancreas (F) from a long-term surviving IL-21R/Fc–treated NOD mouse. (A high-quality digital representation of this figure is available in the online issue.)
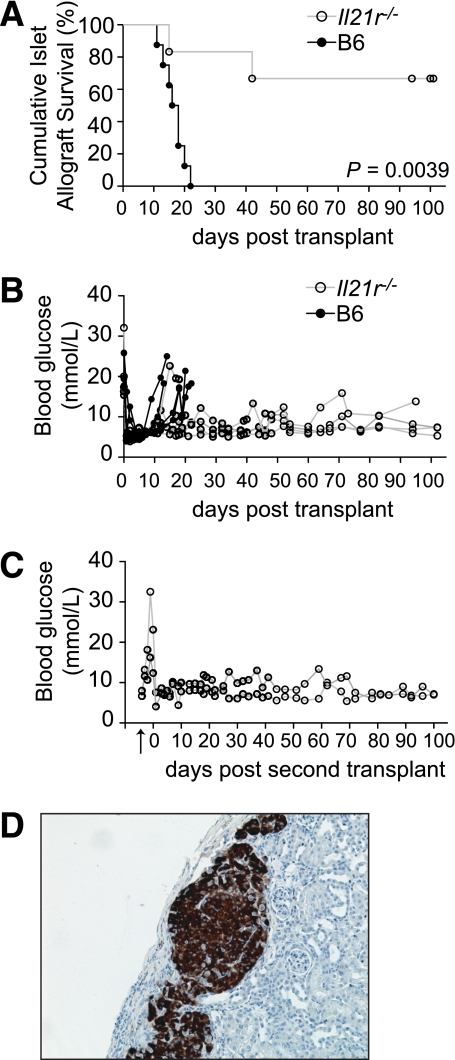
FIG. 5.
Islet allografts exhibit prolonged survival in Il21r−/− mice. A: Percent islet graft survival in B6 and Il21r−/− mice following allogenic islet transplants (BALB/c donors). n = 8 for control group, mean survival time 16.6 days. n = 6 for Il21r−/− group. P = 0.0039 (log-rank test). B: Individual blood glucose readings are shown for a cohort of B6 and Il21r−/− mice. C: As indicated by the arrow, nephrectomies were performed on Il21r−/− mice, which survived long-term after receiving an islet allograft. After the removal of the engrafted kidney, blood glucose sharply rose. On day 0 a subsequent islet allograft was transplanted on the contralateral kidney. D: Representative histological analyses of a long-term surviving islet allograft from an Il21r−/− mouse, with insulin staining in brown. (A high-quality digital representation of this figure is available in the online issue.)
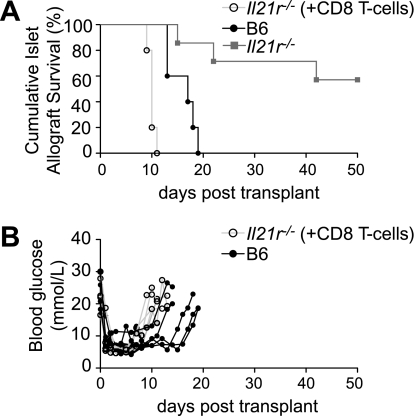
FIG. 6.
Restoring CD8 T-cell responsiveness to IL-21 results in rapid islet allograft rejection. A: Percent islet graft survival in B6 and Il21r−/− mice, and Il21r−/− mice reconstituted with 1 million IL-21R competent CD8+ T-cells on day −4 (+CD8 T-cells), after allogenic islet transplants (day 0) (BALB/c donors). n = 5 for control B6 group, mean survival time of 16.7 days. n = 6 for Il21r−/− group. n = 5 for Il21r−/− (+CD8 T-cells) group. B: Individual blood glucose readings are shown for a cohort of B6 and Il21r−/− (+CD8 T-cells) mice.
RESULTS
Islet inflammation is chronically dependent on IL-21.
We have shown previously that NOD mice express higher levels of IL-21 compared with diabetes-resistant strains (37,39). NOD mice made genetically deficient for IL-21R lack insulitis and do not develop diabetes (10–12). We used an IL-21R/Fc chimeric protein to therapeutically neutralize IL-21 at different periods of time in the disease process. IL-21 was neutralized from 7 to 9 weeks of age in one group, and IL-21 was neutralized from 14 to 16 weeks of age in another group. We also compared groups treated with IL-21R/Fc with NOD mice made genetically deficient in IL-21 that do not develop insulitis (Fig. 1A) or diabetes during the 40 week observation period (Fig. 1B).
Histological analysis of pancreata 5 weeks after treatment of 7-week-old mice revealed islets with mild peri-insulitis, but very few islets exhibited insulitis compared with control mice (Fig. 1C). However, this early short-term treatment was reversible and ultimately had little impact on diabetes incidence (Fig. 1D). The 11 day neutralization of IL-21 in mice treated on the brink of clinical diabetes (14-weeks-of-age) also reduced insulitis in the pancreatic islets compared with control mice (Fig. 1E). Because NOD islets at the start of treatment exhibited a considerably greater degree of insulitis than those at the end of treatment, our results indicate that insulitis was reversed whether we treated with the IL-21R/Fc chimera or an IL-21 neutralizing polyclonal antibody (Fig. 1F). Furthermore, in contrast to the 7-week-old group, the effect of the 11 day treatment was not reversible in mice treated at a late preclinical stage, delaying the onset and reducing the incidence of diabetes to 40%, compared with 90% of control mice by 40 weeks of age (Fig. 1G). These findings indicate that IL-21 was required for the transition from peri-insulitis to insulitis.
Neutralization of IL-21 reduced lymphocytes in the islet lesion.
To further examine the reduction of inflammation caused by neutralization of IL-21, we investigated the effect of IL-21 neutralization on the islet infiltrate from the start of treatment (day 0) and up to 12-weeks after treatment (day 90). One of the early effects of treatment (day 5) was to reduce the percentages and total numbers of lymphocytes in the islet infiltrate comprising CD4+ T-cells, CD8+ T-cells, and B220+ B-cells (Fig. 2A and B, and Supplementary Fig. 2). Analyses of the long-term effects of IL-21 neutralization showed the sustained reduction in CD8+ T-cells and B-cells, but not CD4+ T-cells, 90 days after treatment (Fig. 2A and B, and Supplementary Fig. 2). The percentage of macrophages and dendritic cells were proportionally increased, however, absolute numbers of both subsets remained largely unchanged (Fig. 2C and D, and Supplementary Fig. 2).
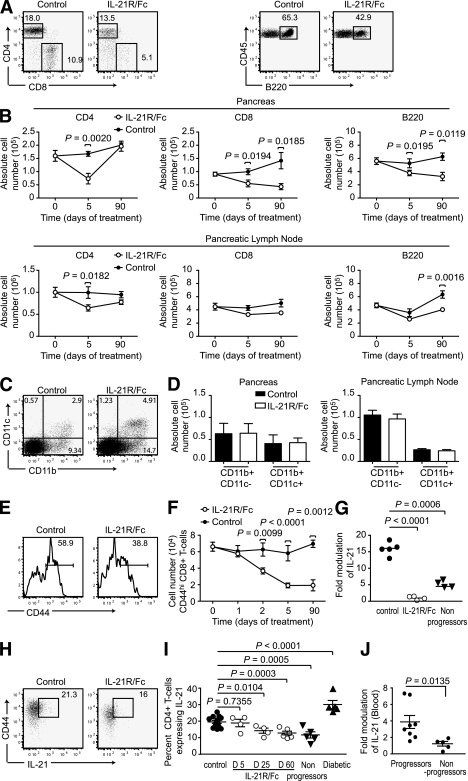
FIG. 2.
IL-21 neutralization reduces lymphocyte populations. NOD mice were treated with IL-21R/Fc or control antibody 2.8 μg/i.v. every other day, with a complete dosing of 20 μg achieved on day 11. Cellular composition of PBS-perfused pancreas preparations, and pancreatic lymph nodes were examined by flow cytometric analysis at time points relative to the start of treatment. A: Representative dot plots of CD4+ T-cells, CD8+ T-cells (CD3+ CD45+ gating), and B220+ cells (CD45+ gating) in the pancreas on day 5 (receiving three doses of 2.8 μg). Numbers represent percentage of total lymphocytes. B: Absolute cell number of CD4+ T-cells, CD8+ T-cells, and B220+ cells in the pancreas and pancreatic lymph nodes on day 5 and day 90 (receiving full 20-μg dose) of treatment with IL-21R/Fc. C: Representative dot plots of CD11b and CD11c staining (CD45+ gating) in the pancreas of IL-21R/Fc–treated mice, day 5. D: Absolute cell number of CD11b+ CD11c- macrophages and CD11b+ CD11c+ dendritic cells in NOD pancreas, measured on day 5 of treatment with IL-21R/Fc. Data are presented as means ± SEM, where n = 5–14 for each group, compiled from four experiments. Representative dot plot (E) and absolute cell number of CD44hi CD8+ T-cells from the pancreata of control and IL-21R/Fc–treated NOD mice (F). G: IL-21 mRNA expression measured in pancreas of age-matched control and treated mice on day 3 of treatment with IL-21R/Fc, and NOD mice remaining diabetes free at 40 weeks, termed nonprogressors, where n = 4–5 for each group, from two experiments. IL-21–expressing cells shown as representative dot plots (H) (CD4+ CD3+ CD45+ gating) day 5, and as a percentage of CD4+ T-cells (I), measured in pancreas of control mice and mice on days of treatment with IL-21R/Fc shown, also compared with NOD mice remaining diabetes free at 35 weeks, termed nonprogressors, and diabetic NOD mice, aged 14–22 weeks, where n = 5–10 for each group, from four experiments. J: IL-21 mRNA expression measured by real-time PCR in blood from 12-week-old NOD mice. The cohort were monitored for diabetes onset until 40 weeks of age, and grouped into those that developed disease (progressor) or nonprogressors.
Analyses of cell surface molecules that distinguish activated T-cells from naïve T-cells indicated that IL-21 neutralization preferentially reduced the number of activated lymphocytes in the islet infiltrate (Table 1). The reduction of CD44hi CD8+ T-cells preceded that of CD4+ T-cells and B-cells (data not shown) and was also sustained until 90 days after treatment (Fig. 2E and F, and Supplementary Fig. 2).
TABLE 1.
Loss of activated lymphocytes in the pancreas following IL21R/Fc treatment
| CD4+ T-cells |
||||
|---|---|---|---|---|
| CD69+ |
CD44+ |
|||
| Percent | Number | Percent | Number | |
| Day 0 | 22.1 ± 1.1 | 37,715 ± 1,916 | 51.8 ± 3.3 | 90,100 ± 5,159 |
| Day 5 | ||||
| Control | 22.3 ± 1.3 | 38,150 ± 209 | 52.3 ± 4.1 | 89,080 ± 5,100 |
| IL-21R/Fc | 14.2 ± 1.0 | 10,682 ± 800 | 41.2 ± 3.1 | 30,967 ± 2,372 |
| P | 0.0014 | <0.0001 | 0.0198 | <0.0001 |
| Day 90 | ||||
| Control | 24.2 ± 1.7 | 42,198 ± 2,416 | 66.0 ± 3.1 | 15,0810 ± 5,672 |
| IL-21R/Fc | 18.2 ± 0.7 | 31,000 ± 1,326 | 51.8 ± 8.2 | 88,100 ± 14,061 |
| P | 0.0128 | 0.0036 | 0.0423 | 0.0033 |
| CD8+ T-cells |
||||
| CD69+ |
CD44+ |
|||
| Percent |
Number |
Percent |
Number |
|
| Day 0 | 24.8 ± 1.7 | 23,564 ± 937 | 60.1 ± 4.9 | 62,250 ± 6,029 |
| Day 5 | ||||
| Control | 26.7 ± 2.0 | 24,760 ± 1,447 | 62.9 ± 5.7 | 59,210 ± 9,313 |
| IL-21R/Fc | 15.7 ± 1.6 | 7,879 ± 718 | 45.6 ± 4.7 | 19,090 ± 2,934 |
| P | 0.0026 | <0.0001 | 0.070 | 0.0034 |
| Day 90 | ||||
| Control | 26.8 ± 1.4 | 24,365 ± 856 | 70.8 ± 3.6 | 93,650 ± 4,428 |
| IL-21R/Fc | 16.7 ± 0.9 | 12,909 ± 465 | 47.7 ± 2.6 | 33,260 ± 6,709 |
| P | 0.0003 | <0.0001 | 0.0052 | 0.0001 |
Percentage and absolute number of activated CD4+ and CD8+ T-cell subsets in NOD pancreas, measured on day 5 and day 90 of treatment with IL-21R/Fc, relative to age-matched control antibody-treated NOD mice. n = 5–9 mice per group, from four experiments.
IL-21 neutralization resulted in a profound reduction in IL-21 expression (Fig. 2G) and IL-21–producing cells (Fig. 2H and I) in the islets. The population of IL-21+ cells had significantly declined after treatment and remained decreased in mice that had not developed diabetes by 40 weeks of age (Fig. 2I). By contrast, mice that did go on to develop diabetes were distinguished from those who remained protected from disease by an elevated fraction of IL-21–producing CD4+ T-cells in the islet infiltrate (Fig. 2I). Measuring IL-21 transcript in secondary lymphoid organs, blood, and the pancreas over the course of disease in the NOD mouse demonstrated that an increase in IL-21 in the blood and pancreas corresponded with the age of heightened destruction of the islets and coincided with the onset of diabetes in our colony (Supplementary Fig. 3). Moreover, the analyses of IL-21 mRNA in blood demonstrated that high IL-21 expression was predictive of the subsequent development of clinical diabetes (Fig. 2J).
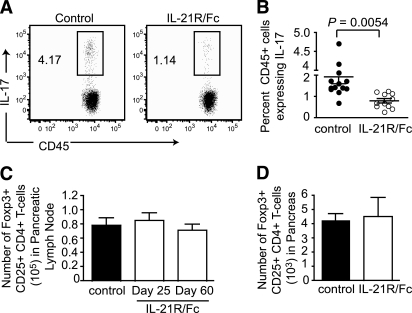
The generation and survival of several TH subsets, including T follicular helper cells (40), TH17 cells and TH2 cells (20,21), are influenced by IL-21. The analyses of pancreata from NOD mice also revealed a small fraction of TH17 cells that were significantly diminished during IL-21R/Fc treatment (Fig. 3A and B). However, a greater than 50% reduction in the fraction of TH17 cells early in the treatment regimen was consistent with an overall loss of activated CD4+ T-cells rather than a specific effect on TH17 cells. By contrast, there was no specific decline or retention of Foxp3+ regulatory T-cells in either the pancreatic lymph nodes or pancreas (Fig. 3C and D).
FIG. 3.
IL-21 neutralization modulates activated TH subsets. IL-17–expressing cells shown as representative dot plots (A) and quantified in the pancreas of treated vs. control mice on day 3 of treatment with IL-21R/Fc (B), as percentage of CD45+ cells, where n = 12 per group compiled from five experiments. Absolute number of Foxp3+ CD25+ CD4+ T-cells in pancreatic lymph node on days of treatment with IL-21R/Fc shown (C), and in pancreas on day 60 after treatment with IL-21R/Fc (D). Data are presented as means ± SEM, where n = 5 per group from two experiments.
Effective combination therapy with IL-21R/Fc and pancreatic islet transplantation.
These data show the merit of neutralizing IL-21 at a late preclinical stage. To be of relevance to human patients with type 1 diabetes, however, it would be more beneficial to reverse diabetes once it is clinically revealed, yet treatment of newly diabetic NOD mice (15–20 weeks of age) with IL-21R/Fc failed to reverse diabetes (data not shown).
Given the slow regeneration of the depleted β-cell mass (41), we assessed whether IL-21 neutralization combined with pancreatic islet transplantation could be a solution. Newly diabetic NOD mice (with two consecutive blood glucose readings above 18 mmol/L) were treated with IL-21R/Fc (10 mg/IV) on day −1, day 0, and every other day until day 12. Pancreatic islets from MHC-matched, lymphocyte deficient NOD/Scid mice were grafted under the kidney capsule of NOD mice (day 0). In mice treated with IL-21R/Fc, destruction of the graft and subsequent diabetes was delayed and reduced (Fig. 4A). The majority of treated mice maintained a stable normal range of blood glucose while undergoing treatment (Fig. 4B) with three of seven mice returning to hyperglycemia, compared with the entire untreated group with a mean survival time of 16.1 days.
To determine whether the graft was still necessary in long-term (100 days) diabetes-free IL-21R/Fc–treated NOD mice, islet grafted kidneys were removed in these mice and blood glucose was measured. No considerable modulation of blood glucose level was observed after the nephrectomy, and mice remained diabetes free for another 100 days (Fig. 4C). This finding raised the possibility that IL-21R/Fc treatment had affected the residual endogenous islets, either by increasing islet mass or restoring function. Assessment of islet mass from histological sections of pancreata immunostained for insulin indicated that IL-21R/Fc–treated NOD mice had equivalent islet mass to that of 15–20-week-old diabetic NOD mice, which were both significantly reduced compared with prediabetic NOD mice (Fig. 4D). Taken together these data indicated that NOD pancreatic islets had regained functionality through the combined effects of IL-21 neutralization and transplantation of syngeneic islets and were capable of maintaining normoglycemia without the islet graft.
Histological analysis of islet grafts from IL-21R/Fc–treated long-term survivors revealed healthy insulin producing grafts free from mononuclear cell infiltration (Fig. 4E). Furthermore, the pancreas revealed islet mass with mild peri-insulitis (Fig. 4F). These findings showed that IL-21R/Fc treatment was capable of inducing tolerance. Furthermore, these data demonstrate that combining islet transplantation (as a source of insulin) with specific immunomodulation of IL-21 could reverse diabetes.
IL-21 influences allograft survival.
These exciting findings have potential application for human type 1 diabetic patients, however this circumstance would have the additional challenge of allograft rejection. Given this challenge, we assessed the role of IL-21 in an islet allograft immune response. A fully mismatched H-2d BALB/c islet allograft was transplanted into H-2b wild type C57BL/6 (WT) and H-2b Il21r−/− streptozotocin-induced diabetic recipients.
All C57BL/6 (H-2b) mice rejected the (H-2d) islet allograft with a mean survival time of day 16.6 (n = 8). In comparison, 66% of Il21r−/− (H-2b) mice failed to acutely reject their H-2d islet allografts (Fig. 5A). As indicated by the individual blood glucose readings (Fig. 5B), the mice that survived long-term consistently maintained steady normoglycemic levels. Nephrectomies were performed on 100 day surviving Il21r−/− mice, resulting in loss of normoglycemia, confirming that the islet allograft was functional and responsible for controlling blood glucose in these mice.
In order to address whether lacking IL-21 responsiveness and an inability to reject the islet allograft was due to a problem in immune priming capacity, we rechallenged the nephrectomized mice with another H-2d BALB/c islet allograft, on the contralateral kidney. These secondary islet allografts remained unchallenged, surviving a further 100 days (Fig. 5C). Histological analyses of the long-term surviving islet allograft in Il21r−/− mice revealed strong insulin production with very mild mononuclear cell infiltrate peripheral to the graft (Fig. 5D). Taken together, these results indicated that responsiveness to IL-21 was crucial for successful rejection of islet allografts.
CD8 T-cell responsiveness to IL-21 is crucial to allograft rejection.
Given the important role IL-21 has in CD8+ T-cell survival and effector function (16,42), and the well documented critical role CD8+ T-cells have in the rejection of pancreatic islet allografts (43), we determined whether IL-21 responsiveness in CD8+ T-cells alone was sufficient to induce allograft rejection. The number and activation status of CD8+ T-cells are not impaired in resting unchallenged Il21r−/− mice (14,33). However, in the absence of IL-21 responsiveness, CD8+ T-cells become “exhausted” in chronic viral infections, exhibiting “impaired self-maintenance” (17–19).
To address whether IL-21 responsiveness in CD8+ T-cells was sufficient to provoke islet allograft rejection, we adoptively transferred IL-21R–sufficient CD8+ T-cells into Il21r−/− recipient mice and repeated the H-2d BALB/c islet allograft. We used MHC class II deficient mice as the source of CD8+ T-cells, thus eliminating the possibility of contaminating CD4+ T-cells. As is shown in Fig. 6, restoring IL-21 signaling to CD8+ T-cells alone resulted in rapid rejection of the allograft.
DISCUSSION
Cytokines can play both destructive and immunomodulatory roles in graft rejection. Roles for cytokines such as IFNγ, IL-1β, and tumor necrosis factor-α in allograft rejection have previously been described (44), but this study is the first to demonstrate a role for IL-21 in islet allograft rejection. IL-21 was important for islet targeted autoimmune inflammation in diabetes, and both autoimmune and allograft responses against islet grafts. Pharmacological neutralization of IL-21 at a late preclinical stage inhibited insulitis and delayed diabetes. Moreover, IL-21 neutralization when combined with syngeneic islet transplantation reversed diabetes. It has previously been shown that diabetic NOD mice retain some islet mass (45,46). Removal of the syngeneic islet graft from long-term (100 days) diabetes-free IL-21R/Fc–treated NOD mice revealed that the residual endogenous pancreatic islets (from previously clinically diabetic NOD mice) had regained functionality and were capable of supporting the insulin requirements without the islet graft.
Short-term neutralization of IL-21 reset the clock of autoimmune infiltration in mice on the brink of clinical disease indicating that the treatment both inhibits the ongoing accumulation of infiltrating immune cells as well as affecting resident lymphocytes, which emphasizes the dynamic nature of the islet lesion. Modulation of IL-21 for the brief time period suggests that the autoimmune inflammation is continually reliant on IL-21. The numbers of IL-21–producing cells in the pancreatic lesion were increased at the preclinical stage and were reduced by neutralization of IL-21. An autocrine role for IL-21 on the survival of IL-21–producing CD4+ T-cells has been demonstrated previously and might offer an explanation as to why the relatively short neutralization treatment regimen could perpetuate a sustained effect at a preclinical stage.
In an effort to explore the therapeutic effect of IL-21 neutralization on islet inflammation, we analyzed the endogenous pancreatic islet infiltrate and observed an immediate reduction in the number of activated lymphocytes. In particular, a sustained reduction in activated/memory-phenotype CD8+ T-cells was observed. Indeed, there was an approximately threefold decline in CD8+ T-cells 90 days following short-term treatment, which indicates that this population was less capable of recovering from treatment than CD4+ T-cells or B-cells. CD8+ T-cells have a well-supported role in both the early stage of disease, and in the final effector stage of diabetes (47,48). The broad effect of IL-21 neutralization on lymphocytes in the islet lesion probably reflects that IL-21 is produced by activated CD4+ T-helper cells and, as well as acting in an autocrine fashion, provides soluble help by facilitating the differentiation and survival of B cells and CD8+ cytotoxic T lymphocytes (33,49,50). Furthermore, our findings suggest that measurement of IL-21 could be a predictive marker for diabetes.
The influence of IL-21 on B-cells and T-cells indicates possible roles in both cellular and humoral rejection. However, IL-21 responsiveness by CD8+ T-cells alone could restore islet allograft destruction. This finding provides insight into the important clinical problem of organ allograft rejection, suggesting a potential role for IL-21 neutralization combined with pancreatic islet grafts for patients with type 1 diabetes. Interestingly, elevated IL-21 and IL-21R mRNA have been shown in biopsies from cardiac allograft recipients, with the highest mRNA expression levels found in rejection specimens (51). These reports support our finding that biological activities of the IL-21 pathway contribute to the graft rejection process.
It remains to be shown in our study how IL-21 promotes destruction of islet allografts by CD8+ T-cells. It is likely that IL-21–mediated rescue from ‘exhaustion’ of the graft specific CD8+ T-cells occurs, by a similar mechanism to that observed in chronic viral infections (17–19). Our finding of a role for IL-21 responsiveness in allograft rejection is in line with previous studies, which found that loss of IL-21 signaling attenuated the pathogenesis of CD4+ T-cell mediated graft versus host disease (GVHD) (23–25). Although these studies vary in the detail of their findings, both groups report a reduction in IFNγ-expressing T-cells (24,25). Even though the majority of Il21r−/− mice accepted islet allografts long-term, these studies indicate a degree of redundancy in the IL-21–IL-21R network, an outcome that can be further explored with combined therapies.
Studies collectively demonstrate that a variety of immunomodulatory reagents can protect the NOD mouse from diabetes. However, there are few examples of reagents that can reverse insulitis (52,53) and only one other reagent, namely anti-CD3 monoclonal antibody (54), that has been shown to prevent the progress of type 1 diabetes once blood glucose levels have begun to rise, leading to clinical trials for type 1 diabetes (55). In contrast to anti-CD3 treatment, neutralization of IL-21 did not reverse disease in overtly diabetic NOD mice (52). However, in accordance with anti-CD3 treatment, syngeneic islets could be transplanted without recurrence of disease or continuous administration of IL-21R/Fc. These influential studies place the blockade of IL-21:IL-21R interactions in an elite group of immunomodulatory agents in type 1 diabetes. The ability of short-term blockade of a single cytokine to have such a profound effect on islet inflammation is unprecedented and raises possibilities for intervention in type 1 diabetes at a late preclinical stage and for the protection of both allogeneic islet graft tissue and transplanted islet tissue during recurrent autoimmunity.
ACKNOWLEDGMENTS
C.K. is supported by grants from the National Health and Medical Research Council of Australia and the Juvenile Diabetes Research Foundation.
No potential conflicts of interest relevant to this article were reported.
H.M.M. researched data and wrote the manuscript. S.W., A.V., and C.M.Y.L. researched data. D.C. reviewed and edited the manuscript. K.E.W., J.S., and S.G. contributed to discussion. C.K. planned experiments and wrote the manuscript.
The authors thank the Australian Cancer Research Fund (ACRF) Unit for the Molecular Genetic of Cancer for use of the ABI Prism 7900 HT Sequence Detection System and the staff at the Biological Testing Facility (Garvan Institute of Medical Research, Sydney) for help with animal breeding and care.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-1157/-/DC1.
See accompanying commentary, p. 697.
REFERENCES
- 1.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol 2005;23:447–485 [DOI] [PubMed] [Google Scholar]
- 2.Bach JF, Mathis D. The NOD mouse. Res Immunol 1997;148:285–286 [DOI] [PubMed] [Google Scholar]
- 3.Green EA, Flavell RA. The initiation of autoimmune diabetes. Curr Opin Immunol 1999;11:663–669 [DOI] [PubMed] [Google Scholar]
- 4.Gazda LS, Charlton B, Lafferty KJ. Diabetes results from a late change in the autoimmune response of NOD mice. J Autoimmun 1997;10:261–270 [DOI] [PubMed] [Google Scholar]
- 5.André-Schmutz I, Hindelang C, Benoist C, Mathis D. Cellular and molecular changes accompanying the progression from insulitis to diabetes. Eur J Immunol 1999;29:245–255 [DOI] [PubMed] [Google Scholar]
- 6.Scharp DW, Lacy PE, Santiago JV, et al. Insulin independence after islet transplantation into type I diabetic patient. Diabetes 1990;39:515–518 [DOI] [PubMed] [Google Scholar]
- 7.Stratta RJ. Review of immunosuppressive usage in pancreas transplantation. Clin Transplant 1999;13:1–12 [DOI] [PubMed] [Google Scholar]
- 8.Castaño L, Eisenbarth GS. Type-I diabetes: a chronic autoimmune disease of human, mouse, and rat. Annu Rev Immunol 1990;8:647–679 [DOI] [PubMed] [Google Scholar]
- 9.Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immunol 2004;4:259–268 [DOI] [PubMed] [Google Scholar]
- 10.Datta S, Sarvetnick NE. IL-21 limits peripheral lymphocyte numbers through T cell homeostatic mechanisms. PLoS ONE 2008;3:e3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spolski R, Kashyap M, Robinson C, Yu Z, Leonard WJ. IL-21 signaling is critical for the development of type I diabetes in the NOD mouse. Proc Natl Acad Sci USA 2008;105:14028–14033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutherland AP, Van Belle T, Wurster AL, et al. Interleukin-21 is required for the development of type 1 diabetes in NOD mice. Diabetes 2009;58:1144–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leonard WJ, Zeng R, Spolski R. Interleukin 21: a cytokine/cytokine receptor system that has come of age. J Leukoc Biol 2008;84:348–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasaian MT, Whitters MJ, Carter LL, et al. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity 2002;16:559–569 [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Lizée G, Lou Y, et al. IL-21 synergizes with IL-7 to augment expansion and anti-tumor function of cytotoxic T cells. Int Immunol 2007;19:1213–1221 [DOI] [PubMed] [Google Scholar]
- 16.Allard EL, Hardy MP, Leignadier J, et al. Overexpression of IL-21 promotes massive CD8+ memory T cell accumulation. Eur J Immunol 2007;37:3069–3077 [DOI] [PubMed] [Google Scholar]
- 17.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science 2009;324:1569–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fröhlich A, Kisielow J, Schmitz I, et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 2009;324:1576–1580 [DOI] [PubMed] [Google Scholar]
- 19.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science 2009;324:1572–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nurieva R, Yang XO, Martinez G, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 2007;448:480–483 [DOI] [PubMed] [Google Scholar]
- 21.Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 2007;448:484–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol 2008;26:57–79 [DOI] [PubMed] [Google Scholar]
- 23.Meguro A, Ozaki K, Oh I, et al. IL-21 is critical for GVHD in a mouse model. Bone Marrow Transplant 2010;45:723–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bucher C, Koch L, Vogtenhuber C, et al. IL-21 blockade reduces graft-versus-host disease mortality by supporting inducible T regulatory cell generation. Blood 2009;114:5375–5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh I, Ozaki K, Meguro A, et al. Altered effector CD4+ T cell function in IL-21R-/- CD4+ T cell-mediated graft-versus-host disease. J Immunol 2010;185:1920–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreuwel HT, Morgan DJ, Krahl T, Ko A, Sarvetnick N, Sherman LA. Comparing the relative role of perforin/granzyme versus Fas/Fas ligand cytotoxic pathways in CD8+ T cell-mediated insulin-dependent diabetes mellitus. J Immunol 1999;163:4335–4341 [PubMed] [Google Scholar]
- 27.Kurrer MO, Pakala SV, Hanson HL, Katz JD. Beta cell apoptosis in T cell-mediated autoimmune diabetes. Proc Natl Acad Sci USA 1997;94:213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnush M, Heitmeier MR, Scarim AL, Marino MH, Manning PT, Corbett JA. IL-1 produced and released endogenously within human islets inhibits beta cell function. J Clin Invest 1998;102:516–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corbett JA, McDaniel ML. Intraislet release of interleukin 1 inhibits beta cell function by inducing beta cell expression of inducible nitric oxide synthase. J Exp Med 1995;181:559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meredith M, Rabaglia ME, Corbett JA, Metz SA. Dual functional effects of interleukin-1beta on purine nucleotides and insulin secretion in rat islets and INS-1 cells. Diabetes 1996;45:1783–1791 [DOI] [PubMed] [Google Scholar]
- 31.Zumsteg U, Frigerio S, Holländer GA. Nitric oxide production and Fas surface expression mediate two independent pathways of cytokine-induced murine beta-cell damage. Diabetes 2000;49:39–47 [DOI] [PubMed] [Google Scholar]
- 32.Serreze DV, Chapman HD, Varnum DS, et al. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD.Ig mu null mice. J Exp Med 1996;184:2049–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozaki K, Spolski R, Feng CG, et al. A critical role for IL-21 in regulating immunoglobulin production. Science 2002;298:1630–1634 [DOI] [PubMed] [Google Scholar]
- 34.Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J Immunol 2007;178:3822–3830 [DOI] [PubMed] [Google Scholar]
- 35.Young DA, Hegen M, Ma HL, et al. Blockade of the interleukin-21/interleukin-21 receptor pathway ameliorates disease in animal models of rheumatoid arthritis. Arthritis Rheum 2007;56:1152–1163 [DOI] [PubMed] [Google Scholar]
- 36.Faveeuw C, Gagnerault MC, Lepault F. Isolation of leukocytes infiltrating the islets of Langerhans of diabetes-prone mice for flow cytometric analysis. J Immunol Methods 1995;187:163–169 [DOI] [PubMed] [Google Scholar]
- 37.McGuire HM, Vogelzang A, Hill N, Flodström-Tullberg M, Sprent J, King C. Loss of parity between IL-2 and IL-21 in the NOD Idd3 locus. Proc Natl Acad Sci USA 2009;106:19438–19443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walters S, Webster KE, Sutherland A, et al. Increased CD4+Foxp3+ T cells in BAFF-transgenic mice suppress T cell effector responses. J Immunol 2009;182:793–801 [DOI] [PubMed] [Google Scholar]
- 39.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell 2004;117:265–277 [DOI] [PubMed] [Google Scholar]
- 40.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity 2008;29:127–137 [DOI] [PubMed] [Google Scholar]
- 41.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes 2005;54:2557–2567 [DOI] [PubMed] [Google Scholar]
- 42.Moroz A, Eppolito C, Li Q, Tao J, Clegg CH, Shrikant PA. IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: comparative evaluation of IL-2, IL-15, and IL-21. J Immunol 2004;173:900–909 [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto H, Monden M, Kawai M, et al. The role of CD8+ and CD4+ cells in islet allograft rejection. Transplantation 1990;50:120–125 [DOI] [PubMed] [Google Scholar]
- 44.Nickerson P, Steiger J, Zheng XX, et al. Manipulation of cytokine networks in transplantation: false hope or realistic opportunity for tolerance? Transplantation 1997;63:489–494 [DOI] [PubMed] [Google Scholar]
- 45.Ryu S, Kodama S, Ryu K, Schoenfeld DA, Faustman DL. Reversal of established autoimmune diabetes by restoration of endogenous beta cell function. J Clin Invest 2001;108:63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chong AS, Shen J, Tao J, et al. Reversal of diabetes in non-obese diabetic mice without spleen cell-derived beta cell regeneration. Science 2006;311:1774–1775 [DOI] [PubMed] [Google Scholar]
- 47.Serreze DV, Gallichan WS, Snider DP, et al. MHC class I-mediated antigen presentation and induction of CD8+ cytotoxic T-cell responses in autoimmune diabetes-prone NOD mice. Diabetes 1996;45:902–908 [DOI] [PubMed] [Google Scholar]
- 48.Kay TW, Parker JL, Stephens LA, Thomas HE, Allison J. RIP-beta 2-microglobulin transgene expression restores insulitis, but not diabetes, in beta 2-microglobulin null nonobese diabetic mice. J Immunol 1996;157:3688–3693 [PubMed] [Google Scholar]
- 49.Good KL, Bryant VL, Tangye SG. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J Immunol 2006;177:5236–5247 [DOI] [PubMed] [Google Scholar]
- 50.Li Y, Bleakley M, Yee C. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J Immunol 2005;175:2261–2269 [DOI] [PubMed] [Google Scholar]
- 51.Baan CC, Balk AH, Dijke IE, et al. Interleukin-21: an interleukin-2 dependent player in rejection processes. Transplantation 2007;83:1485–1492 [DOI] [PubMed] [Google Scholar]
- 52.Lee Y, Chin RK, Christiansen P, et al. Recruitment and activation of naive T cells in the islets by lymphotoxin beta receptor-dependent tertiary lymphoid structure. Immunity 2006;25:499–509 [DOI] [PubMed] [Google Scholar]
- 53.Simon G, Parker M, Ramiya V, et al. Murine antithymocyte globulin therapy alters disease progression in NOD mice by a time-dependent induction of immunoregulation. Diabetes 2008;57:405–414 [DOI] [PubMed] [Google Scholar]
- 54.Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci USA 1994;91:123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isaacs JD. T cell immunomodulation—the Holy Grail of therapeutic tolerance. Curr Opin Pharmacol 2007;7:418–425 [DOI] [PubMed] [Google Scholar]