Abstract
OBJECTIVE
β-Cell and islet endothelial cell destruction occurs during the progression of type 1 diabetes, but, paradoxically, β-cell proliferation is increased during this period. Altered glucose tolerance may affect β-cell mass and its association with endothelial cells. Our objective was to study the effects of glucose and inflammation on islet vascularity and on β function, mass, and insulin in immunologically tolerant anti-CD3 monoclonal antibody (mAb)-treated and prediabetic NOD mice.
RESEARCH DESIGN AND METHODS
The effects of phloridzin or glucose injections on β-cells and endothelial cells were tested in prediabetic and previously diabetic NOD mice treated with anti-CD3 mAbs. Glucose tolerance, immunofluorescence staining, and examination of islet cultures ex vivo were evaluated.
RESULTS
Islet endothelial cell density decreased in NOD mice and failed to recover after anti-CD3 mAb treatment despite baseline euglycemia. Glucose treatment of anti-CD3 mAb–treated mice showed increased islet vascular density and increased insulin content, which was associated with improved glucose tolerance. The increase in the vascular area was dependent on islet inflammation. Increased islet endothelial cell density was associated with increased production of vascular endothelial growth factor (VEGF) by islets from NOD mice. This response was recapitulated ex vivo by the transfer of supernatants from NOD islets cultured in high-glucose levels.
CONCLUSIONS
Our results demonstrate a novel role for glucose and inflammation in the control of islet vasculature and insulin content of β-cells in prediabetic and anti-CD3–treated NOD mice. VEGF production by the islets is affected by glucose levels and is imparted by soluble factors released by inflamed islets.
Studies in NOD mice have described a linear loss of β-cell mass from the time of initiation of insulitis and prediabetes through the presentation with hyperglycemia and afterward, followed by the complete loss of β-cells (1–3). Treatment of diabetic mice with anti-CD3 monoclonal antibodies (mAbs) can reverse hyperglycemia, resulting in the normalization of basal glucose levels. We previously have shown that β-cell regranulation occurs after anti-CD3 mAb treatment (2); however, the factors that improve insulin content in β-cells are poorly understood.
The islet vasculature has long been regarded as playing a crucial role in maintaining β-cell survival and function (4). This is illustrated by the fact that specific deletion of the vascular endothelial growth factor (VEGF) gene in β-cells reduces the islet vasculature and leads to impaired glucose tolerance, reduced glucose sensing, and decreased islet size (5). Similarly, VEGF signaling blockage resulted in a rapid regression of the islet vasculature (6), whereas overexpression of VEGF under the rat insulin promoter improved islet mass after transplantation (7). The ability of the islet vasculature to respond to blood glucose levels by modulating blood flow in the islet (8) suggests that the islet endothelium may respond to changes in metabolic demand and inflammation and affect β-cell function in the setting of diabetes. Moreover, the role of the islet endothelium in type 1 diabetes may be more than providing nutrients because previous reports have shown that islet endothelial cells express costimulatory markers and may function as antigen-presenting cells (9,10).
Inflammation plays a dual role in the progression and remission from type 1 diabetes. β-Cell proliferation increases in NOD.SCID recipients of diabetogenic spleen cells prior to the development of hyperglycemia (2). Three previous studies (1,2,11) have shown that β-cell proliferation increases with islet inflammation during the progression of diabetes in NOD mice because by 4 weeks of age, the proportion of Ki67+insulin+ cells in the islets was significantly increased in mice with insulitis compared with control mice. Anti-inflammatory measures, such as treatment with anti-CD3 mAbs or transfer of Tregs, reduced β-cell proliferation 3 weeks after treatment (2). Similarly, the transfer of diabetogenic spleen cells into NOD.SCID recipients together with CD4+CD25+ regulatory T-cells not only reduced the rate of diabetes but also decreased β-cell proliferation in these mice (2). This paradoxical fall in β-cell proliferation after anti-CD3 treatment and Treg transfer, despite improvement in glucose levels, suggests that inflammation is required for β-cell replication or attempts at recovery of β-cell function.
Impaired metabolic control attributed to deteriorating β-cell mass or function may also affect β-cell function and replication. Interestingly, the highest levels of proliferating Ki67+insulin+ cells are found just prior to the development of hyperglycemia and remain high after hyperglycemia (2). This observation suggests that increased metabolic demand, possibly associated with impaired glucose tolerance prior to the development of hyperglycemia, may contribute to the rate of β-cell proliferation. This hypothesis is supported by the suggestion that increased metabolic demand in obese patients and changes in insulin sensitivity during pregnancy may stimulate β-cell proliferation (12). Pechhold et al. (13) showed that insulin treatment reduced β-cell proliferation in hyperglycemic NOD mice. Alonso et al. (14) found that mice treated with glucose infusions over a period of several weeks showed increased β-cell mass attributed to β-cell proliferation.
The ways in which inflammation and glucose affect β-cell growth and function in the setting of autoimmunity has not been directly studied. We therefore studied the changes in β-cell function, vasculature, and replication after treatment with anti-CD3 in hyperglycemic NOD mice to test whether β-cell function or replication can be enhanced after immune therapy. We show that glucose and inflammation can promote the recovery of insulin content in the islets of anti-CD3–treated mice by stimulating VEGF production in islets.
RESEARCH DESIGN AND METHODS
Female NOD/LtJ and NOD.SCID mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and maintained under pathogen-free conditions. NOD mice were screened for hyperglycemia at 12 weeks of age and diagnosed with diabetes when two consecutive glucose levels were >200 mg/dL (2). Glucose levels were measured in whole blood from the tail vein using a Glucometer Elite XL (Bayer, Elkhart, IN). Animal use was approved by the Yale University Animal Use Committee.
Treatment with phloridzin.
Prediabetic NOD mice were treated with intradermal phloridzin (PHZ) injections at 0.4 mg/kg per day for 14 days (15). Blood glucose and the presence of glucosuria were measured 3 h after PHZ treatment. Glycosuria induced by PHZ was detected for as long as 9 h after the injection. After 14 days, mice were killed and their pancreata were collected.
Treatment with anti-CD3 mAbs.
Diabetic NOD mice were treated with 10 μg/day intraperitoneally (i.p.) of the mAb 145-2C11 (anti-mouse CD3) for 5 days beginning at the onset of hyperglycemia, as previously described (2). Reversal of diabetes was defined when random glucose levels were <200 mg/dL for 2 weeks after diagnosis and persisted for >10 days.
Treatment with glucose.
Glucose was injected i.p. at a dose of 4 g/kg per day. Anti-CD3–treated mice with reversed hyperglycemia for at least 10 days received daily injections of glucose or vehicle for 14 days. On day 14, mice were subjected to an intraperitoneal glucose tolerance test (IPGTT), and sera and pancreata were collected for additional analysis. In a separate set of experiments, nondiabetic 12- to 14-week-old NOD.SCID mice and 8- to 10-week-old NOD mice were subjected to 14 days of either glucose or vehicle treatment.
IPGTT.
Mice undergoing an IPGTT were fasted overnight. They received a 2 g/kg i.p. dextrose injection. Whole-blood glucose levels were measured from the tail vein taken before and 15, 30, 60, and, in some experiments, 120 min after the injection.
Immunofluorescence.
Pancreata were resected and fixed for 24 h in 2% PFA. After fixation, pancreatic tissue was placed in a sucrose gradient and snap frozen in liquid nitrogen. Noncontiguous 14-μm pancreatic sections were stained with antibodies to insulin (Invitrogen, Carlsbad, CA), 4′,6-diamidine-2-phenylindole dihydrochloride (DAPI), CD31 (BD Biosciences, San Jose, CA), and either Ki67 or VEGF (Abcam, Cambridge, MA). The bound antibodies were detected by immunofluorescent secondary antibodies (Jackson Immunoresearch, West Grove, PA). The slides were analyzed by fluorescence microscopy using an Olympus BX-51 microscope. For whole-tissue sections, ×5 images were captured using MosaiX software (Carl Zeiss, Thornwood, NY). Image analysis and postprocessing were performed using ImageJ (http://rsb.info.nih.gov/ij/). Numbers of single-color– and dual-color–labeled cells were counted using functions in ImageJ (colocalization, watershed, and analyze particles). The total pancreatic section area and insulin+ cells were measure using ImageJ (16).
Image capture and automated quantitative analysis.
Insulin concentration was measured on a continuous scale using automated quantitative analysis (AQUA), as has been previously described (17). In brief, a series of high-resolution monochromatic images were captured using an automated microscope platform (PM-2000TM; HistoRx, New Haven, CT). Fluorescent chromagens (DAPI, Cy3-insulin) were used to demarcate insulin+ cells within each whole-section image. DAPI staining of cell nuclei was used to generate the nuclear compartment. Subtracting the generated nuclear compartment from the masked cell area created a nonnuclear compartment, which included both the cytoplasm and the membrane. To generate an AQUA score, each pixel was recorded on a scale of 0 (black) to 255 (white), and pixel intensity was defined using the following equation: (target pixel intensity) × (compartment pixel intensity/255). Using the target and compartment pixel intensities, the following equation was generated: (sum of all target pixel intensities)/(sum of all compartment pixel intensities/255) = the AQUA score. All scores are normalized for image-exposure time and bit depth, allowing direct comparison of different histospot/whole-section scores.
Islet isolation.
Islets were isolated from NOD or NOD.SCID mice after digestion with collagenase (Liberase; Roche, Indianapolis, IN) (18). Islets were handpicked twice with an inverted microscope. To examine the effects of increasing glucose concentrations on VEGF expression, islets were cultured in 10% fetal calf serum glucose-free RPMI (Mediatech, Manassas, VA) supplemented with indicated glucose concentrations for 24 h. In some experiments, preconditioned islet culture media was collected and added to cultures of freshly isolated NOD.SCID islets. After incubation, islets were harvested and total RNA was extracted using an RNAeasy kit according to the manufacturer’s protocol (Qiagen, Valencia, CA). cDNA was generated using SuperScript II (Invitrogen).
Studies of glucose effect on endothelial cell number.
Purified islets from either NOD or NOD.SCID donors were incubated with 5 or 10 mmol/L glucose for 7 days and then dispersed using trypsin digestion. The cells were stained for the endothelial cell–specific marker, BS-1. We used BS-1 rather than CD31 for identification of endothelial cells because of CD31 sensitivity to trypsin digestion. The proportion of BS-1+ cells was evaluated using fluorescence-activated cell sorter analysis.
Real-time PCR.
Examination of VEGF-120, VEGF-164, and VEGF-188 levels was quantitated by real-time PCR using SYBR Green (Qiagen, Valencia, CA) on the iQ-5 multicolor real-time PCR system (Bio-Rad, Hercules, CA). All genes were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. Data analysis was performed using Biorad IQ-5 analyzing software. PCR primer pairs were as follows: GAPDH: forward CTGCACCACCAACTGCTTAG, reverse GATGGCATGGACTGTGGTCAT; and VEGF: exon 3 common forward primer ATCTTCAAGCCGTCCTGTGTGC, 120 reverse TTGGCTTGTCACATTTTTCTGG, 164 reverse CAAGGCTCACAGTGATTTTCTGG, and 188 reverse ATCTTCAAGCCGTCCTGTGTGC.
RESULTS
Islet vasculature density is reduced in prediabetic NOD mice and fails to recover after anti-CD3 treatment.
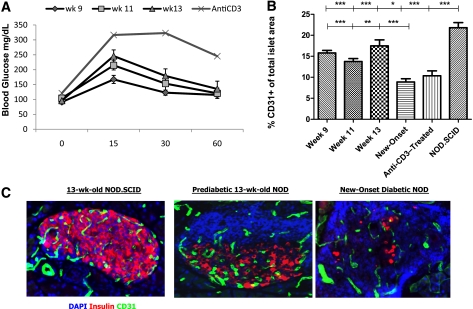
Examination of prediabetic mice showed impaired glucose tolerance in 13-week-old NOD mice when compared with 9-week-old NOD mice (Fig. 1A; area under the curve at 9 weeks = 8,702 vs. at 13 weeks = 11,195; P < 0.05). Previous studies have described a decline in islet vascular area in NOD mice during the progression of diabetes (9), but the relationship between these changes and the impairment of glucose tolerance in NOD mice has not been directly evaluated. We therefore studied the islet vascular area in prediabetic mice and after recovery from hyperglycemia in diabetic NOD mice that were treated with anti-CD3 mAbs. Reduced CD31+ area was evident as early as 9 weeks of age when compared with nonautoimmune NOD.SCID animals (Fig. 1B; P < 0.0001). Although there was not a direct relationship between changes in glucose tolerance and islet vasculature during prediabetes, there was a sharp decline in CD31 area in the islets at the time of diabetes onset (Fig. 1B and C; P < 0.0001).
FIG. 1.
Anti-CD3–treated diabetic NOD mice fail to normalize their IPGTTs and endothelial cell area in the islet. A: IPGTTs of prediabetic mice at the ages of 9, 11, and 13 weeks (n = 5 each group) and after anti-CD3 treatment (n = 12). B: Quantitative histomorphic analysis of CD31+ in pancreatic sections (n = 3 and four mice of islets from prediabetic, diabetic, and anti-CD3–treated NOD mice and n = 3 and four mice at each time point) (mean ± SE). The CD31+ area in the islets was compared with the same in nonautoimmune NOD.SCID mice by ANOVA (top line, P < 0.0001) and by the Dunnett multiple comparison test (*P < 0.05; ***P < 0.001) and with mice with new-onset diabetes (bottom line, ANOVA P < 0.0001 and **P < 0.01; ***P < 0.001). C: Immunofluoresence staining samples of representative islets from 13-week-old nonautoimmune NOD.SCID mice, prediabetic NOD mice, and NOD mice with new-onset diabetes. Blue, DAPI; red, insulin; green, CD31. wk, week. (A high-quality digital representation of this figure is available in the online issue.)
Next, we treated newly diabetic NOD mice with anti-CD3 mAbs and found that the nonfasting glucose levels returned to nondiabetic levels of <200 mg/dL for a period >2 consecutive weeks (154.9 ± 8.2 mg/dL). However, when challenged with an IPGTT, these mice showed glucose intolerance when compared with 9-week-old NOD mice (Fig. 1A; area under the curve at 9 weeks = 8,702 vs. anti-CD3–treated = 16,578; P < 0.0001). In addition, the endothelial cell density was not normalized after anti-CD3 mAb therapy and remained significantly reduced when compared with NOD.SCID or 9-week-old NOD mice (Fig. 1B; P < 0.001). These results show a loss of endothelial cells in the islet during prediabetes that is concomitant with deterioration of glucose tolerance, both of which are not fully corrected following anti-CD3 treatment.
Variations in glucose load affect endothelial cell density in prediabetic and anti-CD3–treated NOD mice.
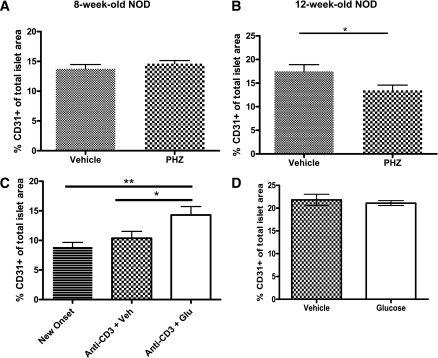
To determine the relationship between changes in glucose and the CD31+ cell density, we treated prediabetic NOD mice with normal (8 weeks old) or impaired (12 weeks old) results from the IPGTT with daily injections of PHZ for a period of 14 days. PHZ increases renal clearance of glucose and reduces glucose load (15). First, we tested PHZ effects on glucose load in normoglycemic BALB/C during the IPGTT. PHZ injection increased clearance of blood glucose, as indicated by a more rapid return to baseline glucose levels (Supplementary Fig. 1A). Daily PHZ injections of 12-week-old NOD mice reduced endothelial cell density when compared with vehicle-treated mice (Fig. 2B and Supplementary Fig. 2), without altering the islet degree of insulitis (Supplementary Fig. 1B). Interestingly, PHZ also decreased the rates of β-cell proliferation (Table 1 and Supplementary Fig. 3), which is consistent with the previously described effects of glucose on β-cell proliferation in prediabetic mice (13). In contrast, PHZ treatment of 8-week-old NOD mice, with normal IPGTT results, did not reduce endothelial cell density (Fig. 2A).
FIG. 2.
Glucose modulates endothelial cell density in prediabetic and anti-CD3–treated NOD mice. Histomorphic analysis of pancreata (n ≥ 3 per group) CD31+ area in 8-week-old (A) and 12-week-old (B) prediabetic NOD mice after daily injections of PHZ for a period of 14 days (*P < 0.04). C: Endothelial cell area in diabetic and anti-CD3–treated NOD mice receiving either vehicle or glucose injections for 14 days. D: As in C but with immunodeficient NOD.SCID mice. *P < 0.05; **P < 0.001. Glu, glucose; Veh, vehicle.
TABLE 1.
Proliferation levels in islets of prediabetic and anti-CD3–treated mice
| Group | Percentage Ki67+Ins+ β-cells | P |
|---|---|---|
| Prediabetic 8 weeks | 0.44 | |
| Vehicle | 0.23 ± 0.07 | |
| PHZ | 0.16 ± 0.06 | |
| Prediabetic 12 weeks | 0.0003 | |
| Vehicle | 0.75 ± 0.11 | |
| PHZ | 0.27 ± 0.07 | |
| Anti-CD3–treated | 0.87 | |
| Vehicle | 0.42 ± 0.13 | |
| PHZ | 0.46 ± 0.18 |
Data are means ± SE. Pancreata from various NOD groups were stained for Ki67 and insulin. The percentage of Ki67+Ins+ cells were analyzed as described in research design and methods.
We also tested whether an increase in glucose in mice with impaired glucose tolerance would have the opposite effect. Recovered anti-CD3–treated NOD mice showing a minimum of 10 consecutive days of basal euglycemia received daily injections of glucose (4 mg/kg i.p.) for 14 days. After treatment, pancreata were collected and islet endothelial cell density was evaluated. Glucose injections significantly increased endothelial cell density to a density similar in prediabetic NOD mice when compared with vehicle-treated controls (Fig. 2A and C; P < 0.04 and P < 0.001). In contrast, similar treatment of NOD.SCID mice with daily glucose injections failed to increase endothelial cell density in islets (Fig. 2D). These data suggest that fluctuation in daily glucose may modulate endothelial cell density under the conditions of islet inflammation.
Increased endothelial cell density after a glucose load is associated with improved glucose tolerance and insulin content in anti-CD3–treated NOD mice.
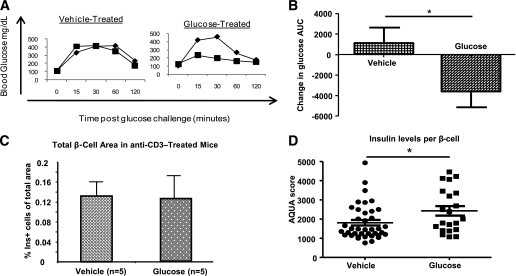
Next, we tested the physiological consequences of increased endothelial cell density in glucose-treated anti-CD3–treated NOD mice. Interestingly, despite the presence of impaired glucose tolerance before treatment, glucose-treated anti-CD3 mice showed improved IPGTT results when compared with vehicle-treated mice (Fig. 3A and B; P < 0.05). Fasting insulin levels in glucose-treated mice were higher than in placebo-treated mice (vehicle = 0.61 ng/mL vs. glucose = 0.90 ng/mL; P < 0.06); however, no increase in β-cell mass was detected (Fig. 3C). Therefore, to examine the effect of glucose on insulin content in individual β-cells, we used the AQUA score to measure the content of insulin. The AQUA score has been validated for protein quantization in histological section of malignant tissues in which good reproducibility and linearity with protein content of tissues has been validated (18–23). AQUA score analysis revealed increased levels of insulin staining per β-cell in glucose-treated mice (Fig. 3D; P < 0.05). Glucose treatment of anti-CD3–treated mice did not increase β-cell proliferation, albeit proliferation was at relatively high levels in both vehicle- and glucose-treated mice (Table 1).These results suggest that increased islet endothelial cell density may promote β-cell function by increasing insulin content rather than β-cell proliferation after treatment with anti-CD3 antibodies.
FIG. 3.
Daily glucose injections improve glucose tolerance and increase insulin content in anti-CD3–treated NOD mice. A: Representative IPGTTs of anti-CD3–treated mice with normal basal glucose levels receiving daily injections of either vehicle (left panel) or glucose (right panel) intraperitoneally (♦, pretreatment; ■, posttreatment). B: Summary of changes in IPGTT results of anti-CD3–treated diabetic mice with normal basal glucose levels after a 14-day challenge of daily injections of either glucose or vehicle. *P < 0.04 (n = 12 per group). Stained pancreatic section of vehicle- and glucose-treated mice for insulin+ cells. C: Total β-cell area. D: AQUA scores of insulin levels per β-cell as described in research design and methods. *P < 0.03. Ins, insulin.
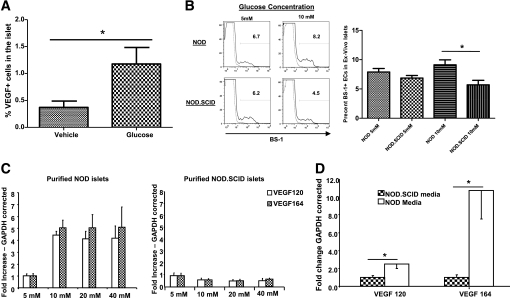
To understand the basis for increased endothelial cell density after glucose treatment, we measured VEGF production in the islets of glucose-treated anti-CD3–treated mice by histomorphic analysis. Preliminary immunofluorescence staining revealed an increase in VEGF levels in prediabetic NOD islets compared with NOD.SCID islets (Supplementary Fig. 4). In anti-CD3–treated mice, daily glucose injections resulted in increased VEGF expression in the islets when compared with vehicle-treated mice (Fig. 4A; P < 0.02), suggesting that VEGF production by resident or infiltrating islet cells can promote islets in response to glucose. To directly examine the effects of glucose on endothelial cell numbers, we incubated purified islets from either NOD or NOD.SCID donors at either 5 mmol/L or 10 mmol/L glucose for 7 days followed by fluorescence-activated cell sorter analysis of BS-1+ endothelial cells. NOD islets incubated at 10 mmol/L glucose showed better maintenance of endothelial cells when compared with islets incubated at 5 mmol/L glucose (Fig. 4B). In contrast, high glucose levels failed to maintain endothelial cell levels in islets derived from NOD/SCID mice when compared with islets incubated at 5 mmol/L glucose (Fig. 4B). Quantitative PCR analysis of purified endothelial cells from NOD islets cultured at 10 mmol/L glucose showed a 4.2-fold increase in the expression of the antiapoptotic BCL-XL gene (cycle difference from GAPDH: NOD = 1.87 ± 0.73 vs. NOD.SCID = 0.44 ± 0.07; P = 0.10), whereas Ki67 gene expression remained unchanged (data not shown).
FIG. 4.
High glucose levels stimulate VEGF expression under the conditions of islet inflammation. A: Histomorphic analysis of islets from the pancreatic section of vehicle- or glucose-treated anti-CD3–treated NOD mice (P < 0.02). B: Purified islets from 8- to 10-week-old NOD or NOD.SCID mice were incubated in either 5 or 10 mmol/L glucose for 7 days. After incubation, islets were dispersed and endothelial cell frequency was analyzed using fluorescence-activated cell sorter of BS-1+ cells as described in research design and methods. Left panel: A representative histogram depicting a sample of two NOD or NOD.SCID islet cultures stained for BS-1. Right panel: Summary of the percentage of BS-1+ cells after culture in 5 or 10 mmol/L glucose. n = 6 per group; *P < 0.013. C: Purified islets from 8- to 10-week-old NOD (left) or NOD.SCID (right) mice as in B were incubated with increasing concentrations of glucose. Quantitative RT-PCR was done for VEGF-120 and VEGF-164 isoforms in the islets. D: Supernatants from NOD or NOD.SCID islets incubated at 10 mmol/L glucose for 72 h were added to freshly purified NOD.SCID islets. Quantitative RT-PCR was done for VEGF-120 and VEGF-164 isoforms in the islets (*P < 0.05). n ≥ 3 per group.
Because VEGF-A is the main regulator of endothelial cell maintenance, we tested whether increased glucose had a differential effect on VEGF production by islets from NOD and NOD.SCID mice ex vivo. Purified islets from 8- to 10-week-old mice were incubated with increasing concentrations of glucose for 24 h followed by mRNA extraction. RT-PCR analysis of VEGF-120, VEGF-164, and VEGF-188 mRNA isoforms revealed a fivefold increase in VEGF-120 and VEGF-164 production in the islets of NOD mice, whereas VEGF-188 was undetectable (Fig. 4C; data not shown and [24]). This increase was evident at glucose concentrations of 5, 10, 20, and 40 mmol/L (Fig. 4C). In contrast, hyperglycemia did not induce VEGF production in cultured islets form NOD.SCID mice (Fig. 4C).
These findings were consistent with the lack of an effect of glucose on vascular area in NOD.SCID mice treated with glucose but left open the possibility that during inflammation, islets could acquire the ability to produce VEGF after direct immune injury or in response to soluble factors produced by the infiltrating cells. To test this, we cultured NOD.SCID islets with supernatants collected after a 72-h incubation of NOD islets in 10 mmol/L glucose and measured VEGF production by real-time PCR. There was a significant increase in VEGF-120 and VEGF-164 after incubation with islets from NOD but not control NOD.SCID mice (Fig. 4D). This increase was more pronounced in the VEGF-164 isoform, suggesting that the VEGF-120 isoform may derive from a non-islet resident cell (Fig. 4D). These data suggest that inflammation can promote the production of VEGF-A in the islet by a yet-unidentified soluble factor.
DISCUSSION
The improvement in insulin secretion that is seen after immune therapy in NOD mice and most likely during the “honeymoon” of type 1 diabetes reflects the functional recovery of existing β-cells, possibly with growth of new cells, but the factor(s) that controls these responses is not well understood (3). In this study, we have confirmed previous findings that in addition to β-cells, the islet vasculature density is reduced prior to the presentation of diabetes and that a significant decrease in β-cell mass and islet endothelial cells after presentation of diabetes in NOD mice further promote glucose intolerance. Moreover, treatment with the anti-inflammatory anti-CD3 mAbs, which reverse frank hyperglycemia, fails to correct the impaired IPGTT results, which are associated with reduced CD31+ cells. In addition, we have found that glucose is a regulator of islet vascular density both in the prediabetic period and after treatment of diabetic mice with anti-CD3 mAbs, where glucose treatment augments CD31 density within the islets. This increase in islet vasculature occurs in conjunction with an increase in VEGF production, resulting in enhanced insulin content and islet vasculature, but not β-cell proliferation, in islets that have recovered or been spared destruction by the immune attack. The increase in endothelial cell density may be a result of decreased rates of cell death because the levels of the antiapoptotic gene BCL-XL were increased in inflamed endothelial cells under the condition of hyperglycemia. Importantly, this effect of glucose is contingent upon the presence of inflammation. Susceptibility to the effects of glucose is induced by soluble factor(s) secreted by the inflamed islets in response to hyperglycemia. This soluble factor produced by inflamed islets controls VEGF production by resident islet cells.
The ongoing immunological assault on insulin-producing β-cells in the islet during the progression of diabetes not only leads to the amplification of the immune response to islet antigens but also results in a gradual deterioration of glucose tolerance in prediabetic NOD mice. This increase in metabolic demand imposes a new challenge for remaining β-cells to compensate for increased glucose levels, as seen in situations of type 2 diabetes and during pregnancy (12). Our finding showing the ability of treatment with the glycosuric agent PHZ to reduce islet vasculature suggests that glucose levels may control the islet vasculature in the prediabetic state by increasing it with hyperglycemia and conversely by contracting it with reduced glucose load. The control of islet capillaries by glucose would be predicted because their unique structure, which consists of a basement membrane and fenestrations and is important for the control of glucose flow to the endocrine cells (25). Islet endothelial cells are highly sensitive to changes in glucose levels because increased glucose levels in GK rats can increase islet capillary blood pressure (26). In addition, Nyman et al. (8) have recently shown that islet blood flow is regulated by glucose in normal mice without insulitis. In their studies (8), blood flow to the islet was dramatically increased after the hyperglycemic clamp.
However, the effects of inflammation on imparting sensitivity of the islet vasculature to glucose had not been previously identified. In anti-CD3 mAb–treated mice, which show improved basal glucose levels but exhibit impaired glucose tolerance, islet vasculature improves modestly following anti-CD3 treatment. This recovery of endothelial cell density is enhanced by daily glucose injections, leading to improved glucose tolerance and increased insulin content per β-cell. Based on our previous studies (27), it is likely that the improved islet vasculature increases the insulin content of recovered degranulated β-cells, which accounts for the majority of β-cell mass recovery after treatment with anti-CD3 (2).
Inflammation has been suggested as a stimulant of β-cell replication because β-cell proliferation is increased in prediabetic NOD mice and is highest at the time of the greatest β-cell destruction (2,28). In addition, the microvasculture is also dependent on inflammation and can increase by a variety of cytokines. These permissive roles of inflammation on β-cell proliferation and islet vasculature are supported by our findings showing no increase in β-cell replication and islet vasculature after glucose treatment of immunodeficient NOD.SCID mice. Our new findings suggest that inflammation also affects the islet vasculature and that soluble products from inflamed islets render islet cells themselves responsive to glucose because NOD.SCID islets cultured with supernatants from NOD islets acquired responsiveness of VEGF production to glucose. One candidate cytokine capable of stimulating VEGF production and vessel remodeling is tumor necrosis factor (TNF)-α (29). The addition of TNF or its closely related family member, lymphotoxin, can lead to increased VEGF production and stimulation of angiogenesis (30). The ability of the islet endothelium to respond to increasing VEGF levels may relate to increased expression of VEGF receptors on endothelial cells during inflammation. The addition of TNF to endothelial cells in culture can indeed result in increased VEGFR2 expression, leading to altered endothelial cell morphology and angiogenesis (31), which, when combined with increased glucose levels, can lead to increased vasculature. The effects of the inflammatory response on the extracellular matrix (ECM) also must be considered. Normal endothelial cell β interactions are largely mediated via integrins, suggesting that inflammation may alter these conditions. Recently, the role of matrix metalloproteinase-9 was documented in mediating the release of VEGF, thus increasing the effective concentration of VEGF-165 at the site of inflammation (32). The breakdown of the ECM may further potentiate angiogenesis and endothelial cell survival under the conditions of inflammation (33). Based on our finding of different proportions of VEGF isoforms produced in response to glucose by NOD islets and NOD.SCID islets cultured with NOD culture supernatants, we speculate that both the islet and immune cells are the sources of VEGF in inflamed islets stimulated with glucose, and the possibility of ECM disturbance and increased accessibility of VEGF to activated endothelial cells require additional investigation.
Our findings with PHZ are consistent with a recent report by Pechhold et al. (13) in which islet transplantation or insulin treatment reduced β-cell proliferation in NOD mice. However, we were unable to detect an increase in β-cell replication when we treated previously diabetic mice with glucose or even in prediabetic mice that were treated with glucose (data not shown). These negative findings are in contrast to studies from Alonso et al. (14) in which chronic hyperglycemia induced β-cell replication in B6 mice. One factor that may account for these discrepancies may be the way in which glucose was administered. We gave a single daily dose rather than a continuous infusion. In addition, the rates of β-cell replication were already high in our prediabetic and postdiabetic mice treated with glucose, and, thus, it may not have been possible to increase the rates of replication further.
Our data suggest a novel role for glucose in the maintenance of islet vasculature during the progression of and recovery from diabetes and the effects of inflammation on imparting sensitivity to these effects. The factor(s) that render the islet vasculature susceptible to glucose may identify a means of increasing β-cell function and/or mass in settings where β-cell mass is compromised.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (Grant no. DK-057846) and the Juvenile Diabetes Research Foundation (Grant nos. 2008-634 and 2008-631).
No potential conflicts of interest relevant to this article were reported.
E.M.A. researched data and wrote, reviewed, and edited the manuscript. M.-T.B., L.W.O.-A., M.A., and E.G. researched data. J.A.K. contributed to discussion and reviewed and edited the manuscript. D.L.R. reviewed and edited the manuscript. K.C.H. reviewed and edited the manuscript and contributed to the discussion.
The authors thank Drs. Diane Krause, Jordan Pober, Steve Strittmatter, and William Cafferty from the Yale University School of Medicine for their technical advice.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-0793/-/DC1.
REFERENCES
- 1.Sreenan S, Pick AJ, Levisetti M, Baldwin AC, Pugh W, Polonsky KS. Increased β-cell proliferation and reduced mass before diabetes onset in the nonobese diabetic mouse. Diabetes 1999;48:989–996 [DOI] [PubMed] [Google Scholar]
- 2.Sherry NA, Kushner JA, Glandt M, Kitamura T, Brillantes AM, Herold KC. Effects of autoimmunity and immune therapy on β-cell turnover in type 1 diabetes. Diabetes 2006;55:3238–3245 [DOI] [PubMed] [Google Scholar]
- 3.Akirav E, Kushner JA, Herold KC. β-Cell mass and type 1 diabetes: going, going, gone? Diabetes 2008;57:2883–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dewitt LM. Morphology and physiology of areas of Langerhans in some vertebrates. J Exp Med 1906;8:193–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai Y, Schneider D, Kidszun A, et al. Vascular endothelial growth factor increases functional beta-cell mass by improvement of angiogenesis of isolated human and murine pancreatic islets. Transplantation 2005;79:1530–1536 [DOI] [PubMed] [Google Scholar]
- 6.Kamba T, Tam BY, Hashizume H, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol 2006;290:H560–H576 [DOI] [PubMed] [Google Scholar]
- 7.Brissova M, Shostak A, Shiota M, et al. Pancreatic islet production of vascular endothelial growth factor-α is essential for islet vascularization, revascularization, and function. Diabetes 2006;55:2974–2985 [DOI] [PubMed] [Google Scholar]
- 8.Nyman LR, Ford E, Powers AC, Piston DW. Glucose-dependent blood flow dynamics in murine pancreatic islets in vivo. Am J Physiol Endocrinol Metab 2010;298:E807–E814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savinov AY, Wong FS, Stonebraker AC, Chervonsky AV. Presentation of antigen by endothelial cells and chemoattraction are required for homing of insulin-specific CD8+ T cells. J Exp Med 2003;197:643–65612615905 [Google Scholar]
- 10.Lozanoska-Ochser B, Klein NJ, Huang GC, Alvarez RA, Peakman M. Expression of CD86 on human islet endothelial cells facilitates T cell adhesion and migration. J Immunol 2008;181:6109–6116 [DOI] [PubMed] [Google Scholar]
- 11.Bone AJ, Walker R, Dean BM, Baird JD, Cooke A. Pre-diabetes in the spontaneously diabetic BB/E rat: pancreatic infiltration and islet cell proliferation. Acta Endocrinol (Copenh) 1987;115:447–454 [DOI] [PubMed] [Google Scholar]
- 12.Bernard-Kargar C, Ktorza A. Endocrine pancreas plasticity under physiological and pathological conditions. Diabetes 2001;50(Suppl. 1):S30–S35 [DOI] [PubMed] [Google Scholar]
- 13.Pechhold K, Koczwara K, Zhu X, et al. Blood glucose levels regulate pancreatic beta-cell proliferation during experimentally-induced and spontaneous autoimmune diabetes in mice. PLoS ONE 2009;4:e4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alonso LC, Yokoe T, Zhang P, et al. Glucose infusion in mice: a new model to induce β-cell replication. Diabetes 2007;56:1792–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossetti L, Lauglin MR. Correction of chronic hyperglycemia with vanadate, but not with phlorizin, normalizes in vivo glycogen repletion and in vitro glycogen synthase activity in diabetic skeletal muscle. J Clin Invest 1989;84:892–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins TJ. ImageJ for microscopy. Biotechniques 2007;43(Suppl.):25–30 [DOI] [PubMed] [Google Scholar]
- 17.Camp RL, Chung GG, Rimm DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med 2002;8:1323–1327 [DOI] [PubMed] [Google Scholar]
- 18.Dolled-Filhart M, McCabe A, Giltnane J, Cregger M, Camp RL, Rimm DL. Quantitative in situ analysis of beta-catenin expression in breast cancer shows decreased expression is associated with poor outcome. Cancer Res 2006;66:5487–5494 [DOI] [PubMed] [Google Scholar]
- 19.Gustavson MD, Bourke-Martin B, Reilly D, et al. Standardization of HER2 immunohistochemistry in breast cancer by automated quantitative analysis. Arch Pathol Lab Med 2009;133:1413–1419 [DOI] [PubMed] [Google Scholar]
- 20.Gould Rothberg BE, Berger AJ, Molinaro AM, et al. Melanoma prognostic model using tissue microarrays and genetic algorithms. J Clin Oncol 2009;27:5772–5780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moeder CB, Giltnane JM, Moulis SP, Rimm DL. Quantitative, fluorescence-based in-situ assessment of protein expression. Methods Mol Biol 2009;520:163–175 [DOI] [PubMed] [Google Scholar]
- 22.McCabe A, Dolled-Filhart M, Camp RL, Rimm DL. Automated quantitative analysis (AQUA) of in situ protein expression, antibody concentration, and prognosis. J Natl Cancer Inst 2005;97:1808–1815 [DOI] [PubMed] [Google Scholar]
- 23.Giltnane JM, Molinaro A, Cheng H, et al. Comparison of quantitative immunofluorescence with conventional methods for HER2/neu testing with respect to response to trastuzumab therapy in metastatic breast cancer. Arch Pathol Lab Med 2008;132:1635–1647 [DOI] [PubMed] [Google Scholar]
- 24.Eldor R, Yeffet A, Baum K, et al. Conditional and specific NF-kappaB blockade protects pancreatic beta cells from diabetogenic agents. Proc Natl Acad Sci USA 2006;103:5072–5077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikolova G, Lammert E. Interdependent development of blood vessels and organs. Cell Tissue Res 2003;314:33–42 [DOI] [PubMed] [Google Scholar]
- 26.Jansson L, Carlsson PO. Graft vascular function after transplantation of pancreatic islets. Diabetologia 2002;45:749–763 [DOI] [PubMed] [Google Scholar]
- 27.Sherry NA, Chen W, Kushner JA, et al. Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of beta-cells. Endocrinology 2007;148:5136–5144 [DOI] [PubMed] [Google Scholar]
- 28.Campbell-Thompson M, Dixon LR, Wasserfall C, et al. Pancreatic adenocarcinoma patients with localised chronic severe pancreatitis show an increased number of single beta cells, without alterations in fractional insulin area. Diabetologia 2009;52:262–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baluk P, Yao LC, Feng J, et al. TNF-alpha drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J Clin Invest 2009;119:2954–2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mounzer RH, Svendsen OS, Baluk P, et al. Lymphotoxin-alpha contributes to lymphangiogenesis. Blood 2010;116:2173–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sainson RC, Johnston DA, Chu HC, et al. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood 2008;111:4997–5007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawinkels LJ, Zuidwijk K, Verspaget HW, et al. VEGF release by MMP-9 mediated heparan sulphate cleavage induces colorectal cancer angiogenesis. Eur J Cancer 2008;44:1904–1913 [DOI] [PubMed] [Google Scholar]
- 33.Bergers G, Brekken R, McMahon G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2000;2:737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]