Abstract
OBJECTIVE
Adiponectin receptor-1 (AdipoR1) expression in skeletal muscle has been suggested to play an important role in insulin resistance and diabetes. We aimed at evaluating the presence of novel AdiopR1 splice variants in human muscle and their regulation under physiological and pathophysiological states.
RESEARCH DESIGN AND METHODS
AdipoR1 5′UTR mRNA transcripts, predicted from bioinformatics data, were evaluated in fetal and adult human tissues. Expression and function of the identified transcripts were assessed in cultured human skeletal muscle cells and in muscle biopsies obtained from individuals with normal glucose tolerance (NGT) and type 2 diabetes (n = 49).
RESULTS
Screening of potential AdipoR1 5′UTR splice variants revealed a novel highly abundant muscle transcript (R1T3) in addition to the previously described transcript (R1T1). Unlike R1T1, R1T3 expression was significantly increased during fetal development and myogenesis, paralleled with increased AdipoR1 protein expression. The 5′UTR of R1T3 was found to contain upstream open reading frames that repress translation of downstream coding sequences. Conversely, AdipoR1 3′UTR was associated with enhanced translation efficiency during myoblast-myotube differentiation. A marked reduction in muscle expression of R1T3, R1T1, and R1T3-to-R1T1 ratio was observed in individuals with type 2 diabetes compared with expression levels of NGT subjects, paralleled with decreased expression of the differentiation marker myogenin. Among NGT subjects, R1T3 expression was positively correlated with insulin sensitivity.
CONCLUSIONS
These results indicate that AdipoR1 receptor expression in human skeletal muscle is subjected to posttranscriptional regulation, including alternative splicing and translational control. These mechanisms play an important role during myogenesis and may be important for whole-body insulin sensitivity.
Adiponectin, an adipocyte-derived abundant plasma protein (1), gained recognition as a potential mechanistic link between obesity, insulin resistance, and diabetes (2). Low serum levels of adiponectin are found in obesity and diabetes (3,4), whereas improvement in insulin sensitivity in obese and diabetic patients, following thiazolidenediones treatment, correlates with a marked increase in adiponectin levels (5,6). The insulin-sensitizing effects of adiponectin are mediated by inhibition of hepatic glucose production and by stimulation of muscle fatty acid oxidation and glucose transport (7–9).
Adiponectin biological effects may depend not only on the relative circulating concentrations of the hormone but also on the expression level and function of its receptors (2). Thus, decreased expression (10–13) or impaired function of the receptors or their downstream effectors (13–16) in obesity and diabetes may lead to reduction in adiponectin bioactivity and in insulin sensitivity (2,11,13). Recent studies have demonstrated that improvement in insulin sensitivity following chronic exercise in obese subjects with type 2 diabetes is associated with enhanced AdipoRs mRNA expression in skeletal muscle and adipose tissue (17–20) and cannot be fully explained by changes in circulating adiponectin levels (17). Taken together, these findings underline the potential importance of human AdipoRs in the pathophysiology of insulin resistance and diabetes. Nevertheless, assessment of the differential mRNA expression of AdipoRs yielded contradicting results. When measured in skeletal muscles, mRNA expression of AdipoR1 (the predominant receptor in this tissue) was found to be lower in subjects with normal glucose tolerance (NGT) than in subjects with impaired glucose tolerance or type 2 diabetes (18). In addition, AdipoR1 expression was found to be upregulated in adipose tissue but downregulated in skeletal muscle following treatment of type 2 diabetic patients with rosiglitazone (21). By contrast, treatment of individuals with type 2 diabetes with pioglitazone resulted in increased mRNA levels for AdipoR1 and -2 in skeletal muscle biopsies, associated with increased whole-body insulin sensitivity (22). Moreover, reduced AdipoR1 mRNA expression was found to exist in muscles of subjects with a positive family history of diabetes (12). Nevertheless, several other reports did not demonstrate any significant changes in AdipoR1 mRNA or protein expression in primary human myotubes (14,23) or muscle biopsies from NGT versus type 2 diabetic patients (24,25). These conflicting results highlight the need for further studies to elucidate the regulation of AdipoR1.
In addition to transcriptional alterations, posttranscriptional mechanisms have a profound role in protein expression regulation. Alternative mRNA splicing is an important mechanism for generating posttranscriptional modulations and structural and functional diversity of proteins (26,27). Another important mechanism is translational regulation of gene expression, which predominantly takes place through untranslated regions (UTRs) located at the 5′ and 3′ ends of the mRNA (28).
In the current study, we have evaluated transcriptional and posttranscriptional regulation of AdipoR1 gene expression in human skeletal muscle and assessed the potential significance of novel muscle AdipoR1 5′UTR splice variants in subjects with NGT or type 2 diabetes.
RESEARCH DESIGN AND METHODS
Database-searching for splice variants of AdipoR1.
The LEADS platform (Compugen, Tel-Aviv, Israel) for clustering and assembly of genomic sequences, cDNAs, and expressed sequence tags (29) was used to search public databases for predicted splice variants of human AdipoR1. The transcripts R1T1–R1T4 are schematically depicted in Fig. 1A.
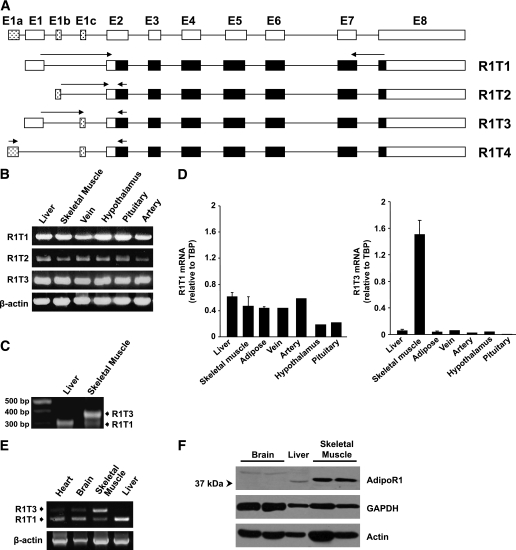
FIG. 1.
Identification and tissue distribution of human AdipoR1 5′UTR splice variants. A: Schematic representations of four predicted transcripts of AdipoR1. The noncoding and coding regions of AdipoR1 transcripts are represented by white and black boxes, respectively. The spotted boxes represent the putative novel exons. The arrows indicate the locations of primers used in the RT-PCR analysis. B: RT-PCR analysis using transcript-specific primers in various human tissues. C: Electrophoresis results of 5′-RACE PCR products from liver and skeletal muscle. 5′-RACE analysis was performed as described in the Supplementary Data. Molecular weight markers are shown in the far left lane. D: qPCR analysis of R1T1 and R1T3 mRNA expression in various human male tissues normalized to TBP. Each sample was measured in triplicates. Data are expressed as the means ± SD of the various tissues (n ≥ 2). E: Semiquantitative RT-PCR analysis of R1T1 and R1T3 expression in human tissues was performed using primers designed from exon 1 and exon 2 of AdipoR1 mRNA, which simultaneously detect both transcripts. F: Western blot analysis of AdipoR1 protein expression in human tissues. Equal amounts (50 μg) of protein lysates were resolved by means of 10% SDS-PAGE and were subjected to Western immunoblotting using anti-AdipoR1 antibody. Anti-GAPDH and anti-actin antibodies were used as loading controls.
Cell cultures.
Primary human skeletal muscle cells (h-SkMcs) derived from two healthy Caucasian donors (male aged 47 years and female aged 25 years, respectively) were purchased from PromoCell (Heidelberg, Germany). Cells were grown according to manufacturers’ instructions and differentiated by changing the growth medium to h-SkMc differentiation medium (PromoCell) supplemented for 10 days. For siRNA experiments, 48 h prior to differentiation induction h-SkMcs were transfected with 100 nmol/L of the following siRNAs: siGENOME SMARTpool AdipoR1-siRNA, siGENOME nontargeted (NT)-siRNA, and R1T3-siRNA (sense, UAUGAUGACAUGAUCUCCAUU, antisense, 5′-PUGGAGAUCAUGUCAUCAUAUU) using DharmaFECT1 transfection reagent (Dharmacon, Lafayette, CO). Human embroyonic kidney (HEK)293 and C2C12 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS. C2C12 myoblast differentiation was induced by incubating an 85% confluent culture in DMEM with 2% horse serum for 4 days.
RNA isolation and RT-PCR analysis.
Total RNA was isolated from cells with a GenElute Mammalian Total RNA Miniprep kit (Sigma, St. Louis, MO) and treated with DNase (Ambion, Austin, TX) to eliminate genomic DNA contamination. Total RNA samples from human, monkey, and mouse tissues (all male) were purchased from BioChain (Hayward, CA). First-strand cDNA was synthesized from 1 μg total RNA using 2.5 units of Super AMV Reverse Transcriptase (CHIMERx, Madison, WI), 10 pmol dT15, and 30 units of RNasin (Promega, Madison, WI) in 20 μL reaction buffer. cDNA was amplified by PCR using ReddyMix PCR Master Mix (Thermo-Scientific, Waltham, MA) in the presence of 250 nmol/L primers. Primers sequences and PCR conditions are detailed in Supplementary Table 1. To ensure quality and purity of cDNA samples, RT-PCR with or without reverse transcriptase was performed for the β-actin cDNA; no PCR products were observed in the negative controls. 5′-rapid amplification of cDNA ends (5′-RACE) was performed as described in the Supplementary Data.
Quantitative real-time PCR analysis.
Gene expression levels were determined using an ABI-Prism 7000 instrument (Applied Biosystems, Carlsbad, CA). cDNA was amplified using SYBR Green PCR Master Mix (Applied Biosystems) supplemented with 250 nmol/L specific primers. Primer sequences and quantitative PCR (qPCR) conditions are detailed in Supplementary Table 2. For 18S rRNA level determination, cDNA synthesis was performed in the presence of 50 pmol random hexamer primers, and the resulting cDNA was assayed using a TaqMan gene expression assay (Hs99999901-s1; Applied Biosystems). Target gene expression levels were normalized to mRNA levels of TATA-binding protein (TBP), and results were analyzed using the 2−(∆Ct) method (∆Ctsample = Cttarget gene − Ctreference gene). When human muscle biopsies were used, gene expression levels were normalized to β-actin mRNA levels. To account for between runs, one skeletal muscle cDNA sample was analyzed in each run as a calibrator and relative quantification of gene expression was performed by analyzing the qPCR data using the 2−(∆∆Ct) method (∆∆Ct = ∆Ctsample − ∆Ctcalibrator). Mouse AdipoR1 levels were normalized to 18S rRNA levels, and relative quantification of gene expression was performed by data analysis using the 2−(∆∆Ct) method.
Immunoblot analysis.
Protein extracts from cells and tissues were prepared by solubilization with extraction buffer (50 mmol/L Tris-HCl pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mmol/L NaCl, 1 mmol/L EGTA, 1 mmol/L Na3Vo4, 1 mmol/L NaF, protease inhibitor cocktail [1:1000], and phosphatase inhibitor cocktail 1 and 2 [1:100]) at 4°C. Supernatants (12,000 g) were resolved by SDS-PAGE and immunoblotted with the following antibodies: AdipoR1, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Abcam, Cambridge, MA), myosin heavy chain (MyHC) (Millipore, Lake Placid, NY), myogenin (Santa Cruz, CA), tubulin (Sigma), and actin (MP Biomedicals, CA).
Reporter assays.
The distinct AdipoR1–5′UTRs and AdipoR1–3′UTR were cloned into pGL3-promoter vector (see Supplementary Data). Chimeric reporter gene (firefly luciferase) constructs were mixed with an expression vector for Renilla luciferase (pRL-TK; Promega) in a 50:1 mass ratio, and the two plasmids were cotransfected into cells using Lipofectamine 2000 (Invitrogen, CA). Cells were harvested 24 h after transfection (HEK293 and C2C12 myoblasts) or incubated with C2C12 differentiation medium for another 4 days (C2C12 myotubes). Cell lysates were analyzed for firefly and Renilla luciferase activity levels using the Dual-Luciferase Assay System (Promega). Firefly luciferase mRNA levels were quantified by qPCR and normalized to Renilla mRNA levels. Relative quantification of gene expression was performed by analyzing data using the 2−(∆∆Ct) method.
Human studies.
The study population consisted of 49 Caucasian men: 29 with NGT and 20 with overt type 2 diabetes. Patients with malignant diseases, alcohol or drug abuse, or diabetic retinopathy or nephropathy or any acute or chronic inflammatory disease were excluded from the study. The study was approved by the ethics committee of the University of Leipzig (Leipzig, Germany), and all study participants gave written informed consent. Blood samples and skeletal muscle samples were collected between 0800 and 1000 h after an overnight fast. Plasma insulin, glucose, leptin, and adiponectin concentrations were determined as previously described (18). Insulin sensitivity was assessed with the euglycemic-hyperinsulinemic clamp method as previously described (18). Skeletal muscle biopsies were obtained under local anesthesia from the right vastus lateralis muscle and were immediately snap-frozen in liquid nitrogen. mRNA expression levels in muscle samples were determined by qPCR as described above.
Statistical analyses.
Statistical analyses were conducted using SPSS (version 14; SPSS, Chicago, IL). Variables were tested for normal distribution using the Kolmogorov-Smirnov and Shapiro-Wilk tests. Data are expressed as means ± SD or median (interquartile range). Comparison of AdipoR1 transcripts levels between different tissues was performed using one-way ANOVA. Comparison of descriptive characteristics between the NGT and type 2 diabetes groups was conducted using Student t test or Mann-Whitney U test (for normally and non–normally distributed variables, respectively). We calculated Spearman correlation coefficients between variables to examine associations of adiponectin and its receptors with anthropometric and metabolic parameters. Analyses were stratified according to the presence or absence of the diagnosis of diabetes. Univariate and multivariate linear regression models produced crude and age- and BMI-adjusted odds ratios for R1T1/actin and R1T3/actin levels (after log transformation) and R1T3-to-R1T1 ratio among subjects with NGT and type 2 diabetes. P < 0.05 was considered significant, and 0.05 < P < 0.10 was considered representative of a trend.
RESULTS
Identification of human AdipoR1 5′UTR splice variants.
The human AdipoR1 gene consists of eight exons (E1–E8) (Fig. 1A) and seven introns, and the 5′UTR includes exon 1 and part of exon 2 (8). Using the LEADS Human Transcriptosome Database, four predicted 5′UTR splice variants that encode the AdipoR1 protein were identified (Fig. 1A and Supplementary Table 4). R1T1 corresponds to the published transcript (8), whereas the other potential transcripts result from alternative 5′ start (R1T2), alternative additional 5′ exon (R1T3), or both (R1T4), the latter of which has been reported in the public domain (NM_001127687). RT-PCR analysis revealed the expression of the known R1T1 transcript in addition to the novel suggested R1T2 and R1T3 transcripts, but not of R1T4, in the various tissues assessed (Fig. 1B). To further analyze the transcription start sites of the newly identified transcripts and assess the existence of potential additional AdipoR1 transcripts, we performed 5′-RACE using two of the main adiponectin target tissues: liver and skeletal muscle (Fig. 1C). Whereas the liver exhibited a single 5′-RACE product identified by sequence analysis as R1T1, two major products, corresponding to R1T1 and R1T3, were detected in skeletal muscle. The transcription start site was identical for both transcripts, indicating that they are not derived from alternative promoter usage but from differential splicing. The 5′-RACE analysis and additional qPCR analysis demonstrated relatively low abundance of R1T2 (data not shown). Therefore, we further characterized the expression of R1T1 and RIT3 under physiological and pathological conditions.
Tissue distribution of R1T1 and R1T3.
qPCR analysis was conducted to estimate the relative distribution of the two transcripts in various human tissues relevant to adiponectin biological activities (liver, skeletal muscle, and adipose tissue, insulin sensitizing effects; artery and vein, antiatherogenic effects; and hypothalamus, food intake regulation) (7,30,31). According to this analysis, R1T1 is abundant in most of the studied tissues, whereas R1T3 is highly expressed in skeletal muscle (Fig. 1D). Similarly, PCR analysis using primers designed from exons 1 and 2 of AdipoR1, which simultaneously detects both transcripts, demonstrated that R1T3 is the muscle predominant transcript (Fig. 1E). These findings indicate that the alternatively spliced variants of AdipoR1 are expressed in a tissue-specific manner.
When AdipoR1 protein expression was assessed, a significantly higher protein level of AdipoR1 could be observed in skeletal muscle compared with other adiponectin target tissues including liver and brain (Fig. 1F). Interestingly, differences in AdipoR1 mobility on SDS-PAGE were detected between the various tissues, suggesting that AdipoR1 may undergo tissue-specific posttranslational modifications.
Expression of R1T3 in mammals.
The R1T3 transcript results from an insertion of a 67-bp exon (exon 1c) (Fig. 1A) between exons 1 and 2 in the 5′UTR of AdipoR1 mRNA. To examine whether this additional exon is conserved among other species, we screened the public databases. The genomic organization of exon 1c was identified primates (macaque and chimpanzee) and Laurasiatheria (dog and horse) but not in rodents (such as mouse) (Supplementary Fig. 1A). Indeed, similar to the observation in human tissues (Fig. 1E), a high level of the Macaca-Mulatta R1T3 homolog could be detected in skeletal muscle but not in liver, brain, or heart (Supplementary Fig. 1B and C). An R1T3 mouse homolog could not be detected in the mouse tissues assessed, and the faint band observed in mouse muscle (Supplementary Fig. 1B) was found to represent an AdipoR1 transcript, resulting from insertion of an alternative exon distinct from human 1c exon (Supplementary Table 4).
Developmental regulation of R1T1 and R1T3 transcripts.
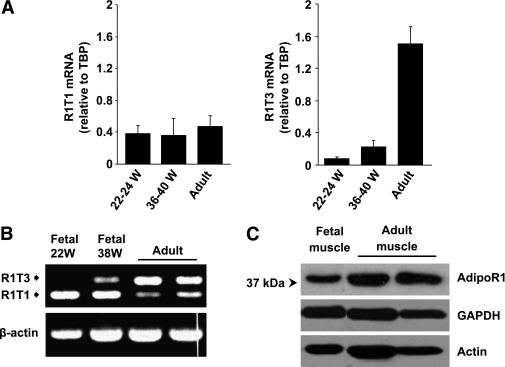
Because alternative splicing is often developmentally regulated, we next evaluated the expression pattern of AdipoR1 transcripts during embryogenesis. As demonstrated in Fig. 2A and B, the predominant transcript in skeletal muscle during embryo development is R1T1, whereas R1T3, the main transcript in adult muscle, was detected at low levels only in the mature, 38-week-old fetus. Correspondingly, protein expression of AdipoR1 was low during embryogenesis and increased significantly in adult skeletal muscle (Fig. 2C).
FIG. 2.
Developmental regulation of R1T1 and R1T3 transcripts. A: qPCR analysis of R1T1 and R1T3 mRNA expression normalized to TBP in fetal and adult human tissues. Each sample was measured in triplicate. Data are expressed as means ± SD (n ≥ 2). B: Semiquantitative RT-PCR analysis of R1T1 and R1T3 expression (as described in Fig. 1) in fetal and adult human skeletal muscles. C: Western blot analysis of AdipoR1 protein expression in fetal and adult human skeletal muscles. Equal amounts (50 μg) of protein lysates were resolved by means of 10% SDS-PAGE and were subjected to Western immunoblotting using anti-AdipoR1 antibody. Anti-actin and anti-GAPDH were used as loading controls.
Regulation of R1T1 and R1T3 transcripts during muscle differentiation.
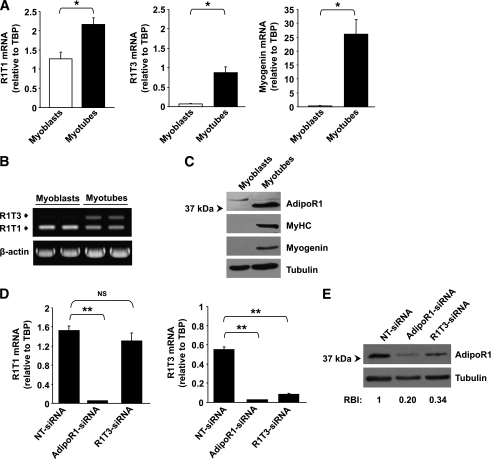
To assess whether expression of R1T1 and R1T3 transcripts is subjected to differentiation-dependent modulation, we next compared the frequency of R1T1 and R1T3 and AdipoR1 protein expression in primary culture of human skeletal muscle cells (h-SkMcs) before and following differentiation. RT-PCR and Western blot analyses demonstrated that, similar to embryo development, differentiation of myoblasts to myotubes was associated with robust increase in R1T3 expression (Fig. 3A and B) and a marked increase in AdipoR1 protein expression (Fig. 3C). The increase in R1T3 transcript and in AdipoR1 protein level paralleled with cell differentiation as indicated by increased expression of myogenin and MyHC (Fig. 3A and C).
FIG. 3.
Alterations in AdipoR1 splice variants and AdipoR1 protein expression levels during h-SkMc differentiation and in knockdown experiments. A: qPCR analysis of R1T1, R1T3, and myogenin mRNA expression normalized to TBP in h-SkMc myoblasts and myotubes. Each sample was measured in triplicate. Data are expressed as the means ± SD of three independent experiments. B: Semiquantitative RT-PCR analysis of R1T1 and R1T3 expression (as described in Fig. 1) in h-SkMc myoblasts and myotubes. C: Western blot analysis of AdipoR1 protein expression in h-SkMc myoblasts and myotubes. Equal amounts (50 μg) of protein lysates were resolved by means of 10% SDS-PAGE and were subjected to Western immunoblotting using anti-AdipoR1, anti-MyHC, anti-myogenin (as differentiation markers), and anti-tubulin (as loading control) antibodies. D: qPCR analysis of R1T1 and R1T3 expression in h-SkMc transfected with siRNAs (n = 3). The cells were transfected with 100 nmol/L NT-siRNA, pool of siRNAs targeting different sites of the AdipoR1 ORF (AdipoR1-siRNA), or with siRNA targeting exon 1c (R1T3-siRNA). Forty-eight hours after transfection, cell differentiation was induced by incubation with differentiation medium for 10 days and mRNA expression was measured. Data are expressed as means ± SD. E: Western blot analysis of AdipoR1 protein expression in h-SkMc transfected with siRNAs as described above (n = 3). Equal amounts (40 μg) of protein lysates were resolved by means of 10% SDS-PAGE and were subjected to Western immunoblotting using anti-AdipoR1 and anti-tubulin (as loading control) antibodies. The relative band intensity (RBI) was determined by densitometry of AdipoR1 band intensity, normalized to tubulin band intensity. An average RBI value from three independent experiments is shown. P values were evaluated using Student t test. *P < 0.05; **P < 0.005. NS, nonsignificant.
Knockdown of AdipoR1 expression by R1T3-siRNA.
To evaluate the competence of R1T3 transcript to produce AdipoR1 protein, we employed RNAi to downregulate either R1T3, using siRNA specific to the human 1c exon (R1T3-siRNA), or the expression of both R1T1 and R1T3 transcripts, using an siRNA pool targeting different sites of AdipoR1 open reading frame (ORF) (AdipoR1-siRNA). As depicted in Fig. 3D, R1T3-siRNA efficiently silenced R1T3 without affecting R1T1 expression, whereas AdipoR1-siRNA downregulated both transcripts. Transfection with either R1T3-siRNA or AdipoR1-siRNA resulted in substantial reduction of AdipoR1 protein (Fig. 3E) (P < 0.001 for both, compared with NT-siRNA), indicating that R1T3 is a functional transcript important for AdipoR1 protein expression in differentiated h-SkMc.
Evaluation of AdipoR1 transcripts in human skeletal muscle of subjects with NGT and type 2 diabetes.
The abundance of AdipoR1 transcripts in skeletal muscle of subjects with NGT (n = 29) and type 2 diabetes (n = 20) was next studied. Basal characteristics of both groups are demonstrated in Table 1. As can be observed, the type 2 diabetic patients were significantly older, had higher BMI values, and had significantly decreased plasma adiponectin concentrations and increased plasma leptin levels.
TABLE 1.
Baseline anthropometrics and metabolic characteristics of men with NGT and type 2 diabetes
| NGT | Type 2 diabetes | |
|---|---|---|
| n | 29 | 20 |
| Age (years) | 30.8 ± 10.5 | 53.1 ± 7.0* |
| BMI (kg/m2) | 25.4 ± 3.0 | 34.7 ± 4.3* |
| Fat mass (%) | 14.1 ± 8.1 | 35.6 ± 5.8* |
| FPG (mmol/L) | 5.1 ± 0.7 | 6.4 ± 0.3* |
| FPI (pmol/L) | 13.7 (6.6–18) | 159 (130–239)* |
| GIR (mg/kg/min) | 101 (96.5–119) | 48.5 (24.3–55.7)* |
| Adiponectin (μg/mL) | 13.4 ± 3.6 | 4.3 ± 2.2* |
| Leptin (pg/mL) | 3.6 (2.0–8.5) | 16.4 (13.8–18.8)* |
Data are expressed as means ± SD or as median (interquartile range) unless otherwise indicated. FPG, fasting plasma glucose; FPI, fasting plasma insulin.
*P < 0.001.
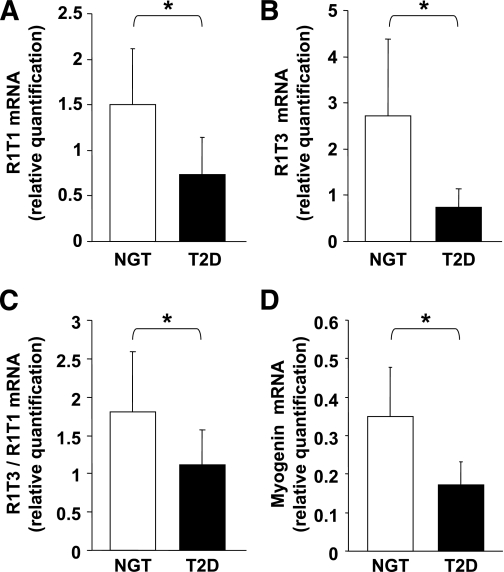
qPCR analysis of cDNA obtained from skeletal muscle of these subjects demonstrated decreased expression of R1T1 (P < 0.001) and R1T3 (P < 0.001) in the type 2 diabetic group compared with the NGT group (Fig. 4A and B). The reduction in R1T3 transcript levels was more profound than the reduction observed in R1T1 levels among the type 2 diabetic group (by fourfold and by 2.2-fold compared with the NGT group, respectively). Accordingly, R1T3-to-R1T1 ratio was found to be 37% lower in the type 2 diabetic group than in the NGT group (1.11 vs. 1.76, respectively; P < 0.001) (Fig. 4C). In a multivariate linear regression analysis, adjusted for both age and BMI differences, type 2 diabetes remained independently associated with decreased expression of R1T1 (β = −0.45; P = 0.018), R1T3 (β = −0.40; P = 0.037), and R1T3-to-R1T1 ratio (β = −0.29; P = 0.006) compared with the NGT group. A statistically significant correlation was found between R1T3 expression levels and the degree of insulin sensitivity, as assessed by glucose infusion rate (GIR), within the NGT subjects (r = 0.39, P = 0.03) (Table 2). In addition, there was a trend toward a negative correlation between the expression of both transcripts and fasting plasma insulin levels in these subjects (R1T1, r = −0.35, P = 0.06; R1T3, r = −0.34, P = 0.06) (Table 2). Because our results in the h-SkMcs supported a differentiation-dependent modulation of R1T3 transcript, we next assayed the expression of the muscle differentiation factor myogenin within the two groups. A significant decreased myogenin expression was observed in the type 2 diabetic group compared with the NGT group (P < 0.001) (Fig. 4D).
FIG. 4.
Evaluation of AdipoR1 transcripts and myogenin in human skeletal muscle of subjects with NGT and type 2 diabetes (T2D). R1T1 mRNA levels (A), R1T3 mRNA levels (B) and R1T3 mRNA relative to R1T1 mRNA expression (C) in subjects with NGT (n = 29) and type 2 diabetes (n = 20). D: Myogenin mRNA levels in subjects with NGT (n = 25) and type 2 diabetes (n = 12). Data are results of qPCR analysis expressed as means ± SD. Gene expression levels were normalized to β-actin mRNA levels. Relative quantification of gene expression was calculated as described under research design and methods. The P values were evaluated using Student t test. *P < 0.001.
TABLE 2.
Spearman correlation matrix of adiponectin, R1T1, and R1T3 mRNA levels with clinical, biochemical, and hormonal parameters
| NGT (n = 29) | Type 2 diabetes (n = 20) | |||||||
|---|---|---|---|---|---|---|---|---|
| Adiponectin | R1T1 | R1T3 | T3/T1 | Adiponectin | R1T1 | R1T3 | T3/T1 | |
| Age | −0.27 | −0.12 | −0.22 | −0.22 | 0.29 | −0.27 | −0.19 | −0.02 |
| BMI | −0.17 | −0.01 | −0.05 | −0.01 | 0.02 | 0.18 | 0.14 | 0.09 |
| Fat mass % | −0.35* | −0.14 | −0.25 | −0.15 | 0.15 | −0.12 | −0.01 | 0.17 |
| FPG | 0.06 | 0.22 | 0.08 | 0.06 | −0.59† | 0.05 | 0.02 | 0.05 |
| FPI | −0.28 | −0.35* | −0.34* | −0.22 | −0.49‡ | 0.38 | 0.29 | 0.07 |
| GIR | 0.53† | 0.29 | 0.39‡ | 0.24 | 0.58† | 0.32 | 0.13 | −0.28 |
| Leptin | −0.28 | −0.12 | −0.25 | −0.18 | 0.04 | −0.29 | −0.10 | 0.35 |
| Adiponectin | — | 0.16 | 0.24 | 0.24 | — | 0.01 | −0.04 | −0.08 |
| R1T1 | — | — | 0.63† | 0.18 | — | — | 0.73† | −0.23 |
FPG, fasting plasma glucose; FPI, fasting plasma insulin; T3/T1, R1T3-to-R1T1 ratio.
†P < 0.01;
‡P < 0.05;
*P < 0.07.
Effect of 5′UTR of R1T1 and R1T3 on translation efficiency.
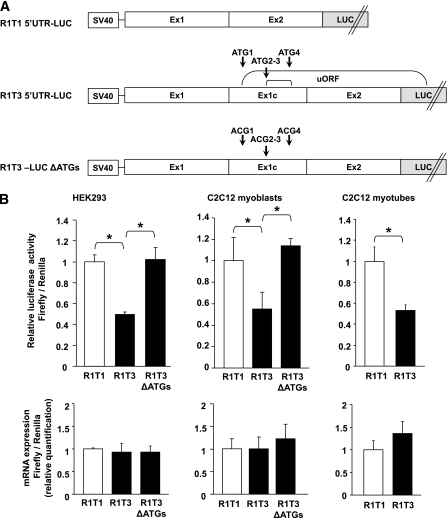
Alternative splicing in the 5′UTR could affect posttranscriptional regulation of gene expression due to differences in the translation efficiency of distinct splice variants (32,33). To evaluate whether introduction of an additional 5′ exon in R1T3 affects translational efficiency, we cloned the distinct 5′UTRs of R1T3 and R1T1 upstream of a luciferase reporter gene (Fig. 5A). HEK293 cells were transfected with the respective constructs, and after 24 h cells were assayed for luciferase activity. These experiments demonstrated that exon 1c insertion in the 5′UTR of R1T3 caused a significant decrease in luciferase activity without a significant change in transcript levels (Fig. 5B). Similar results were obtained using C2C12 myoblasts and myotubes (Fig. 5B). Analysis of the R1T3–5′UTR nucleotide sequence revealed that exon 1c insertion introduces four novel upstream AUG (uAUG) start codons, creating new upstream ORFs (uORFs) that may reduce the efficient translation of the downstream coding sequences (Fig. 5A and Supplementary Fig. 2). Indeed, elimination of the new uORFs by mutating the four uAUG initiation codons to ACG significantly enhanced translation efficiency (Fig. 5B). Altogether, these findings indicate that the 5′UTR of R1T3 attenuates the translation of downstream coding sequences compared with the 5′UTR of R1T1, probably due to introduction of uORFs.
FIG. 5.
Effect of 5′UTR of R1T1 and R1T3 on translation efficiency. A: Schematic representation of the generated constructs containing the alternative AdipoR1 5′UTRs. R1T1 and R1T3 5′UTR were fused upstream to a firefly luciferase reporter gene (R1T1–5′UTR-LUC and R1T3–5′UTR-LUC, respectively). The uAUG initiation codons (denoted as ATG) in the R1T3–5′UTR-LUC construct were altered to ACG codons by site-directed mutagenesis to generate an R1T3-LUC ΔATGs construct. B: Luciferase activity and mRNA expression of the constructs transfected in HEK293 and C2C12 cells. Cells were transiently cotransfected with each of the cloned firefly reporter plasmids (R1T1–5′UTR-LUC [R1T1], R1T3–5′UTR-LUC [R1T3], or R1T3-LUC ΔATGs [R1T3 ΔATGs]) and with the Renilla plasmid as a control for transfection efficiencies. Twenty-four hours after transfection, the cells were harvested (HEK293 and C2C12 myoblasts) or incubated with C2C12 differentiation medium for another 4 days (C2C12 myotubes). Firefly luciferase activities were assessed and normalized to Renilla values. The results are expressed relative to R1T1–5′UTR-LUC luciferase activity. Firefly luciferase mRNA levels in the transfected cells were quantified using qPCR and normalized to Renilla mRNA levels (lower panel). Data are expressed as the means ± SD of three independent experiments. P values were evaluated using Student t test. *P < 0.002.
Regulation of AdipoR1 translation through its 3′UTR during muscle differentiation.
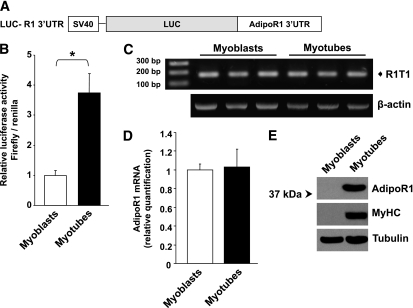
In addition to the 5′UTR, the 3′UTR is known to play an important role in regulation of mRNA translation (28) and may be part of the posttranscriptional mechanisms contributing to the marked increase of AdipoR1 protein expression during muscle differentiation (Fig. 3C). To address this possibility, we cloned the human AdipoR1 3′UTR (identical for both R1T1 and R1T3) downstream of the luciferase coding region of a pGL3 reporter vector (LUC-R1–3′UTR) (Fig. 6A). As depicted in Fig. 6B, a significant increase in luciferase activity of LUC-R1–3′UTR construct was detected in C2C12 myotubes compared with C2C12 myoblasts, suggesting that alterations in endogenous factors during muscle differentiation enhance the translation of human AdipoR1 through its 3′UTR. In support of this observation were the findings that differentiation of C2C12 cells, which express the mouse homolog of R1T1, is associated with a marked increase in AdipoR1 protein levels in the absence of significant differences in total mRNA levels (Fig. 6C–E).
FIG. 6.
Regulation of AdipoR1 translation through its 3′UTR during muscle differentiation. A: Schematic representation of the generated construct containing the human AdipoR1 3′UTR fused downstream to the firefly luciferase gene (LUC-R1–3′UTR). B: Luciferase activity of LUC-R1–3′UTR construct in C2C12 myoblasts and myotubes; firefly luciferase activities were assessed and normalized to Renilla values as a control for transfection efficiencies. Activity of LUC-R1–3′UTR construct was normalized to activity of pGL3 control vector transfected in parallel plates. The results are expressed relative to LUC-R1–3′UTR activity in myoblasts. C: RT-PCR analysis to detect possible exon inclusion in the mouse AdipoR1 5′UTR in C2C12 myoblasts and myotubes. The PCR was performed using primers designed from exon 1 and exon 2 of the mouse AdipoR1 mRNA. D: qPCR analysis of AdipoR1 mRNA expression normalized to 18S rRNA in C2C12 myoblasts and 4 day–differentiated myotubes. E: Western blot analysis of mouse AdipoR1 protein expression in C2C12 myoblasts and myotubes. Equal amounts (70 μg) of protein lysates were resolved by means of 10% SDS-PAGE and were subjected to Western immunoblotting using anti-AdipoR1, anti-MyHC (as differentiation marker), and anti-tubulin (as loading control) antibodies. Data are expressed as the means ± SD of three independent experiments. P values were evaluated using Student t test. *P < 0.001.
DISCUSSION
In the current study, we demonstrate an important role for posttranscriptional mechanisms, including alternative splicing and translational control, in regulation of AdipoR1 expression in human skeletal muscle. We identified a novel AdipoR1 splice variant (R1T3) derived from an insertion of additional short exon located in intron 1 at the 5′UTR of human AdipoR1 gene. R1T3 is abundantly and predominantly expressed in adult human muscle, contrary to the previously described human AdipoR1 mRNA transcript R1T1, which is ubiquitously expressed in adult human tissues including muscle (8). The two distinct transcripts are controlled by the same promoter, and both encode AdipoR1 receptor. R1T3 is subjected to developmental regulation, and its expression is considerably increased during human myoblast-myotube differentiation and during fetal development and adaptation to extrauterine environment. Comparison of translational efficiency of the discrete transcripts revealed that exon 1c insertion in the 5′UTR of R1T3 introduces uORFs, which suppress translation of the downstream coding region in a cell type–independent mechanism. Conversely, the 3′UTR of AdipoR1 is associated with enhanced translation efficiency during myoblast-myotube differentiation.
Posttranscriptional regulation is often controlled by short sequence elements located at the 5′ and 3′ ends of the mRNA. Our findings imply that AdipoR1 3′UTR has a significant role in the robust increase of AdipoR1 protein expression observed during differentiation. MicroRNAs and RNA-binding proteins are known to regulate mRNA translation by interacting with specific elements in their 3′UTR. Thus, the translational regulation of AdipoR1 3′UTR may be linked to alterations in specific microRNAs or RNA-binding proteins that occur during myoblast-myotube differentiation. Recent studies suggest that 5′UTR uORFs reduce the protein expression of thousands of mammalian genes, and variation in these elements can influence human phenotype and disease (34). In line with these studies is our finding that introduction of uORFs in R1T3 reduces its ability to translate downstream coding sequences. The introduction of uORFs, which is upregulated by alternative splicing during muscle differentiation, may serve as a negative control mechanism to attenuate the concomitant marked increase in protein translation mediated by the 3′UTR during differentiation. On the other hand, in subsets of genes uORFs are known to have a positive regulatory role. Introduction of uORFs in genes with important roles in cell growth and differentiation (35,36) has been shown to enable their selective translation under conditions that inhibit the translation of most cellular transcripts such as terminal differentiation and stress (37–40). Therefore, it may be hypothesized that such a mechanism has evolved to enable adaptation of human muscle AdipoR1 receptor expression to diverse physiological and pathological stress conditions.
Studying AdipoR1 transcript expression in subjects with NGT and type 2 diabetes demonstrated decreased expression of R1T1 and R1T3 in the type 2 diabetic group. Because the study is cross-sectional in nature, we cannot conclude a cause-and-effect relationship from this study. Nevertheless, the correlation between decreased expression of the predominant human skeletal muscle transcript R1T3 and reduced insulin sensitivity (assessed by GIR) among healthy subjects may suggest that inhibition in AdipoR1 mRNA expression precedes the development of the metabolic abnormalities observed in insulin resistant and overtly diabetic patients. In support of these findings is the observation of decreased AdipoR1 mRNA expression in skeletal muscle of normoglycemic subjects with a positive family history of diabetes compared with expression in age- and BMI-matched control subjects with a negative family history of the disease (12), as well as the observation that specific disruption of AdipoR1 in muscle had led to significantly decreased glucose disposal rate and GIR, indicating decreased muscle insulin sensitivity (41). In line with our findings in human skeletal muscle are several studies utilizing different animal models for obesity and diabetes, which almost unanimously observed reduced muscle AdipoR1 mRNA expression in various diabetic phenotypes such as ob/ob mice (11), db/db mice (10), KKAy mice (42), C57Bl/6 mice fed a high-fat and high-sucrose diet (43), and obese Zucker rats (44).
Putative mechanism for the decreased expression of AdipoR1 mRNA in diabetes had been raised following the observations that insulin itself acted as a negative regulator of AdipoR1 mRNA expression (10,11). It had been suggested that this negative regulation is mediated via the phosphatidylinositol 3-kinase/Foxo1 pathway (11) and that AdipoR1 promoter contains a putative insulin-responsive element (45). Consistent with this proposition is our finding of a negative association between the expression of AdipoR1 transcripts and fasting plasma insulin levels among subjects with NGT. Nevertheless, our observation of a significant decrease in R1T3-to-R1T1 ratio in the type 2 diabetic group compared with the NGT group cannot be explained solely by regulation of the AdipoR1 promoter but, rather, may indicate diabetes-specific repression of AdipoR1 alternative splicing. This raises the possibility that R1T3 repression in diabetes may represent a compensatory mechanism to enhance AdipoR1 biosynthesis when AdipoR1 gene transcription is significantly reduced.
An additional putative mechanism to explain the differences in AdipoR1 transcript expression may be alteration in fiber type composition and extent of differentiation in skeletal muscle of the type 2 diabetic patients, which is supported by the finding of decreased expression of myogenin in the diabetic muscles. Other than as a marker for myoblast-myotube differentiation, myogenin had also been suggested to play a role in regulation of slow oxidative phenotype (Type I) of human muscle fibers (46). Furthermore, insulin-resistant skeletal muscle cells had been reported to have repressed myogenin expression and to display oxidative stress and lower mitochondrial capacity (47). Indeed, as previously described, skeletal muscle of type 2 diabetic patients represents reduced oxidative capacity in parallel with increased glycolytic and altered muscle fiber composition (48), and a positive correlation between the proportion of type I myocytes and insulin sensitivity had been demonstrated even in normoglycemic subjects (49). The robust increase in the expression of RIT3 late in gestation, when a very rapid muscle differentiation and transition in muscle fiber type occurs favoring oxidative over glycolytic capacity (50), may support the notion that R1T3 is a specific transcript expressed in oxidative and differentiated muscle fibers.
In summary, the work reported here describes a novel skeletal muscle–specific AdipoR1 transcript that is significantly increased during myoblast-myotube differentiation and fetal development and demonstrates an important role for posttranscriptional mechanisms in the regulation of AdipoR1 biosynthesis in skeletal muscle. Further studies will be required to sort out the cellular factors involved in the regulation of R1T3 mRNA expression and AdipoR1 transcript translation and to understand their potential influence on AdipoR1 protein expression and adiponectin biological action in muscle.
ACKNOWLEDGMENTS
This work was supported by research grants from the Israeli Association for the Study of Diabetes (to R.H. and H.K.), the D-Cure Foundation for Diabetes Care in Israel (to A.K. and H.K.), and the Deutsche Forschungsgemeinschaft (to the clinical research group “Atherobesity” KFO 152) (project BL 833/1-1 [to M.B.]).
No potential conflicts of interest relevant to this article were reported.
R.A. and R.H. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. A.T. contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. R.G. and E.Y. researched data. A.C.-D. and A.R. researched data and reviewed and edited the manuscript. A.K. contributed to discussion. M.B. researched data, contributed to discussion, and reviewed and edited the manuscript. H.K. contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript.
Parts of this study were presented in abstract form at Keystone Symposia: Type 2 Diabetes, Insulin Resistance and Metabolic Dysfunction, Keystone, Colorado, 12–17 January 2011.
The authors thank Angela Chetrit (Sheba Medical Center) and Dr. Rachel Levy-Drummer (Bar Ilan University) for their assistance in the statistical analyses. The authors thank Dr. Michael Walker and Keren Bahar (Weizmann Institute of Science), Dr. Orna Elroy-Stein (Tel-Aviv University), Dr. Assaf Rudich (Ben Gurion University), Dr. Shlomo Sasson, and Dr. Arie Gruzman (Hebrew University in Jerusalem) for helpful discussions.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db09-0532/-/DC1.
REFERENCES
- 1.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 1995;270:26746–26749 [DOI] [PubMed] [Google Scholar]
- 2.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 2006;116:1784–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 2000;20:1595–1599 [DOI] [PubMed] [Google Scholar]
- 4.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 2001;86:1930–1935 [DOI] [PubMed] [Google Scholar]
- 5.Maeda N, Takahashi M, Funahashi T, et al. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 2001;50:2094–2099 [DOI] [PubMed] [Google Scholar]
- 6.Combs TP, Wagner JA, Berger J, et al. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: a potential mechanism of insulin sensitization. Endocrinology 2002;143:998–1007 [DOI] [PubMed] [Google Scholar]
- 7.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 2002;8:1288–1295 [DOI] [PubMed] [Google Scholar]
- 8.Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003;423:762–769 [DOI] [PubMed] [Google Scholar]
- 9.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 2001;7:947–953 [DOI] [PubMed] [Google Scholar]
- 10.Inukai K, Nakashima Y, Watanabe M, et al. Regulation of adiponectin receptor gene expression in diabetic mice. Am J Physiol Endocrinol Metab 2005;288:E876–E882 [DOI] [PubMed] [Google Scholar]
- 11.Tsuchida A, Yamauchi T, Ito Y, et al. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem 2004;279:30817–30822 [DOI] [PubMed] [Google Scholar]
- 12.Civitarese AE, Jenkinson CP, Richardson D, et al. Adiponectin receptors gene expression and insulin sensitivity in non-diabetic Mexican Americans with or without a family history of type 2 diabetes. Diabetologia 2004;47:816–820 [DOI] [PubMed] [Google Scholar]
- 13.Lin HV, Kim JY, Pocai A, et al. Adiponectin resistance exacerbates insulin resistance in insulin receptor transgenic/knockout mice. Diabetes 2007;56:1969–1976 [DOI] [PubMed] [Google Scholar]
- 14.Chen MB, McAinch AJ, Macaulay SL, et al. Impaired activation of AMP-kinase and fatty acid oxidation by globular adiponectin in cultured human skeletal muscle of obese type 2 diabetics. J Clin Endocrinol Metab 2005;90:3665–3672 [DOI] [PubMed] [Google Scholar]
- 15.Bruce CR, Mertz VA, Heigenhauser GJ, Dyck DJ. The stimulatory effect of globular adiponectin on insulin-stimulated glucose uptake and fatty acid oxidation is impaired in skeletal muscle from obese subjects. Diabetes 2005;54:3154–3160 [DOI] [PubMed] [Google Scholar]
- 16.Barnea M, Shamay A, Stark AH, Madar Z. A high-fat diet has a tissue-specific effect on adiponectin and related enzyme expression. Obesity (Silver Spring) 2006;14:2145–2153 [DOI] [PubMed] [Google Scholar]
- 17.Vu V, Riddell MC, Sweeney G. Circulating adiponectin and adiponectin receptor expression in skeletal muscle: effects of exercise. Diabetes Metab Res Rev 2007;23:600–611 [DOI] [PubMed] [Google Scholar]
- 18.Blüher M, Bullen JW, Jr, Lee JH, et al. Circulating adiponectin and expression of adiponectin receptors in human skeletal muscle: associations with metabolic parameters and insulin resistance and regulation by physical training. J Clin Endocrinol Metab 2006;91:2310–2316 [DOI] [PubMed] [Google Scholar]
- 19.O’Leary VB, Jorett AE, Marchetti CM, et al. Enhanced adiponectin multimer ratio and skeletal muscle adiponectin receptor expression following exercise training and diet in older insulin-resistant adults. Am J Physiol Endocrinol Metab 2007;293:E421–E427 [DOI] [PubMed] [Google Scholar]
- 20.Blüher M, Williams CJ, Klöting N, et al. Gene expression of adiponectin receptors in human visceral and subcutaneous adipose tissue is related to insulin resistance and metabolic parameters and is altered in response to physical training. Diabetes Care 2007;30:3110–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan GD, Debard C, Funahashi T, et al. Changes in adiponectin receptor expression in muscle and adipose tissue of type 2 diabetic patients during rosiglitazone therapy. Diabetologia 2005;48:1585–1589 [DOI] [PubMed] [Google Scholar]
- 22.Coletta DK, Sriwijitkamol A, Wajcberg E, et al. Pioglitazone stimulates AMP-activated protein kinase signalling and increases the expression of genes involved in adiponectin signalling, mitochondrial function and fat oxidation in human skeletal muscle in vivo: a randomised trial. Diabetologia 2009;52:723–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAinch AJ, Steinberg GR, Mollica J, et al. Differential regulation of adiponectin receptor gene expression by adiponectin and leptin in myotubes derived from obese and diabetic individuals. Obesity (Silver Spring) 2006;14:1898–1904 [DOI] [PubMed] [Google Scholar]
- 24.Kuoppamaa H, Skrobuk P, Sihvo M, et al. Globular adiponectin stimulates glucose transport in type 2 diabetic muscle. Diabetes Metab Res Rev 2008;24:554–562 [DOI] [PubMed] [Google Scholar]
- 25.Jang C, Inder WJ, Obeyesekere VR, Alford FP. Adiponectin, skeletal muscle adiponectin receptor expression and insulin resistance following dexamethasone. Clin Endocrinol (Oxf) 2008;69:745–750 [DOI] [PubMed] [Google Scholar]
- 26.Modrek B, Resch A, Grasso C, Lee C. Genome-wide detection of alternative splicing in expressed sequences of human genes. Nucleic Acids Res 2001;29:2850–2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kan Z, Rouchka EC, Gish WR, States DJ. Gene structure prediction and alternative splicing analysis using genomically aligned ESTs. Genome Res 2001;11:889–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatterjee S, Pal JK. Role of 5′- and 3′-untranslated regions of mRNAs in human diseases. Biol Cell 2009;101:251–262 [DOI] [PubMed] [Google Scholar]
- 29.Xie H, Zhu WY, Wasserman A, Grebinskiy V, Olson A, Mintz L. Computational analysis of alternative splicing using EST tissue information. Genomics 2002;80:326–330 [DOI] [PubMed] [Google Scholar]
- 30.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem 2004;279:28670–28674 [DOI] [PubMed] [Google Scholar]
- 31.Kubota N, Yano W, Kubota T, et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 2007;6:55–68 [DOI] [PubMed] [Google Scholar]
- 32.Wang G, Guo X, Floros J. Differences in the translation efficiency and mRNA stability mediated by 5′-UTR splice variants of human SP-A1 and SP-A2 genes. Am J Physiol Lung Cell Mol Physiol 2005;289:L497–L508 [DOI] [PubMed] [Google Scholar]
- 33.Lundell K, Thulin P, Hamsten A, Ehrenborg E. Alternative splicing of human peroxisome proliferator-activated receptor delta (PPAR delta): effects on translation efficiency and trans-activation ability. BMC Mol Biol 2007;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calvo SE, Pagliarini DJ, Mootha VK. Upstream open reading frames cause widespread reduction of protein expression and are polymorphic among humans. Proc Natl Acad Sci USA 2009;106:7507–7512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris DR, Geballe AP. Upstream open reading frames as regulators of mRNA translation. Mol Cell Biol 2000;20:8635–8642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meijer HA, Thomas AA. Control of eukaryotic protein synthesis by upstream open reading frames in the 5′-untranslated region of an mRNA. Biochem J 2002;367:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harding HP, Novoa I, Zhang Y, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 2000;6:1099–1108 [DOI] [PubMed] [Google Scholar]
- 38.Gerlitz G, Jagus R, Elroy-Stein O. Phosphorylation of initiation factor-2 alpha is required for activation of internal translation initiation during cell differentiation. Eur J Biochem 2002;269:2810–2819 [DOI] [PubMed] [Google Scholar]
- 39.Noon LA, Bakmanidis A, Clark AJ, O’Shaughnessy PJ, King PJ. Identification of a novel melanocortin 2 receptor splice variant in murine adipocytes: implications for post-transcriptional control of expression during adipogenesis. J Mol Endocrinol 2006;37:415–420 [DOI] [PubMed] [Google Scholar]
- 40.Raveh-Amit H, Maissel A, Poller J, et al. Translational control of protein kinase Ceta by two upstream open reading frames. Mol Cell Biol 2009;29:6140–6148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iwabu M, Yamauchi T, Okada-Iwabu M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature 2010;464:1313–1319 [DOI] [PubMed] [Google Scholar]
- 42.Huang H, Iida KT, Sone H, Yokoo T, Yamada N, Ajisaka R. The effect of exercise training on adiponectin receptor expression in KKAy obese/diabetic mice. J Endocrinol 2006;189:643–653 [DOI] [PubMed] [Google Scholar]
- 43.Bonnard C, Durand A, Vidal H, Rieusset J. Changes in adiponectin, its receptors and AMPK activity in tissues of diet-induced diabetic mice. Diabetes Metab 2008;34:52–61 [DOI] [PubMed] [Google Scholar]
- 44.Chang SP, Chen YH, Chang WC, Liu IM, Cheng JT. Increase of adiponectin receptor gene expression by physical exercise in soleus muscle of obese Zucker rats. Eur J Appl Physiol 2006;97:189–195 [DOI] [PubMed] [Google Scholar]
- 45.Sun X, He J, Mao C, et al. Negative regulation of adiponectin receptor 1 promoter by insulin via a repressive nuclear inhibitory protein element. FEBS Lett 2008;582:3401–3407 [DOI] [PubMed] [Google Scholar]
- 46.Vissing K, Andersen JL, Harridge SD, et al. Gene expression of myogenic factors and phenotype-specific markers in electrically stimulated muscle of paraplegics. J Appl Physiol 2005;99:164–172 [DOI] [PubMed] [Google Scholar]
- 47.Sell H, Eckardt K, Taube A, et al. Skeletal muscle insulin resistance induced by adipocyte-conditioned medium: underlying mechanisms and reversibility. Am J Physiol Endocrinol Metab 2008;294:E1070–E1077 [DOI] [PubMed] [Google Scholar]
- 48.Oberbach A, Bossenz Y, Lehmann S, et al. Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care 2006;29:895–900 [DOI] [PubMed] [Google Scholar]
- 49.Coen PM, Dubé JJ, Amati F, et al. Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes 2010;59:80–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colling-Saltin AS. Enzyme histochemistry on skeletal muscle of the human foetus. J Neurol Sci 1978;39:169–185 [DOI] [PubMed] [Google Scholar]