Abstract
OBJECTIVE
The contribution of innate immunity responsible for aggressive β-cell destruction in human fulminant type 1 diabetes is unclear.
RESEARCH DESIGN AND METHODS
Islet cell expression of Toll-like receptors (TLRs), cytoplasmic retinoic acid–inducible gene I (RIG-I)-like receptors, downstream innate immune markers, adaptive immune mediators, and apoptotic markers was studied in three autopsied pancreata obtained 2 to 5 days after onset of fulminant type 1 diabetes.
RESULTS
RIG-I was strongly expressed in β-cells in all three pancreata infected with enterovirus. Melanoma differentiation–associated gene-5 was hyperexpressed in islet cells, including β- and α-cells. TLR3 and TLR4 were expressed in mononuclear cells that infiltrated islets. Interferon (IFN)-α and IFN-β were strongly expressed in islet cells. Major histocompatibility complex (MHC)-class I, IFN-γ, interleukin-18, and CXC motif ligand 10 were expressed and colocalized in affected islets. CD11c+ MHC-class II+ dendritic cells and macrophage subsets infiltrated most islets and showed remarkable features of phagocytosis of islet cell debris. CD4+ forkhead box P3+ regulatory T cells were not observed in and around the affected islets. Mononuclear cells expressed the Fas ligand and infiltrated most Fas-expressing islets. Retinoic acid–receptor responder 3 and activated caspases 8, 9, and 3 were preferentially expressed in β-cells. Serum levels of IFN-γ were markedly increased in patients with fulminant type 1 diabetes.
CONCLUSIONS
These findings demonstrate the presence of specific innate immune responses to enterovirus infection connected with enhanced adoptive immune pathways responsible for aggressive β-cell toxicity in fulminant type 1 diabetes.
Fulminant type 1 diabetes is a unique subtype of type 1 diabetes and is characterized by an abrupt onset of severe hyperglycemia/ketoacidosis and severe β-cell damage that is preceded by flu-like symptoms (1–3). Recently, we have reported unique enterovirus-induced mechanisms for β-cell destruction involving CXC chemokine ligand 10 (CXCL10) and chemokine receptor CXCR3 in fulminant type 1 diabetes (4).
In this study, we examined the in situ status of innate and adaptive immunity of enterovirus-induced fulminant type 1 diabetes. This includes expression of Toll-like receptors (TLRs) TLR3 and TLR4 and cytoplasmic retinoic acid–inducible gene I (RIG-I)-like receptors (RLRs) RIG-I and melanoma differentiation–associated gene-5 (MDA5). TLRs and RLRs are major receptor systems for detecting RNA viruses like enterovirus (5). As interferon (IFN)-α and -β potentially inhibit viral replication and enhance cytotoxic β-cell immunity (6), their expression, cytokine/chemokine expression, and activity of dendritic cells (DCs)/macrophages, CD4+ forkhead box P3 (Foxp3)+ regulatory T cells (Tregs) in affected islet cells were examined.
RESEARCH DESIGN AND METHODS
Patients.
Clinical profiles of three autopsied patients with fulminant type 1 diabetes have been reported (4). Briefly, case 1 was a 14-year-old boy who died from diabetic ketoacidosis, following flu-like symptoms 5 days earlier. Case 2 was a 25-year-old man who died from diabetic ketoacidosis, following sudden symptoms of nausea and epigastralgia 2 days earlier. Case 3 was a 29-year-old man who died from diabetic ketoacidosis, following slight fever, nausea, and vomiting 2 days earlier.
Control subjects.
Pancreatic tissues from 10 nondiabetic men (mean age ± SD, 62 ± 10 years) with gastric carcinoma who had undergone partial pancreatectomy and from five autopsied nondiabetic men (65 ± 11 years) were used as nondiabetic control subjects for immunohistochemical analysis. Pancreatic tissues from four autopsied type 1 diabetic patients (44 ± 9 years) who had histopathological insulitis and glutamic acid decarboxylase autoantibodies (titer: 3.0 ± 1.5 U/mL, cutoff <1.5) were examined as type 1 diabetic control subjects.
Immunostaining.
Methods for immunohistochemical analyses have been reported previously (4). Primary antibodies used in this study are listed in Supplementary Table 1. The definition of insulitis and frequencies of insulitis and mononuclear cell (MNC) phenotypes in islets of cases 1–3 have been documented previously (4). The number of pancreatic acinar cells surrounded by CD8+ T cells was counted in the randomly selected 60 photos of pancreatic section in each case. A confocal laser-scanning microscope, Fluoview FV1000 (Olympus, Tokyo, Japan), was also used.
Measurement of serum IFN-γ.
We obtained sera from 18 patients with fulminant type 1 diabetes (age [range]: 32.3 ± 13.5 [17–58] years, sex [man/woman]: 12/6, duration: 31.0 ± 64.1 [0–240] days), 27 patients with typical type 1 diabetes (age: 31.4 ± 14.7 [6–55] years, sex: 12/15, duration: 12.5 ± 25.4 [0.1–108] months), and 30 nondiabetic control subjects (age: 33.3 ± 13.3 [20–60] years, sex: 17/13). Serum level of IFN-γ was measured by ELISA (PBL Biomedical Laboratories, R&D Systems, Piscataway, NJ).
Ethics.
The Ethics Committee of the University of Yamanashi approved all of the procedures performed in this study. All patients gave informed consent for measuring serum IFN-γ.
Statistical analysis.
Differences in variables between groups were compared using Student t test and ANOVA. Fisher exact test was used to compare frequencies of islets. Values are expressed as means ± SD unless otherwise mentioned.
RESULTS
MDA5, RIG-I, and enterovirus-capsid protein expression.
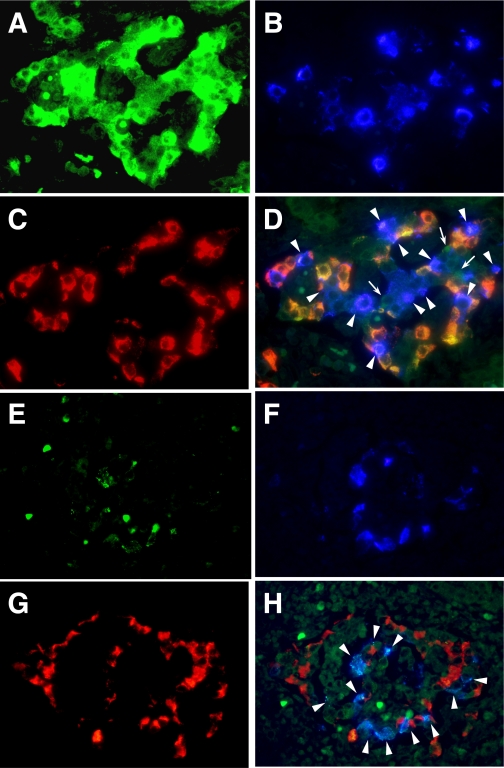
MDA5 was strongly expressed in β-cells, α-cells, and other types of islet cells of fulminant type 1 diabetic pancreata (Fig. 1A–D). In nondiabetic control and type 1 diabetic control subjects, weak MDA5 expression was observed in a few α-cells (Supplementary Fig. 1). Significant expression of RIG-I was observed preferentially in β-cells in all three patients with fulminant type 1 diabetes (Fig. 1E–H), yet it was not expressed in nondiabetic and type 1 diabetic control subjects (Supplementary Fig. 1). Enterovirus-capsid protein (VP1) was detected in β- and non–β-cells of fulminant type 1 diabetic pancreata confirming our previous report (Supplementary Fig. 2) (4) but not type 1 diabetic control and nondiabetic control subjects.
FIG. 1.
Intracytoplasmic double-stranded virus RNA receptor expression in enterovirus-associated human fulminant type 1 diabetes. A–C: Triple-immunostaining of MDA5 (A), insulin (B), and glucagon (C). The merged image (D) demonstrates hyperexpression of MDA5 in β-cells (light blue, arrowheads), α-cells (orange), and other types of islet cells (green, arrows) (×400, case 2). Triple-immunostaining of RIG-I (E), insulin (F), and glucagon (G) is also shown. The merged image (H) demonstrates specific expression of RIG-I in β-cells (light blue, arrowheads) (×400, case 2). (A high-quality digital representation of this figure is available in the online issue.)
TLR3 and TLR4 expression.
Both TLR3 and TLR4 were expressed in MNCs that had infiltrated islets of fulminant type 1 diabetic pancreata but not nondiabetic and type 1 diabetic control subjects (Table 1).
TABLE 1.
Frequency of islets with MNCs that express TLR3, TLR4, CD11c, CD4+Foxp3, and FasL in three fulminant type 1 diabetic cases, and nondiabetic and type 1 diabetic control subjects
| Frequency of TLR3+ MNCs (%) | Frequency of TLR4+ MNCs (%) | Frequency of CD11c+ cells (%) | Frequency of CD4+ Foxp3+ cells (%) | Frequency of FasL+ MNCs (%) | |
|---|---|---|---|---|---|
| Case | |||||
| 1 | 11.3 (7/62) | 1.6 (1/62) | 95.9 (70/73) | 0 (0/75) | 82.6 (38/46) |
| 2 | 5.0 (2/40) | 1.8 (1/56) | 100 (64/64) | 0 (0/63) | 90.0 (36/40) |
| 3 | 18.8 (6/32) | 9.4 (5/53) | 91.2 (52/57) | 0 (0/70) | 95.2 (40/42) |
| Mean | |||||
| FT1D (n = 3) | 11.7 ± 6.9 (15/134) | 4.3 ± 4.4 (7/171) | 95.7 ± 4.4 (186/194) | 0 (0/208) | 89.3 ± 6.3 (114/128) |
| Nondiabetic control subjects (n = 15) | 0 (0/652) | 0.3 ± 0.6 (0–1.7) (3/742) | 0.2 ± 0.5 (0–1.4) (2/863) | 0 (0/692) | 0.3 ± 0.7 (0–1.9) (3/763) |
| Type 1 diabetic control subjects (n = 4) | 0 (0/228) | 0 (0/284) | 2.4 ± 4.2 (0–9.8) (10/392) | 0 (0/351) | 18.2 ± 16.9 (3.8–46.6) (81/417) |
| P value FT1D vs. nondiabetes | <0.0001 | <0.003 | <0.0001 | NS | <0.0001 |
| FT1D vs. type 1 diabetes | <0.002 | NS | <0.0001 | NS | =0.0005 |
| Nondiabetes vs. type 1 diabetes | NS | NS | NS | NS | =0.001 |
Values are means ± SD unless otherwise indicated. FT1D, fulminant type 1 diabetes.
IFN-α, IFN-β, interferon regulatory factor-7, and major histocompatibility complex class I expression.
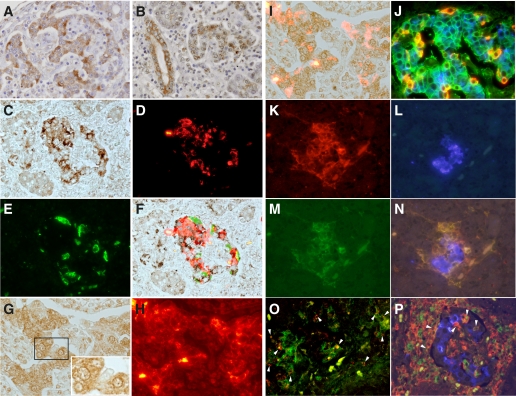
In all three pancreata of the patients with fulminant type 1 diabetes, IFN-α and -β1 were strongly expressed (Fig. 2A–F). Some MNCs that had infiltrated around or in islets and pancreatic acinar and ductal cells also expressed theses cytokines in fulminant type 1 diabetes (Fig. 2A and B) but not in either control subject. The numbers of pancreatic acinar cells surrounded by CD8+ T cells were 11/mm2, 24/mm2, and 22/mm2, respectively, in the pancreatic sections of cases 1, 2, and 3. Most IFN-α–expressing cells were β-cells, α-cells, and islet non–β- and non–α-cells (Fig. 2C–F). Interferon regulatory factor (IRF)-7 (7) was strongly expressed in β- and α-cells (Fig. 2G–I) and mostly stained around and in the nucleus of the islet cells (Fig. 2G). Major histocompatibility complex class I (MHC-I) was hyperexpressed in all islet cell subsets of fulminant type 1 diabetic pancreata (Fig. 2J). Nondiabetic control and type 1 diabetic control subjects did not show expression of IFN-α, IFN-β1, or IRF-7 and hyperexpression of MHC-I in their islets.
FIG. 2.
Immunohistochemical staining of IFN-α, IFN-β1, IRF-7, and MHC-class I in a pancreas with fulminant type 1 diabetes (×200, case 2). A and B: Immunostaining of IFN-α (A) and IFN-β1 (B). C–F: Triple-immunohistochemical staining of IFN-α (C), insulin (D), and glucagon (E). A merged image (F) demonstrates a high proportion of β-cells and α-cells expressing IFN-α. Color balance of F has been adjusted. G–I: Double-immunohistochemical staining of IRF-7 (G) and insulin (H). Insert in G demonstrates strong peri- and intranuclear staining of IRF-7, indicating translocation of IRF-7 from the cytoplasm to the nuclease, thus acting as an activated transcription factor. The merged image (I) shows strong expression of IRF-7 in both islet β-cells and islet non–β-cells. Color balance of I has been adjusted. J: Triple-immunostaining shows MHC-class I molecules are hyperexpressed at the cell surface (green) in β-cells (blue), α-cells (orange), and non–β-/non–α- (nonstained for cytoplasm) islet cells. K–N: Triple-immunostaining of CD11c (K), insulin (L), and MHC-II (M). Merged image (N) demonstrates that CD11c+ cells expressing MHC-II migrate around and into the islets (×200, case 1). O: Confocal microscopic demonstration of intraislet CD11c+ cells (green), showing phagocytosis of the unprocessed β-cell antigen, insulin (red; arrowheads) (×400, case 1). P: Merged image of triple-immunostaining of CD11c (red), CD68 (green), and insulin (blue). Arrowheads indicate positive cells (yellow) both for CD11c and CD68 (×200, case 1). (A high-quality digital representation of this figure is available in the online issue.)
CD11c+ cells in islets.
Remarkable CD11c+ cells migration to the islets was observed in most islets of fulminant type 1 diabetic pancreata (Table 1). Intraislet CD11c+ cells expressed MHC class-II molecules (Fig. 2K–N). Confocal microscopy showed that some CD11c+ cells contained β-cell debris positive for insulin (Fig. 2O). Such findings were not observed in islets of nondiabetic or type 1 diabetic control subjects. Most CD11c+ cells were also positive for CD1a and some for CD68 (Fig. 2P), likely representing DCs and macrophage subsets. CD56+ or CD57+ NK cells and Tregs (CD4+ Foxp3+ cells) were not detected in or around islets of fulminant type 1 diabetic pancreata and either control. Tregs, CD4+ Foxp3+ cells, were not detected in or around the islets or in exocrine regions of the pancreas in fulminant type 1 diabetic, nondiabetic control, or type 1 diabetic control subjects (Table 1).
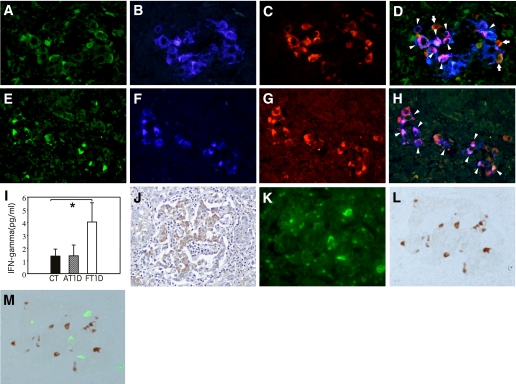
IL-18, IFN-γ, and CXCL10 expression.
IL-18 was expressed in islet cells in all three fulminant type 1 diabetes (Fig. 3A and E). Most residual β-cells expressed both IFN-γ and IL-18 (Fig. 3G and H). IL-18, IFN-γ, and CXCL10 colocalized in most β- and islet non–β-cells (Fig. 3A–H). IL-12 was not expressed in any cells in affected pancreata. A few islets of type 1 diabetic control subjects (mean [range]: 2.8% [0–5.2]) expressed IL-18 and IFN-γ but not CXCL10. Nondiabetic control subjects did not express IL-18, IFN-γ, and CXCL10.
FIG. 3.
Immunohistochemical staining of IL-18, insulin, CXCL10, IFN-γ, and FasL. A–D: Triple-immunostaining of IL-18 (A), insulin (B), and CXCL10 (C). The merged image (D) shows that most β-cells express both IL-18 and CXCL10 (arrowheads). Some islet non–β-cells also express IL-18 and CXCL10 (orange, arrows) (×400, case 2). E–H: Triple-immunostaining of IL-18 (E), insulin (F), and IFN-γ (G). The merged image (H) shows that most β-cells express both IL-18 and IFN-γ (arrowheads) and that some islet non–β-cells also express IFN-γ (red) (×400, case 2). I: Serum levels of IFN-γ in patients with fulminant type 1 diabetes (FT1D) or with typical acute-onset type 1 diabetes (AT1D) and nondiabetic control (CT) subjects. Values are expressed as means ± SE; *P < 0.05. J: Immunostaining of Fas in islets affected by fulminant type 1 diabetes (brown) demonstrates strong expression of Fas in islet cells (×200, case 2). K–M: Double-immunofluorescent staining of insulin (K) and FasL (L). The merged image (M) shows that FasL-positive cells infiltrate the islets (×400, case 3). Color balance of M has been adjusted. (A high-quality digital representation of this figure is available in the online issue.)
Serum IFN-γ levels in patients with fulminant type 1 diabetes.
Serum levels of IFN-γ in patients with fulminant type 1 diabetes were approximately three times higher than those in nondiabetic and type 1 diabetic control subjects (Fig. 3I).
Fas expression in islet cells and infiltration of islet Fas-ligand–bearing MNCs.
Elevated expression of Fas in islet cells coincided with marked MNC infiltration in fulminant type 1 diabetic pancreata (Fig. 3J). The subsets of islet cells with Fas expression were mostly β-cells. Fas-ligand (FasL)-bearing MNCs infiltrated most islets of fulminant type 1 diabetic pancreata (Fig. 3K–M) (Table 1). In islet cells of nondiabetic control and type 1 diabetic control subjects, Fas was not expressed (Supplementary Fig. 3). FasL-bearing MNC infiltration of islets was observed in type 1 diabetic but not nondiabetic control subjects (Table 1) (Supplementary Fig. 3).
Expression of retinoic acid–receptor responder 3 and activated caspases 8, 9, and 3 in islet β-cells.
Retinoic acid–receptor responder 3 (RARRES3) (8,9) was expressed in β-cells of fulminant type 1 diabetic pancreata (Supplementary Fig. 4). Cleaved caspase 8, a marker of the Fas-mediated extrinsic apoptotic pathway, cleaved caspase 9, a marker of the activated non–Fas-mediated apoptotic pathway, and activated caspases 3, a marker of the end stage of β-cell apoptosis, were expressed specifically in islet β-cells (Supplementary Fig. 4). In islets of autopsied nondiabetic control subjects, RARRES3, cleaved caspases 8, -9, and -3 were not expressed (Supplementary Fig. 5). In type 1 diabetic control subjects, RARRES3, cleaved caspases 8, -9, and -3 were expressed weakly in some islet β-cells (Supplementary Fig. 5).
DISCUSSION
Both RIG-I and MDA5 were strongly expressed in β-cells of fulminant type 1 diabetic pancreata. MDA5 was also hyperexpressed in α-cells and non−β-/non–α-cells in affected islets. Hyperexpression of RIG-I and MDA5 with expression of IFN-α and -β1 in β-cells suggests a crucial role of RIG-I and MDA5 for sensing and responding to enterovirus infection in the pancreas of patients with fulminant type 1 diabetes. Mutations of MDA5 genes have been implicated in reducing the risk of type 1 diabetes (10). Reports also noted RIG-I mRNA expression in human islets infected with Coxsackievirus B3 and B5 (11,12). We showed that IRF-7, a master transcription factor of IFN-α and -β (7), translocated to the nucleus and that IFN-α and -β, essential factors that protect β-cells against viral infection (6), were strongly expressed in both β- and α-cells. These results indicate that all islet cells are in an activated state of innate immunity in response to enterovirus in patients with fulminant type 1 diabetes.
Increased TLR3+ MNCs that infiltrate affected islets should participate in sensing viral RNA and subsequently destroy β-cells with RIG-I– and MDA5-initiated proinflammatory signal axes in the innate immune response against Coxsackievirus B3 (13). Intra- and peri-islet DCs and macrophage subsets drastically increased in number and showed active phagocytosis of enterovirus-infected β-cells, whereas MHC-I was hyperexpressed in all islet subsets. Some DCs and macrophage subsets also expressed MHC-II molecules. Activated innate immune responses including virus sensing by RIG-I and MDA5 with subsequent IFN-α and -β production and DC and macrophage activation will not only protect for enteroviral infection by upregulating RIG-I and MDA5 (12,14) but will also enhance the adaptive immunity cascades for islet cell destruction (6,15,16). Indeed, patients with fulminant type 1 diabetes showed elevated serum levels of IFN-γ.
CD4+ Foxp3+ cells, which represent a pivotal subset of Tregs, were not observed in or around the islets of fulminant type 1 diabetic pancreata, suggesting that the extremely polarized local condition to predominance for Th1 in response to enteroviral infection suppresses Treg differentiation from naive T CD4+ precursors (17). In turn, the Treg-depleted islet condition enhances Th1 cytokine (i.e., IFN-γ) generation.
Notably, IL-18, an IFN-γ–inducing factor, was extensively expressed in islet cells of infected fulminant type 1 diabetic pancreata. In response to viral infection, IL-18 is promptly secreted from virus-activated macrophages, DCs, and T cells (18), stimulating production of IFN-γ synergistically with IFN-α and -β through a unique pathway that sometimes occurs independently of IL-12 or NK cells (19). Conversely, IL-18 can be induced by IFN-γ alone or in combination with other cytokines in islet β-cells (20). Thus, for fulminant type 1 diabetes, enterovirus itself or enterovirus-activated T cells and macrophages most likely infiltrate islets to induce IL-18 production in these cells. In addition, IFN-α and -β, produced in islet cells and islet-infiltrating MNCs, can enhance IL-18–mediated signaling (21). Subsequently, islet-secreted IL-18 may induce IFN-γ production via receptors on the islet cells or islet stromal cells in an autocrine/paracrine manner. Once this positive autocrine/paracrine circuit for production of IL-18, IFN-γ, and CXCL10 is established in islet cells, destructive mechanisms involving CXCR3+ T cells and macrophages might persist until complete destruction of the β-cells (4).
We found that Fas was highly expressed in affected islet β-cells and islet-infiltrating FasL+ cells. Taken together with the finding that MHC-I and IFN-α, -β, and -γ were strongly expressed in affected islet cells, effecter mechanisms for β-cell apoptosis in fulminant type 1 diabetes are likely mediated in part by MHC-I and by the Fas-FasL pathway (22). Inflammation-induced Fas-FasL expression in β-cells was reported to lead to rapid and massive β-cell destruction (23). Other apoptotic mechanisms through the IFN-γ–dependent JAK/STAT pathway (24) and innate immune pathway (25) will also exert β-cell destruction.
ACKNOWLEDGMENTS
This study was partly supported by grants from the Ministry of Education, Science, Sports and Culture and Ministry of Health, Labor and Welfare (Japan).
No potential conflicts of interest relevant to this article were reported.
K.A. and Y.N. conducted immunohistochemical staining, RT-PCR data analysis, and discussed, reviewed, and edited the article. S.T. contributed to planning and discussion and edited the article. T.M. and A.S. sampled autopsied pancreas and participated in discussion. T.A., M.S., and H.S. contributed to discussion and reviewed and edited the article. S.T., T.M., M.I., D.A., and T.A. recruited serum samples, measured serum levels of IFN-γ, and participated in discussion. F.F., A.K., and M.K. contributed to analysis of immunostained sample data. J.I., H.F., and T.E. contributed to analysis of nondiabetic pancreas and Treg+ lymph nodes. T.K. analyzed data and wrote and edited the article.
The authors thank K. Hosaka, C. Imai, and S. Takei for excellent secretarial work; Drs. T. Momotsu and E. Okazaki (Niigata City General Hospital) for generous assistance; and Professors A. Nakao (Department of Immunology) and R. Kato (Department of Pathology) at the University of Yamanashi for critical assessment and assistance of the article.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-0795/-/DC1.
REFERENCES
- 1.Kobayashi T. Immunology and immunogenetics of type 1 diabetes in Japan. IDF Bull 1990;35:34–37 [Google Scholar]
- 2.Imagawa A, Hanafusa T, Miyagawa J, Matsuzawa Y, Osaka IDDM Study Group A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies. N Engl J Med 2000;342:301–307 [DOI] [PubMed] [Google Scholar]
- 3.Tanaka S, Kobayashi T, Momotsu T. A novel subtype of type 1 diabetes mellitus. N Engl J Med 2000;342:1835–1837 [PubMed] [Google Scholar]
- 4.Tanaka S, Nishida Y, Aida K, et al. Enterovirus infection, CXC chemokine ligand 10 (CXCL10), and CXCR3 circuit: a mechanism of accelerated beta-cell failure in fulminant type 1 diabetes. Diabetes 2009;58:2285–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeuchi O, Akira S. MDA5/RIG-I and virus recognition. Curr Opin Immunol 2008;20:17–22 [DOI] [PubMed] [Google Scholar]
- 6.von Herrath M. Diabetes: A virus-gene collaboration. Nature 2009;459:518–519 [DOI] [PubMed] [Google Scholar]
- 7.Honda K, Yanai H, Negishi H, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 2005;434:772–777 [DOI] [PubMed] [Google Scholar]
- 8.Sturniolo MT, Dashti SR, Deucher A, et al. A novel tumor suppressor protein promotes keratinocyte terminal differentiation via activation of type I transglutaminase. J Biol Chem 2003;278:48066–48073 [DOI] [PubMed] [Google Scholar]
- 9.Sarkar SA, Wong R, Hackl SI, et al. Induction of indoleamine 2,3-dioxygenase by interferon-gamma in human islets. Diabetes 2007;56:72–79 [DOI] [PubMed] [Google Scholar]
- 10.Nejentsev S, Walker N, Riches D, Egholm M, Todd JA. Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science 2009;324:387–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ylipaasto P, Kutlu B, Rasilainen S, et al. Global profiling of coxsackievirus- and cytokine-induced gene expression in human pancreatic islets. Diabetologia 2005;48:1510–1522 [DOI] [PubMed] [Google Scholar]
- 12.Schulte BM, Kramer M, Ansems M, et al. Phagocytosis of enterovirus-infected pancreatic beta-cells triggers innate immune responses in human dendritic cells. Diabetes 2010;59:1182–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Negishi H, Osawa T, Ogami K, et al. A critical link between Toll-like receptor 3 and type II interferon signaling pathways in antiviral innate immunity. Proc Natl Acad Sci USA 2008;105:20446–20451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hultcrantz M, Hühn MH, Wolf M, et al. Interferons induce an antiviral state in human pancreatic islet cells. Virology 2007;367:92–101 [DOI] [PubMed] [Google Scholar]
- 15.Le Bon A, Durand V, Kamphuis E, et al. Direct stimulation of T cells by type I IFN enhances the CD8+ T cell response during cross-priming. J Immunol 2006;176:4682–4689 [DOI] [PubMed] [Google Scholar]
- 16.Blanco P, Palucka AK, Pascual V, Banchereau J. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev 2008;19:41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci USA 2007;104:18169–18174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okamura H, Tsutsi H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 1995;378:88–91 [DOI] [PubMed] [Google Scholar]
- 19.Pien GC, Nguyen KB, Malmgaard L, Satoskar AR, Biron CA. A unique mechanism for innate cytokine promotion of T cell responses to viral infections. J Immunol 2002;169:5827–5837 [DOI] [PubMed] [Google Scholar]
- 20.Frigerio S, Holländer GA, Zumsteg U. Functional IL-18 Is produced by primary pancreatic mouse islets and NIT-1 beta cells and participates in the progression towards destructive insulitis. Horm Res 2002;57:94–104 [DOI] [PubMed] [Google Scholar]
- 21.Sareneva T, Julkunen I, Matikainen S. IFN-alpha and IL-12 induce IL-18 receptor gene expression in human NK and T cells. J Immunol 2000;165:1933–1938 [DOI] [PubMed] [Google Scholar]
- 22.Nagata S. Apoptosis by death factor. Cell 1997;88:355–365 [DOI] [PubMed] [Google Scholar]
- 23.Christen U, Darwiche R, Thomas HE, et al. Virally induced inflammation triggers fratricide of Fas-ligand-expressing beta-cells. Diabetes 2004;53:591–596 [DOI] [PubMed] [Google Scholar]
- 24.Eizirik DL, Mandrup-Poulsen T. A choice of death—the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia 2001;44:2115–2133 [DOI] [PubMed] [Google Scholar]
- 25.Besch R, Poeck H, Hohenauer T, et al. Proapoptotic signaling induced by RIG-I and MDA-5 results in type I interferon-independent apoptosis in human melanoma cells. J Clin Invest 2009;119:2399–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]