Abstract
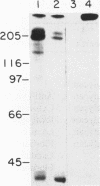
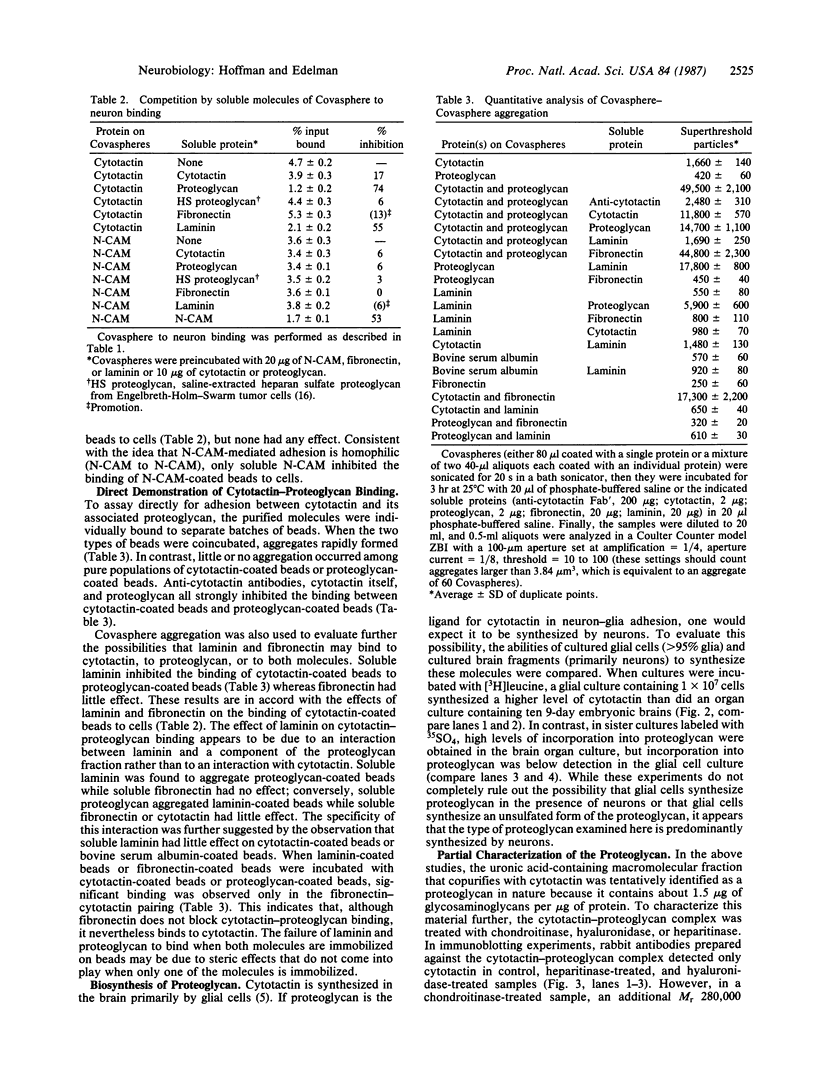
Cytotactin is an extracellular matrix protein that is involved in neuron-glia adhesion and is found in both neural and nonneural sites. It is synthesized by glia but not by neurons. In this study, we have examined the binding of cytotactin to a variety of extracellular matrix components using uniform microscopic beads (Covaspheres) that could be labeled and then linked to purified molecules. Cytotactin-coated beads bound well to neurons, and this binding was strongly inhibited by anti-cytotactin antibodies but not by anti-neural cell adhesion molecule (anti-N-CAM) antibodies. In contrast, the binding of N-CAM-coated beads to neurons was inhibited by anti-N-CAM antibodies and not by anti-cytotactin antibodies. To identify a neuronal ligand for cytotactin, we tested several molecules for their ability to block the binding of cytotactin-coated beads to cells. A proteoglycan-containing fraction that copurified with cytotactin from brain extracts strongly inhibited binding, whereas neither a heparan sulfate proteoglycan from Engelbreth-Holm-Swarm tumor cells nor soluble cytotactin itself had a significant inhibitory effect. The neural proteoglycan also inhibited the binding of cytotactin-coated beads to fibroblasts. Digestion with chondroitinase, heparitinase, and hyaluronidase as well as immunological analyses suggested that the predominant species in the active fraction was a chondroitin sulfate proteoglycan with a Mr280,000 core protein bearing HNK-1 antigenic determinants and also indicated that hyaluronic acid was present in this fraction. In experiments on in vitro synthesis, it was found that the proteoglycan was synthesized in culture by embryonic chicken brain tissue but not by embryonic chicken glial cells. A series of binding experiments was performed on appropriately derivatized beads to confirm that the proteoglycan is a ligand for cytotactin and to check for the possibility that other extracellular matrix proteins might interact with one or the other member of this binding couple. Proteoglycan-coated beads and cytotactin-coated beads coaggregated readily. The aggregation was inhibitable by anti-cytotactin antibodies, soluble cytotactin, or soluble proteoglycan. Addition of laminin inhibited the binding of cytotactin-coated beads to proteoglycan-coated beads or to cells; this is consistent with data indicating that laminin interacts with a component of the proteoglycan-containing fraction. In contrast, fibronectin bound to cytotactin, but it did not bind to proteoglycan or interfere with the binding of cytotactin to proteoglycan. The results of this study are in accord with the idea that the functions of extracellular matrix components during neural and nonneural development may be modulated both by competition for shared cell surface receptors and by a network of molecular interactions among the matrix components themselves.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J. P., Ray D. A., Steck T. L. Electrophoretic determinations of hyaluronate produced by cells in culture. Biochim Biophys Acta. 1972 Mar 30;264(1):73–84. doi: 10.1016/0304-4165(72)90118-3. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Brackenbury R., Rutishauser U., Edelman G. M. Distinct calcium-independent and calcium-dependent adhesion systems of chicken embryo cells. Proc Natl Acad Sci U S A. 1981 Jan;78(1):387–391. doi: 10.1073/pnas.78.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet M., Fambrough D. M. Chick myotendinous antigen. I. A monoclonal antibody as a marker for tendon and muscle morphogenesis. J Cell Biol. 1984 Jun;98(6):1926–1936. doi: 10.1083/jcb.98.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou K. H., Ilyas A. A., Evans J. E., Quarles R. H., Jungalwala F. B. Structure of a glycolipid reacting with monoclonal IgM in neuropathy and with HNK-1. Biochem Biophys Res Commun. 1985 Apr 16;128(1):383–388. doi: 10.1016/0006-291x(85)91690-0. [DOI] [PubMed] [Google Scholar]
- Crossin K. L., Hoffman S., Grumet M., Thiery J. P., Edelman G. M. Site-restricted expression of cytotactin during development of the chicken embryo. J Cell Biol. 1986 May;102(5):1917–1930. doi: 10.1083/jcb.102.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham B. A., Hoffman S., Rutishauser U., Hemperly J. J., Edelman G. M. Molecular topography of the neural cell adhesion molecule N-CAM: surface orientation and location of sialic acid-rich and binding regions. Proc Natl Acad Sci U S A. 1983 May;80(10):3116–3120. doi: 10.1073/pnas.80.10.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules in the regulation of animal form and tissue pattern. Annu Rev Cell Biol. 1986;2:81–116. doi: 10.1146/annurev.cb.02.110186.000501. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules. Science. 1983 Feb 4;219(4584):450–457. doi: 10.1126/science.6823544. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., Inglesias J. L. A six-armed oligomer isolated from cell surface fibronectin preparations. Nature. 1984 Sep 20;311(5983):267–269. doi: 10.1038/311267a0. [DOI] [PubMed] [Google Scholar]
- Grumet M., Edelman G. M. Heterotypic binding between neuronal membrane vesicles and glial cells is mediated by a specific cell adhesion molecule. J Cell Biol. 1984 May;98(5):1746–1756. doi: 10.1083/jcb.98.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet M., Hoffman S., Crossin K. L., Edelman G. M. Cytotactin, an extracellular matrix protein of neural and non-neural tissues that mediates glia-neuron interaction. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8075–8079. doi: 10.1073/pnas.82.23.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet M., Hoffman S., Edelman G. M. Two antigenically related neuronal cell adhesion molecules of different specificities mediate neuron-neuron and neuron-glia adhesion. Proc Natl Acad Sci U S A. 1984 Jan;81(1):267–271. doi: 10.1073/pnas.81.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C. Interaction of cartilage proteoglycans with hyaluronic acid. J Supramol Struct. 1977;7(1):101–120. doi: 10.1002/jss.400070110. [DOI] [PubMed] [Google Scholar]
- Hassell J. R., Leyshon W. C., Ledbetter S. R., Tyree B., Suzuki S., Kato M., Kimata K., Kleinman H. K. Isolation of two forms of basement membrane proteoglycans. J Biol Chem. 1985 Jul 5;260(13):8098–8105. [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Greggs R., Decker C., Buck C. The cell substrate attachment (CSAT) antigen has properties of a receptor for laminin and fibronectin. J Cell Biol. 1985 Dec;101(6):2134–2144. doi: 10.1083/jcb.101.6.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse J., Keilhauer G., Faissner A., Timpl R., Schachner M. The J1 glycoprotein--a novel nervous system cell adhesion molecule of the L2/HNK-1 family. Nature. 1985 Jul 11;316(6024):146–148. doi: 10.1038/316146a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liesi P. Laminin-immunoreactive glia distinguish regenerative adult CNS systems from non-regenerative ones. EMBO J. 1985 Oct;4(10):2505–2511. doi: 10.1002/j.1460-2075.1985.tb03963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Wewer U. M. Biochemical interactions of tumor cells with the basement membrane. Annu Rev Biochem. 1986;55:1037–1057. doi: 10.1146/annurev.bi.55.070186.005133. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Timpl R., Fujiwara S., Dziadek M., Aumailley M., Weber S., Engel J. Laminin, proteoglycan, nidogen and collagen IV: structural models and molecular interactions. Ciba Found Symp. 1984;108:25–43. doi: 10.1002/9780470720899.ch3. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]