Abstract
OBJECTIVE
Chronically elevated free fatty acids contribute to insulin resistance and pancreatic β-cell failure. Among numerous potential factors, the involvement of endoplasmic reticulum (ER) stress has been postulated to play a mechanistic role. Here we examined the efficacy of the chemical chaperone, sodium phenylbutyrate (PBA), a drug with known capacity to reduce ER stress in animal models and in vitro, on lipid-induced insulin resistance and β-cell dysfunction in humans.
RESEARCH DESIGN AND METHODS
Eight overweight or obese nondiabetic men underwent four studies each, in random order, 4 to 6 weeks apart. Two studies were preceded by 2 weeks of oral PBA (7.5 g/day), followed by a 48-h i.v. infusion of intralipid/heparin or saline, and two studies were preceded by placebo treatment, followed by similar infusions. Insulin secretion rates (ISRs) and sensitivity (SI) were assessed after the 48-h infusions by hyperglycemic and hyperinsulinemic-euglycemic clamps, respectively.
RESULTS
Lipid infusion reduced SI, which was significantly ameliorated by pretreatment with PBA. Absolute ISR was not affected by any treatment; however, PBA partially ameliorated the lipid-induced reduction in the disposition index (DI = ISR × SI), indicating that PBA prevented lipid-induced β-cell dysfunction.
CONCLUSIONS
These results suggest that PBA may provide benefits in humans by ameliorating the insulin resistance and β-cell dysfunction induced by prolonged elevation of free fatty acids.
Insulin resistance and pancreatic β-cell failure are hallmarks of type 2 diabetes (1). Many factors contribute to the development of insulin resistance and β-cell dysfunction, including chronically elevated circulating free fatty acids (FFAs). Prolonged elevation of FFAs has been consistently demonstrated to induce insulin resistance in animals and humans (2). FFAs are essential in maintaining basal insulin secretion in the fasted state (3), and acute elevation of FFAs enhances glucose-stimulated insulin secretion (GSIS) (4). Although the role of prolonged elevation of FFAs on insulin secretion is more controversial, mounting evidence points to detrimental effects of prolonged elevation of FFAs on β-cell function (5). β-Cell function in vivo cannot be inferred only by absolute insulin secretion because β-cells compensate for insulin resistance so that the product of insulin secretion and sensitivity is a constant (disposition index [DI]) in normal individuals (6,7). Because FFAs induce insulin resistance, which would be expected to lead to compensatory insulin hypersecretion, even an unchanged or mildly elevated absolute GSIS that is not at the level anticipated for that degree of insulin resistance indicates β-cell dysfunction, and it is the DI, not the absolute GSIS, that correctly evaluates β-cell function. In previous studies, we and others have shown in humans, particularly in predisposed individuals, that prolonged elevation of FFAs lowers DI, indicating impaired β-cell function (8–16).
The mechanisms whereby chronically elevated FFAs impair insulin action and β-cell function are not fully understood. Several hypotheses have been proposed, including tissue accumulation of lipids and intermediate metabolites (17), protein kinase C activation (18–20), proinflammatory nuclear factor (NF)-κB pathway activation (20–22), and oxidative stress (15,23).
Recent studies in animals and humans suggest an important role of endoplasmic reticulum (ER) stress in the development of insulin resistance and type 2 diabetes (24). ER is a major site for newly synthesized proteins to undergo posttranslational modifications and folding, a process facilitated by chaperones and foldases. Accumulation of unfolded proteins triggers several signal transduction pathways (unfolded protein response) to maintain ER homeostasis, and excessive accumulation of unfolded proteins beyond the processing capacity of the ER leads to ER stress (25).
In rodents, the development of insulin resistance is accompanied by increased ER stress in various tissues and is prevented with alleviation of ER stress with chemical chaperones or genetic manipulation of the unfolded protein response pathway (26,27). In obese, insulin-resistant humans, ER stress markers are activated in the adipose tissue (28,29), which is reduced with weight loss after gastric bypass surgery (30). Prolonged in vitro exposure to palmitate induces β-cell apoptosis and impairs GSIS, possibly through induction of ER stress (31–33). In type 2 diabetic patients, ER stress markers are activated in the β-cells (34).
Although these studies suggest an important role of ER stress in the development of insulin resistance and β-cell failure, it remains unclear whether chronically elevated FFAs exert their detrimental effects through induction of ER stress in humans, and more importantly, whether currently available chemical chaperones with shown capability to reduce ER stress in animal models and in vitro can alleviate such effects. The objective of this study, therefore, was to examine the potential efficacy of sodium phenylbutyrate (PBA), a drug with known ability to reduce ER stress, in amelioration of lipid-induced insulin resistance and β-cell dysfunction in humans. Overweight and obese individuals were studied because they are more susceptible to FFA-induced β-cell dysfunction (9). The study was specifically designed to examine the effects of FFAs independently of other factors inducing insulin resistance and β-cell dysfunction in obesity.
RESEARCH DESIGN AND METHODS
Subjects.
Eight overweight or obese nondiabetic men participated in the study (Table 1). None of the participants was taking any medication or had any known systemic illness. All subjects had normal oral glucose tolerance in response to a 75-g oral glucose tolerance test and had a negative family history of type 2 diabetes. Informed, written consent was obtained from all participants in accordance with the guidelines of the Research Ethics Board of the University Health Network, University of Toronto.
TABLE 1.
Characteristics of the subjects (N = 8)
| Variable | Mean ± SE |
|---|---|
| Age, years | 44.3 ± 3.1 |
| Weight, kg | 93.6 ± 3.3 |
| Height, cm | 176.3 ± 2.1 |
| BMI, kg/m2 | 30.1 ± 0.9 |
| Fasting plasma levels | |
| Glucose, mmol/L | 5.11 ± 0.11 |
| Insulin, pmol/L | 89.52 ± 11.61 |
| C-peptide, nmol/L | 0.65 ± 0.09 |
| TG, mmol/L | 1.34 ± 0.28 |
| FFA, mmol/L | 0.52 ± 0.05 |
All subjects are male.
Experimental protocol.
Each participant was studied on four occasions, in random order, 4 to 6 weeks apart: 1) 2-week oral placebo, followed by intravenous infusion of normal saline for 48 h (SAL); 2) 2-week oral placebo followed by intravenous infusion of intralipid (20%, 40 mL/h) plus heparin (250 U/h) for 48 h to raise plasma FFAs by about twofold (IH); 3) 2-week oral PBA (7.5 g/day; 500-mg Buphenyl tablet, Ucyclyd Pharma, Scottsdale, AZ; five tablets three times daily), followed by 48-h IH infusion as above (IH + PBA); and 4) 2-week oral PBA, followed by 48-h saline infusion (PBA). PBA was well tolerated by all subjects (no side effects were reported), and compliance (>95%) was ascertained by pill counting. The dose was in the lower end of therapeutic range for treatment of urea cycle disorder as specified in the drug monograph.
After 2 weeks of placebo or PBA, subjects were admitted to the Metabolic Test Centre of the Toronto General Hospital for infusion of saline or intralipid plus heparin and testing of β-cell function and insulin sensitivity. During the hospital stay, participants were fed an American Heart Association phase 1 diet and refrained from exercise. On each occasion, on day 1 of the admission to the Metabolic Test Centre, an intravenous catheter was placed in a superficial vein of one forearm for infusion of saline or intralipid plus heparin, and for infusion of glucose and insulin during the glycemic clamps.
On day 3, after an overnight fast, a second intravenous catheter was placed in the opposite forearm, which was maintained in a heating blanket (65°C) to “arterialize” venous blood for blood sampling. At approximately 0800 h, a 30-min baseline sampling period was started, followed by a 2-h, 20-mmol/L hyperglycemic clamp, as previously described (8). Urine glucose loss was assumed to be equal between studies for the same individual because plasma glucose levels were similar. At the end of the 2-h hyperglycemic clamp, the intravenous dextrose infusion was slowly tapered while avoiding hypoglycemia, allowing the blood glucose to return to basal level. Three hours after the end of the hyperglycemic clamp, a hyperinsulinemic-euglycemic clamp was started with a primed infusion of 40 mU ⋅ m−2 ⋅ min−1 of insulin and 20% dextrose, as previously described (35).
GSIS, insulin sensitivity, insulin clearance, and DI.
Insulin secretion rate (ISR) was derived from deconvolution of plasma C-peptide concentrations during the last 30 min of the hyperglycemic clamp (36). The insulin sensitivity index (SI) was derived from the last 30 min of the hyperinsulinemic-euglycemic clamp by normalization of glucose infusion rate (Ginf) for glucose and insulin, which takes into account the experimental variability, in particular insulin variability, which is affected by insulin clearance. Insulin clearance (ClI) was derived as insulin infusion rates divided by insulin concentrations during the last 30 min of the hyperinsulinemic-euglycemic clamp. The DI was a product of the insulin secretion rate and insulin sensitivity (DI = ISR × SI), where ISR and SI were derived from the hyperglycemic clamp and the hyperinsulinemic-euglycemic clamp, respectively, as described.
Laboratory analysis.
Plasma glucose was assayed at the bedside using a Beckman Glucose Analyzer II (Beckman Instruments Corp., Fullerton, CA). Plasma insulin and C-peptide were measured with radioimmunoassay kits (Millipore, Billerica, MA). Plasma triglycerides (TG; Roche Diagnostics, Laval, QC, Canada) and FFA (Wako Industrials, Osaka, Japan) were analyzed with enzymatic colorimetric kits.
Statistics.
Plasma glucose, insulin, C-peptide, TG, and FFAs were analyzed by two-way ANOVA for repeated measures with the Tukey test to detect differences between treatments at each time and between times within treatment during the 48-h infusion period and differences between treatments during the last 30 min of the clamps. A value of P < 0.05 was considered significant. All statistical analyses were performed with SAS 8.0 software (SAS Institute, Cary, NC).
RESULTS
Preclamp data.
Plasma levels of TG and FFAs were similar before infusion of either lipid or saline. After the 48-h lipid infusion, plasma TG and FFAs in both lipid infusion groups (IH and IH + PBA) increased by approximately twofold compared with preinfusion levels (P < 0.05). No significant effects of the 2-week prior treatment with oral PBA (7.5 g/day) on TG and FFAs were observed when compared with placebo, with and without lipid infusion. Therefore, the protocol achieved similar circulating FFA levels between SAL and PBA, and between IH and IH + PBA, with the latter being significantly elevated. Plasma glucose, insulin, and C-peptide concentrations were comparable in all treatment groups before and after lipid/saline infusion (Table 2).
TABLE 2.
Fasting levels of plasma markers before and after 48-h infusion of saline or intralipid plus heparin*
| SAL | IH | IH + PBA | PBA | ||
|---|---|---|---|---|---|
| Glucose, mmol/L | Preinfusion | 5.23 ± 0.14 | 5.03 ± 0.14 | 5.09 ± 0.17 | 5.13 ± 0.15 |
| Preclamp | 5.23 ± 0.15 | 5.43 ± 0.22 | 5.50 ± 0.34 | 5.29 ± 0.22 | |
| Insulin, pmol/L | Preinfusion | 107.10 ± 15.67 | 86.40 ± 12.41 | 81.47 ± 11.36 | 83.11 ± 7.02 |
| Preclamp | 93.40 ± 11.33 | 102.69 ± 11.15 | 78.24 ± 5.33 | 87.67 ± 18.83 | |
| C-peptide, nmol/L | Preinfusion | 0.75 ± 0.12 | 0.66 ± 0.08 | 0.59 ± 0.08 | 0.59 ± 0.09 |
| Preclamp | 0.63 ± 0.10 | 0.77 ± 0.12 | 0.74 ± 0.13 | 0.68 ± 0.08 | |
| Triglyceride, mmol/L | Preinfusion | 1.60 ± 0.46 | 1.28 ± 0.19 | 1.47 ± 0.32 | 1.40 ± 0.36 |
| Preclamp | 1.58 ± 0.24 | 2.63 ± 0.36†‡§ | 3.04 ± 0.44†‡§ | 1.63 ± 0.26 | |
| Free fatty acid, mmol/L | Preinfusion | 0.42 ± 0.08 | 0.43 ± 0.06 | 0.48 ± 0.07 | 0.50 ± 0.05 |
| Preclamp | 0.38 ± 0.05 | 0.74 ± 0.04†‡§ | 0.73 ± 0.07†‡§ | 0.38 ± 0.05 |
Data are means ± SE; N = 8.
Clamps were performed on subjects in four randomized visits after a 48-h SAL infusion; a 48-h IH infusion; 2-week oral PBA (7.5 g/day), followed by a 48-h IH infusion; or 2-week oral PBA, followed by a 48-h SAL infusion. Preinfusion, before start of SAL or IH infusion. Preclamp, 48-h after start of SAL or IH infusion and before start of the clamp.
†P, 0.05 vs. SAL;
‡P, 0.05 vs. PBA;
§P, 0.05 vs. preinfusion.
Hyperglycemic clamp.
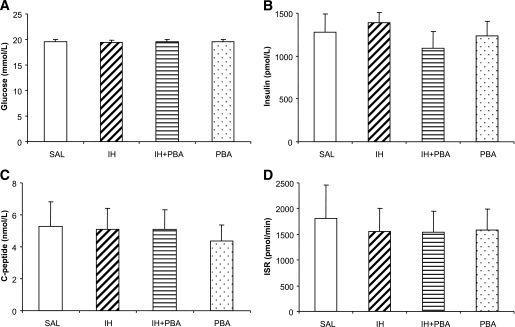
Plasma glucose concentrations were elevated to approximately 20 mmol/L and were well matched during the last 30 min steady state of the 2-h hyperglycemic clamp (Fig. 1A). There was a nonsignificant trend for insulin concentrations to be slightly higher in IH and slightly lower in IH + PBA compared with SAL and PBA (Fig. 1B). Plasma C-peptide concentrations were comparable between treatments (Fig. 1C), and in accordance, insulin secretion rates calculated by deconvolution of C-peptide were not significantly different between treatments (SAL = 1801.3 ± 658.5, IH = 1560.7 ± 439.2, IH + PBA = 1539.9 ± 403.4, and PBA = 1588.9 ± 409.3 pmol/min, respectively; Fig. 1D).
FIG. 1.
Plasma concentrations of glucose (A), insulin (B), and C-peptide (C), and calculated insulin secretion rates (D) during the last 30 min of the hyperglycemic clamp. Clamps were performed on subjects in four randomized visits after a 48-h SAL infusion; a 48-h IH infusion; 2-week oral PBA (7.5 g/day), followed by a 48-h IH infusion; or 2-week oral PBA, followed by a 48-h SAL infusion. Data are means ± SE.
Hyperinsulinemic-euglycemic clamp.
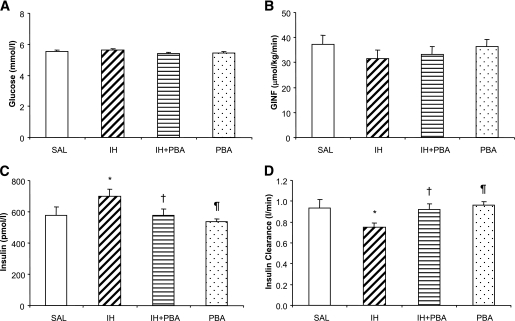
During the last 30 min of the hyperinsulinemic-euglycemic clamp, plasma glucose levels were maintained at ∼5.5 mmol/L and were well matched between studies (Fig. 2A). To maintain plasma glucose at this euglycemic level, a slightly lower, although not statistically significant, glucose infusion rate was required in IH (31.6 ± 3.2 μmol/kg/min) compared with SAL (37.2 ± 3.7 μmol/kg/min; Fig. 2B). Glucose infusion rates in IH + PBA (33.1 ± 3.1 μmol/kg/min) and PBA (36.4 ± 2.7 μmol/kg/min) were not significantly different from SAL. IH was associated with a higher plasma insulin concentration (700.1 ± 44.1 pmol/L) versus SAL (576.8 ± 54.9 pmol/L, P < 0.05; Fig. 2C). Pretreatment with PBA eliminated this effect, and PBA alone had similar insulin concentrations to SAL (IH + PBA = 575.1 ± 43 pmol/L and PBA = 537.2 ± 17.3 pmol/L). Insulin clearance was decreased with lipid infusion (P < 0.05 IH vs. SAL), which was reversed by PBA pretreatment in IH + PBA, whereas PBA alone did not affect insulin clearance (Fig. 2D). C-peptide levels during the hyperinsulinemic-euglycemic clamps were not statistically different among treatments (SAL = 1.36 ± 0.16, IH = 1.78 ± 0.11, IH + PBA = 1.67 ± 0.12, and PBA = 1.66 ± 0.11 nmol/L, P = NS). Insulin clearance was also reduced by IH and restored by PBA during the hyperglycemic clamps (SAL = 1.4 ± 0.2, IH = 1.1 ± 0.3, IH + PBA = 1.4 ± 0.3, and PBA = 1.3 ± 0.4 L/min, P < 0.05 IH vs. others). Therefore, the higher insulin levels in IH during the hyperinsulinemic-euglycemic clamps were unlikely due to endogenous insulin secretion.
FIG. 2.
Plasma concentrations of glucose (A), glucose infusion rates (B), plasma concentrations of insulin (C), and calculated insulin clearance (D) during the last 30 min of the hyperinsulinemic-euglycemic clamp. Clamps were performed on subjects in four randomized visits after a 48-h SAL infusion; a 48-h IH infusion; 2-week oral PBA (7.5 g/day), followed by a 48-h IH infusion; or 2-week oral PBA, followed by a 48-h SAL infusion. Data are means ± SE. *P < 0.05 vs. SAL, †P < 0.05 vs. IH, ¶P < 0.01 vs. IH.
SI and DI.
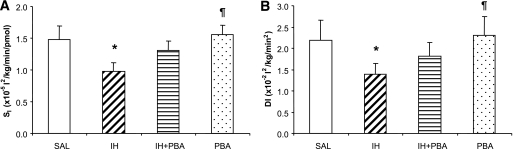
SI calculated during the last 30 min of the hyperinsulinemic-euglycemic clamp indicated a significant 35% reduction in IH versus SAL (P < 0.05), in accordance with slightly lower Ginf and higher insulin (Fig. 3A). In IH + PBA, this reduction was partially prevented (P = NS vs. SAL and IH), whereas SI was unaffected in PBA (P = NS vs. SAL and IH + PBA, P < 0.05 vs. IH). Because the absolute insulin secretion rates were not significantly different in the various treatments (Fig. 1D), the DI followed a similar pattern as SI, with a significant 36% lower DI in IH (1.4 × 10−2 vs. 2.2 × 10−2 L2/kg/min, IH vs. SAL P < 0.05) that was partly prevented by PBA in IH + PBA (1.8 × 10−2 L2/kg/min, P = NS vs. SAL, IH, and PBA; Fig. 3B). PBA alone did not affect DI (2.3 × 10−2 L2/kg/min, P = NS vs. SAL).
FIG. 3.
Insulin sensitivity index calculated from the last 30 min of the hyperinsulinemic-euglycemic clamp (A) and disposition index (B). Clamps were performed on subjects in four randomized visits after a 48-h SAL infusion; a 48-h IH infusion; 2-week oral PBA (7.5 g/day), followed by a 48-h IH infusion; or 2-week oral PBA, followed by a 48-h SAL infusion. Data are means ± SE. *P < 0.01 vs. SAL, ¶P < 0.05 vs. IH.
DISCUSSION
The mechanism of lipid-induced insulin resistance and pancreatic β-cell dysfunction is complex, and a number of interacting mechanisms have been proposed. Chronically elevated circulating FFAs in the setting of obesity-associated insulin resistance or high-fat feeding, or by lipid infusion experimental protocols, may lead to oxidative stress (15,23) and inflammation (20–22,37,38). Recent studies have implicated ER stress as a possibly unifying mechanism in the development of insulin resistance and type 2 diabetes in experimental models (24,25). However, the potential involvement of ER stress in lipid-induced insulin resistance and β-cell dysfunction has not been examined in humans. Here we demonstrate that PBA, a drug with the capability to reduce ER stress in animal models (27) and in vitro (32,39), partially prevents lipid-induced impairment in insulin sensitivity and β-cell function in humans. To our knowledge, this is the first study in humans to administer PBA in the prolonged lipid infusion setting and to demonstrate protection against lipotoxicity. We did not prove that PBA reduced ER stress in humans and we cannot exclude that the protective effects of PBA were unrelated to ER stress relief. However, these studies raise the possibility that agents known to relieve ER stress may prove useful in the prevention and treatment of type 2 diabetes.
ER stress has been implicated in the development of insulin resistance and β-cell dysfunction in animal and cellular models. For instance, in mice with high-fat feeding or on a genetic obesity background, development of insulin resistance is associated with increased expression of ER stress markers in various tissues (26,27). In obese individuals with insulin resistance, ER stress markers are increased in adipose tissue (28,29). Furthermore, gastric bypass surgery in obese patients is associated with reduced ER stress in adipose tissues along with weight loss and improved insulin sensitivity (30). ER stress markers are increased in the islets of db/db mice (34). Increased ER stress markers are also detected in islets of type 2 diabetic patients (34). Very limited information exists regarding the potential role of ER stress in insulin secretion per se. A recent study showed that Wolfram syndrome 1 protein is present in β-cell secretory granules and plays an important role in maintaining β-cell function (40). Palmitate has been shown to exert lipotoxic/apoptotic effects on β-cells through the induction of ER stress (31,34,39,41,42). In INS-1 cells and primary rat islets, palmitate-induced inhibition of GSIS is ameliorated by PBA (32).
Although modulating ER function via the use of several small molecular, chemical chaperones, or genetic modification of ER chaperone proteins improved insulin sensitivity or secretion in animal models and in vitro (27,32,43), the feasibility and applications in humans has remained unstudied until recently. Of the chemical chaperones, sodium phenylbutyrate or PBA stabilizes protein folding (44,45) and is currently used clinically for the treatment of urea-cycle disorders, thalassemia, and cystic fibrosis, with a satisfactory safety profile.
The finding in the current study that PBA partially prevented lipid-induced insulin resistance is in line with studies in animal models and in vitro (27). Lipid infusion for longer than 6 h, a protocol shown to induce hepatic insulin resistance and impair β-cell function in rodents (23,32,38), induced ER stress in the liver of mice (46). It is therefore possible that the effect of PBA on insulin sensitivity may be through amelioration of ER stress. Although most studies indicate that ER stress may affect β-cell survival, 48-h lipid infusion, as was used in the current study, does not affect β-cell mass in rodents (47), which have a higher β-cell turnover, unlike humans.
As previously discussed, palmitate-induced decrease in β-cell function in vitro was prevented by PBA (32). It is possible that the differing composition of fatty acids may have differential effects on ER status at the β-cell level (48). Intralipid, which was used in the present experiments, consists predominantly of polyunsaturated fatty acids. Although intralipid or PBA did not affect absolute insulin secretion, amelioration of the lipid-induced reduction in the DI, which represents insulin secretion in relation to insulin resistance, by PBA pretreatment of lipid-infused humans, indicates benefits to β-cell function.
Because the difference in SI and DI in the IH and IH + PBA groups was not statistically significant, PBA did not completely reverse the deleterious effects of prolonged FFA elevation, which may be due to the dose and duration of PBA treatment or multiple mechanisms of lipotoxicity. PBA alone did not improve insulin sensitivity and β-cell function in this study. It is possible that the effects of PBA can only be detected under exacerbated conditions, such as prolonged lipid infusion in the current study or increased obesity; thus, ER stress has been reported in tissues of individuals with increased obesity (29,30).
The current study does not allow us to exclude the possibility that the beneficial effects of PBA may be due to an unknown action of the drug beyond its known anti-ER stress properties. For instance, PBA has been reported to activate peroxisome proliferator–activated receptor-γ and inhibit histone deacetylase activities (49). Currently, there are no reliable plasma ER stress markers to assess tissue ER status. Biopsy specimens, such as in adipose tissue, may provide such information, but biopsy per se may cause ER stress (50). In a recent study, tauroursodeoxycholic acid, another chemical chaperone with ER stress reduction capability in animals, improved insulin sensitivity in morbidly obese subjects without detectable changes in ER stress markers in extrahepatic tissues (51). This suggests that in humans, reduction of ER stress may be in tissues, such as the liver, that are not easily accessible to biopsy.
PBA also prevented a decline in insulin clearance caused by IH infusion in this study. Insulin clearance is mediated by similar and partly overlapping pathways affecting insulin sensitivity, including insulin receptor but not insulin receptor substrate (52–54). It is therefore possible that PBA, through ER stress reduction or mechanisms independent of ER function, may have affected insulin clearance along these pathways. The mechanism by which PBA prevents lipid-induced reduction in insulin clearance warrants further study.
In conclusion, the current study demonstrates that PBA may provide clinical benefits in humans against insulin resistance and β-cell dysfunction induced by prolonged exposure to elevated circulating FFAs. Future studies are needed to explore the mechanism of such effects and to establish the long-term benefit and safety of compounds such as PBA in prediabetic and type 2 diabetic individuals.
ACKNOWLEDGMENTS
This work was supported by an operating grant from the Canadian Diabetes Association. G.F.L. holds a Canada Research Chair in Diabetes and the Drucker Family Chair in Diabetes Research and is a Career Investigator of the Heart and Stroke Foundation of Canada.
No potential conflicts of interest relevant to this article were reported.
C.X. performed the studies, analyzed data, and wrote the manuscript. A.G. contributed intellectually to data interpretation, and reviewed and edited the manuscript. G.F.L. obtained grant funding to support the research, designed the study, analyzed data, interpreted data, and edited the manuscript.
The authors thank Pat Harley of Toronto General Hospital and Linda Szeto of University of Toronto for technical assistance.
Footnotes
Clinical trial reg. no. NCT00533559, clinicaltrials.gov.
REFERENCES
- 1.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444:840–846 [DOI] [PubMed] [Google Scholar]
- 2.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest 2002;32(Suppl. 3):14–23 [DOI] [PubMed] [Google Scholar]
- 3.Stein DT, Esser V, Stevenson BE, et al. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J Clin Invest 1996;97:2728–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes 2006;55(Suppl. 2):S16–S23 [DOI] [PubMed] [Google Scholar]
- 5.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev 2008;29:351–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes 2002;51(Suppl. 1):S212–S220 [DOI] [PubMed] [Google Scholar]
- 7.Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 1993;42:1663–1672 [DOI] [PubMed] [Google Scholar]
- 8.Carpentier A, Mittelman SD, Lamarche B, Bergman RN, Giacca A, Lewis GF. Acute enhancement of insulin secretion by FFA in humans is lost with prolonged FFA elevation. Am J Physiol 1999;276:E1055–E1066 [DOI] [PubMed] [Google Scholar]
- 9.Carpentier A, Mittelman SD, Bergman RN, Giacca A, Lewis GF. Prolonged elevation of plasma free fatty acids impairs pancreatic beta-cell function in obese nondiabetic humans but not in individuals with type 2 diabetes. Diabetes 2000;49:399–408 [DOI] [PubMed] [Google Scholar]
- 10.Carpentier A, Zinman B, Leung N, et al. Free fatty acid-mediated impairment of glucose-stimulated insulin secretion in nondiabetic Oji-Cree individuals from the Sandy Lake community of Ontario, Canada: a population at very high risk for developing type 2 diabetes. Diabetes 2003;52:1485–1495 [DOI] [PubMed] [Google Scholar]
- 11.Cusi K, Kashyap S, Gastaldelli A, Bajaj M, Cersosimo E. Effects on insulin secretion and insulin action of a 48-h reduction of plasma free fatty acids with acipimox in nondiabetic subjects genetically predisposed to type 2 diabetes. Am J Physiol Endocrinol Metab 2007;292:E1775–E1781 [DOI] [PubMed] [Google Scholar]
- 12.Kashyap S, Belfort R, Gastaldelli A, et al. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes 2003;52:2461–2474 [DOI] [PubMed] [Google Scholar]
- 13.Leung N, Sakaue T, Carpentier A, Uffelman K, Giacca A, Lewis GF. Prolonged increase of plasma non-esterified fatty acids fully abolishes the stimulatory effect of 24 hours of moderate hyperglycaemia on insulin sensitivity and pancreatic beta-cell function in obese men. Diabetologia 2004;47:204–213 [DOI] [PubMed] [Google Scholar]
- 14.Xiao C, Giacca A, Carpentier A, Lewis GF. Differential effects of monounsaturated, polyunsaturated and saturated fat ingestion on glucose-stimulated insulin secretion, sensitivity and clearance in overweight and obese, non-diabetic humans. Diabetologia 2006;49:1371–1379 [DOI] [PubMed] [Google Scholar]
- 15.Xiao C, Giacca A, Lewis GF. Oral taurine but not N-acetylcysteine ameliorates NEFA-induced impairment in insulin sensitivity and beta cell function in obese and overweight, non-diabetic men. Diabetologia 2008;51:139–146 [DOI] [PubMed] [Google Scholar]
- 16.Xiao C, Giacca A, Lewis GF. The effect of high-dose sodium salicylate on chronically elevated plasma non-esterified fatty acid-induced insulin resistance and beta-cell dysfunction. Am J Physiol Endocrinol Metab 2009;297:E1205–E1211 [DOI] [PubMed] [Google Scholar]
- 17.Yu C, Chen Y, Cline GW, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 2002;277:50230–50236 [DOI] [PubMed] [Google Scholar]
- 18.Kim JK, Fillmore JJ, Sunshine MJ, et al. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest 2004;114:823–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam TK, Yoshii H, Haber CA, et al. Free fatty acid-induced hepatic insulin resistance: a potential role for protein kinase C-delta. Am J Physiol Endocrinol Metab 2002;283:E682–E691 [DOI] [PubMed] [Google Scholar]
- 20.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 2002;51:2005–2011 [DOI] [PubMed] [Google Scholar]
- 21.Yuan M, Konstantopoulos N, Lee J, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 2001;293:1673–1677 [DOI] [PubMed] [Google Scholar]
- 22.Hundal RS, Petersen KF, Mayerson AB, et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest 2002;109:1321–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oprescu AI, Bikopoulos G, Naassan A, et al. Free fatty acid-induced reduction in glucose-stimulated insulin secretion: evidence for a role of oxidative stress in vitro and in vivo. Diabetes 2007;56:2927–2937 [DOI] [PubMed] [Google Scholar]
- 24.Hotamisligil GS. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int J Obes (Lond) 2008;32(Suppl. 7):S52–S54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem 2005;74:739–789 [DOI] [PubMed] [Google Scholar]
- 26.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004;306:457–461 [DOI] [PubMed] [Google Scholar]
- 27.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006;313:1137–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma NK, Das SK, Mondal AK, et al. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J Clin Endocrinol Metab 2008;93:4532–4541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boden G, Duan X, Homko C, et al. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 2008;57:2438–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gregor MF, Yang L, Fabbrini E, et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes 2009;58:693–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology 2006;147:3398–3407 [DOI] [PubMed] [Google Scholar]
- 32.Choi SE, Lee YJ, Jang HJ, et al. A chemical chaperone 4-PBA ameliorates palmitate-induced inhibition of glucose-stimulated insulin secretion (GSIS). Arch Biochem Biophys 2008;475:109–114 [DOI] [PubMed] [Google Scholar]
- 33.Cnop M, Ladrière L, Igoillo-Esteve M, Moura RF, Cunha DA. Causes and cures for endoplasmic reticulum stress in lipotoxic β-cell dysfunction. Diabetes Obes Metab 2010;12(Suppl. 2):76–82 [DOI] [PubMed] [Google Scholar]
- 34.Laybutt DR, Preston AM, Akerfeldt MC, et al. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 2007;50:752–763 [DOI] [PubMed] [Google Scholar]
- 35.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 36.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41:368–377 [DOI] [PubMed] [Google Scholar]
- 37.Boden G, She P, Mozzoli M, et al. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappaB pathway in rat liver. Diabetes 2005;54:3458–3465 [DOI] [PubMed] [Google Scholar]
- 38.Park E, Wong V, Guan X, Oprescu AI, Giacca A. Salicylate prevents hepatic insulin resistance caused by short-term elevation of free fatty acids in vivo. J Endocrinol 2007;195:323–331 [DOI] [PubMed] [Google Scholar]
- 39.Akerfeldt MC, Howes J, Chan JY, et al. Cytokine-induced beta-cell death is independent of endoplasmic reticulum stress signaling. Diabetes 2008;57:3034–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hatanaka M, Tanabe K, Ohta Y, et al. Wolfram syndrome 1 gene (wfs1) product localizes to secretory granule and contributes to maintenance of granular acidification in pancreatic beta cells. Diabetes 2010;59:A76. [DOI] [PubMed] [Google Scholar]
- 41.Lai E, Bikopoulos G, Wheeler MB, Rozakis-Adcock M, Volchuk A. Differential activation of ER stress and apoptosis in response to chronically elevated free fatty acids in pancreatic beta-cells. Am J Physiol Endocrinol Metab 2008;294:E540–E550 [DOI] [PubMed] [Google Scholar]
- 42.Gwiazda KS, Yang TL, Lin Y, Johnson JD. Effects of palmitate on ER and cytosolic Ca2+ homeostasis in beta-cells. Am J Physiol Endocrinol Metab 2009;296:E690–E701 [DOI] [PubMed] [Google Scholar]
- 43.Ozawa K, Miyazaki M, Matsuhisa M, et al. The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes 2005;54:657–663 [DOI] [PubMed] [Google Scholar]
- 44.Cohen FE, Kelly JW. Therapeutic approaches to protein-misfolding diseases. Nature 2003;426:905–909 [DOI] [PubMed] [Google Scholar]
- 45.Vilatoba M, Eckstein C, Bilbao G, et al. Sodium 4-phenylbutyrate protects against liver ischemia reperfusion injury by inhibition of endoplasmic reticulum-stress mediated apoptosis. Surgery 2005;138:342–351 [DOI] [PubMed] [Google Scholar]
- 46.Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest 2008;118:316–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magnan C, Collins S, Berthault MF, et al. Lipid infusion lowers sympathetic nervous activity and leads to increased beta-cell responsiveness to glucose. J Clin Invest 1999;103:413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diakogiannaki E, Morgan NG. Differential regulation of the ER stress response by long-chain fatty acids in the pancreatic beta-cell. Biochem Soc Trans 2008;36:959–962 [DOI] [PubMed] [Google Scholar]
- 49.Lea MA, Sura M, Desbordes C. Inhibition of cell proliferation by potential peroxisome proliferator-activated receptor (PPAR) gamma agonists and antagonists. Anticancer Res 2004;24:2765–2771 [PubMed] [Google Scholar]
- 50.Boden G, Silviera M, Smith B, Cheung P, Homko C. Acute tissue injury caused by subcutaneous fat biopsies produces endoplasmic reticulum stress. J Clin Endocrinol Metab 2010;95:349–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kars M, Yang L, Gregor MF, et al. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes 2010;59:1899–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choice CV, Howard MJ, Poy MN, Hankin MH, Najjar SM. Insulin stimulates pp120 endocytosis in cells co-expressing insulin receptors. J Biol Chem 1998;273:22194–22200 [DOI] [PubMed] [Google Scholar]
- 53.Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev 1998;19:608–624 [DOI] [PubMed] [Google Scholar]
- 54.Poy MN, Yang Y, Rezaei K, et al. CEACAM1 regulates insulin clearance in liver. Nat Genet 2002;30:270–276 [DOI] [PubMed] [Google Scholar]